Abstract
The proinflammatory cytokine interleukin 1 (IL-1) activates the transcription of many genes encoding acute phase and proinflammatory proteins, a function mediated primarily by the transcription factor NF-κB. An early IL-1 signaling event is the recruitment of the Ser/Thr kinase IRAK to the type I IL-1 receptor (IL-1RI). Here we describe the function of a previously identified IL-1 receptor subunit designated IL-1 receptor accessory protein (IL-1RAcP). IL-1 treatment of cells induces the formation of a complex containing both IL-1RI and IL-1RAcP. IRAK is recruited to this complex through its association with IL-1RAcP. Overexpression of an IL-1RAcP mutant lacking its intracellular domain, the IRAK-binding domain, prevented the recruitment of IRAK to the receptor complex and blocked IL-1-induced NF-κB activation.
Interleukin 1 (IL-1) plays a central role in the generation of inflammatory responses (1). After binding to the cell surface receptor IL-1RI, IL-1 activates intracellular signaling cascades leading to the activation of the transcription factor NF-κB, which in turn up-regulates the expression of many proinflammatory genes in the nucleus (1, 2). Although considerable progress has been made recently in delineating the IL-1 signaling mechanism, our understanding of the process(es) remains incomplete. Within seconds after cells are exposed to IL-1, IRAK is recruited in the IL-1 receptor complex, where it becomes highly phosphorylated (3). Subsequently, IRAK interacts with TRAF6, a TRAF family member required for IL-1 mediated NF-κB activation (4). Two other members of the TRAF family, TRAF2 and TRAF5, have been implicated in NF-κB activation signaled through the tumor necrosis factor (TNF) receptor superfamily (5–7). One gap in our understanding of IL-1 signaling is the mechanism by which IRAK is recruited to the receptor. IRAK and IL-IRI do not interact directly with one another in mammalian cells when the two proteins are coexpressed, nor do they interact in the yeast two hybrid system (8) (data not shown). These observations suggest that IRAK may be recruited to the receptor complex through interaction with other components.
A molecule that might fulfill the role as an adapter able to recruit IRAK is the IL-1 receptor accessory protein (IL-1RAcP) (9). IL-1RAcP is a transmembrane protein that shares 25% sequence identity with IL-1RI and is required for IL-1 signal transduction (10). IL-1RAcP does not bind directly to IL-1, but it can be cross-linked to radiolabeled ligand in the presence of IL-1RI (9). To address the role of IL-1RAcP, we isolated the human IL-1RAcP cDNA from a placenta cDNA library that encodes a protein 89% identical to its murine counterpart (GenBank accession no. AF029213). In this paper, we report the biochemical characterization of interactions among IL-1RAcP, IL-1RI, and IRAK and the significance of this complex formation in IL-1 signal transduction.
EXPERIMENTAL PROCEDURES
Isolation of Human IL-1RI cDNA.
A cDNA fragment encoding the intracellular portion of the mouse IL-1RAcP was obtained by PCR and used as a hybridization probe to isolate the human IL-1RAcP cDNA from a λ phage cDNA library made from placenta (CLONTECH).
Construction of Expression Plasmids.
The expression plasmids for IL-1RAcP and AcP(1–403) were constructed by inserting PCR-generated cDNA fragments lacking coding sequence for the signal peptide into the mammalian expression vector pFlag-CMV-1 (Kodak), which contains the preprotrypsin leader sequence for transient expression of amino-terminal Flag fusion proteins on the surface of mammalian cells.
Transfection, Electrophoretic Mobility-Shift Assay (EMSA), and Luciferase Reporter Assay.
For EMSA, 2 × 106 293 cells were plated in 100-mm dishes and transfected with 5 μg of the indicated plasmid DNA by calcium phosphate precipitation method (11). Nuclear extracts were prepared 24 h after transfection (12) and tested for activity that would shift the electrophoretic mobility of a [32P]-radiolabeled oligonucleotide containing an NF-κB binding site derived from the human ELAM-1 promoter (13). For luciferase reporter assay, 2 × 105 293 or HeLa cells were seeded on 35-mm dishes and transfected on the following day with indicated expression plasmids, together with 0.5 μg of ELAM-1 luciferase reporter plasmid (13) and 0.05 μg of a cytomegalovirus–β-galactosidase plasmid for monitoring transfection efficiency. Twenty four hours after transfection, cells were treated with 10 ng/ml of IL-1β or TNFα for 6 h, and luciferase activity was measured using reagents from Promega.
