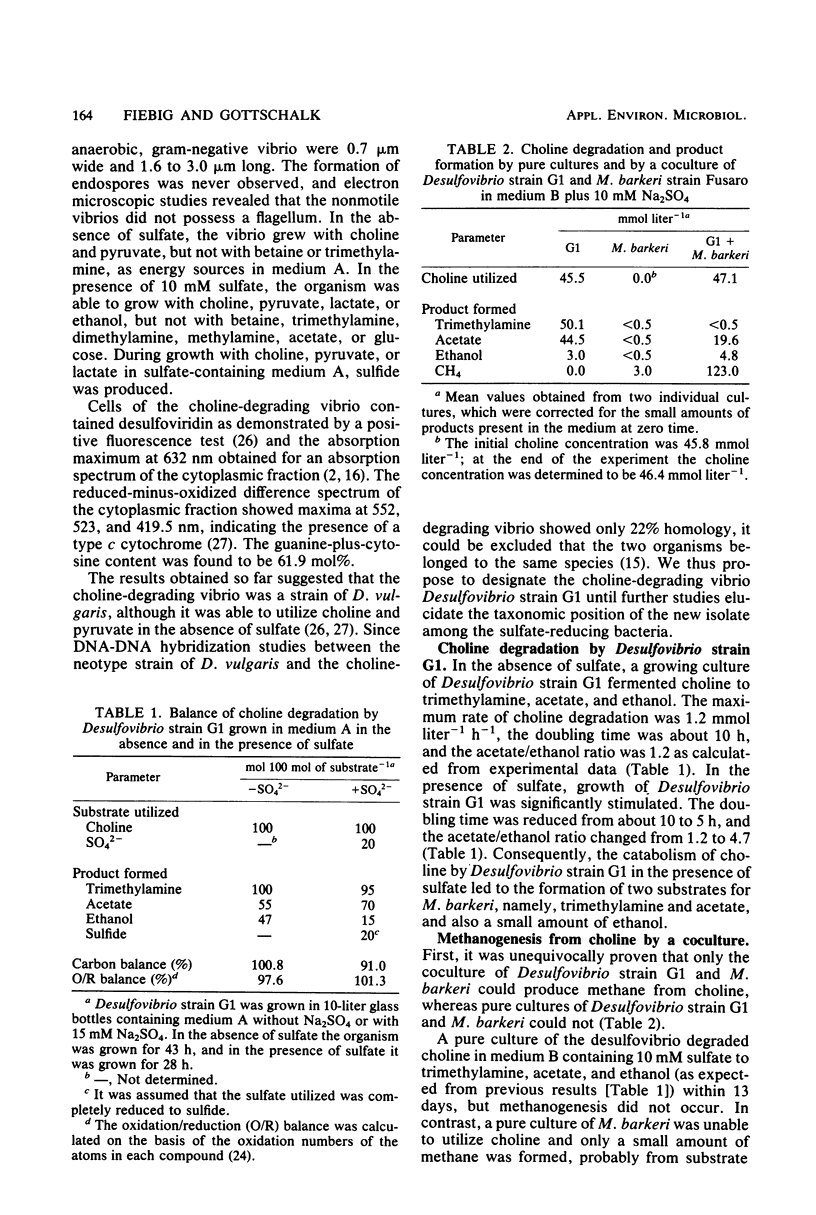
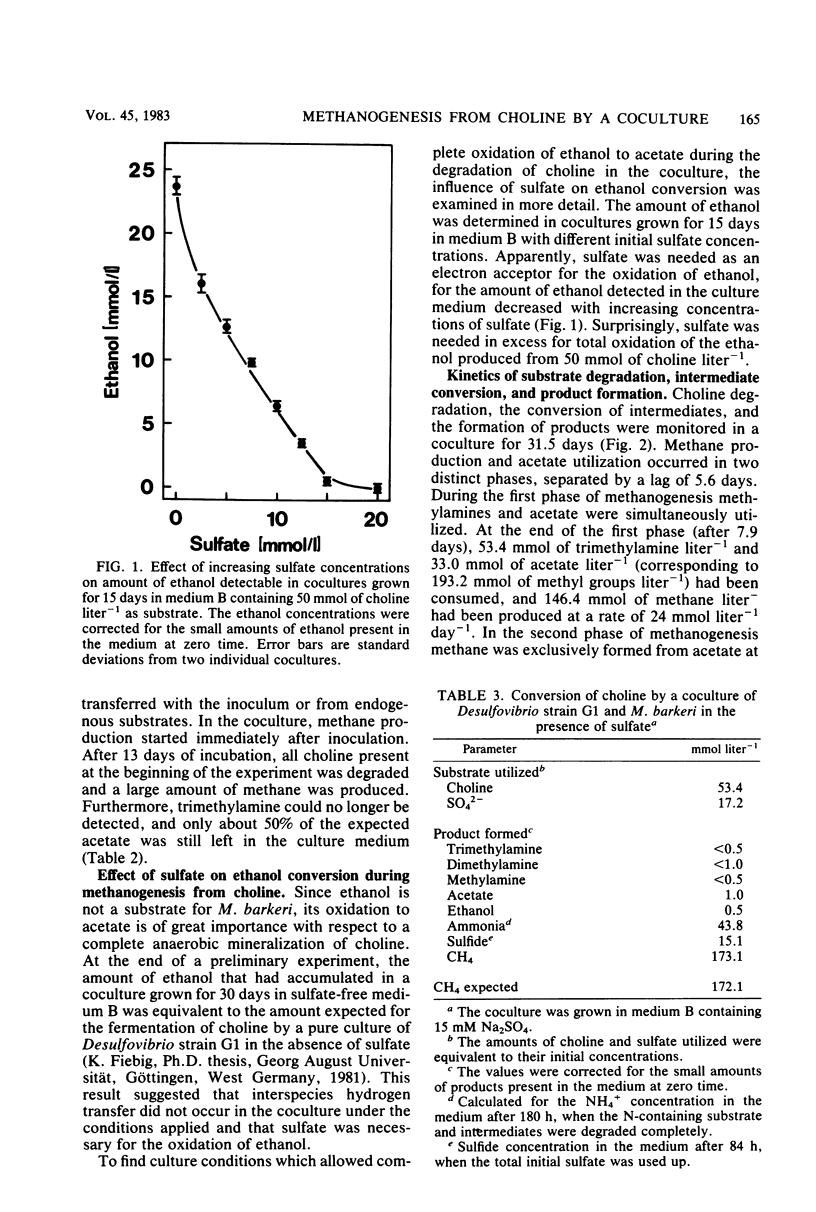
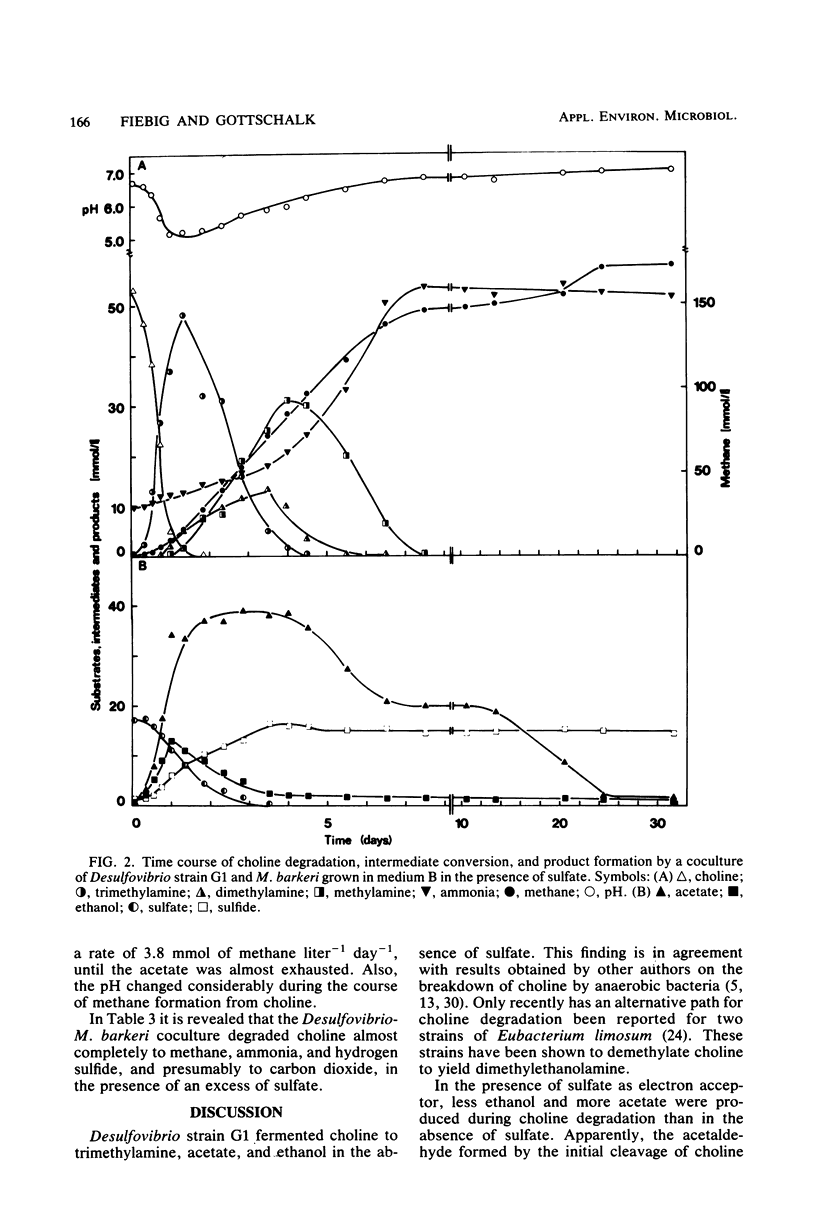
Abstract
A sulfate-reducing vibrio was isolated from a methanogenic enrichment with choline as the sole added organic substrate. This organism was identified as a member of the genus Desulfovibrio and was designated Desulfovibrio strain G1. In a defined medium devoid of sulfate, a pure culture of Desulfovibrio strain G1 fermented choline to trimethylamine, acetate, and ethanol. In the presence of sulfate, more acetate and less ethanol were formed from choline than in the absence of sulfate. When grown in a medium containing sulfate, a coculture of Desulfovibrio strain G1 and Methanosarcina barkeri strain Fusaro degraded choline almost completely to methane, ammonia, and hydrogen sulfide and presumably to carbon dioxide. Methanogenesis occurred in two distinct phases separated by a lag of about 6 days. During the first phase of methanogenesis choline was completely converted to trimethylamine, acetate, hydrogen sulfide, and traces of ethanol by the desulfovibrio. M. barkeri fermented trimethylamine to methane, ammonia, and presumably carbon dioxide via dimethyl- and methylamine as intermediates. Simultaneously, about 60% of the acetate expected was metabolized. In the second phase of methanogenesis, the residual acetate was almost completely catabolized.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badziong W., Thauer R. K., Zeikus J. G. Isolation and characterization of Desulfovibrio growing on hydrogen plus sulfate as the sole energy source. Arch Microbiol. 1978 Jan 23;116(1):41–49. doi: 10.1007/BF00408732. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbeer C. The clostridial fermentations of choline and ethanolamine. 1. Preparation and properties of cell-free extracts. J Biol Chem. 1965 Dec;240(12):4669–4674. [PubMed] [Google Scholar]
