Abstract
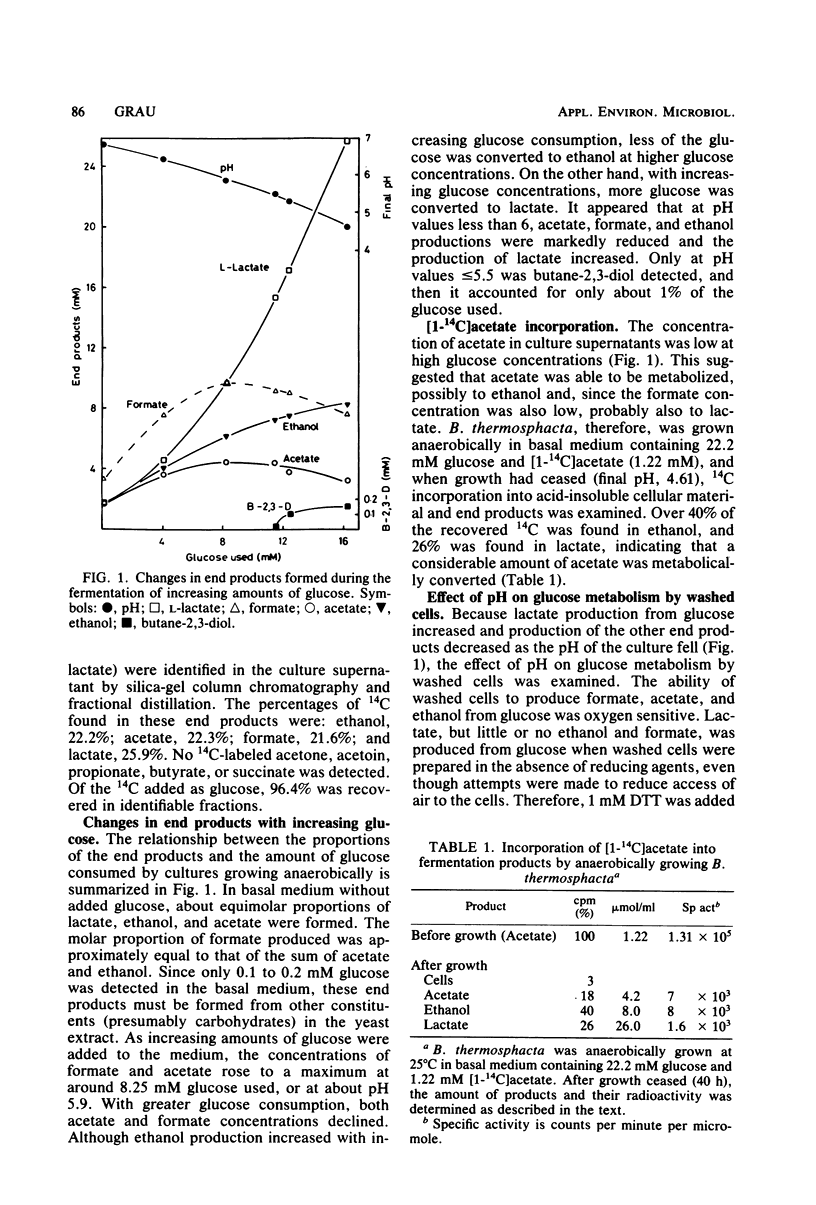
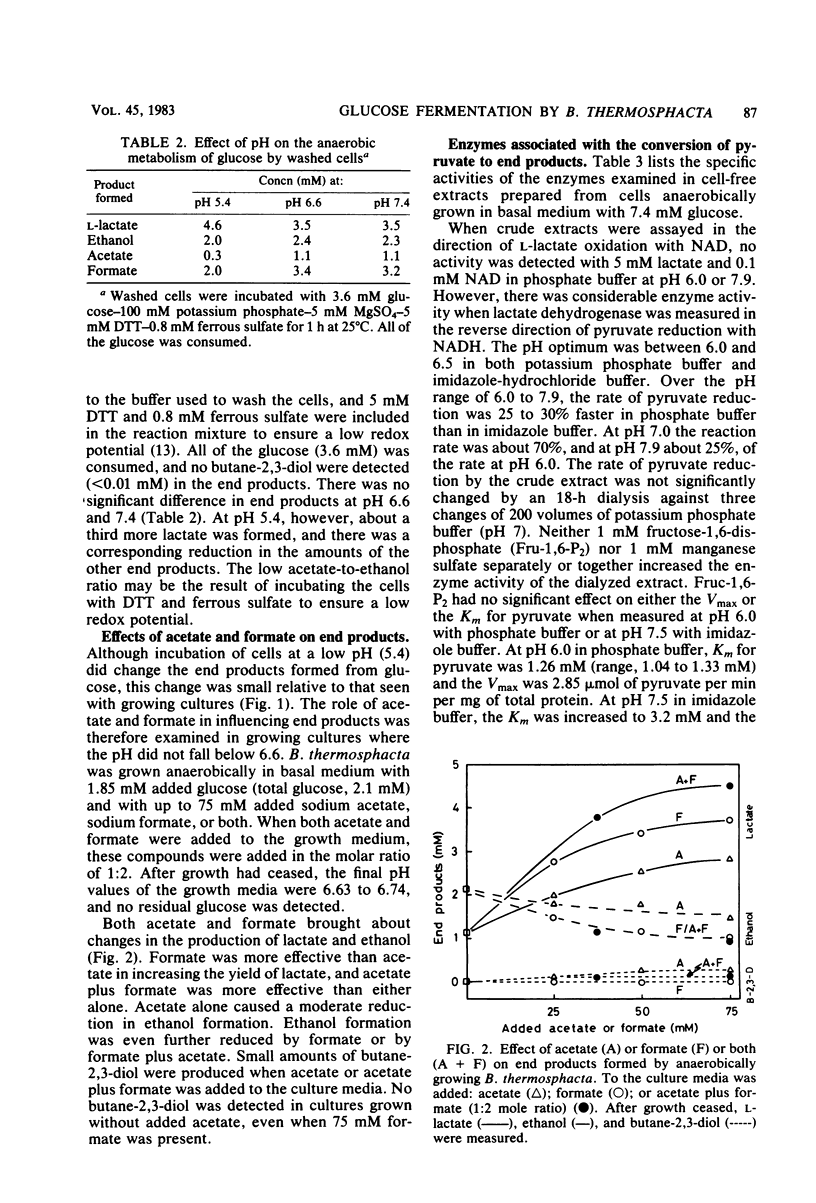
Anaerobically, Brochothrix thermosphacta fermented glucose primarily to l-lactate, acetate, formate, and ethanol. The ratio of these end products varied with growth conditions. Both the presence of acetate and formate and a pH below about 6 increased l-lactate production from glucose. Small amounts of butane-2,3-diol were also produced when the pH of the culture was low (≤5.5) or when acetate was added to the growth medium. Radioactive label from [1-14C]acetate was incorporated into ethanol and l-lactate, implying reversibility of pyruvate-formate lyase. In crude extracts, the following enzymes involved in pyruvate metabolism were demonstrated: lactate dehydrogenase, phosphotransacetylase, acetate kinase, acetaldehyde dehydrogenase (coenzyme A acetylating), ethanol dehydrogenase, pH 6 acetolactate-forming enzyme, and diacetyl (acetoin) reductase. The lactate dehydrogenase did not require fructose-1,6-disphosphate or Mn2+ for activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark D. P., Cronan J. E., Jr Acetaldehyde coenzyme A dehydrogenase of Escherichia coli. J Bacteriol. 1980 Oct;144(1):179–184. doi: 10.1128/jb.144.1.179-184.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainty R. H., Hibbard C. M. Aerobic metabolism of Brochothrix thermosphacta growing on meat surfaces and in laboratory media. J Appl Bacteriol. 1980 Jun;48(3):387–396. doi: 10.1111/j.1365-2672.1980.tb01027.x. [DOI] [PubMed] [Google Scholar]
- Davidson C. M., Mobbs P., Stubbs J. M. Some morphological and physiological properties of Microbacterium thermosphactum. J Appl Bacteriol. 1968 Dec;31(4):551–559. doi: 10.1111/j.1365-2672.1968.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Garvie E. I. Bacterial lactate dehydrogenases. Microbiol Rev. 1980 Mar;44(1):106–139. doi: 10.1128/mr.44.1.106-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau F. H. Inhibition of the Anaerobic Growth of Brochothrix thermosphacta by Lactic Acid. Appl Environ Microbiol. 1980 Sep;40(3):433–436. doi: 10.1128/aem.40.3.433-436.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau F. H. Nutritional Requirements of Microbacterium thermosphactum. Appl Environ Microbiol. 