Coimmunoprecipitation and Immunoblotting Analysis.
293IL-1RI (3) or HeLa cells (4 × 107 each) were used for coimmunoprecipitation of endogenous proteins. For coprecipitation of transfected proteins, 2 × 106 293 or 293IL-1RI cells were plated on 100-mm dishes and transfected with 5 μg of expression plasmids. After 20–24 h, the cells were collected, induced with IL-1β (100 ng/ml) for 3–5 min, and lysed as described (3). The immunoprecipitates were prepared using the appropriate antiserum and protein A or G conjugated to Sepharose beads for 2–4 h at 4°C. The beads were washed six times with cell lysis buffer (3) and boiled in 20 μl of SDS sample buffer. Solubilized proteins were fractionated on 8% SDS–polyacrylamide gels and immunoblotted with polyclonal antiserum to IRAK or to IL-1RI. The reactive proteins were detected with enhanced chemiluminescence reagents (Amersham).
RESULTS AND DISCUSSION
Transient Coexpression of IL-1RI and IL-1RAcP Activates NF-κB.
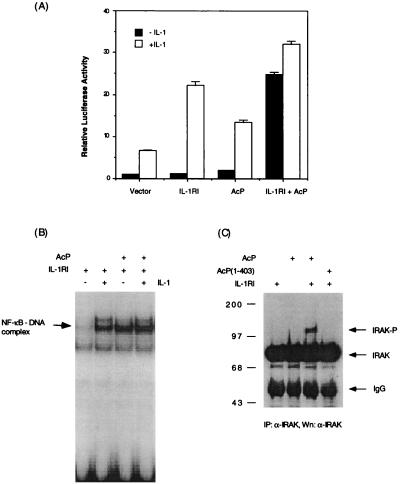
Unlike the TNF receptor 1 or 2, which signals through ligand-induced homotrimerization (14), overexpression of IL-1RI does not result in ligand-independent NF-κB activation (15), suggesting that aggregation of IL-1RI alone is insufficient for signaling. To determine if IL-1RAcP might be the missing component, we tested whether coexpression of IL-1RI and IL-1RAcP would activate NF-κB. Transient expression of either IL-1RI or IL-1RAcP alone did not result in ligand-independent induction of an NF-κB-dependent luciferase reporter gene in 293 cells. However, coexpression of both proteins resulted in a 20-fold activation of NF-κB activity (Fig. 1A). The reporter assay results were confirmed by EMSA. In cells that coexpressed IL-1RI and IL-1RAcP, NF-κB DNA binding activity was elevated to a level comparable to that induced by IL-1 (Fig. 1B). These data suggest that aggregation of IL-1RI and IL-1RAcP as a result of protein overexpression can elicit a signaling pathway leading to NF-κB activation.
Figure 1.
Coexpression of IL-1RI and IL-1RAcP activates NF-κB. (A) Activation of NF-κB by coexpression of IL-1RI and IL-1RAcP in 293 cells determined by luciferase reporter assay; 293 cells were transfected with a luciferase reporter gene driven by the ELAM-1 promoter (13) (0.5 μg/35-mm plate) along with IL-1RI or IL-1RAcP expression plasmids (1 μg/35 mm plate), as indicated. Twenty hours after transfection, the cells were treated for 6 h with IL-1β (10 ng/ml) or left untreated, then lysed for measurement of luciferase activity. (B) Activation of NF-κB DNA binding activity in 293 cells coexpressing IL-1RI and IL-1RAcP; 293 cells were transfected with indicated expression plasmids for 24 h and induced with IL-1β (10 ng/ml) for 1 h. Nuclear extracts were prepared for testing NF-κB-DNA binding activity by EMSA (12). (C) Coexpression of IL-1RI and IL-1RAcP activates IRAK. IRAK protein was immunoprecipitated from lysate of 293 cells transfected with indicated expression plasmids, separated by SDS gel electrophoresis, and immunoblotted with antiserum to IRAK. Molecular size markers are in kilodaltons.
