Abstract
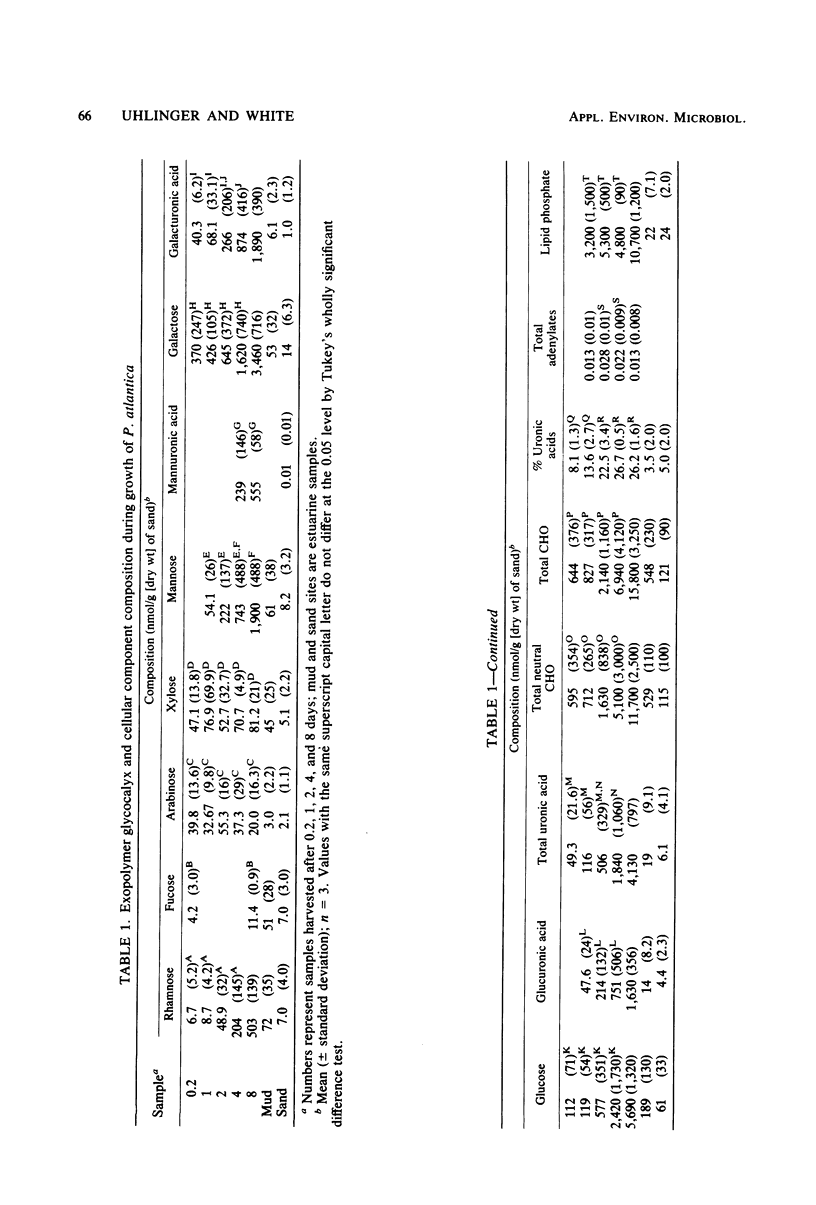
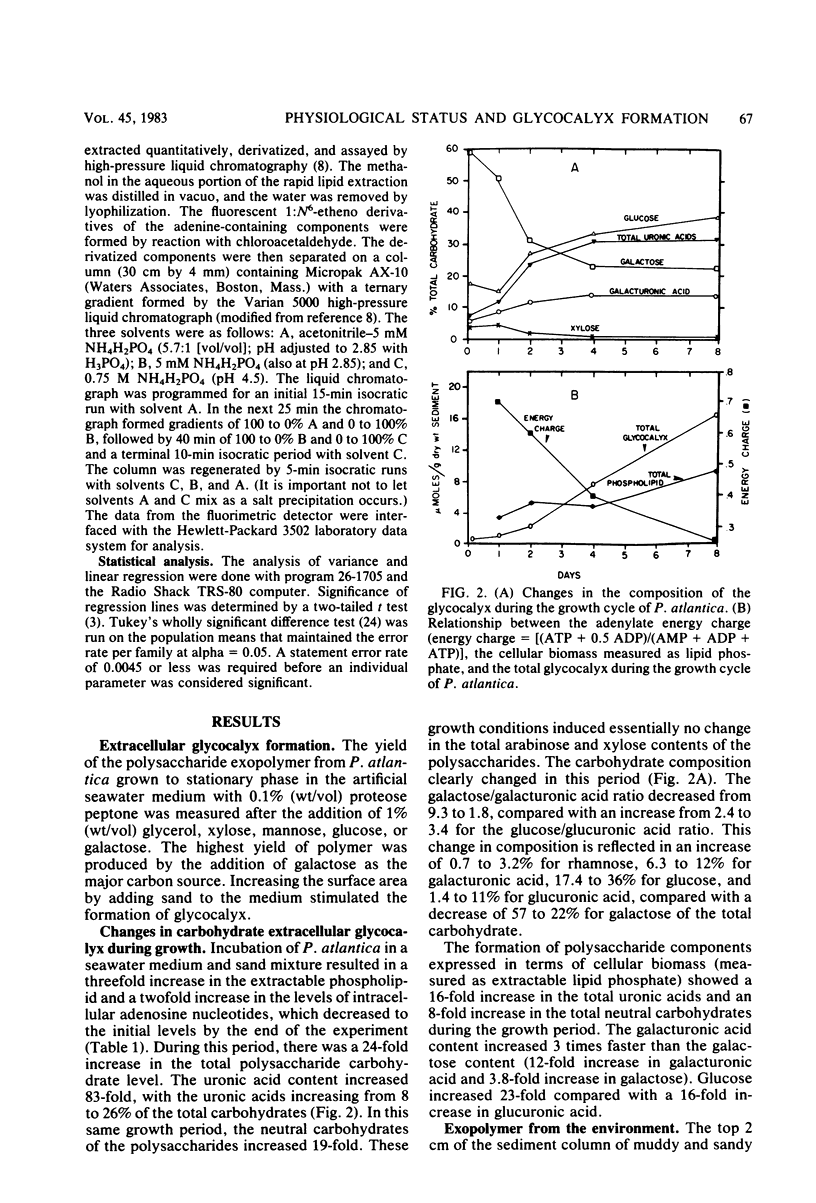
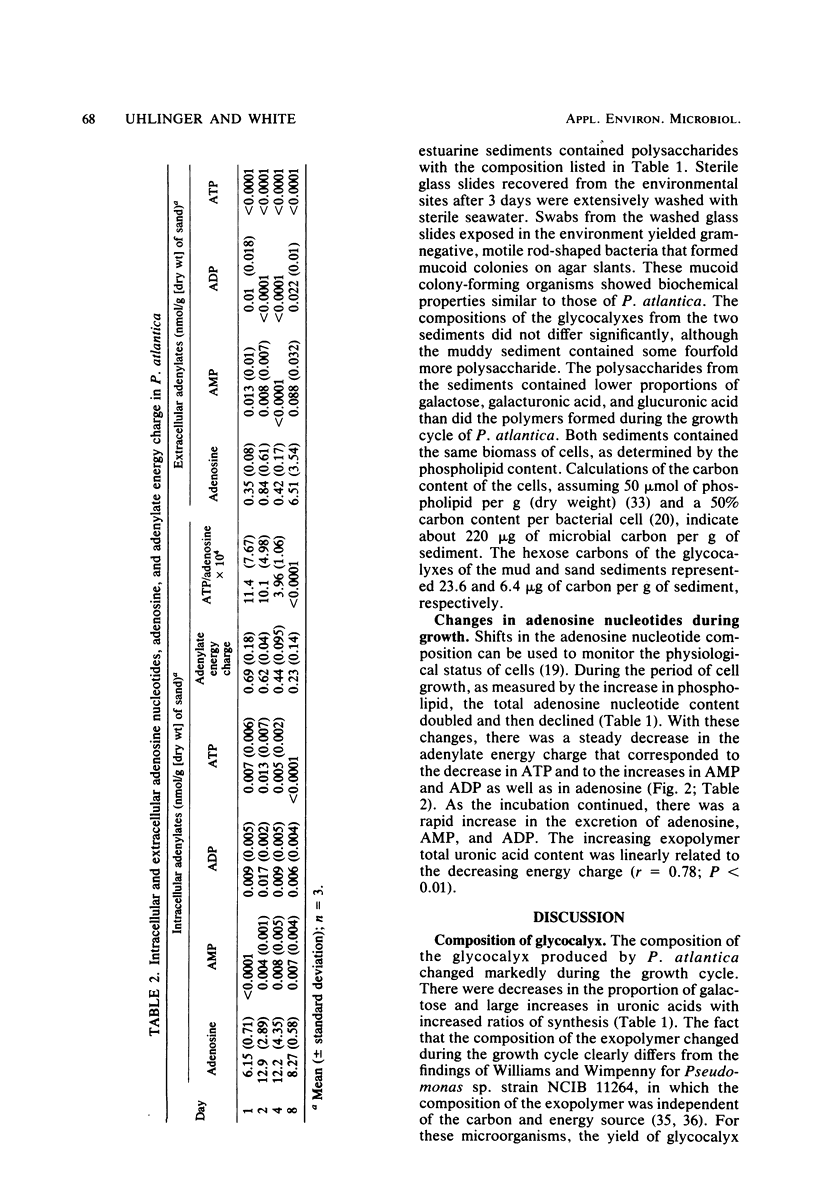
Marine pseudomonads, such as Pseudomonas atlantica, are readily isolated from sediments. These organisms form extracellular polysaccharide polymers (glycocalyx). The factors affecting the composition and amount of glycocalyx in batch culture of these organisms were examined. The formation of glycocalyx was stimulated by the inclusion of galactose as the carbon source and by increased surface area resulting from addition of sand to the medium. The composition of the glycocalyx changed during the growth cycle, with a marked increase in the proportions and absolute amounts of uronic acids as the rate of synthesis increased. In estuarine sediments, the glycocalyx contained a carbon content at least as great as in the microbes themselves. The greatest accumulation of these polymers occurred late in the stationary phase when the physiological status of the cells, as measured by the adenylate energy charge, showed maximal stress. Maximal formation of glycocalyx possibly could be used as an estimate of the nutritional status of these microbes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Geesey G. G., Cheng K. J. How bacteria stick. Sci Am. 1978 Jan;238(1):86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Irvin R. T., Cheng K. J. The bacterial glycocalyx in nature and disease. Annu Rev Microbiol. 1981;35:299–324. doi: 10.1146/annurev.mi.35.100181.001503. [DOI] [PubMed] [Google Scholar]
