Abstract
Lysyl oxidase (EC 1.4.3.13) oxidizes peptidyl lysine to peptidyl aldehyde residues within collagen and elastin, thus initiating formation of the covalent cross-linkages that insolubilize these extracellular proteins. Recent findings raise the possibility that this enzyme may also function intracellularly. The present study provides evidence by immunocytochemical confocal microscopy, Western blot analysis, enzyme assays, and chemical analyses for lysyl oxidase reaction products that this enzyme is present and active within rat vascular smooth muscle cell nuclei. Confocal microscopy indicates its presence within nuclei of 3T3 fibroblasts, as well.
Lysyl oxidase (LO; EC 1.4.3.13) catalyzes the post-translational modification of elastin and collagen, by oxidizing selected lysine residues within these proteins to peptidyl α-aminoadipic-δ-semialdehyde. Subsequent spontaneous reactions of the peptidyl aldehydes yield covalent cross-linkages (1). LO is synthesized as a 46-kDa preproenzyme by fibrogenic cells. After signal peptide cleavage and N-glycosylation, the resulting 50-kDa N-glycosylated proenzyme is secreted (2) and proteolytically processed in the extracellular space to a mature enzyme of 31 ± 1 kDa (3).
Although initiation of the cross-linking of elastin and collagen is a critical function of LO, there is evidence that it may have additional biological roles. The mature enzyme isolated from bovine aorta is chemotactic for monocytes and lymphocytes in assays in vitro, with the chemotactic effect requiring a functional active site (4). Moreover, LO expression is negligibly low in several neoplastically transformed cell lines (5), including fibroblasts transformed with the Ha-ras oncogene (6, 7). It is of particular interest in this regard that a murine ras recision gene, the expression of which appears to suppress the tumorigenic effect of Ha-ras, encodes LO (6–9). The basis of this apparent effect of LO has yet to be understood. However, a recent report notes that transfection of revertants derived from ras-transformed NIH 3T3 cells with LO antisense but not LO sense constructs induced a change in the relatively “loose” chromatin packing state of the revertants to the tighter chromatin packing state of the original transformants (10). These results raise the possibility that LO may directly or indirectly exert effects on nuclear components. In the present study, we provide evidence that LO occurs and catalytically functions within the nuclei of fibrogenic cells.
EXPERIMENTAL PROCEDURES
Cell Culture.
Neonatal rat aorta smooth muscle cells (NRASMCs), explanted from 2- to 3-day-old rat pups as described (11, 12), were used in first passage at or just prior to confluency. Swiss 3T3 fibroblasts (American Type Culture Collection) were used at confluency after 3–4 days of culture in passages 9–12. Cells were cultured in DMEM containing 3.7 g of NaHCO3 per l, 100 units of penicillin per ml, 100 μg of streptomycin per ml, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate (GIBCO/BRL), and 10% fetal bovine serum (FBS) at 37°C in a 5% CO2/95% air atmosphere.
Isolation of Nuclei.
NRASMCs were cultured in 10% FBS/DMEM for 6 days. The conditioned medium was removed, the cell layers were rinsed with phosphate-buffered saline (PBS; 10 mM Na2HPO4/1.77 mM KH2PO4/0.14 M NaCl, pH 7.4), and the cells were suspended by incubation for 5 min in Hanks’ balanced salt solution containing 0.05% trypsin and 0.53 mM EDTA. After addition of an equal volume of 10% FBS/DMEM, the cells were sedimented at 500 × g, and the cell pellet was resuspended and washed twice in PBS. Cells were lysed by incubation in buffered solutions containing either the cationic detergent cetylpyridinium chloride (CPC) or the neutral detergent Nonidet P-40, depending upon the specific analyses to be performed. Biochemical analyses of nuclei isolated with the Nonidet P-40 buffer revealed the presence of cytosolic contaminants consistent with previous descriptions (13). Nuclei prepared with the CPC buffer were negative for these markers, consistent with the earlier description of the use of this cationic detergent for isolation of nuclei apparently free of cytosolic components (14). Lysis with the CPC buffer was performed by suspension of the cell pellets in 0.2% CPC/0.25 M sucrose/1 mM MgCl2/10 mM Tris⋅HCl, pH 7.4, followed by incubation of the suspension for 10 min at room temperature with gentle oscillation of the mixture. The nuclear pellet was sedimented at 600 × g for 5 min and washed twice by suspension in and sedimentation through 0.25 M sucrose/1 mM MgCl2, pH 7.4, yielding the final nuclear pellet. Lysis in Nonidet P-40 buffer was performed by suspension of the cell pellet in 0.32 M sucrose/3 mM CaCl2/2 mM magnesium acetate/0.1 mM EDTA/10 mM Tris⋅HCl, pH 8.0/1 mM DTT/0.5 mM phenylmethylsulfonyl fluoride/0.5% Nonidet P-40 (15). The nuclear pellet was sedimented, then treated with the lysis buffer once again, and sedimented, and the pellet was washed three times in this buffer lacking Nonidet P-40. Microscopic examination of nuclei isolated by either method established that cell lysis occurred and that the nuclei appeared to remain intact. SDS/PAGE of nuclear pellets was carried out by suspending pellets in 62.5 mM Tris⋅HCl, pH 6.8/10% glycerol/2% SDS/5% 2-mercaptoethanol, followed by heating at 100°C for 5 min, centrifugation, and electrophoresis of aliquots as described (16). Western blot analyses were performed as described (17). LO isozymes were resolved by urea gel electrophoresis (18) of purified 32-kDa bovine aorta LO and of extracts of nuclei in 8 M urea/16 mM potassium phosphate, pH 7.8. Resolved LO bands were identified by Western blot analysis with anti-LO. Nuclei were isolated by the Nonidet P-40 method for this purpose to prevent artifactual band migration that might otherwise occur in the presence of the cationic detergent in urea gel electrophoresis.