To assess if overexpression of IL-1RI and IL-1RAcP activates NF-κB by engaging the same signaling pathway that is activated by IL-1, we examined if IRAK was activated under these experimental conditions. Evidence for IRAK’s activation is its own phosphorylation, which retards its mobility in an SDS gel (3). In cells coexpressing IL-1RI and IL-1RAcP, two species of IRAK protein were detected by immunoblotting, an 80-kDa form that corresponds to unphosphorylated IRAK and an 100-kDa form that corresponds to phosphorylated IRAK (Fig. 1C). The phosphorylated form was not detected in cells that expressed IL-1RI or IL-1RAcP alone, nor was it detected in the cells coexpressing IL-1RI and a truncated form of IL-1RAcP [AcP(1–403)] in which most of the intracellular domain of the protein has been deleted (Fig. 1C). Compared with the full length IL-1RAcP, similar amounts of AcP(1–403) were expressed on the cell surface as indicated by flow cytometry (data not shown). Thus, coexpression of IL-1RI and IL-1RAcP appears to trigger a pathway similar to that activated by IL-1, and this IRAK activation requires the intracellular domain of IL-1RAcP.
IL-1 Induces the Association of IL-1RI and IL-1RAcP.
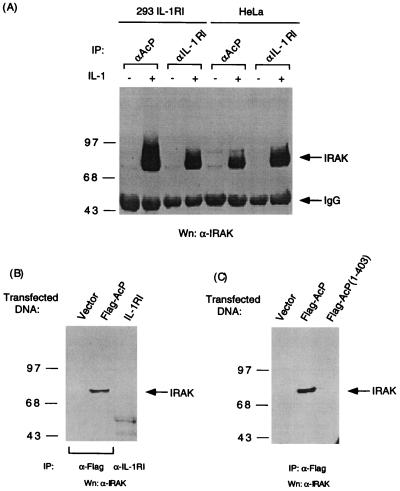
We next examined if IL-1RI and IL-1RAcP could form a complex upon IL-1 treatment of cells. Rabbit polyclonal antiserum raised to the extracellular domain of IL-1RAcP can immunoprecipitate IL-1RAcP from protein extracts of 293 cells stably transfected with expression plasmid for IL-1RI (293IL-1RI) (Fig. 2A). IL-1RI was detected by immunoblotting in the IL-1RAcP immunocomplex obtained from IL-1 treated, but not untreated, 293IL-1RI cells (Fig. 2B), indicating that the association of the endogenous IL-1RAcP with IL-1RI is ligand-dependent. We then asked if the intracellular portion of the IL-1RAcP is necessary for its association with IL-1RI. 293IL-1RI cells were transiently transfected with expression vectors for epitope-tagged IL-1RAcP or AcP(1–403) and treated briefly with IL-1 or left untreated.
Figure 2.
IL-1 induces complex formation of IL-1RI and IL-1RAcP. (A) Immunoblot analysis to detect IL-1RAcP precipitated from protein extract of 293IL-1RI cells (10-cm dish) with rabbit preimmune serum or antiserum raised to the extracellular domain of human IL-1RAcP. (B) IL-1-dependent coprecipitation of IL-1RI and IL-1RAcP. Immunoblot analysis was performed to detect IL-1RI in the immunocomplex precipitated with antiserum to the extracellular domain of IL-1RAcP from IL-1-treated (100 ng/ml for 5 min) or untreated 293IL-1RI cells. Rabbit preimmune serum was used as control for immunoprecipitation. (C) Interaction of transiently expressed IL-1RAcP and IL-1RI. 293IL-1RI cells were transfected for 20 h with vector alone, expression plasmids for Flag epitope-tagged IL-1RAcP, or AcP(1–403) and treated with IL-1 (100 ng/ml) for 5 min or left untreated. The immunocomplexes precipitated with anti-Flag antibody were subjected to immunoblot analysis to detect coprecipitating IL-1RI.