- Davis W. M., White D. C. Fluorometric determination of adenosine nucleotide derivatives as measures of the microfouling, detrital, and sedimentary microbial biomass and physiological status. Appl Environ Microbiol. 1980 Sep;40(3):539–548. doi: 10.1128/aem.40.3.539-548.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio S. A., Uhlinger D. J., Parker J. H., White D. C. Estimations of uronic acids as quantitative measures of extracellular and cell wall polysaccharide polymers from environmental samples. Appl Environ Microbiol. 1982 May;43(5):1151–1159. doi: 10.1128/aem.43.5.1151-1159.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federle T. W., White D. C. Preservation of estuarine sediments for lipid analysis of biomass and community structure of microbiota. Appl Environ Microbiol. 1982 Nov;44(5):1166–1169. doi: 10.1128/aem.44.5.1166-1169.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto S., Murakawa T., Kuwahara S. Slime production by Pseudomonas aeruginosa. II. A new synthetic medium and cultural conditions suitable for slime production by Pseudomonas aeruginosa. Jpn J Microbiol. 1973 Jan;17(1):45–51. doi: 10.1111/j.1348-0421.1973.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Marshall K. C., Stout R., Mitchell R. Selective sorption of bacteria from seawater. Can J Microbiol. 1971 Nov;17(11):1413–1416. doi: 10.1139/m71-225. [DOI] [PubMed] [Google Scholar]
