Abstract
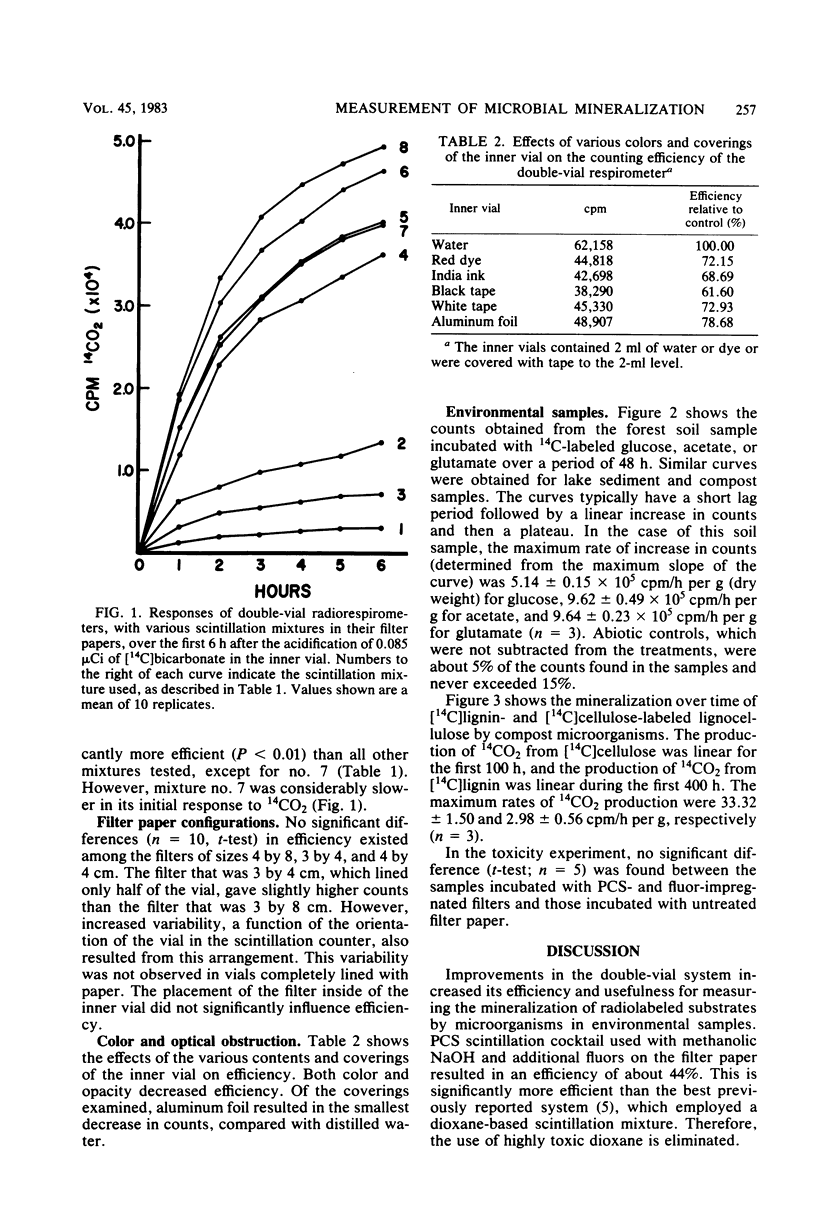
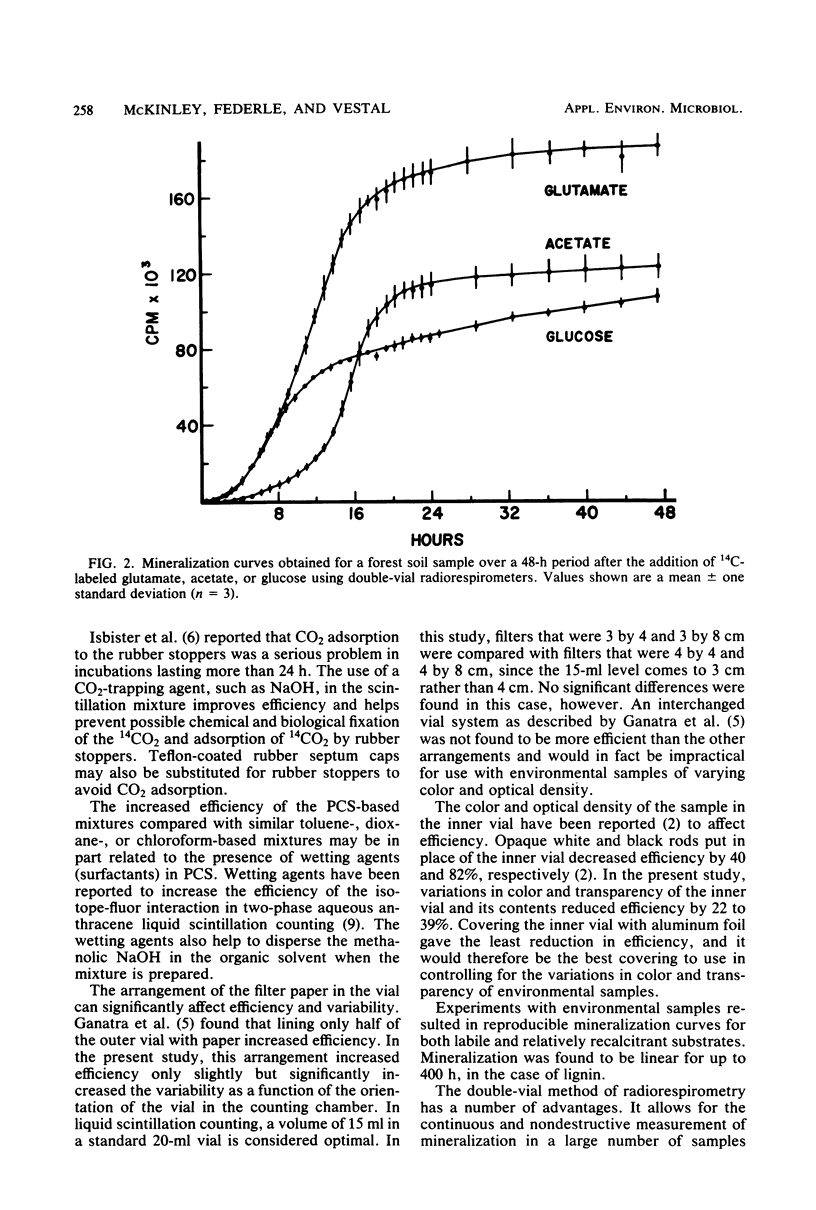
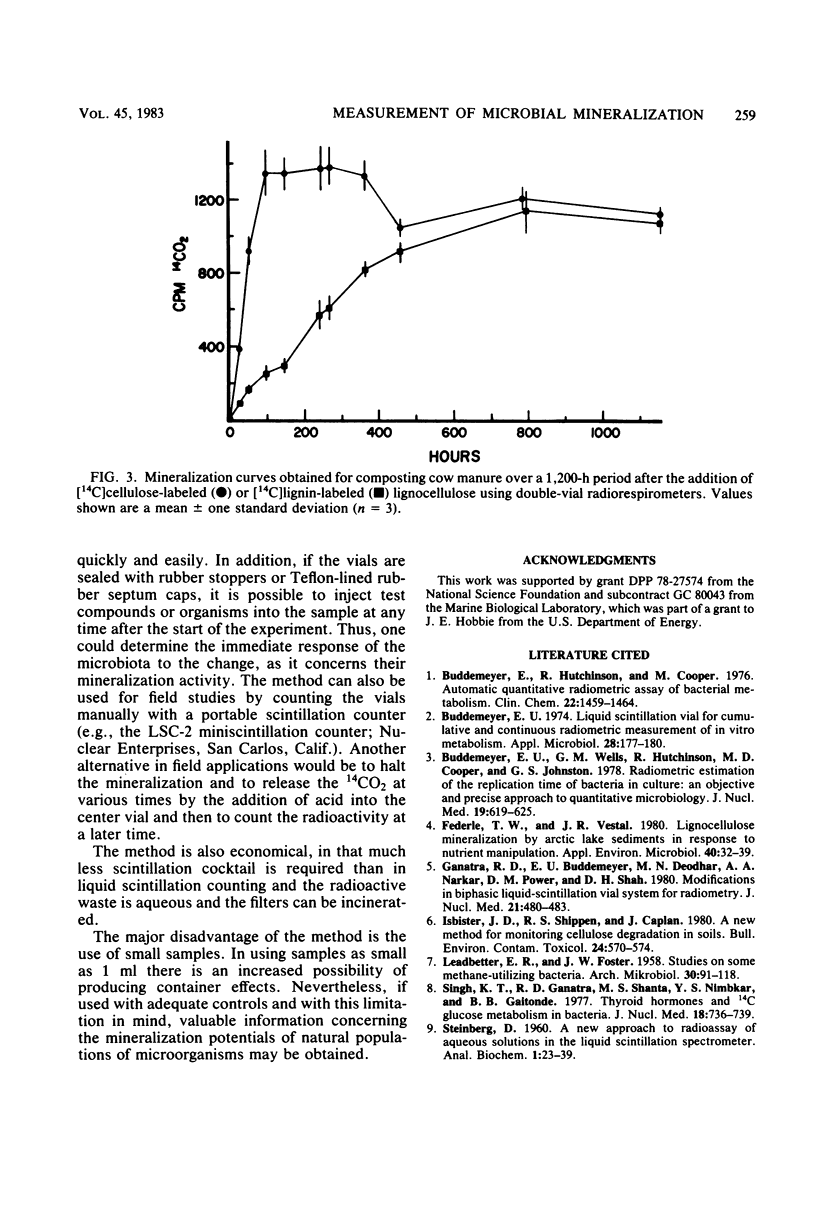
Several variations in the scintillation mixture and the filter paper arrangements for double-vial radiorespirometry were compared. Improved efficiencies (44%) and shorter response times were found by adding wetting agents and methanolic NaOH to the scintillation mixture in the filter paper. The scintillation chemicals used did not contain dioxane and were found to be nontoxic to the test microbiota in this system. Covering the inner reaction vial with aluminum foil minimized the reduction in counting efficiency when testing colored or dense environmental samples. Mineralization rates were determined with 14C-labeled glucose, acetate, and glutamate and [14C]cellulose- and [14C]lignin-labeled lignocellulose for composting cow manure, forest soil, and arctic lake sediment microbiota. This improved method can be used in a variety of procedures involving the measurement of