Abstract
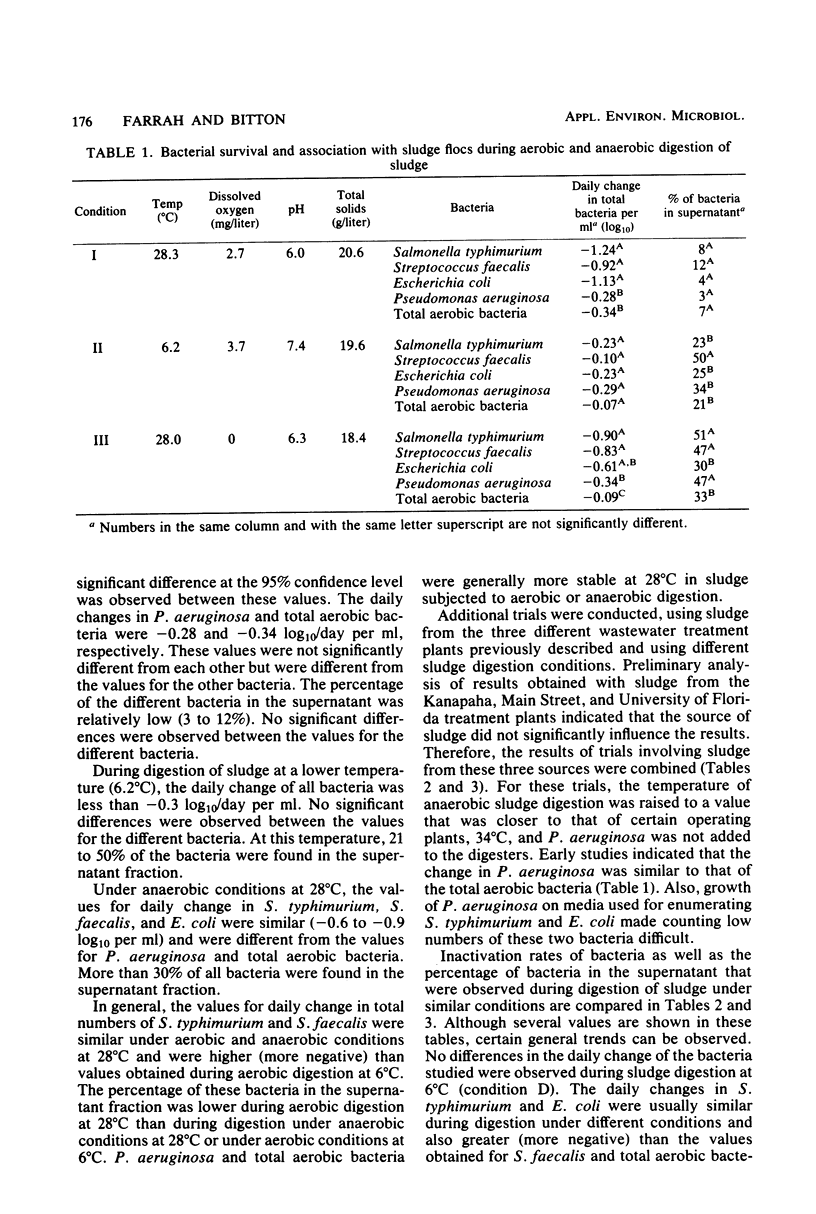
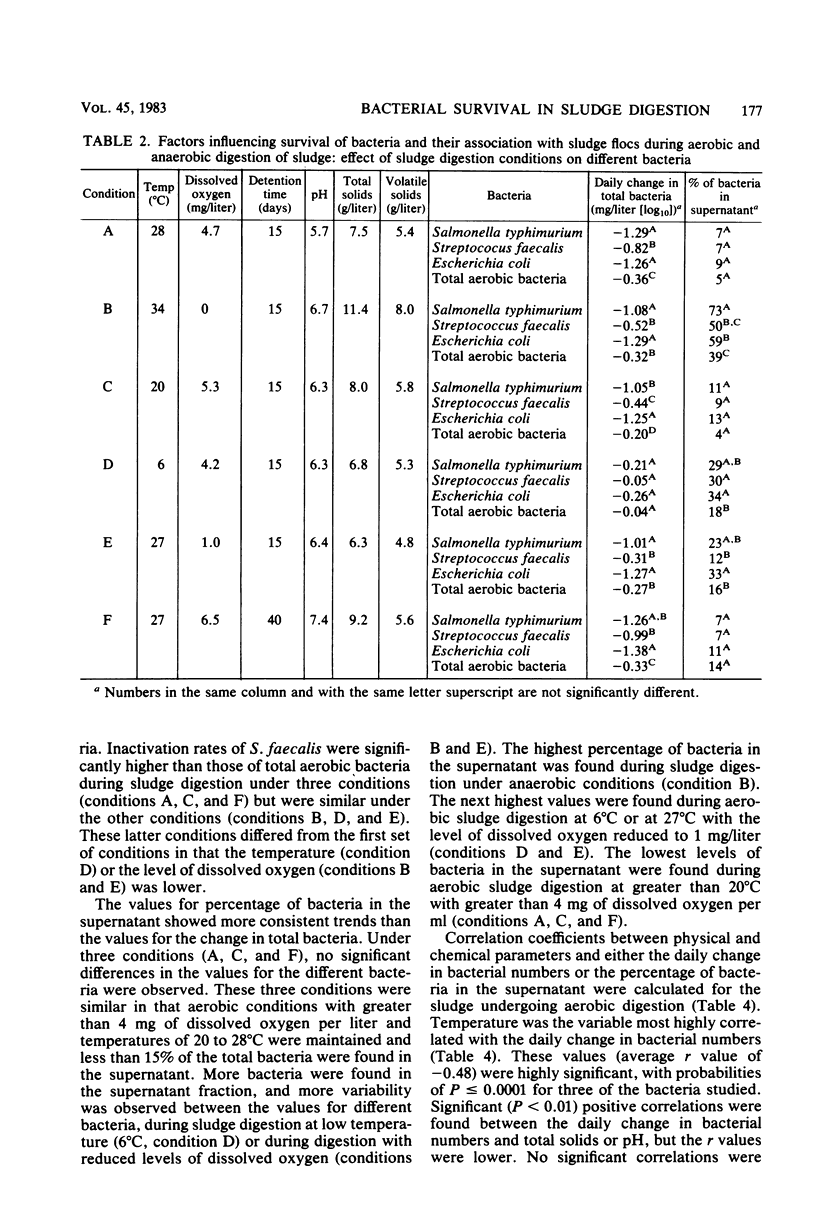
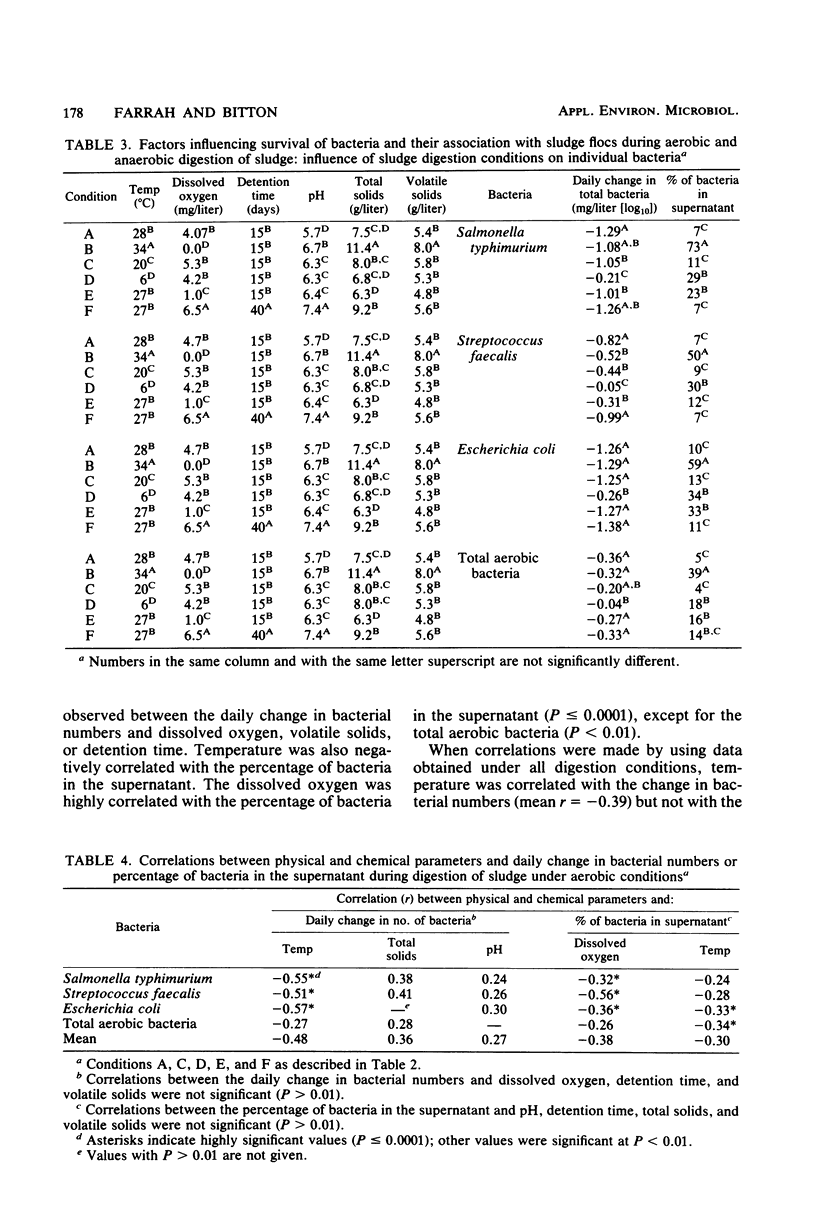
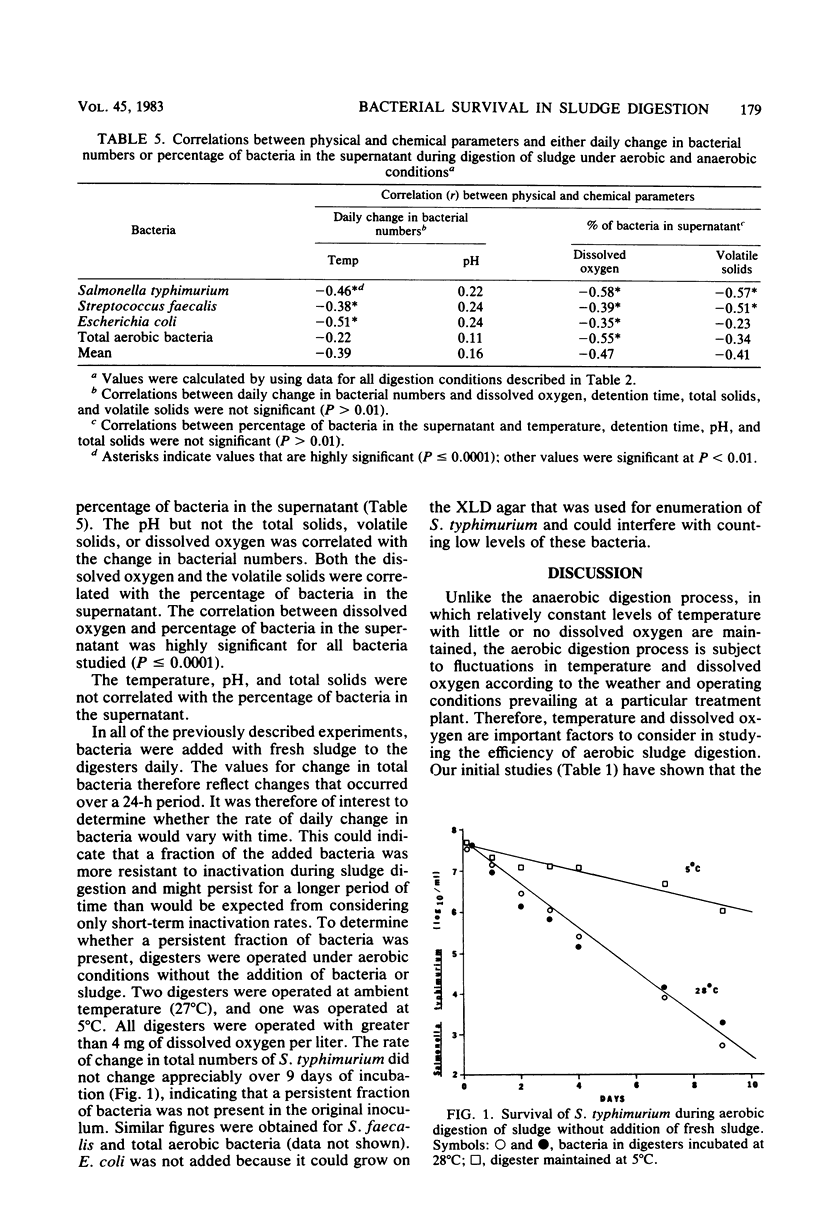
The fate of indicator bacteria, a bacterial pathogen, and total aerobic bacteria during aerobic and anaerobic digestion of wastewater sludge under laboratory conditions was determined. Correlation coefficients were calculated between physical and chemical parameters (temperature, dissolved oxygen, pH, total solids, and volatile solids) and either the daily change in bacterial numbers or the percentage of bacteria in the supernatant. The major factor influencing survival of Salmonella typhimurium and indicator bacteria during aerobic digestion was the temperature of sludge digestion. At 28 degrees C with greater than 4 mg of