Abstract
We developed a biphasic culture system consisting of 4 ml of brucella agar (BA) and 6 ml of brucella broth (BB) in 25-cm2 tissue culture flasks, which were incubated in air (BB/BAa) or in a gas mixture of 5% O2, 10% CO2, and 85% N2 (BB/BAg). These media were also used with a supplement consisting of ferrous sulfate, sodium metabisulfite, and sodium pyruvate and incubated as above (FB/FAa and FB/FAg, respectively). Highly satisfactory growth of Campylobacter jejuni 301 was obtained with all medium-gas phase combinations provided that the number of viable cells in the inoculum was large (greater than or equal to 10(6)/ml). The use of FB/FAa permitted the inoculum to be reduced to 100 cells per ml. With an adjusted gas phase (BB/BAg and FB/FAg), near-optimal growth was obtained from an inoculum of 1 to 10 cells per ml. Under most of these conditions the generation time was approximately 90 min. During the logarithmic growth phase, the cells retained their typical spiral morphology and high motility. These media also proved to be highly satisfactory for the cultivation of fresh isolates as well as other stock strains of Campylobacter. When the broth phase of the cultures, after addition of 15% glycerol, was quickly frozen and maintained at -70 degrees C, all strains thus far examined were readily recoverable and satisfactorily cultivated without additional passage.
Full text
PDF

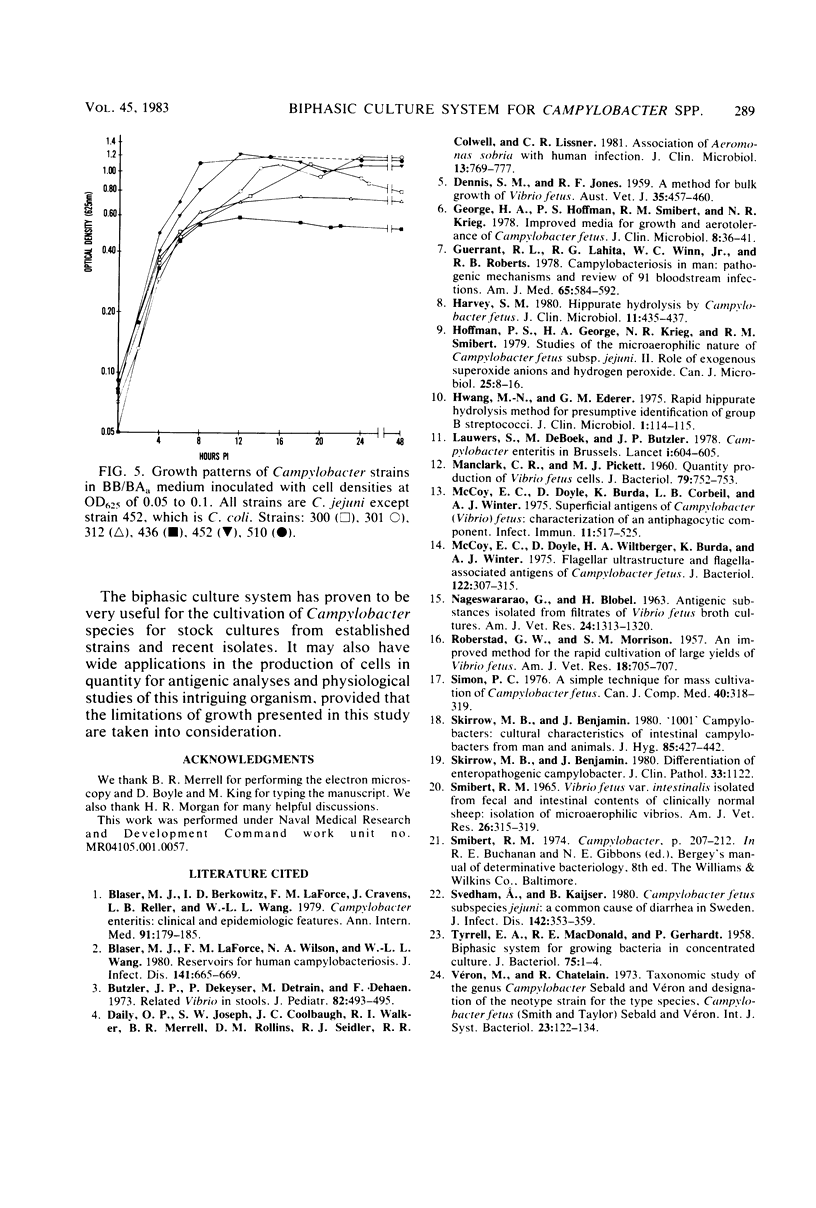
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser M. J., Berkowitz I. D., LaForce F. M., Cravens J., Reller L. B., Wang W. L. Campylobacter enteritis: clinical and epidemiologic features. Ann Intern Med. 1979 Aug;91(2):179–185. doi: 10.7326/0003-4819-91-2-179. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., LaForce F. M., Wilson N. A., Wang W. L. Reservoirs for human campylobacteriosis. J Infect Dis. 1980 May;141(5):665–669. doi: 10.1093/infdis/141.5.665. [DOI] [PubMed] [Google Scholar]
- Butzler J. P., Dekeyser P., Detrain M., Dehaen F. Related vibrio in stools. J Pediatr. 1973 Mar;82(3):493–495. doi: 10.1016/s0022-3476(73)80131-3. [DOI] [PubMed] [Google Scholar]