- Nickels J. S., Bobbie R. J., Lott D. F., Martz R. F., Benson P. H., White D. C. Effect of manual brush cleaning on biomass and community structure of microfouling film formed on aluminum and titanium surfaces exposed to rapidly flowing seawater. Appl Environ Microbiol. 1981 Jun;41(6):1442–1453. doi: 10.1128/aem.41.6.1442-1453.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickels J. S., King J. D., White D. C. Poly-beta-Hydroxybutyrate Accumulation as a Measure of Unbalanced Growth of the Estuarine Detrital Microbiota. Appl Environ Microbiol. 1979 Mar;37(3):459–465. doi: 10.1128/aem.37.3.459-465.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons A. B., Dugan P. R. Production of extracellular polysaccharide matrix by Zoogloea ramigera. Appl Microbiol. 1971 Apr;21(4):657–661. doi: 10.1128/am.21.4.657-661.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W. Bacterial exopolysaccharides. Adv Microb Physiol. 1972;8:143–213. doi: 10.1016/s0065-2911(08)60190-3. [DOI] [PubMed] [Google Scholar]
- Unz R. F., Farrah S. R. Exopolymer production and flocculation by zoogloea mp6. Appl Environ Microbiol. 1976 Apr;31(4):623–626. doi: 10.1128/aem.31.4.623-626.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. G., Wimpenny J. W. Exopolysaccharide production by Pseudomonas NCIB11264 grown in batch culture. J Gen Microbiol. 1977 Sep;102(1):13–21. doi: 10.1099/00221287-102-1-13. [DOI] [PubMed] [Google Scholar]
- Williams A. G., Wimpenny J. W. Exopolysaccharide production by Pseudomonas NCIB11264 grown in continuous culture. J Gen Microbiol. 1978 Jan;104(1):47–57. doi: 10.1099/00221287-104-1-47. [DOI] [PubMed] [Google Scholar]