Immunoprecipitation of the transiently expressed IL-1RAcP coprecipitated IL-1RI in the absence of IL-1 induction (Fig. 2C). However, the amount of coprecipitating IL-1RI was enhanced significantly by IL-1. The formation of IL-1RI and IL-1RAcP complex before IL-1 treatment is likely caused by overexpression of the two transmembrane proteins. This observation is consistent with the activation of IRAK and NF-κB observed in cells that transiently coexpress the two receptor chains (Fig. 1). The association of IL-1RI and AcP(1–403) was not evident in untreated cells but was readily detectable in IL-1-treated cells (Fig. 2B). These results suggest that both extra- and intracellular domains of IL-1RAcP may play a role in the formation of the active receptor complex. Because IL-1 binds directly to IL-1RI (16) and can be cross-linked to IL-1RAcP (4), the initial step of IL-1 signaling may be a ligand-mediated association of IL-1RI and IL-1RAcP through their extracellular domains. The complexes may then be further stabilized through their cytoplasmic domains, which could be accomplished by a direct interaction of these two proteins or by other associated molecules.
Immunoprecipitation of IL-1RAcP Coprecipitates IRAK.
We have shown previously that IRAK coimmunoprecipitates with IL-1RI after IL-1 induction (3). If IL-1RAcP forms a complex with IL-1RI after IL-1 treatment, then immunoprecipitation of IL-1RAcP should also coprecipitate IRAK. IRAK was indeed readily detected in immunoprecipitates of both IL-1RAcP and IL-1RI from IL-1-treated, but not untreated, 293IL-1RI and HeLa cells (Fig. 3A). We then explored the possibility that IL-1RAcP might be responsible for recruiting IRAK to the receptor complex. IL-1RAcP or IL-1RI was expressed transiently in 293 cells and tested for ability to coprecipitate endogenous IRAK. By immunoblotting, IRAK was found to coprecipitate only with IL-1RAcP but not with IL-1RI (Fig. 3B). IL-1RAcP(1–403), lacking most of the intracellular domain, failed to coprecipitate IRAK (Fig. 3C), a result consistent with the inability of this mutant to activate IRAK (Fig. 1C). Thus, when overexpressed and aggregated by antibody, IL-1RAcP, but not IL-1RI, is capable of binding to IRAK. Although the stoichiometry of the receptor components is not clear at the moment, IL-1 signaling requires the oligomerization of IL-1RI as determined by fluorescence resonance energy transfer analysis (17). It is reasonable, therefore, to deduce that the signaling receptor complex may also contain aggregated IL-1RAcP that can bring in IRAK.
Figure 3.
IL-1RAcP interacts with IRAK. (A) IL-1-dependent coprecipitation of IRAK with IL-1RAcP in 293IL-1RI and HeLa cells. Protein extracts from 293IL-1RI or HeLa cells untreated or treated with IL-1 (100 ng/ml for 5 min) were immunoprecipitated with antiserum to the extracellular domain of IL-1RAcP or IL-1RI. The immunocomplexes were analyzed by immunoblotting to detect IRAK. (B) Interaction of IRAK with transiently expressed IL-1RAcP. IL-1RI or Flag epitope-tagged IL-1RAcP was expressed in 293 cells by transient transfection and immunoprecipitated with antiserum to IL-1RI or with anti-Flag antibody, respectively. Anti-Flag antibody was used for immunoprecipitation of extracts of cells transfected with the vector alone. After separation by SDS/PAGE, the immunocomplexes were blotted with antiserum to IRAK. Reactive proteins were detected with horseradish peroxide conjugated to protein A. (C) Interaction of IRAK with the cytoplasmic domain of IL-1RAcP. Flag-tagged IL-1RAcP or AcP(1–403) transiently expressed in 293 cells was immunoprecipitated with anti-Flag antibody and immunoblotted to detect coprecipitating IRAK.
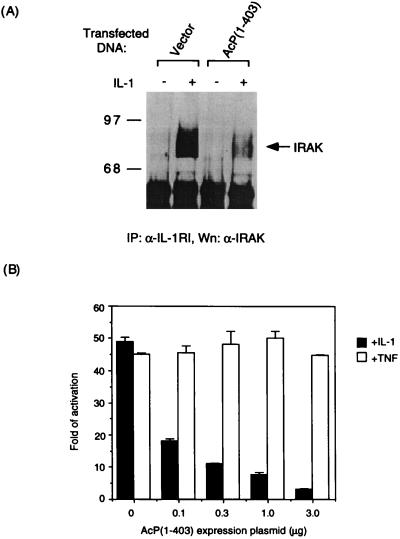
IL-1RAcP(1–403) Blocks Recruitment of IRAK to the Receptor and IL-1 Signaling.