1979 Nov;38(5):818–820. doi: 10.1128/aem.38.5.818-820.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins T. E., Johnson M. J. Pathways of anaerobic acetate utilization in Escherichia coli and Aerobacter cloacae. J Bacteriol. 1970 Mar;101(3):885–891. doi: 10.1128/jb.101.3.885-891.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchener B. J., Egan A. F., Rogers P. J. Energetics of Microbacterium thermosphactum in glucose-limited continuous culture. Appl Environ Microbiol. 1979 Jun;37(6):1047–1052. doi: 10.1128/aem.37.6.1047-1052.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen A. R., Walker N. J. Separation of diacetyl, acetoin, and 2,3-butylene glycol by ion-exchange chromatography. Anal Biochem. 1973 Apr;52(2):475–481. doi: 10.1016/0003-2697(73)90051-1. [DOI] [PubMed] [Google Scholar]
- Knappe J., Blaschkowski H. P. Pyruvate formate-lyase from Escherischia coli and its activation system. Methods Enzymol. 1975;41:508–518. doi: 10.1016/s0076-6879(75)41107-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamed R., Zeikus J. G. Glucose fermentation pathway of Thermoanaerobium brockii. J Bacteriol. 1980 Mar;141(3):1251–1257. doi: 10.1128/jb.141.3.1251-1257.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S. H., Stormer F. C. Diacetyl (acetoin) reductase from Aerobacter aerogenes. Kinetic mechanism and regulation by acetate of the reversible reduction of acetoin to 2,3-butanediol. Eur J Biochem. 1973 Apr 2;34(1):100–106. doi: 10.1111/j.1432-1033.1973.tb02734.x. [DOI] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Suzuki T., Kameda K. Y., Abiko Y. Phosphotransacetylase of Escherichia coli B, purification and properties. Biochim Biophys Acta. 1969;191(3):550–558. doi: 10.1016/0005-2744(69)90348-9. [DOI] [PubMed] [Google Scholar]
- Stormer F. C. 2,3-Butanediol biosynthetic system in Aerobacter aerogenes. Methods Enzymol. 1975;41:518–532. doi: 10.1016/s0076-6879(75)41108-9. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Kirchniawy F. H., Jungermann K. A. Properties and function of the pyruvate-formate-lyase reaction in clostridiae. Eur J Biochem. 1972 May 23;27(2):282–290. doi: 10.1111/j.1432-1033.1972.tb01837.x. [DOI] [PubMed] [Google Scholar]



