Abstract
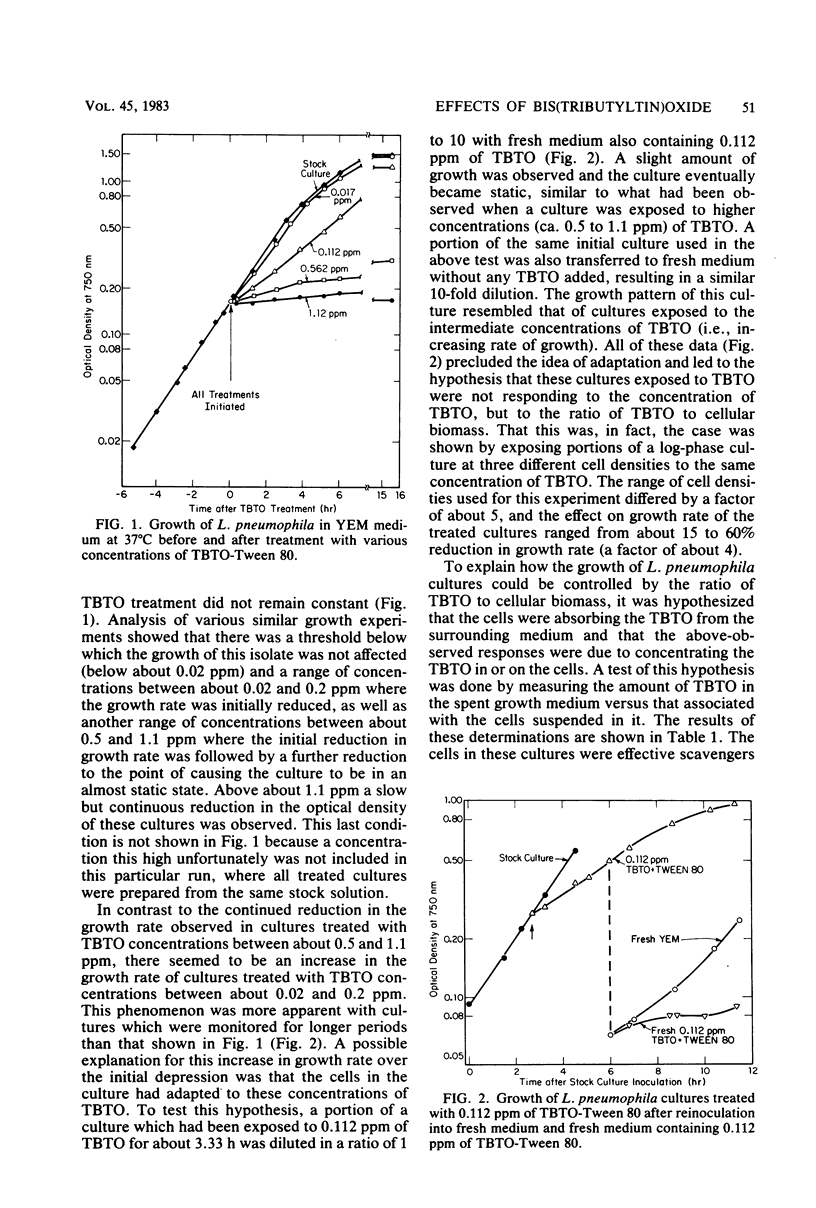
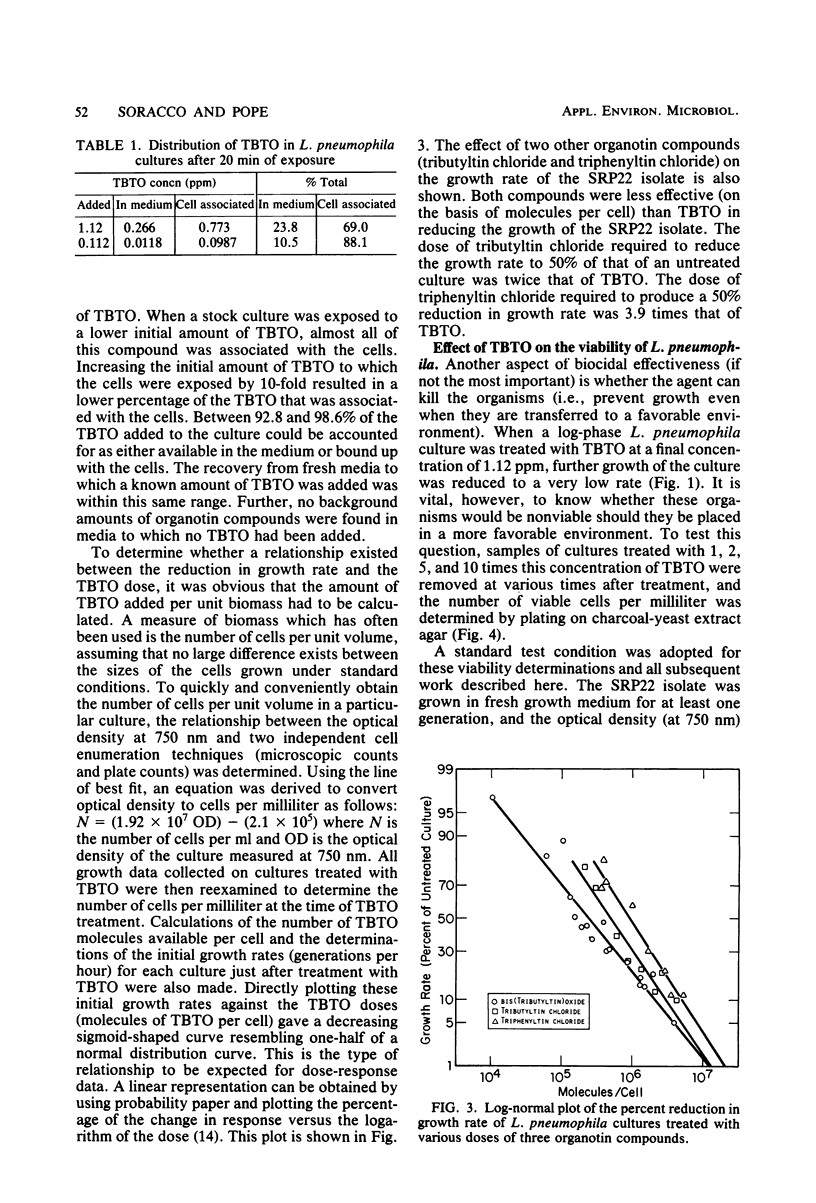
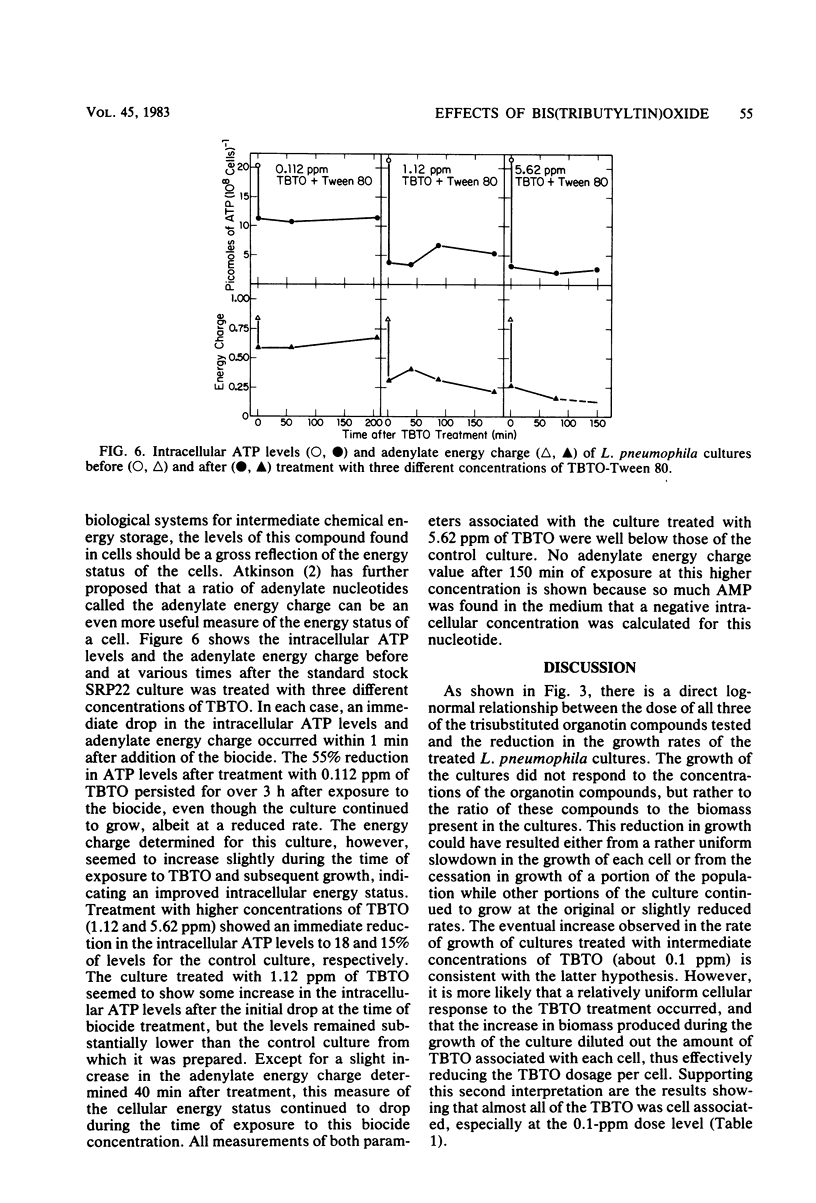
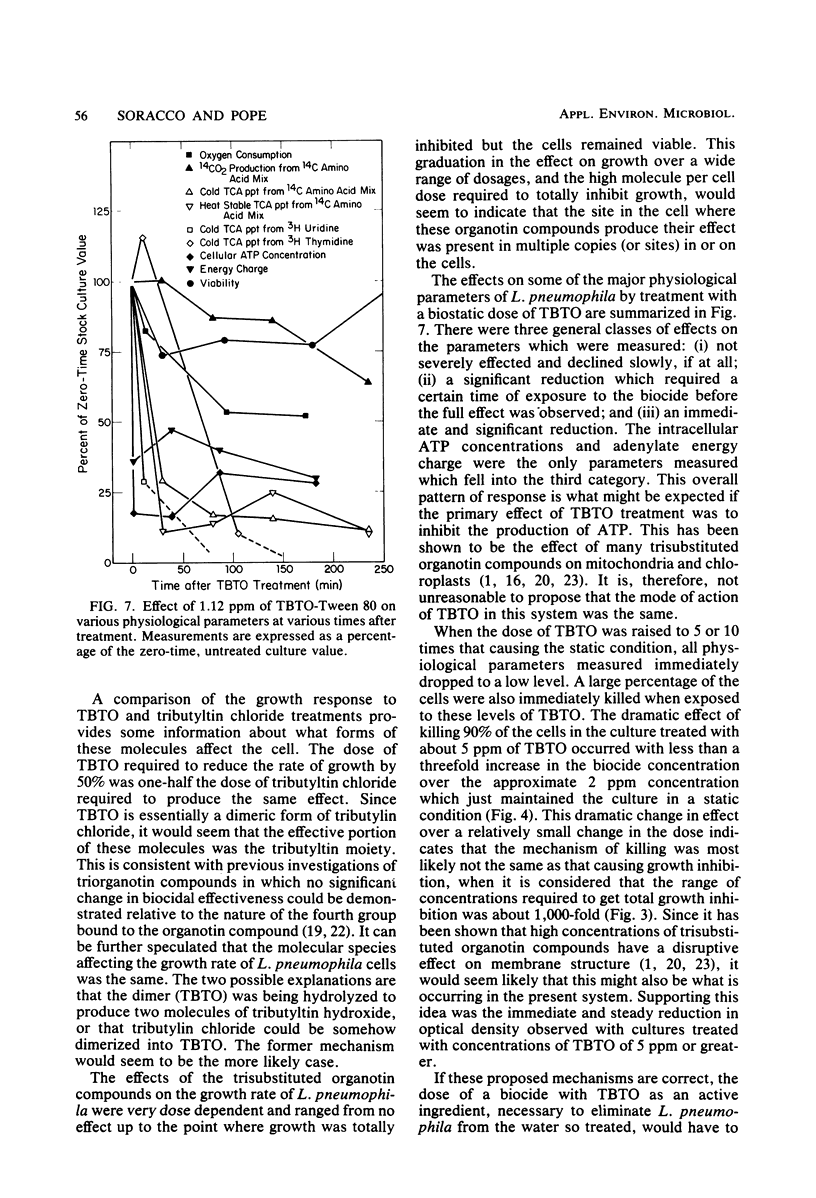
The modes of action of bis(tributyltin)oxide (TBTO) doses between 1 X 10(4) and 6 X 10(7) molecules per cell on a single environmental isolate of Legionella pneumophila were studied by monitoring the following parameters: (i) growth, (ii) cell viability, (iii) 14C-amino acid incorporation, (iv) 14CO2 production from 14C-amino acids, (v) [3H]uridine incorporation, (vi) [3H]thymidine incorporation, (vii) oxygen consumption, (viii) cellular ATP levels, and (ix) adenylate energy charge. The amount of TBTO associated with the cells in these laboratory cultures was also compared with that remaining in the suspending medium. Most of the TBTO (68 to 88%) was found to be associated with the cells. This result explained why the cellular responses which were measured did not correlate with the TBTO concentration, but rather with the dose of TBTO to which the cells were exposed. At the lower TBTO doses tested (10(4) to 10(7) molecules per cell) a log-normal relationship was observed between the reduction in growth rate and the TBTO concentration. At intermediate TBTO doses (ca. 10(7) molecules per cell) growth