Antibodies.
Polyclonal anti-LO, previously shown to be specific for LO forms immunoprecipitated from vascular smooth muscle cells (2) and Swiss 3T3 fibroblasts (19), was raised in rabbits against pure 32-kDa bovine aorta LO (2). The antiserum was affinity-purified against purified bovine aorta LO (20) immobilized on agarose, according to the instructions provided with the AminoLink immobilization kit (Pierce Chemicals). As confirmed in the present study, Western blotting of crude urea extracts of bovine aorta with this antibody visualizes only one prominent band at 32 kDa, the molecular weight of purified LO, indicative of its specificity for this catalyst. Monoclonal antibodies specific for α-smooth muscle actin or 58-kDa Golgi-associated protein (Sigma) and polyclonal anti-calreticulin (Affinity Bioreagents, Neshanic Station, NJ) were obtained from commercial sources.
Confocal Microscopy.
To avoid artifacts in the microscopic assessment of the intracellular distribution of LO, an immunocytochemical protocol was used that involved fixation of cells in situ prior to detergent extraction, as described (21). Briefly, cells were cultured on glass coverslips in 10% FBS/DMEM for 3 days followed by incubation in 0.3% FBS/DMEM for 72 h. The cultures were washed with PBS, fixed with 3.4% formaldehyde in 0.1 M Pipes, pH 6.9/1 mM Mg2SO4/2 M glycerol/2 mM EGTA, washed, and incubated with buffered 0.3% Nonidet P-40 and RNase (1 mg/ml in PBS), washed, and then incubated with rabbit anti-LO (1:40 dilution). After removal of the primary antibody and washing with PBS, the cells were incubated with goat anti-rabbit IgG conjugated to fluorescein isothiocyanate. Cell preparations were also stained with the fluorescent DNA marker propidium iodide (1 μg/ml). Fluorescence of fluorescein isothiocyanate-coupled IgG and propidium iodide from cultured cell preparations was observed by confocal microscopy exciting at 488 nm or 536 nm, respectively, and employing a Leica Diaplan light microscope equipped with an argon ion laser light source. Scanning cell samples through a series of focal points by confocal microscopy confirmed that the confocal micrographs shown in this report represent intranuclear planes of focus. Replacing anti-LO with preimmune serum, preadsorbing anti-LO with pure 32-kDa bovine aorta LO, and omitting the primary antibody were conditions used as negative controls, each of which yielded unstained cellular or nuclear preparations.
Assays for LO Activity.
NRASMCs were cultured for 4 days in 10% FBS/DMEM and incubated for 30 min in lysine-deficient medium and then for 16 h in a fresh aliquot of this medium supplemented with dialyzed 10% FBS and l-[4,5-3H]lysine (50 μCi/ml; 108 mCi/mmol; 1 Ci = 37 GBq; NEN) in the presence or absence of 100 μM β-aminopropionitrile (BAPN), an irreversible inhibitor of LO (22). Pulse labeling in the presence of BAPN was performed to selectively and irreversibly inhibit LO that might be endogenous to the nucleus. Subsequent incubation under LO assay conditions in vitro of nuclei isolated from cells pulsed in the absence but not in the presence of BAPN should then permit the detection of endogenous nuclear LO acting upon endogenous nuclear substrates. Nuclei were isolated from these cells by the CPC method and then incubated intact or after lysis to assess for activity of functional LO–substrate complexes by determining the LO-dependent release of tritium as the index of LO activity (23). Nuclei were lysed by gentle suspension in 50 mM sodium borate/0.15 M NaCl, pH 8.2 (final volume, 800 μl), at 37°C. Intact nuclei were suspended in PBS. Aliquots containing 2 × 106 cpm of tritium of the intact nuclei (100 μg of protein), nuclear lysate (100 μg of protein), or the cytosol fraction (1 mg of protein) were diluted to 800 μl with 50 mM sodium borate/0.15 M NaCl, pH 8.0, and incubated at 37°C for 4 h in the presence or absence of 50 μM BAPN. Enzyme activities are expressed as BAPN-inhibitable cpm of tritium released per 1 × 106 cpm of cell fraction.
