Abstract
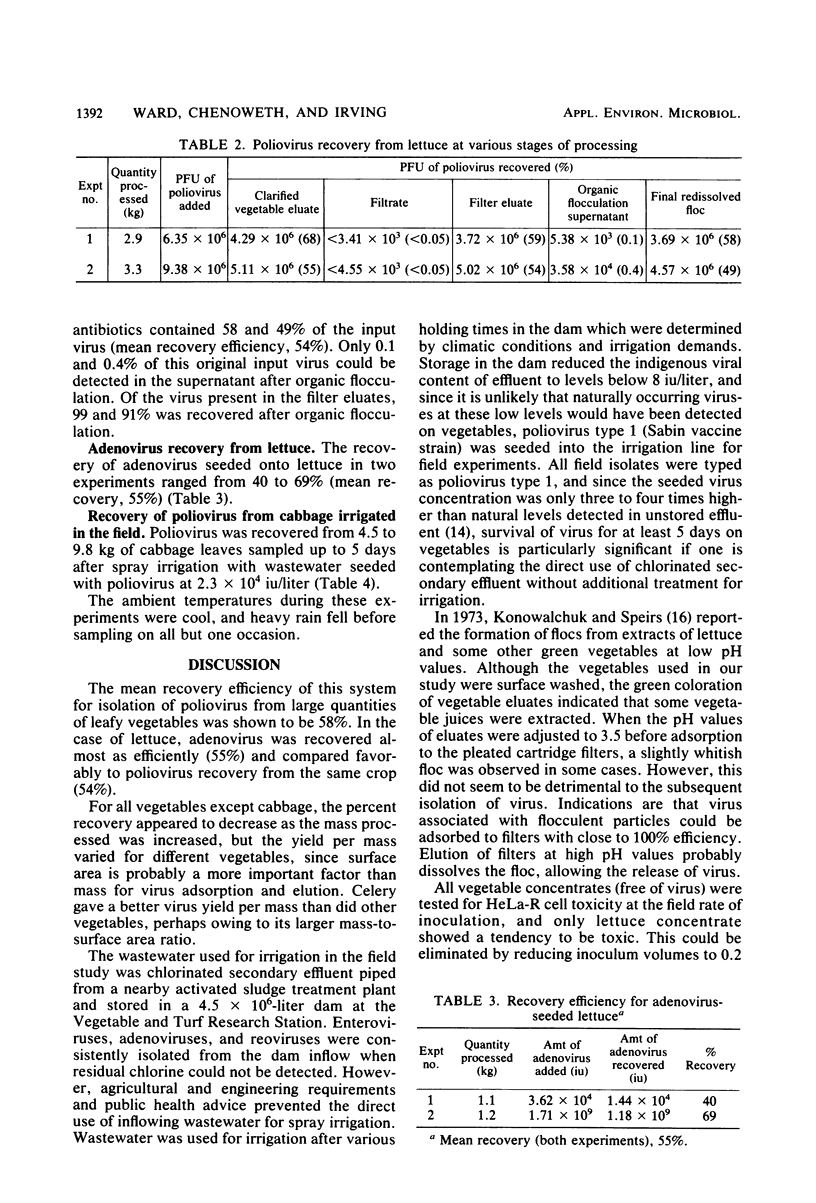
The efficiency of a system developed for the recovery of viruses contaminating large quantities of vegetables was investigated in the laboratory and tested in the field. Viruses seeded onto a number of leafy vegetables in the laboratory were eluted with a phosphate-buffered saline solution (pH 9.0). The eluate was clarified by glass wool filtration, and any viruses present were concentrated by adsorption to a Filterite pleated cartridge filter, eluted with 3% beef extract (pH 9.0), and further concentrated by organic flocculation. At least 24 liters of vegetable eluate could be concentrated to 70 to 80 ml, equivalent to a greater than 99.5% reduction in volume. With this system, poliovirus was recovered with a mean efficiency of 58% for all vegetables tested. Adenovirus was recovered from lettuce with a slightly lower mean efficiency (55%). Poliovirus was recovered from large quantities of cabbage for up to 5 days in the field after spray irrigation of relatively low levels of virus, even when heavy rain fell before sampling.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dahling D. R., Berg G., Berman D. BGM, a continuous cell line more sensitive than primary rhesus and African green kidney cells for the recovery of viruses from water. Health Lab Sci. 1974 Oct;11(4):275–282. [PubMed] [Google Scholar]
- Ellis A. W., McKinnon G. T., Lewis F. A., Gust I. D. Adenovirus type 4 in Melbourne, 1969-1971. Med J Aust. 1974 Feb 16;1(7):209–211. doi: 10.5694/j.1326-5377.1974.tb93113.x. [DOI] [PubMed] [Google Scholar]
- Farrah S. R., Gerba C. P., Wallis C., Melnick J. L. Concentration of viruses from large volumes of tap water using pleated membrane filters. Appl Environ Microbiol. 1976 Feb;31(2):221–226. doi: 10.1128/aem.31.2.221-226.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feachem R., Garelick H., Slade J. Enteroviruses in the environment. Trop Dis Bull. 1981 Mar;78(3):185–230. [PubMed] [Google Scholar]
- Gwaltney J. M., Jr, Calhoun A. M. Viral aggregation resulting in the failure to correctly identify an unknown rhinovirus. Appl Microbiol. 1970 Sep;20(3):390–392. doi: 10.1128/am.20.3.390-392.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J. E., Cliver D. O. Methods for detecting food-borne enteroviruses. Appl Microbiol. 1968 Oct;16(10):1564–1569. doi: 10.1128/am.16.10.1564-1569.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. F., Jr, Jakubowski W., Akin E. W., Clarke N. A. Detection of virus in water: sensitivity of the tentative standard method for drinking water. Appl Environ Microbiol. 1976 Feb;31(2):254–261. doi: 10.1128/aem.31.2.254-261.