Abstract
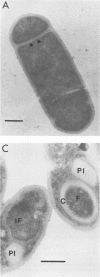
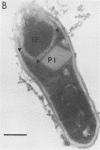
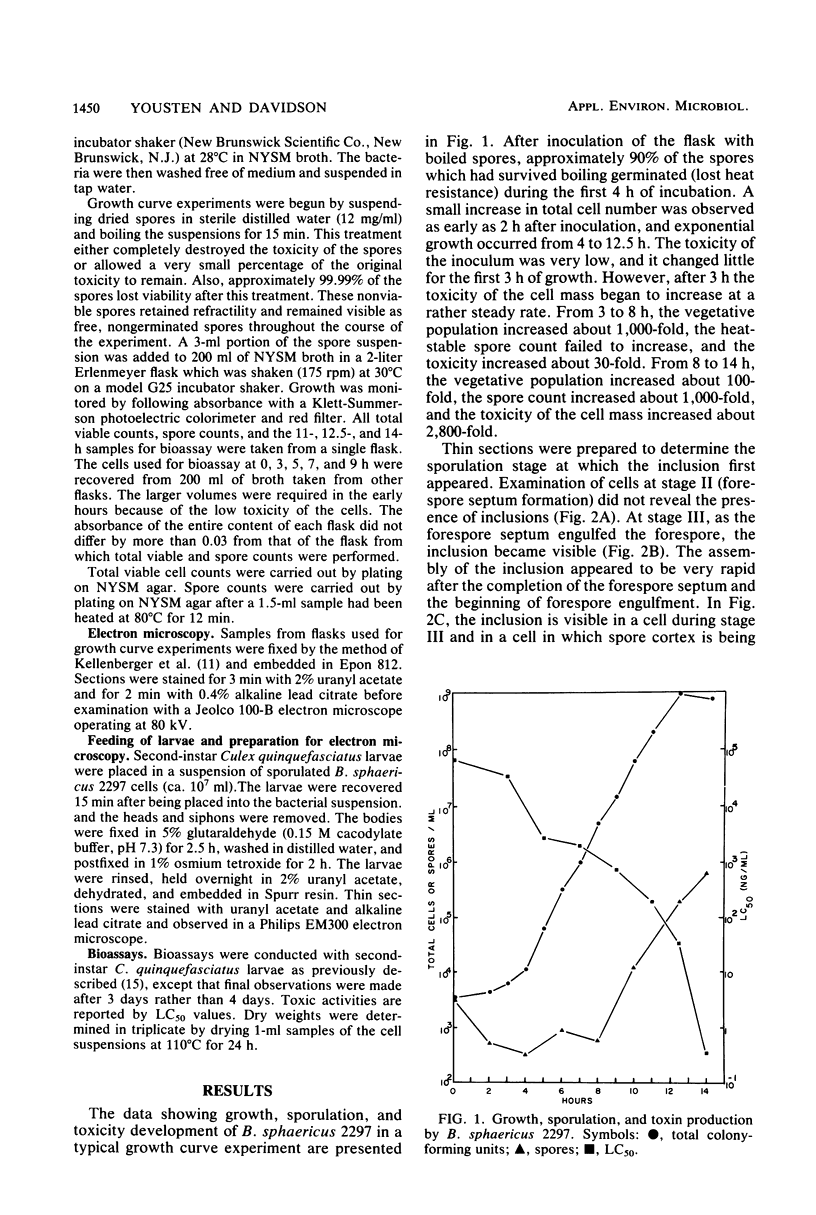
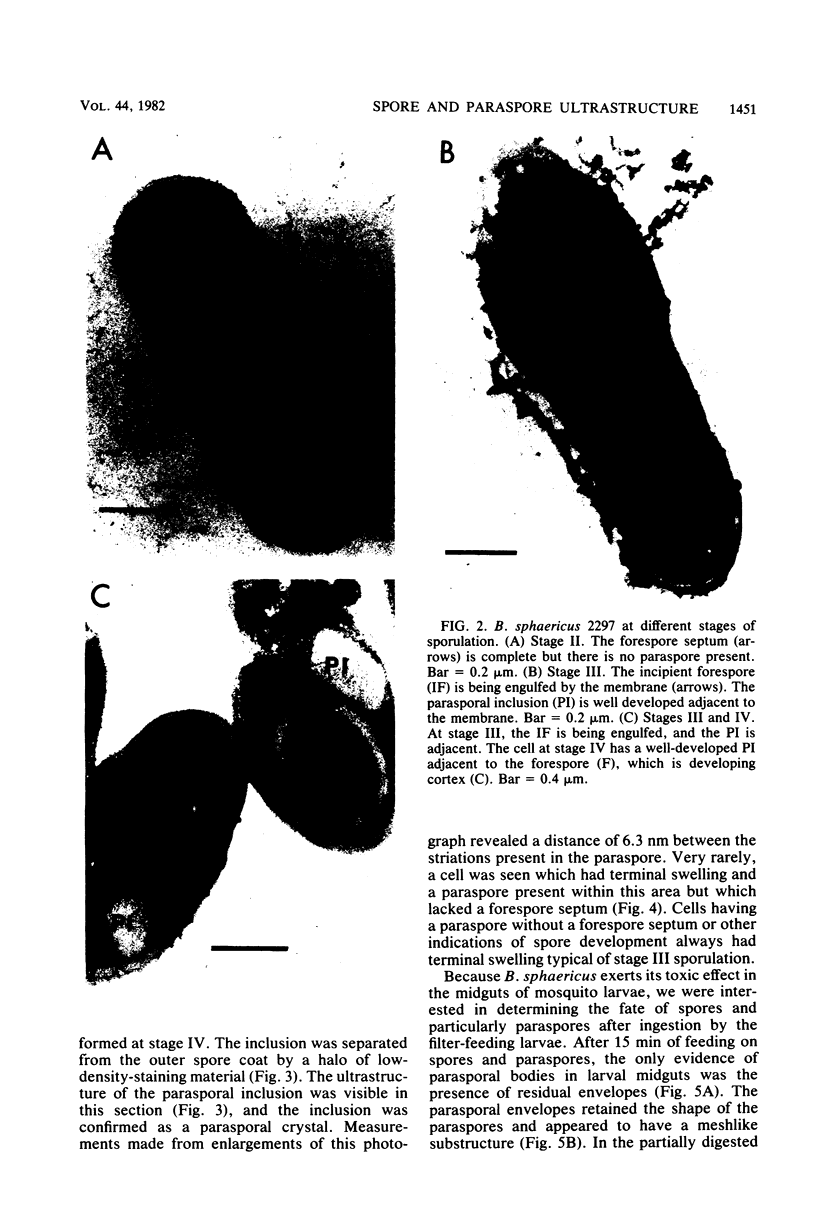
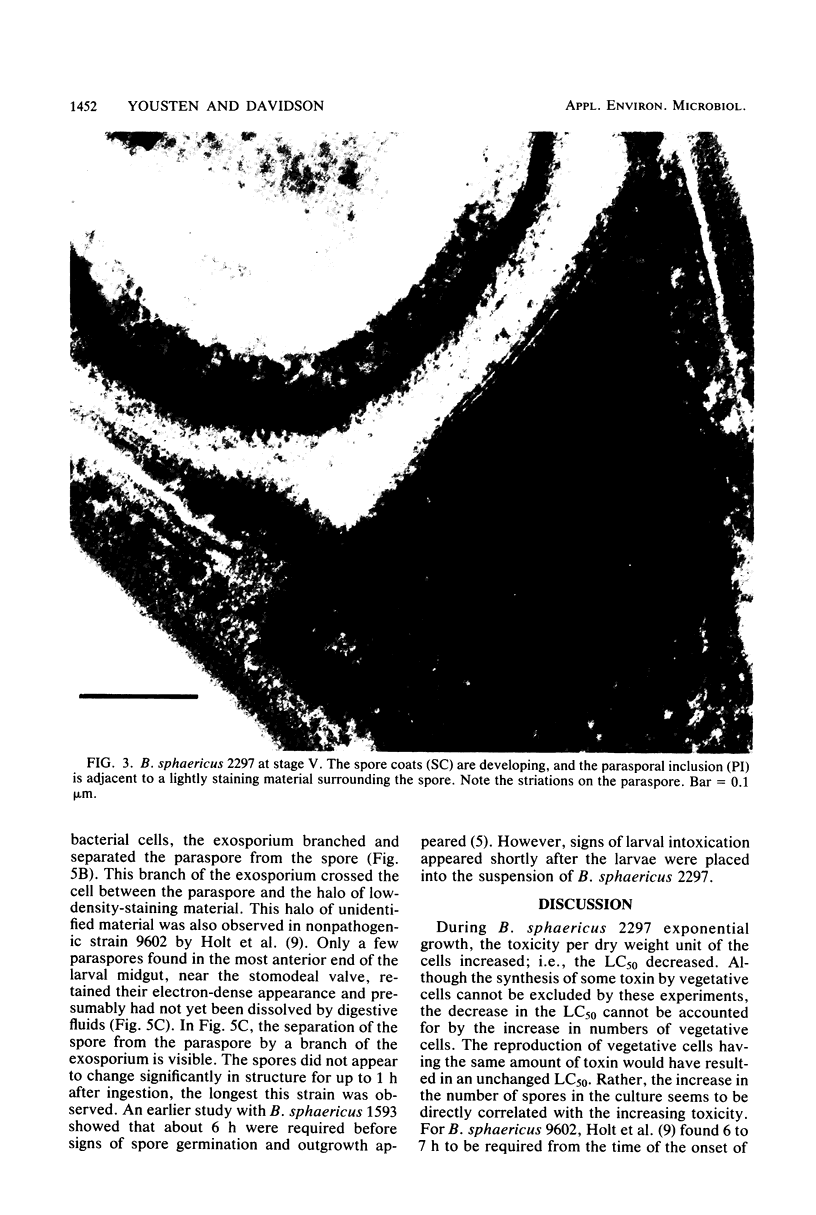
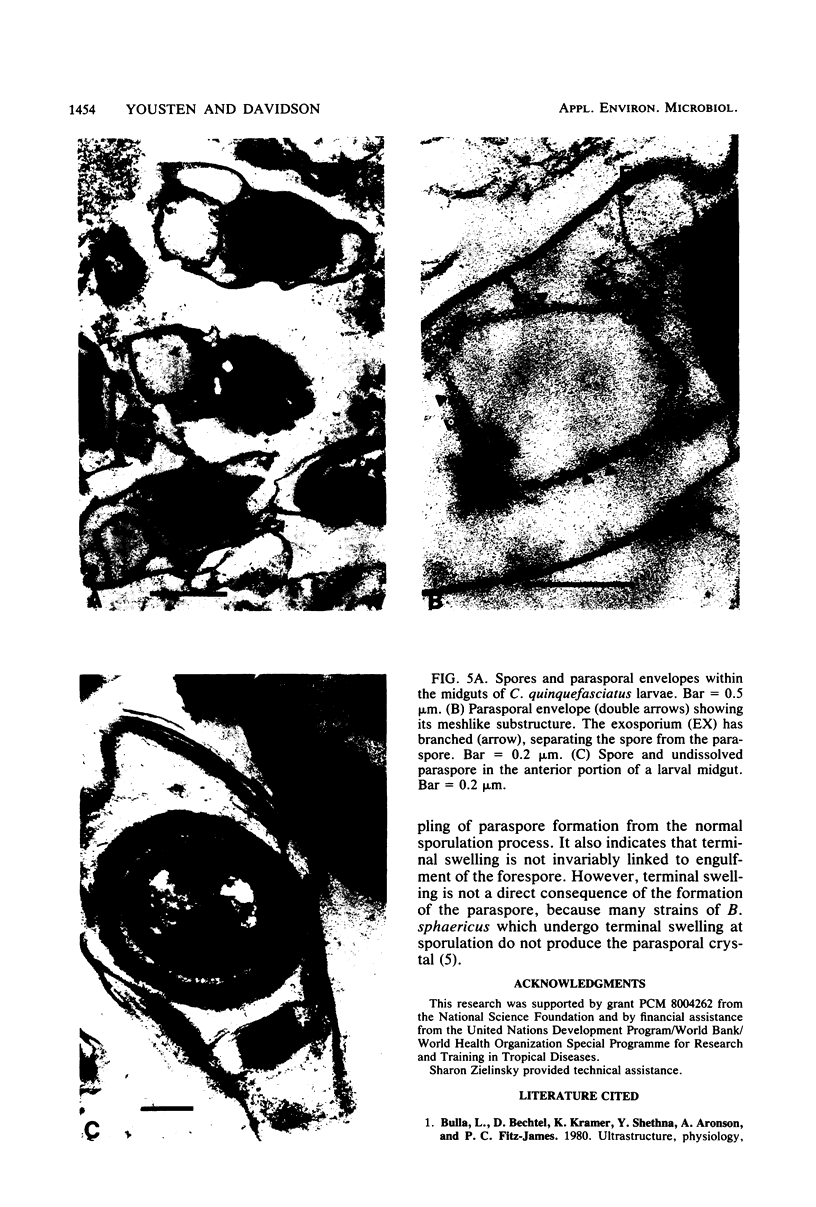
Bacillus sphaericus 2297, growing from a boiled, relatively nontoxic spore inoculum, increased about 30-fold in toxicity for mosquito larvae during early exponential growth but showed an approximately 1,000-fold toxicity increase during the late-exponential phase, as spores began to appear in the culture. The development of spores in the bacterial cells was accompanied by the formation of parasporal crystals. These parasporal crystals appeared during stage III as the forespore septum engulfed the incipient forespore. The paraspores were separated from