Analysis for Lysine-Derived Cross-Links.
Nuclei were isolated by the CPC method from first-passage NRASMCs that had been cultured initially for 5 days in 10% FBS/DMEM and then further incubated in fresh aliquots of this medium in the presence and absence of 10−4 M BAPN for 10 days. Nuclei were isolated as described, and the initial cell lysate was saved as the cytosol fraction. The isolated nuclei were incubated with DNase I and lysed by addition of 10 vol of 0.2 M sodium phosphate (pH 7.4), and the lysate was dialyzed against that phosphate buffer. Reduction of the dialyzed lysate with NaB3H4 and diphenylborinic acid (DPBA) analysis for borotritide-reduced cross-links were performed as described (24). In brief, groups of monofunctional amino acids and cross-linking amino acids were initially resolved by Bio-Gel P2 chromatography. The P2 fractions were then reacted with DPBA and the DPBA derivatives were resolved by HPLC (24). Authentic lysinonorleucine (LNL) was prepared by incubation of poly-(l-lysine) at 2 mg/ml with 0.01 mM pyrroloquinoline quinone in 0.1 M sodium borate (pH 10.35) at 37°C for 3 h (25). The oxidized polylysine was dialyzed against 1% acetic acid, lyophilized, and reduced with NaB3H4. Acid hydrolysis gave a single radioactive product that coeluted with LNL from bone collagen by DPBA cross-link analysis and ion-exchange HPLC analysis (25).
The results of each experiment described in this report were reproducibly obtained and are representative of the results of at least two and, in most cases, three experiments.
RESULTS
Immunocytochemistry of Cultured Fibrogenic Cells.
The distribution of LO was explored in cultured NRASMCs and 3T3 fibroblasts by using immunocytochemistry. As shown (Fig. 1A), confocal laser scanning microscopy reveals that the NRASMC nuclei are stained positively when the cells are treated with anti-LO followed by incubation with goat anti-rabbit IgG conjugated to fluorescein isothiocyanate, with punctate staining seen in the cytosol. An enlargement of the cell in the center of Fig. 1A is shown in Fig. 1B, revealing that distribution of the enzyme protein is not uniform throughout the nucleus because three prominent vacuoles are seen that appear to lack immunoreactive sites. 3T3 fibroblasts also exhibit intranuclear and prominent perinuclear staining (Fig. 1C). A representative negative control is shown for the NRASMCs in which anti-LO was replaced with preimmune serum (Fig. 1D). The intranuclear localization of LO was confirmed by its codistribution with DNA-bound propidium iodide within the intranuclear planes of focus seen in Fig. 1 A–C (data not shown). The cells shown in Fig. 1 were cultured an insufficiently long time to permit the accumulation of extracellular fibers of collagen and elastin and thus of the matrix to which extracellular LO normally adheres (1), accounting for the apparent deficiency of immunodetectable LO in the extracellular space.
Figure 1.
Detection of intracellular proteins reactive with anti-LO by laser scanning confocal microscopy. (A) NRASMCs at ×400 magnification. (B) NRASMCs at ×1000 magnification. (C) 3T3 fibroblasts at ×400 magnification. (D) NRASMCs, preimmune (control) serum, at ×400 magnification.
Western Blotting of Nuclear Extracts.
NRASMCs were cultured in 10% FBS/DMEM for 6 days, the nuclei were isolated and extracted, nuclear proteins were resolved by SDS/PAGE, and bands were identified by Western blot analysis with anti-LO. As shown in Fig. 2A, a prominent immunoreactive band of the nuclear extract was seen at 32 kDa (lane 3), migrating in the same position as the purified 32-kDa LO (lane 1). The band at 25–28 kDa in the nuclear extract (lane 3) may represent alternatively processed forms of the enzyme and/or breakdown products of the 32-kDa species, with precedent for the latter possibility previously noted (26). A band approximating 50 kDa, the size of the proenzyme, was seen in the cytosol (lane 4), whereas a band at 32 kDa was not detected. The single band at 32 kDa seen in the crude bovine aorta extract (lane 2) illustrates the specificity of this antibody for LO. Because the purity of the isolated nuclei is critical to the interpretation of these Western blots, the reaction of nuclear and cytosolic fractions of the NRASMCs with antibodies specific for proteins predominantly found in the cytosol was examined. As shown in Fig. 2B, the nuclei isolated by the CPC method did not contain actin, calreticulin, or a 58-kDa protein specific to Golgi (lanes 2, 4, and 6), whereas the cytosolic fractions of these cells were clearly positive for each of these markers (lanes 1, 3, and 5).
