Abstract
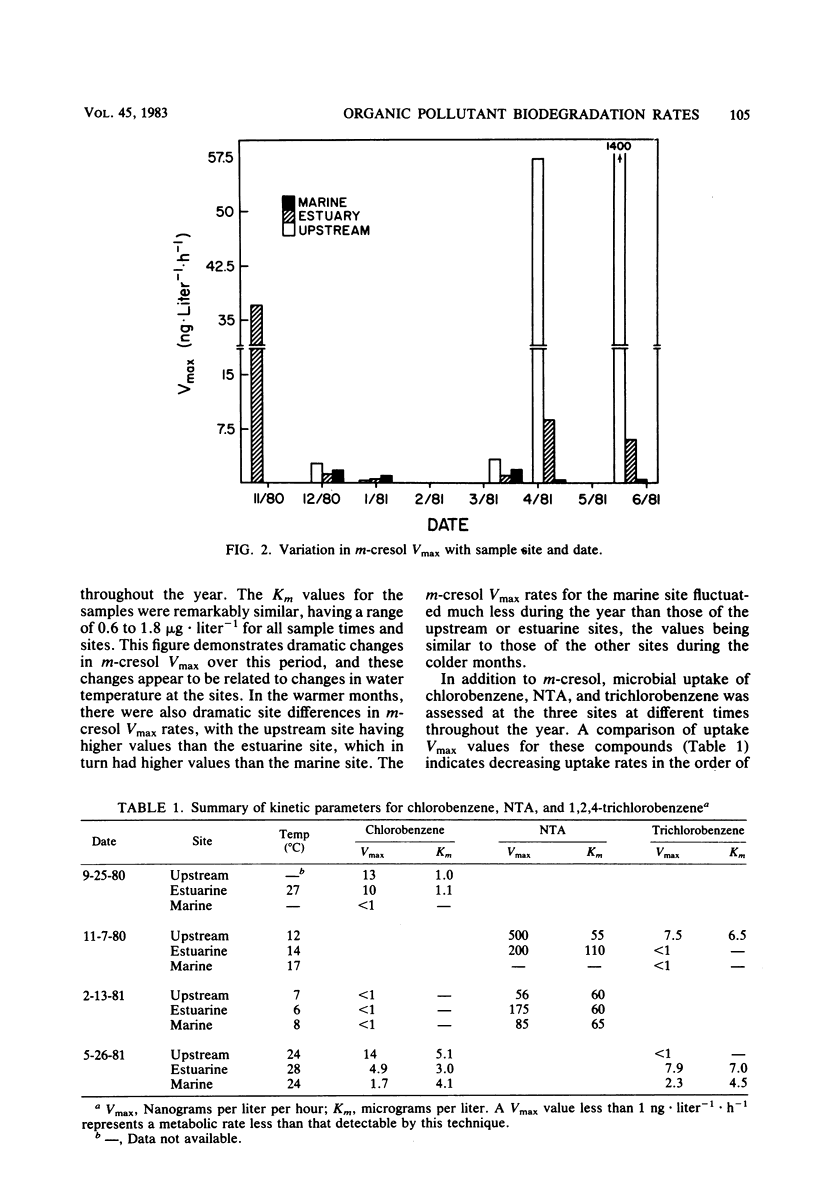
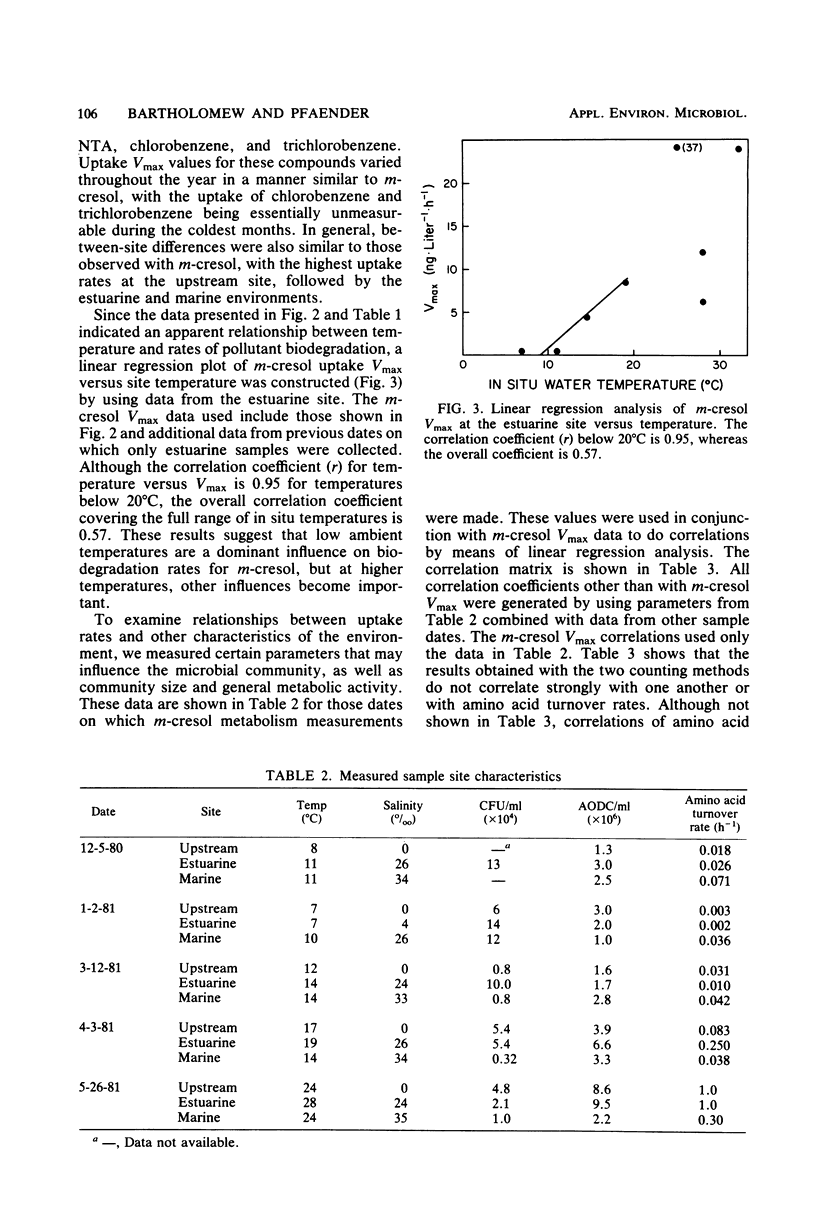
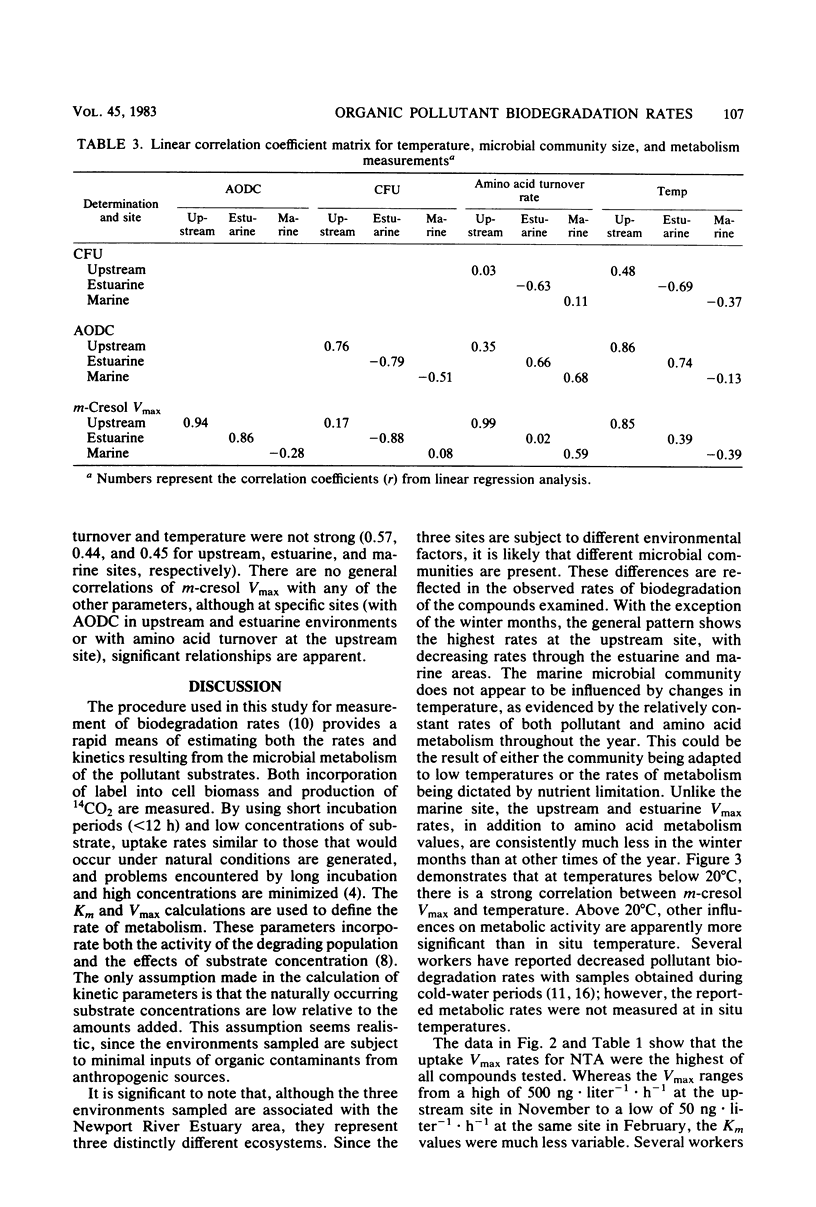
The influence of spatial and temporal environmental variations on rates of organic pollutant biodegradation were assessed by using heterotrophic uptake kinetics. These studies were conducted at three sites, representing the gradient from freshwater to estuarine to marine systems. Of the compounds tested, total uptake Vmax rates decreased in the order of nitrilotriacetic acid, m-cresol, chlorobenzene, and 1,2,4-trichlorobenzene. In general, the freshwater site exhibited the highest uptake rates, with somewhat lower rates at the estuarine site. Rates at the marine site were much lower than at the other sites, except during the winter. Metabolic rates at both the freshwater and estuarine areas were significantly decreased during periods of low water temperature. Rates at the marine site were relatively uniform throughout the year. Linear regression analysis was used