the forespores by a branch of the exosporium across the cell. Measurements of the parasporal substructure revealed a 6.3-nm distance between the striations. When spores and paraspores were fed to mosquito larvae and the larvae were fixed 15 min after feeding, it was found that the spores remained relatively unchanged but that the matrix of the paraspores was dissolved. After dissolution of the paraspore matrix, a meshlike envelope remained which retained the paraspore shape and which was often in contact with the cross-cell portion of the exosporium. The parasporal crystals may be a source of the mosquito larval toxin in this strain of B. sphaericus, but proof will require their isolation from other cellular components.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bulla L. A., Jr, Bechtel D. B., Kramer K. J., Shethna Y. I., Aronson A. I., Fitz-James P. C. Ultrastructure, physiology, and biochemistry of Bacillus thuringiensis. Crit Rev Microbiol. 1980;8(2):147–204. doi: 10.3109/10408418009081124. [DOI] [PubMed] [Google Scholar]
- Charles J. F., de Barjac H. Sporulation et cristallogenèse de Bacillus thuringiensis var. Israelensis en microscopie électronique. Ann Microbiol (Paris) 1982 May-Jun;133(3):425–442. [PubMed] [Google Scholar]
- Dadd R. H. Alkalinity within the midgut of mosquito larvae with alkaline-active digestive enzymes. J Insect Physiol. 1975 Nov;21(11):1847–1853. doi: 10.1016/0022-1910(75)90252-8. [DOI] [PubMed] [Google Scholar]
- Davidson E. W., Singer S., Briggs J. D. Pathogenesis of Bacillus sphaericus strain SSII-1 infections in Culex pipiens quinquefasciatus (equal to C. pipiens fatigans) larvae. J Invertebr Pathol. 1975 Mar;25(2):179–184. doi: 10.1016/0022-2011(75)90066-x. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Gauther J. J., Tipper D. J. Ultrastructural studies of sporulation in Bacillus sphaericus. J Bacteriol. 1975 Jun;122(3):1322–1338. doi: 10.1128/jb.122.3.1322-1338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers P., Yousten A. A., Davidson E. W. Comparative studies of the mosquito-larval toxin of Bacillus sphaericus SSII-1 and 1593. Can J Microbiol. 1979 Nov;25(11):1227–1231. doi: 10.1139/m79-193. [DOI] [PubMed] [Google Scholar]
- Myers P., Yousten A. A. Toxic activity of Bacillus sphaericus SSII-1 for mosquito larvae. Infect Immun. 1978 Mar;19(3):1047–1053. doi: 10.1128/iai.19.3.1047-1053.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]