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving L. G., Smith F. A. One-year survey of enteroviruses, adenoviruses, and reoviruses isolated from effluent at an activated-sludge purification plant. Appl Environ Microbiol. 1981 Jan;41(1):51–59. doi: 10.1128/aem.41.1.51-59.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenelson E., Fattal B., Hostovesky T. Organic flocculation: an efficient second-step concentration method for the detection of viruses in tap water. Appl Environ Microbiol. 1976 Oct;32(4):638–639. doi: 10.1128/aem.32.4.638-639.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konowalchuk J., Speirs J. I. Enterovirus recovery with vegetable floc. Appl Microbiol. 1973 Oct;26(4):505–507. doi: 10.1128/am.26.4.505-507.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostenbader K. D., Jr, Cliver D. O. Filtration methods for recovering enteroviruses from foods. Appl Microbiol. 1973 Aug;26(2):149–154. doi: 10.1128/am.26.2.149-154.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis F. A., Kennett M. L. Comparison of rhinovirus-sensitive HeLa cells and human embryo fibroblasts for isolation of rhinoviruses from patients with respiratory disease. J Clin Microbiol. 1976 May;3(5):528–532. doi: 10.1128/jcm.3.5.528-532.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURPHY W. H., Jr, SYVERTON J. T. Absorption and translocation of mammalian viruses by plants. II. Recovery and distribution of viruses in plants. Virology. 1958 Dec;6(3):623–636. doi: 10.1016/0042-6822(58)90111-9. [DOI] [PubMed] [Google Scholar]
- Sadovski A. Y., Fattal B., Goldberg D., Katzenelson E., Shuval H. I. High levels of microbial contamination of vegetables irrigated with wastewater by the drip method. Appl Environ Microbiol. 1978 Dec;36(6):824–830. doi: 10.1128/aem.36.6.824-830.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub S. A., Sagik B. P. Association of enteroviruses with natural and artificially introduced colloidal solids in water and infectivity of solids-associated virions. Appl Microbiol. 1975 Aug;30(2):212–222. doi: 10.1128/am.30.2.212-222.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney J. T., Sullivan R., Larkin E. P., Peeler J. T. Comparison of methods for the recovery of virus inoculated into ground beef. Appl Microbiol. 1973 Oct;26(4):497–501. doi: 10.1128/am.26.4.497-501.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney J. T., Sullivan R., Larkin E. P. Persistence of poliovirus 1 in soil and on vegetables grown in soil previously flooded with inoculated sewage sludge or effluent. Appl Environ Microbiol. 1977 Jan;33(1):109–113. doi: 10.1128/aem.33.1.109-113.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westaway E. G., Reedman B. M. Proteins of the group B arbovirus Kunjin. J Virol. 1969 Nov;4(5):688–693. doi: 10.1128/jvi.4.5.688-693.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