Figure 2.
(A) Detection of nuclear LO proteins by Western blot analysis. Lanes: 1, purified bovine aorta LO; 2, crude extract (16 mM potassium phosphate/6 M urea, pH 7.8) of bovine aorta; 3, extract of NRASMC nuclei; 4, NRASMC cytosol. (B) Western blots of cytosol fractions (lanes 1, 3, and 5) and nuclear extracts (lanes 2, 4, and 6) of NRASMCs probed with monoclonal antibodies to cytosol proteins. Probes used were as follows. Lanes: 1 and 2, anti-actin; 3 and 4, anti-calreticulin; 5 and 6, anti-Golgi-specific protein (58 kDa).
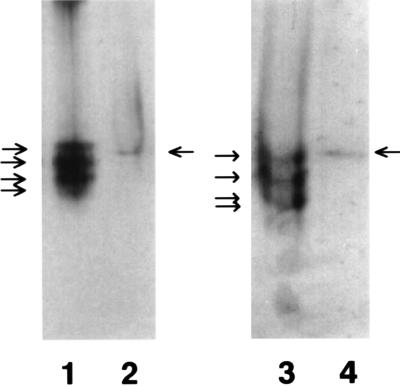
Isolation of mature LO from several connective tissues yields a protein migrating as an apparently single species in SDS/PAGE, with molecular weight values of these isolates from 28 to 32 kDa, depending upon the tissue of origin (1). Nevertheless, this purified protein resolves into three or four ionic variants, as indicated by anion-exchange chromatography (26) or urea gel electrophoresis in the absence of disulfide reductants and cationic or anionic detergents (18). Urea gel electrophoresis was performed to assess whether one or more such variants appear in the nucleus. As shown (Fig. 3, lane 1), purified bovine aorta LO resolves into three or four bands, as reported (18, 26). In contrast (lane 2), the 8 M urea extract of NRASMC nuclei contains only one band reactive with anti-LO migrating between the positions of the first (top) and second bands of the purified bovine enzyme. To test the possibility that the neutral detergent or other components in the nuclear extract may influence migration of individual variants, the bovine enzyme was mixed with the nuclear extract and again resolved by urea gel electrophoresis. As shown (lane 3), the multiple variants of the bovine enzyme again resolve with slightly different migration as a group. The nuclear extract again contains one visible immunoreactive band that now migrates similarly to the top band of the bovine enzyme.
Figure 3.
Urea gel electrophoresis-Western blot of purified 32-kDa bovine aorta and of extract of NRASMC nuclei probed with anti-LO. Lanes: 1, bovine aorta LO in 16 mM potassium phosphate (pH 7.8); 2, nuclei extracted into Nonidet P-40 buffer; 3, bovine aorta LO mixed with Nonidet P-40 extract of NRASMC nuclei before electrophoresis; 4, nuclei extracted into Nonidet P-40 buffer.
Functional Analyses with Isolated Nuclei.
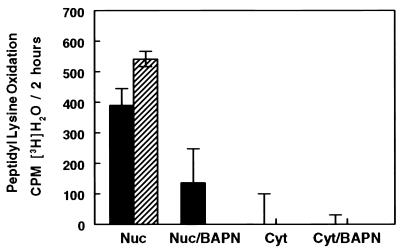
The possibility was addressed that LO activity is expressed within the nucleus. NRASMCs were pulse-labeled with l-[4,5-3H]lysine in the presence or absence of 100 μM BAPN in culture. Oxidation of peptidyl [4,5-3H]lysine by LO labilizes tritium from carbon 5 upon the production of the aldehyde, which is then isolated and measured as [3H]H2O (23). Intact or lysed nuclei isolated from cells labeled in this fashion were incubated in assay buffer at 37°C for 3 h in the presence and absence of 100 μM BAPN, assessing for endogenous productive LO–substrate complexes within the nuclei. As shown (Fig. 4), comparable and significant levels of lysine oxidation occur with both the intact and lysed nuclear preparations, whereas there was either significantly less or no LO-dependent tritium release from lysed or intact nuclei obtained from cells that had been pulse-labeled in the presence of BAPN. These results are consistent with the presence of catalytically functional LO and of substrates of LO within the nucleus. The greater release from nuclei obtained from cells labeled in the absence of BAPN is consistent with the irreversible inhibition of nuclear LO-like activity by BAPN during the culture period.
Figure 4.
