Abstract
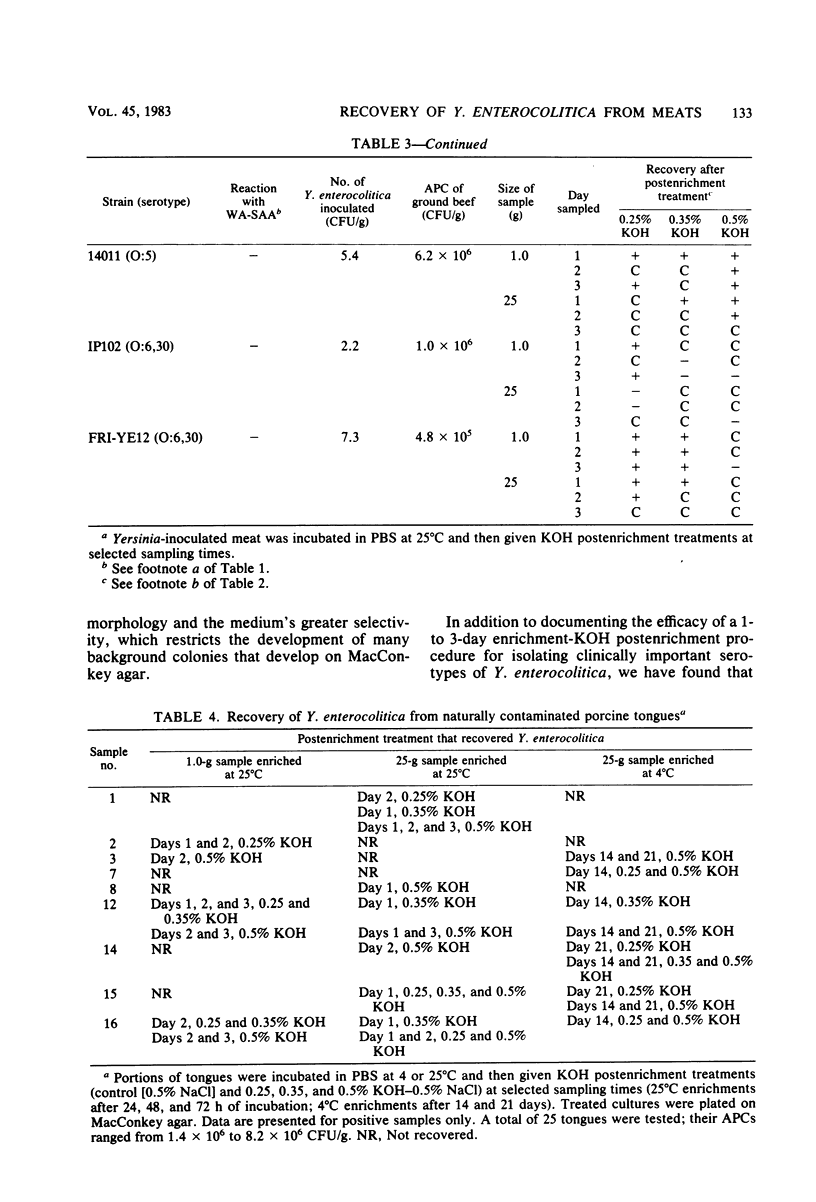
A 1- to 3-day enrichment-KOH postenrichment procedure was evaluated and found to be as effective in recovering Yersinia enterocolitica from meats as a 14- to 21-day cold enrichment procedure, with or without KOH postenrichment. The shortened procedure consists of enriching 1.0- and 25-g samples of meat in phosphate-buffered saline (pH 7.2) at 25 degrees C. After incubation (48 and 72 h for 1.0-g samples and 24 and 48 h for 25-g samples); 0.5-ml portions of enrichment culture were treated with 4.5 ml of 0.25% KOH-0.5% NaCl for 2 min and 0.5% KOH-0.5% NaCl for 15 s, and 0.1-ml portions of treated culture were plated onto MacConkey or CIN agars or both. The procedure effectively recovered 2 to 12 cells of a number of both mouse-virulent and avirulent strains per g of ground beef with aerobic plate counts of approximately 10(6) to 10(7) CFU/g. Similarly, the procedure isolated both likely virulent and avirulent strains from porcine tongues (aerobic plate counts of 10(5) to 10(7) CFU/g) naturally contaminated with Y. enterocolitica. The organism was isolated from the tongues at similar rates by both shortened enrichment and cold enrichment procedures. Eight tongues were positive for serotype O:5,27 strains that agglutinate with WA-specific absorbed antiserum, an antiserum specific for mouse-virulent Y. enterocolitica (Doyle et al., Infect. Immun. 37:1234-1240, 1982), indicating that the oral cavity of swine is a reservoir of likely virulent serotype O:5,27 strains.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aulisio C. C., Mehlman I. J., Sanders A. C. Alkali method for rapid recovery of Yersinia enterocolitica and Yersinia pseudotuberculosis from foods. Appl Environ Microbiol. 1980 Jan;39(1):135–140. doi: 10.1128/aem.39.1.135-140.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett M. L. Yersiniosis in California. Contrib Microbiol Immunol. 1979;5:159–168. [PubMed] [Google Scholar]
- Bottone E. J., Robin T. Prevalence of unique Yersinia enterocolitica in the area of the Mount Sinai Hospital, New York, N.Y. Contrib Microbiol Immunol. 1979;5:95–105. [PubMed] [Google Scholar]
- Carter P. B. Pathogenecity of Yersinia enterocolitica for mice. Infect Immun. 1975 Jan;11(1):164–170. doi: 10.1128/iai.11.1.164-170.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M. P., Hugdahl M. B., Chang M. T., Beery J. T. Serological relatedness of mouse-virulent Yersinia enterocolitica. Infect Immun. 1982 Sep;37(3):1234–1240. doi: 10.1128/iai.37.3.1234-1240.