stasis occurred, with nearly 100% of the cells in these cultures remaining viable for at least 5 h after treatment. The cellular function which seemed to be primarily affected at these levels of TBTO was the energy conversion mechanism, since the decline in the rates of CO2 production, oxygen consumption, and macromolecular synthesis was preceded by an immediate (within 1 min) drop in the intracellular levels of ATP and the adenylate energy charge. At the higher TBTO doses greater than 10(7) molecules per cell) an initial, precipitous, drop in the number of viable cells was observed, which was followed by a further exponential reduction of viable cells in the treated culture. This dramatic increase in bactericidal activity with a slight increase in the TBTO dose indicated that the modes of bacteriostatic and bactericidal action of TBTO were different.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge W. N., Street B. W. Oxidative phosphorylation. Biochemical effects and properties of trialkyltins. Biochem J. 1964 May;91(2):287–297. doi: 10.1042/bj0910287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes L. G., Fraser D. W., Skaliy P., Perlino C. A., Elsea W. R., Mallison G. F., Hayes P. S. Legionnaires' disease outbreak at an Atlanta, Georgia, Country Club: evidence for spread from an evaporative condenser. Am J Epidemiol. 1980 Apr;111(4):425–431. doi: 10.1093/oxfordjournals.aje.a112917. [DOI] [PubMed] [Google Scholar]