- Bradley S. G. Relationships among mycobacteria and nocardiae based upon deoxyribonucleic acid reassociation. J Bacteriol. 1973 Feb;113(2):645–651. doi: 10.1128/jb.113.2.645-651.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P., Campbell L. L., Reddy C. A., Crabill M. R. Growth of desulfovibrio in lactate or ethanol media low in sulfate in association with H2-utilizing methanogenic bacteria. Appl Environ Microbiol. 1977 May;33(5):1162–1169. doi: 10.1128/aem.33.5.1162-1169.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Bryant M. P., Wolin E. A., Wolin M. J., Wolfe R. S. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch Mikrobiol. 1967;59(1):20–31. doi: 10.1007/BF00406313. [DOI] [PubMed] [Google Scholar]
- De Ley J., Cattoir H., Reynaerts A. The quantitative measurement of DNA hybridization from renaturation rates. Eur J Biochem. 1970 Jan;12(1):133–142. doi: 10.1111/j.1432-1033.1970.tb00830.x. [DOI] [PubMed] [Google Scholar]
- HAYWARD H. R., STADTMAN T. C. Anaerobic degradation of choline. I. Fermentation of choline by an anaerobic, cytochrome-producing bacterium, Vibrio cholinicus n. sp. J Bacteriol. 1959 Oct;78:557–561. doi: 10.1128/jb.78.4.557-561.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippe H., Caspari D., Fiebig K., Gottschalk G. Utilization of trimethylamine and other N-methyl compounds for growth and methane formation by Methanosarcina barkeri. Proc Natl Acad Sci U S A. 1979 Jan;76(1):494–498. doi: 10.1073/pnas.76.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. E. Cytochromes and other pigments of dissimilatory sulphate-reducing bacteria. Arch Mikrobiol. 1972;84(3):207–224. doi: 10.1007/BF00425199. [DOI] [PubMed] [Google Scholar]