Catalytic expression in vitro of endogenous enzyme–substrate complexes in NRASMC nuclei. Solid bars, extract of isolated nuclei; hatched bars, intact nuclei. Nuc, nuclei; Cyt, cytosol. Cells were incubated in culture in the absence (Nuc or Cyt) or presence (Nuc/BAPN or Cyt/BAPN) of 100 μM BAPN. Results are presented as the means (bar heights) ± SD (error bars) of triplicate assays.
Identification of LO-Derived Cross-Links in Nuclear Protein.
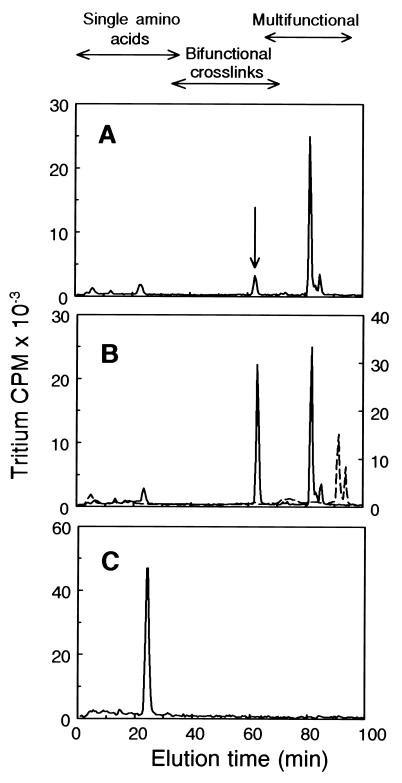
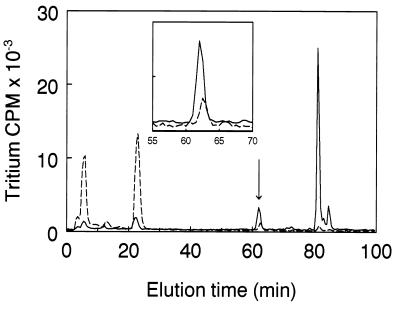
NRASMCs were incubated in culture for 10 days to allow the accumulation of possible intranuclear products of LO action, the nuclei and cytosol fractions were isolated, and total protein of each fraction was reduced with NaB3H4 and acid hydrolyzed. The higher molecular weight components of the hydrolyzate (eluting from Bio-Gel P2 at or earlier than the position of the bifunctional reducible collagen cross-links) were then subjected to DPBA cross-link analysis (24). Fig. 5A shows the DPBA analysis of NRASMC nuclear protein. A prominent peak appears at 63 min in the bifunctional cross-link region at the elution position of the derivative of LNL, the reduced Schiff base cross-link. Its identity was confirmed by mixing this sample with the DPBA derivative of synthetic LNL prior to HPLC analysis (Fig. 5B). Moreover, the underivatized radioactive peak coeluted with authentic LNL by chromatography (25) on an amino acid analysis ion-exchange column (data not shown). Notably, cross-links derived from hydroxylysine, which are always the major reducible cross-links in collagen (27), are absent from nuclear protein, whereas LNL, which is a minor component in collagen (27), is a significant reducible cross-link in nuclear protein. (Dihydroxylysinonorleucine and hydroxylysinonorleucine elute at 52 and 58 min, respectively, in this system.) An apparently novel multifunctional cross-link is also present in this sample, eluting at 82 min. Its elution position is earlier than those of reduced desmosine or isodesmosine standards, the tetrafunctional elastin cross-links, which elute at 91.5 and 94 min, respectively (Fig. 5B). In Fig. 5C, DPBA analysis of the reduced cytosol fraction of NRASMCs shows that the only reducible species present is a molecule eluting in the position of a monofunctional amino acid. Hydrolyzates of nuclear fractions isolated from BAPN-treated cells contained only 22% as much LNL per cell and 2% as much of the novel multifunctional cross-link (Fig. 6), consistent with the requirement for LO in their formation. Two minor peaks eluting at 6 min and 23 min are significantly increased in the presence of BAPN. The identities of these peaks are not known, although their elution properties indicate that they stem from monofunctional amino acid residues. The elution of these amino acids from the Bio-Gel P2 gel filtration column within the higher molecular weight cross-link fraction (data not shown) excludes the possibility that either of them represents the unsubstituted reduced lysine-derived aldehyde. These peaks may be glycosylated derivatives containing reducible linkages to individual amino acid residues. Notably, the radioactive peak from the cytosol was not lessened by BAPN treatment. Although its identity is also unknown, this result indicates that its formation is independent of LO action (data not shown).
Figure 5.
DPBA cross-link analysis of NaB3H4-reduced proteins of NRASMC nuclei and cytosol. (A) Nuclear protein. The arrow indicates the elution position of reduced LNL. (B) Solid line, the same sample mixed with the DPBA derivative of synthetic reduced LNL; dashed line, reduced desmosine and isodesmosine standards. (C) Cytosol.