AcP(1–403) can be recruited to IL-1RI by the ligand (Fig. 2B), yet is unable to bind to IRAK (Fig. 3C), so we predicted that, when overexpressed, this polypeptide would impede the association of endogenous IL-1RAcP with IL-1RI and, thereby, prevent the recruitment of IRAK to the receptor complex. Indeed, the amount of IRAK protein detected in IL-1RI immunoprecipitates was markedly reduced in HeLa cells transiently transfected with expression plasmid for IL-1RAcP(1–403) (Fig. 4A). Consistently, transfecting HeLa cells with the expression plasmid for IL-1RAcP (1–403) resulted in a dose-dependent inhibition of IL-1-induced NF-κB activation as determined by luciferase reporter assay (Fig. 4B). Under the same conditions, AcP(1–403) overexpression had no effect on TNF-mediated NF-κB activation, indicating that the observed inhibition is specific to IL-1 signal transduction.
Figure 4.
Inhibition of recruitment of IRAK to the receptor complex and IL-1-induced NF-κB activation by AcP(1–403). (A) Interference of IRAK’s recruitment to the receptor complex by AcP(1–403). Immunocomplex precipitated with antiserum to IL-1RI from HeLa cells transfected for 40 h with expression plasmid for AcP(1–403) (10 μg/100-mm plate) or vector alone were immunoblotted to detect IRAK. Half of the cells transfected with each plasmid were induced for 5 min with IL-1β (100 ng/ml). (B) Specific inhibition of IL-1-induced NF-κB activation by AcP(1–403). HeLa cells were transfected for 40 h with a luciferase reporter gene controlled by the ELAM-1 promoter (13) along with indicated amounts of the expression plasmid for AcP(1–403). The cells were treated with IL-1β or TNF-α (10 ng/ml) for 6 h before being lysed for determination of luciferase activity. Fold activation in untreated cells transfected with an empty vector was 1.
We have shown that IL-1 signals by aggregating the cell surface molecules IL-1RI and IL-1RAcP. Although the former conveys high affinity ligand binding (16), the latter plays a role in recruiting IRAK to the receptor complex, an event that correlates strictly with IL-1-induced NF-κB activation (15). Although when overexpressed and aggregated by antibody, IL-1RI AcP coprecipitated IRAK in unstimulated cells, we believe that IRAK is not associated with IL-1RAcP before IL-1 treatment. In both HeLa and 293IL-1RI cells in which IL-1RAcP is not overexpressed, the association of IRAK with IL-1RAcP is dependent on IL-1 treatment (Fig. 3). It is not clear, however, if IL-1RAcP is the functional equivalent of the Drosophila protein tube, a speculated adapter molecule that functions upstream of pelle, a Ser/Thr kinase most related by sequence to IRAK (3, 18, 19). Tube and pelle are components in a Drosophila signaling pathway analogous to the mammalian IL-1 pathway, and both are required for the activation of the NF-κB homolog dorsal, which controls dorsal–ventral polarity during embryogenesis (20). However, tube is not a transmembrane protein like IL-1RAcP, and there is no apparent sequence homology found in the two proteins. Even though IL-1RI alone is insufficient for binding IRAK and for signaling, its intracellular domain is required for IL-1-mediated NF-κB activation (15, 21–24). It is possible that, when complexed with IL-1RAcP, IL-1RI may also make contact with IRAK, or it may provide docking sites for other signaling molecules. The observation that aggregated IL-1RAcP, but not IL-1RI, can bind to IRAK indicates that IL-1RAcP is the primary component responsible for recruiting the kinase.
Acknowledgments
We thank K. Williamson for DNA sequencing, and D. Goeddel and T. Hoey for comments on the manuscript.