- Cordes L. G., Goldman W. D., Marr J. S., Friedman S. M., Band J. D., Rothschild E. O., Kravitz H., Feeley J. C., Fraser D. W. Legionnaires' disease in New York City, August-September 1978. Bull N Y Acad Med. 1980 Jun;56(5):467–482. [PMC free article] [PubMed] [Google Scholar]
- Dondero T. J., Jr, Rendtorff R. C., Mallison G. F., Weeks R. M., Levy J. S., Wong E. W., Schaffner W. An outbreak of Legionnaires' disease associated with a contaminated air-conditioning cooling tower. N Engl J Med. 1980 Feb 14;302(7):365–370. doi: 10.1056/NEJM198002143020703. [DOI] [PubMed] [Google Scholar]
- Eickhoff T. C. Epidemiology of Legionnaires' disease. Ann Intern Med. 1979 Apr;90(4):499–502. doi: 10.7326/0003-4819-90-4-499. [DOI] [PubMed] [Google Scholar]
- Feeley J. C., Gibson R. J., Gorman G. W., Langford N. C., Rasheed J. K., Mackel D. C., Baine W. B. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol. 1979 Oct;10(4):437–441. doi: 10.1128/jcm.10.4.437-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliermans C. B., Cherry W. B., Orrison L. H., Smith S. J., Tison D. L., Pope D. H. Ecological distribution of Legionella pneumophila. Appl Environ Microbiol. 