to compare m-cresol biodegradation rates to characteristics of the microbial community, which included direct microscopic counts, CFU counts, and cellular incorporation of amino acids. The observed rates did not consistently correlate well with any of the measured characteristics of the microbial community.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. Biodegradation: problems of molecular recalcitrance and microbial fallibility. Adv Appl Microbiol. 1965;7:35–80. doi: 10.1016/s0065-2164(08)70383-6. [DOI] [PubMed] [Google Scholar]
- Button D. K., Schell D. M., Robertson B. R. Sensitive and accurate methodology for measuring the kinetics of concentration-dependent hydrocarbon metabolism rates in seawater by microbial communities. Appl Environ Microbiol. 1981 Apr;41(4):936–941. doi: 10.1128/aem.41.4.936-941.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D. F., Steen W. C., Baughman G. L., Barnett J. T. Second-order model to predict microbial degradation of organic compounds in natural waters. Appl Environ Microbiol. 1981 Mar;41(3):603–609. doi: 10.1128/aem.41.3.603-609.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaender F. K., Bartholomew G. W. Measurement of aquatic biodegradation rates by determining heterotrophic uptake of radiolabeled pollutants. Appl Environ Microbiol. 1982 Jul;44(1):159–164. doi: 10.1128/aem.44.1.159-164.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill T. W., Sayler G. S. Phenanthrene biodegradation in freshwater environments. Appl Environ Microbiol. 1980 Jan;39(1):172–178. doi: 10.1128/aem.39.1.172-178.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. E., Duthie J. R. The biodegradability and treatability of NTA. J Water Pollut Control Fed. 1968 Feb;40(2):306–319. [PubMed] [Google Scholar]
- Yordy J. R., Alexander M. Microbial metabolism of N-nitrosodiethanolamine in lake water and sewage. Appl Environ Microbiol. 1980 Mar;39(3):559–565. doi: 10.1128/aem.39.3.559-565.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