ABBREVIATIONS
- IL-1
interleukin 1
- IL-1RI
type I IL-1 receptor
- TNF
tumor necrosis factor
- IL-1RAcP
IL-1 receptor accessory protein
- EMSA
electrophoretic mobility-shift assay
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF029213).
References
- 1.Dinarello C A. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 2.Baeuerle P A, Henkel T. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 3.Cao Z, Henzel W J, Gao X. Science. 1996;271:1128–1131. doi: 10.1126/science.271.5252.1128. [DOI] [PubMed] [Google Scholar]
- 4.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel D V. Nature (London) 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 5.Rothe M, Sarma V, Dixit V M, Goeddel D V. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi M, Rothe M, Goeddel D V. J Biol Chem. 1996;271:19935–19942. doi: 10.1074/jbc.271.33.19935. [DOI] [PubMed] [Google Scholar]
- 7.Nakano H, Oshima H, Chung W, Williams-Abbott L, Ware C F, Yagita H, Okumura K. J Biol Chem. 1996;271:14661–14664. doi: 10.1074/jbc.271.25.14661. [DOI] [PubMed] [Google Scholar]
- 8.Fields S, Song O. Nature (London) 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 9.Greenfeder S A, Nunes P, Kwee L, Labow M, Chizzonite R A, Ju G. J Biol Chem. 1995;270:13757–13765. doi: 10.1074/jbc.270.23.13757. [DOI] [PubMed] [Google Scholar]
- 10.Wesche H, Korherr C, Kracht M, Falk W, Resch K, Martin M U. J Biol Chem. 1997;272:7727–7731. doi: 10.1074/jbc.272.12.7727. [DOI] [PubMed] [Google Scholar]
- 11.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Curr Prot Mol Biol. 1994;1:9.1.1–9.1.3. [Google Scholar]
- 12.Schütze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Kronke M. Cell. 1992;71:765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- 13.Schindler U, Baichwal V R. Mol Cell Biol. 1994;14:5820–5831. doi: 10.1128/mcb.14.9.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tartagalia L A, Goeddel D V. Immunol Today. 1992;13:151–153. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- 15.Croston G E, Cao Z, Goeddel D V. J Biol Chem. 1995;270:16514–16517. doi: 10.1074/jbc.270.28.16514. [DOI] [PubMed] [Google Scholar]
- 16.Sims J E, March C J, Cosman D, Widmer M B, MacDonald H R, McMahan C J, Grubin C E, Wignall J M, Jackson J L, Call S M, Friend D, Alpert A R, Gillis S, Urdal D L, Dower S K. Science. 1988;241:585–589. doi: 10.1126/science.2969618. [DOI] [PubMed] [Google Scholar]
- 17.Guo C, Dower S K, Holowka D, Baird B. J Biol Chem. 1995;270:27562–27568. doi: 10.1074/jbc.270.46.27562. [DOI] [PubMed] [Google Scholar]
- 18.GroBhans J, Bergmann A, Haffter P, Nusslein-Volhard C. Nature (London) 1994;372:563–566. doi: 10.1038/372563a0. [DOI] [PubMed] [Google Scholar]
- 19.Galindo R L, Edwards D N, Gillespie S K H, Wasserman S A. Development. 1995;121:2209–2218. doi: 10.1242/dev.121.7.2209. [DOI] [PubMed] [Google Scholar]
- 20.Morisato D, Anderson K V. Annu Rev Genet. 1995;29:371–399. doi: 10.1146/annurev.ge.29.120195.002103. [DOI] [PubMed] [Google Scholar]
- 21.Curtis B M, Gallis B, Overell R W, McMahan C J, De Roos P, Ireland R, Eisenman J, Dower S K, Sims J E. Proc Natl Acad Sci USA. 1989;86:3045–3049. doi: 10.1073/pnas.86.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heguy A, Baldari C T, Macchia G, Telford J L, Melli M. J Biol Chem. 1992;267:2605–2609. [PubMed] [Google Scholar]
- 23.Kuno K, Okamoto S, Hirose K, Murakami S, Matsushima K. J Biol Chem. 1993;268:13510–13518. [PubMed] [Google Scholar]
- 24.Leung K, Betts J C, Xu L, Nabel G. J Biol Chem. 1994;269:1579–1582. [PubMed] [Google Scholar]