- Krzycki J. A., Wolkin R. H., Zeikus J. G. Comparison of unitrophic and mixotrophic substrate metabolism by acetate-adapted strain of Methanosarcina barkeri. J Bacteriol. 1982 Jan;149(1):247–254. doi: 10.1128/jb.149.1.247-254.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn W., Fiebig K., Walther R., Gottschalk G. Presence of a cytochrome b559 in Methanosarcina barkeri. FEBS Lett. 1979 Sep 15;105(2):271–274. doi: 10.1016/0014-5793(79)80627-4. [DOI] [PubMed] [Google Scholar]
- Madigan M. T., Cox J. C., Gest H. Physiology of dark fermentative growth of Rhodopseudomonas capsulata. J Bacteriol. 1980 Jun;142(3):908–915. doi: 10.1128/jb.142.3.908-915.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah R. A., Smith M. R., Baresi L. Studies on an acetate-fermenting strain of Methanosarcina. Appl Environ Microbiol. 1978 Jun;35(6):1174–1184. doi: 10.1128/aem.35.6.1174-1184.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney M. J., Bryant M. P. Anaerobic Degradation of Lactate by Syntrophic Associations of Methanosarcina barkeri and Desulfovibrio Species and Effect of H(2) on Acetate Degradation. Appl Environ Microbiol. 1981 Feb;41(2):346–354. doi: 10.1128/aem.41.2.346-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller E., Fahlbusch K., Walther R., Gottschalk G. Formation of N,N-Dimethylglycine, Acetic Acid, and Butyric Acid from Betaine by Eubacterium limosum. Appl Environ Microbiol. 1981 Sep;42(3):439–445. doi: 10.1128/aem.42.3.439-445.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill A. R., Grime D. W., Dawson R. M. Conversion of choline methyl groups through trimethylamine into methane in the rumen. Biochem J. 1978 Mar 15;170(3):529–535. doi: 10.1042/bj1700529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT K., LIAAENJENSEN S., SCHLEGEL H. G. DIE CAROTINOIDE DER THIORHODACEAE. I. OKENON ALS HAUPTEAROTINOID VON CHROMATIUM OKENII PERTY. Arch Mikrobiol. 1963 Aug 1;46:117–126. [PubMed] [Google Scholar]