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M. P., Hugdahl M. B., Taylor S. L. Isolation of virulent Yersinia enterocolitica from porcine tongues. Appl Environ Microbiol. 1981 Oct;42(4):661–666. doi: 10.1128/aem.42.4.661-666.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. H., Harris M. E., McClain D., Smith R. E., Johnston R. W. Two modified selenite media for the recovery of Yersinia enterocolitica from meats. Appl Environ Microbiol. 1980 Jan;39(1):205–209. doi: 10.1128/aem.39.1.205-209.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. H. Testing for the recovery of Yersinia enterocolitica in foods and their ability to invade HeLa cells. Contrib Microbiol Immunol. 1979;5:228–233. [PubMed] [Google Scholar]
- Marks M. I., Pai C. H., Lafleur L., Lackman L., Hammerberg O. Yersinia enterocolitica gastroenteritis: a prospective study of clinical, bacteriologic, and epidemiologic features. J Pediatr. 1980 Jan;96(1):26–31. doi: 10.1016/s0022-3476(80)80318-0. [DOI] [PubMed] [Google Scholar]
- Schiemann D. A. Development of a two-step enrichment procedure for recovery of Yersinia enterocolitica from food. Appl Environ Microbiol. 1982 Jan;43(1):14–27. doi: 10.1128/aem.43.1.14-27.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann D. A. Enrichment methods for recovery of Yersinia enterocolitica from foods and raw milk. Contrib Microbiol Immunol. 1979;5:212–227. [PubMed] [Google Scholar]
- Schiemann D. A. Synthesis of a selective agar medium for Yersinia enterocolitica. Can J Microbiol. 1979 Nov;25(11):1298–1304. doi: 10.1139/m79-205. [DOI] [PubMed] [Google Scholar]
- Toma S., Lafleur L., Deidrick V. R. Canadian experience with Yersinia enterocolitica (1966--1977). Contrib Microbiol Immunol. 1979;5:144–149. [PubMed] [Google Scholar]
- Toma S., Lafleur L. Survey on the incidence of Yersinia enterocolitica infection in Canada. Appl Microbiol. 1974 Sep;28(3):469–473. doi: 10.1128/am.28.3.469-473.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Noyen R., Vandepitte J. L'isolement de Yersinia enterocolitica par une technique usuelle de coproculture. Ann Inst Pasteur (Paris) 1968 Apr;114(4):463–467. [PubMed] [Google Scholar]
- Van Noyen R., Vandepitte J., Wauters G. Nonvalue of cold enrichment of stools for isolation of Yersinia enterocolitica serotypes 3 and 9 from patients. J Clin Microbiol. 1980 Feb;11(2):127–131. doi: 10.1128/jcm.11.2.127-131.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandepitte J., Wauters G. Epidemiological and clinical aspects of human Yersinia enterocolitica infections in Belgium. Contrib Microbiol Immunol. 1979;5:150–158. [PubMed] [Google Scholar]