Figure 6.
DPBA cross-link analysis of NaB3H4-reduced proteins of NRASMC nuclei isolated from cells cultured in the presence (dashed line) or absence (solid line) of BAPN. (Inset) Profile of fractions 55–70 presented at an expanded scale.
DISCUSSION
Extracellular forms of elastin and collagen are widely accepted as the principal natural substrates of LO. Nevertheless, the potential of this enzyme to oxidize a variety of proteins purified from various intracellular compartments has been demonstrated in vitro (28). The present study provides immunological, catalytic, and chemical evidence that this catalyst occurs and appears to function within the nuclei of fibrogenic cells. Thus, fixing the cells in situ prior to disruption of cell membranes by detergent for immunocytochemistry is expected to prevent contamination of nuclei by enzyme originating from nonnuclear compartments. Moreover, confocal microscopy, as used herein, permits the selection of fields of LO localized at intranuclear layers. The BAPN-inhibitable formation of the LNL cross-link in NRASMC nuclear protein also supports this conclusion whereas the absence of hydroxylysine-derived or desmosine cross-linkages in the nuclear protein preparation argues against contamination of the isolated nuclei by collagen or elastin. Further support for the unique intracellular distribution of LNL within the nuclear compartment comes from the observation that LNL was not detected in the cytosol. Similarly, the Western blot indicating an immunoreactive nuclear protein at 32 kDa in the nuclear extract and the expression of LO-like activity in intact nuclei supports the presence of the enzyme within the nuclear compartment. Importantly, Western blots indicated that the nuclei used for these analyses lacked demonstrable cytosol-derived contaminants.
The expression of BAPN-inhibitable catalytic activity and the presence of the 32-kDa immunoreactive band within the isolated nuclei argue strongly for the presence of the mature proteolytically processed catalyst. Although the Western blot did not reveal the presence of the 50-kDa N-glycosylated proenzyme or the nonglycosylated 46-kDa form of the proenzyme (2), the possibility that pro-LO species may enter the nucleus and be rapidly converted to the mature 32-kDa catalyst is not presently excluded. However, because LO is synthesized as a preproprotein (2, 19), it is expected to be directed by its signal peptide to enter the endoplasmic reticulum in preparation for secretion from the cell. Nevertheless, there are precedents for both extracellular and intranuclear distribution of products of preproproteins, including nucleoside triphosphatase associated with chromatin of pea nuclei (29) and fibroblast growth factor 3 (30). It should also be noted that various secreted proteins find their way into nuclei of apparently intact cells including, for example, granzyme (31) and lactoferrin (32), raising the possibility that extracellular enzyme may be taken up and incorporated into the nuclei.
Multiple ionic variants of mature LO isolated from connective tissues have long been known (26), although the origin and biological significance of these variants remains to be established. As shown in the present study, only one such variant was detectable in the NRASMC nuclei, corresponding most closely to the most slowly migrating species of the multiple variants of the 32-kDa bovine enzyme. Similarly, urea gel electrophoresis/Western blot analyses of urea extracts of the intact cell layer, containing the bulk of the mature catalyst accumulating in these cultures, also revealed the presence of this but not other LO bands (data not shown). Thus, the enzyme of the cultured NRASMCs does not display the heterogeneity of ionic species characteristic of enzyme isolated from animal connective tissues. It is consistent, therefore, that such multiple ionic species are not found in the nuclei of these cells. It is possible that the enzyme heterogeneity arises in tissues by alternative processing of pro-LO by proteases derived from the variety of cell types present at the tissue level. In addition to charge heterogeneity, there also appears to be more than one gene giving rise to LO-like proteins. Thus, the human LO gene occurs at chromosome 5q23, and a human LO-like gene occurs at 15q24–q25. The cDNA sequence of the LO-like gene predicts a prepro-LO species similar in size to the rat enzyme. Although its predicted signal peptide and propeptide sequences differ significantly from other pro-LO species, the protein sequence of the mature enzyme that is predicted to be derived from the LO-like precursor is highly homologous to that of the mature rat, chicken, and human LO enzymes (33) and thus should be reactive with the antibody raised against the mature rat enzyme used in these studies. An additional human gene has been reported that codes for an 87-kDa LO-related protein that exhibits 48% identity with the LO and LO-like proteins described above. Sequences consistent with those of members of the scavenger-receptor cysteine-rich family are also present. The sequence of the active site region of LO appears to be fully conserved in this protein, suggesting that it has catalytic potential, although products smaller than 87 kDa were not observed. Expression of the mRNA of this gene correlates with the ability of tumor cells to adhere to culture dish surfaces and is increased in cellular senescence (34). The nuclear LO found in the present study displays the immunoreactivity, molecular mass, and catalytic function and sensitivity to BAPN characteristic of extracellular LO conventionally associated with elastin and collagen cross-linking. Further studies are required to test its origin from the same gene as that catalyst.