- Smith M. R., Mah R. A. Acetate as sole carbon and energy source for growth of methanosarcina strain 227. Appl Environ Microbiol. 1980 May;39(5):993–999. doi: 10.1128/aem.39.5.993-999.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., Mah R. A. Growth and methanogenesis by Methanosarcina strain 227 on acetate and methanol. Appl Environ Microbiol. 1978 Dec;36(6):870–879. doi: 10.1128/aem.36.6.870-879.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strøm A. R., Larsen H. Anaerobic fish spoilage by bacteria. I. Biochemical changes in herring extracts. J Appl Bacteriol. 1979 Jun;46(3):531–543. doi: 10.1111/j.1365-2672.1979.tb00852.x. [DOI] [PubMed] [Google Scholar]
- TRUEPER H. G., SCHLEGEL H. G. SULPHUR METABOLISM IN THIORHODACEAE. I. QUANTITATIVE MEASUREMENTS ON GROWING CELLS OF CHROMATIUM OKENII. Antonie Van Leeuwenhoek. 1964;30:225–238. doi: 10.1007/BF02046728. [DOI] [PubMed] [Google Scholar]
- Winter J. U., Wolfe R. S. Methane formation from fructose by syntrophic associations of Acetobacterium woodii and different strains of methanogens. Arch Microbiol. 1980 Jan;124(1):73–79. doi: 10.1007/BF00407031. [DOI] [PubMed] [Google Scholar]
- Winter J., Wolfe R. S. Complete degradation of carbohydrate to carbon dioxide and methane by syntrophic cultures of Acetobacterium woodii and Methanosarcina barkeri. Arch Microbiol. 1979 Apr;121(1):97–102. doi: 10.1007/BF00409211. [DOI] [PubMed] [Google Scholar]
- Zinder S. H., Mah R. A. Isolation and Characterization of a Thermophilic Strain of Methanosarcina Unable to Use H(2)-CO(2) for Methanogenesis. Appl Environ Microbiol. 1979 Nov;38(5):996–1008. doi: 10.1128/aem.38.5.996-1008.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