dissolved oxygen per liter, the daily change in numbers of these bacteria was approximately -1.0 log10/ml. At 6 degrees C, the daily change was less than -0.3 log10/ml. Most of the bacteria were associated with the sludge flocs during aerobic digestion of sludge at 28 degrees C with greater than 2.4 mg of dissolved oxygen per liter. Lowering the temperature or the amount of dissolved oxygen decreased the fraction of bacteria associated with the flocs and increased the fraction found in the supernatant.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg G., Berman D. Destruction by anaerobic mesophilic and thermophilic digestion of viruses and indicator bacteria indigenous to domestic sludges. Appl Environ Microbiol. 1980 Feb;39(2):361–368. doi: 10.1128/aem.39.2.361-368.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliver D. O. Virus association with wastewater solids. Environ Lett. 1975;10(3):215–223. doi: 10.1080/00139307509435823. [DOI] [PubMed] [Google Scholar]
- Dudley D. J., Guentzel M. N., Ibarra M. J., Moore B. E., Sagik B. P. Enumeration of potentially pathogenic bacteria from sewage sludges. Appl Environ Microbiol. 1980 Jan;39(1):118–126. doi: 10.1128/aem.39.1.118-126.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayford C. G., Richards J. P. Isolation and enumeration of aerobic heterotrophic bacteria in activated sludge. J Appl Bacteriol. 1970 Jun;33(2):342–350. doi: 10.1111/j.1365-2672.1970.tb02205.x. [DOI] [PubMed] [Google Scholar]
- Hess E., Breer C. Salmonellenepidemiologie und Grünlanddüngung mit Klärschlamm. Zentralbl Bakteriol Orig B. 1975 Sep;161(1):54–60. [PubMed] [Google Scholar]
- PRAMER D., HEUKELEKIAN H., RAGOTZKIE R. A. Survival of tubercle bacilli in various sewage treatment processes; development of a method for the quantitative recovery of Mycobacteria from sewage. Public Health Rep. 1950 Jul 7;65(27):851–859. [PMC free article] [PubMed] [Google Scholar]
- Schaub S. A., Sagik B. P. Association of enteroviruses with natural and artificially introduced colloidal solids in water and infectivity of solids-associated virions. Appl Microbiol. 1975 Aug;30(2):212–222. doi: 10.1128/am.30.2.212-222.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. L., Ashley C. S. Inactivation of poliovirus in digested sludge. Appl Environ Microbiol. 1976 Jun;31(6):921–930. doi: 10.1128/aem.31.6.921-930.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