Several nuclear proteins contain cationic sequence elements that are essential for their uptake into nuclei. Such signals include four basic residues (lysine and/or arginine) within a hexapeptide sequence, as well as bipartite signals containing two groups of cationic charge separated by 10–16 residues of various chemistry (35, 36). Although there are only 5 lysines in the entire prepro-LO molecule, 39 arginine residues occur within the 411 residues of rat prepro-LO (37), several of which appear to be components of potential nuclear localization signals. For example, the disposition of arginines in residues 62–81 of rat pro-LO, RRRDSATAPRADGNAAAQPR, is similar to the nuclear localization signal identified in N-myc, ERRRNHNILERQRRND (36). Moreover, the N-terminal region of the mature protein appearing after proteolytic processing of the precursor contains several vicinal arginines. Thus, both precursor and mature forms of LO appear to have the potential to facilitate nuclear entry.
The biological role of nuclear LO has yet to be defined, although the report of Mello et al. (10) indicates that it may have an impact upon the organization of chromatin. It is of interest that electrostatic interactions between positively charged amino acid side chains of nucleosomal proteins with oxyanions of DNA appear to influence DNA transcription (38). The reaction catalyzed by LO most immediately results in the loss of the ɛ-amino group and, thus, the positive charge of the substrate lysine residue. Thus, among other possible roles for nuclear LO, the oxidative deamination of peptidyl lysine within nuclear proteins might influence regulatory phenomena within nuclei by perturbation of nucleic acid–protein interactions.
Acknowledgments
W.L., K.N., T.S., L.G., K.M.T., and H.M.K. contributed equally to the development of this study. We thank Katherine Svoboda, Ph.D., for the confocal microscopy and Iih-Nan Chou, Ph.D., for helpful discussions and for the use of the Nikon fluorescence microscope employed in these studies. This research was supported by National Institutes of Health Grants HL-13262 and R37-AR-18880 (to H.M.K.).
ABBREVIATIONS
- BAPN
β-aminopropionitrile
- CPC
cetylpyridinium chloride
- DPBA
diphenylborinic acid
- FBS
fetal bovine serum
- LNL
lysinonorleucine
- LO
lysyl oxidase
- NRASMC
neonatal rat aorta smooth muscle cell
References
- 1.Kagan H M. In: Biology of the Extracellular Matrix. Mecham R P, editor. l. Orlando, FL: Academic; 1986. pp. 321–398. [Google Scholar]
- 2.Trackman P C, Bedell-Hogan D, Tang J, Kagan H M. J Biol Chem. 1992;267:8666–8671. [PubMed] [Google Scholar]
- 3.Panchenko M V, Stetler-Stevenson W G, Trubetskoy O V, Gacheru S N, Kagan H M. J Biol Chem. 1996;271:7113–7119. doi: 10.1074/jbc.271.12.7113. [DOI] [PubMed] [Google Scholar]
- 4.Lazarus H M, Cruikshank W W, Narasimhan N, Kagan H M, Center D M. Matrix Biol. 1995;14:727–731. doi: 10.1016/s0945-053x(05)80015-0. [DOI] [PubMed] [Google Scholar]
- 5.Kuivaniemi H, Korhonen R M, Vaheri A, Kivirikko K I. FEBS Lett. 1986;195:261–264. doi: 10.1016/0014-5793(86)80172-7. [DOI] [PubMed] [Google Scholar]
- 6.Kenyon K, Contente S, Trackman P C, Tang J, Kagan H M, Friedman R M. Science. 1991;253:802. doi: 10.1126/science.1678898. [DOI] [PubMed] [Google Scholar]
- 7.Contente S, Kenyon K, Rimoldi D, Friedman R M. Science. 1990;249:796–798. doi: 10.1126/science.1697103. [DOI] [PubMed] [Google Scholar]
- 8.Krzyzosiak W J, Shindo-Okada N, Teshima H, Nakajima K, Nishimura S. Proc Natl Acad Sci USA. 1992;89:4879–4883. doi: 10.1073/pnas.89.11.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajnal A, Klemenz R, Schafer R. Cancer Res. 1993;53:4670–4675. [PubMed] [Google Scholar]