microbial mineralizations of organic compounds. Since no liquid scintillation cocktail is used for counting, the radioactive wastes are aqueous or can be incinerated, making disposal easy.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buddemeyer E. U. Liquid scintillation vial for cumulative and continuous radiometric measurement of in vitro metabolism. Appl Microbiol. 1974 Aug;28(2):177–180. doi: 10.1128/am.28.2.177-180.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buddemeyer E. U., Wells G. M., Hutchinson R., Cooper M. D., Johnston G. S. Radiometric estimation of the replication time of bacteria in culture: an objective and precise approach to quantitative microbiology. J Nucl Med. 1978 Jun;19(6):619–625. [PubMed] [Google Scholar]
- Federle T. W., Vestal J. R. Lignocellulose mineralization by arctic lake sediments in response to nutrient manipulation. Appl Environ Microbiol. 1980 Jul;40(1):32–39. doi: 10.1128/aem.40.1.32-39.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganatra R. D., Buddemeyer E. U., Deodhar M. N., Narkar A. A., Powar D. M., Shah D. H. Modifications in biphasic liquid-scintillation vial system for radiometry. J Nucl Med. 1980 May;21(5):480–483. [PubMed] [Google Scholar]
- Isbister J. D., Shippen R. S., Caplan J. A new method for monitoring cellulose and starch degradation in soils. Bull Environ Contam Toxicol. 1980 Apr;24(4):570–574. doi: 10.1007/BF01608157. [DOI] [PubMed] [Google Scholar]
- LEADBETTER E. R., FOSTER J. W. Studies on some methane-utilizing bacteria. Arch Mikrobiol. 1958;30(1):91–118. doi: 10.1007/BF00509229. [DOI] [PubMed] [Google Scholar]
- STEINBERG D. A new approach to radioassay of aqueous solutions in the liquid scintillation spectrometer. Anal Biochem. 1960 Jun;1:23–39. doi: 10.1016/0003-2697(60)90016-6. [DOI] [PubMed] [Google Scholar]
- Singh K. T., Ganatra R. D., Shanta M. S., Nimbkar Y. S., Gaitonde B. B. Thyroid hormones and [14C] glucose metabolism in bacteria. J Nucl Med. 1977 Jul;18(7):736–739. [PubMed] [Google Scholar]