1981 Jan;41(1):9–16. doi: 10.1128/aem.41.1.9-16.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliermans C. B., Cherry W. B., Orrison L. H., Thacker L. Isolation of Legionella pneumophila from nonepidemic-related aquatic habitats. Appl Environ Microbiol. 1979 Jun;37(6):1239–1242. doi: 10.1128/aem.37.6.1239-1242.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace R. D., Dewar N. E., Barnes W. G., Hodges G. R. Susceptibility of Legionella pneumophila to three cooling tower microbicides. Appl Environ Microbiol. 1981 Jan;41(1):233–236. doi: 10.1128/aem.41.1.233-236.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi B. D., Fraser D. W., Mallison G. F., Mohatt J. V., Morris G. K., Patton C. M., Feeley J. C., Telle R. D., Bennett J. V. A major focus of Legionnaires' disease in Bloomington, Indiana. Ann Intern Med. 1979 Apr;90(4):587–591. doi: 10.7326/0003-4819-90-4-587. [DOI] [PubMed] [Google Scholar]
- Selwyn M. J., Dunnett S. J., Philo R. D., Dawson A. P. Factors affecting the inhibition of mitochondrial adenosine triphosphatase by trialkyltin compounds. Biochem J. 1972 Apr;127(3):66P–67P. doi: 10.1042/bj1270066pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L. R., Carlson T. L. A modified phenylfluorone method for determining organotin compounds in the ppb and sub-ppb range. J Anal Toxicol. 1980 Jan-Feb;4(1):31–33. doi: 10.1093/jat/4.1.31. [DOI] [PubMed] [Google Scholar]