- 10.Mello M L S, Contente S, Vidal B C, Planding W, Schenck U. Exp Cell Res. 1995;220:374–382. doi: 10.1006/excr.1995.1328. [DOI] [PubMed] [Google Scholar]
- 11.Oakes B W, Batty A C, Handley C J, Sandberg L B. Eur J Biochem. 1982;27:34–46. [PubMed] [Google Scholar]
- 12.Barone L M, Faris B, Chipman S, Toselli P, Oakes B W, Franzblau C. Biochim Biophys Acta. 1985;840:245–254. doi: 10.1016/0304-4165(85)90125-4. [DOI] [PubMed] [Google Scholar]
- 13.Staufenbiel M, Deppert W. Exp Cell Res. 1982;138:207–214. doi: 10.1016/0014-4827(82)90107-0. [DOI] [PubMed] [Google Scholar]
- 14.Nakaya K, Manabe H, Ushiwata A, Shibayama T, Nakamura Y. Canc Res. 1977;37:3701–3706. [PubMed] [Google Scholar]
- 15.Dyer R B, Herzog N K. BioTechniques. 1995;19:192–196. [PubMed] [Google Scholar]
- 16.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4355. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams M A, Kagan H M. Anal Biochem. 1985;149:430–437. doi: 10.1016/0003-2697(85)90594-9. [DOI] [PubMed] [Google Scholar]
- 19.Li W, Chou I-N, Boak A, Kagan H M. Am J Respir Cell Mol Biol. 1995;13:418–425. doi: 10.1165/ajrcmb.13.4.7546771. [DOI] [PubMed] [Google Scholar]
- 20.Kagan H M, Cai P. Methods Enzymol. 1995;258:122–132. doi: 10.1016/0076-6879(95)58041-7. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Kagan H M, Chou I-N. Toxicol Appl Pharmacol. 1994;126:114–127. doi: 10.1006/taap.1994.1097. [DOI] [PubMed] [Google Scholar]
- 22.Tang S S, Trackman P C, Kagan H M. J Biol Chem. 1983;258:4331–4338. [PubMed] [Google Scholar]
- 23.Kagan H M, Sullivan K A. Methods Enzymol. 1982;82A:637–649. doi: 10.1016/0076-6879(82)82092-2. [DOI] [PubMed] [Google Scholar]
- 24.Graham L, Gallop P M. Anal Biochem. 1994;217:298–305. doi: 10.1006/abio.1994.1122. [DOI] [PubMed] [Google Scholar]
- 25.Shah M A, Bergethon P R, Boak A, Crombie G D, Gallop P M, Kagan H M. Biochim Biophys Acta. 1992;1159:311–318. doi: 10.1016/0167-4838(92)90061-h. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan K A, Kagan H M. J Biol Chem. 1982;257:13520–13526. [PubMed] [Google Scholar]
- 27.Eyre D R, Paz M A, Gallop P M. Annu Rev Biochem. 1984;53:717–748. doi: 10.1146/annurev.bi.53.070184.003441. [DOI] [PubMed] [Google Scholar]
- 28.Kagan H M, Williams M A, Williamson P R, Anderson J M. J Biol Chem. 1984;259:11203–11207. [PubMed] [Google Scholar]
- 29.Hsieh H L, Tong C G, Thomas C, Roux S J. Plant Mol Biol. 1996;30:135–147. doi: 10.1007/BF00017808. [DOI] [PubMed] [Google Scholar]
- 30.Kiefer P, Acland P, Pappin D, Peters G, Dickson C. EMBO J. 1994;13:4126–4136. doi: 10.1002/j.1460-2075.1994.tb06730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jans D A, Jans P, Briggs L J, Sutton V, Trapani J A. J Biol Chem. 1996;271:30781–30789. doi: 10.1074/jbc.271.48.30781. [DOI] [PubMed] [Google Scholar]
- 32.Fleet J C. Nutr Rev. 1995;53:226–227. doi: 10.1111/j.1753-4887.1995.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 33.Kenyon K, Modi W S, Contente S, Friedman R M. J Biol Chem. 1993;268:18435–18437. [PubMed] [Google Scholar]
- 34.Saito H, Papaconstantinou J, Sato H, Goldstein S. J Biol Chem. 1997;272:8157–8160. doi: 10.1074/jbc.272.13.8157. [DOI] [PubMed] [Google Scholar]
- 35.Dingwall C, Laskey R A. Trends Biochem Sci. 1991;16:478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- 36.Boulikas T. J Cell Biochem. 1994;55:32–58. doi: 10.1002/jcb.240550106. [DOI] [PubMed] [Google Scholar]
- 37.Kagan H M, Reddy V B, Narasimhan N, Csizsar K. The Molecular Biology and Pathology of Elastic Tissues, Ciba Foundation Symposium 192. Chichester, U.K.: Wiley; 1995. pp. 100–121. [DOI] [PubMed] [Google Scholar]
- 38.Wolffe A P. Science. 1996;272:371–372. doi: 10.1126/science.272.5260.371. [DOI] [PubMed] [Google Scholar]