- Skaliy P., Thompson T. A., Gorman G. W., Morris G. K., McEachern H. V., Mackel D. C. Laboratory studies of disinfectants against Legionella pneumophila. Appl Environ Microbiol. 1980 Oct;40(4):697–700. doi: 10.1128/aem.40.4.697-700.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockdale M., Dawson A. P., Selwyn M. J. Effects of trialkyltin and triphenyltin compounds on mitochondrial respiration. Eur J Biochem. 1970 Aug;15(2):342–351. doi: 10.1111/j.1432-1033.1970.tb01013.x. [DOI] [PubMed] [Google Scholar]
- Stratton G. W., Burrell R. E., Kurp M. L., Corke C. T. Interactions between the solvent acetone and the pyrethroid insecticide permethrin on activities of the blue-green alga Anabaena. Bull Environ Contam Toxicol. 1980 Apr;24(4):562–569. doi: 10.1007/BF01608156. [DOI] [PubMed] [Google Scholar]
- Watling-Payne A. S., Selwyn M. J. Inhibition and uncoupling of photophosphorylation in isolated chloroplasts by organotin, organomercury and diphenyleneiodonium compounds. Biochem J. 1974 Jul;142(1):65–74. doi: 10.1042/bj1420065. [DOI] [PMC free article] [PubMed] [Google Scholar]