- Daily O. P., Joseph S. W., Coolbaugh J. C., Walker R. I., Merrell B. R., Rollins D. M., Seidler R. J., Colwell R. R., Lissner C. R. Association of Aeromonas sobria with human infection. J Clin Microbiol. 1981 Apr;13(4):769–777. doi: 10.1128/jcm.13.4.769-777.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George H. A., Hoffman P. S., Smibert R. M., Krieg N. R. Improved media for growth and aerotolerance of Campylobacter fetus. J Clin Microbiol. 1978 Jul;8(1):36–41. doi: 10.1128/jcm.8.1.36-41.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Lahita R. G., Winn W. C., Jr, Roberts R. B. Campylobacteriosis in man: pathogenic mechanisms and review of 91 bloodstream infections. Am J Med. 1978 Oct;65(4):584–592. doi: 10.1016/0002-9343(78)90845-8. [DOI] [PubMed] [Google Scholar]
- Harvey S. M. Hippurate hydrolysis by Campylobacter fetus. J Clin Microbiol. 1980 Apr;11(4):435–437. doi: 10.1128/jcm.11.4.435-437.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. S., George H. A., Krieg N. R., Smibert R. M. Studies of the microaerophilic nature of Campylobacter fetus subsp. jejuni. II. Role of exogenous superoxide anions and hydrogen peroxide. Can J Microbiol. 1979 Jan;25(1):8–16. doi: 10.1139/m79-002. [DOI] [PubMed] [Google Scholar]
- Hwang M. N., Ederer G. M. Rapid hippurate hydrolysis method for presumptive identification of group B streptococci. J Clin Microbiol. 1975 Jan;1(1):114–115. doi: 10.1128/jcm.1.1.114-115.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwers S., De Boeck M., Butzler J. P. Campylobacter enteritis in Brussels. Lancet. 1978 Mar 18;1(8064):604–605. doi: 10.1016/s0140-6736(78)91045-0. [DOI] [PubMed] [Google Scholar]
- MANCLARK C. R., PICKETT M. J. Quantity production of Vibrio fetus cells. J Bacteriol. 1960 May;79:752–753. doi: 10.1128/jb.79.5.752-753.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy E. C., Doyle D., Burda K., Corbeil L. B., Winter A. J. Superficial antigens of Campylobacter (Vibrio) fetus: characterization of antiphagocytic component. Infect Immun. 1975 Mar;11(3):517–525. doi: 10.1128/iai.11.3.517-525.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy E. C., Doyle D., Wiltberger H., Burda K., Winter A. J. Flagellar ultrastructure and flagella-associated antigens of Campylobacter fetus. J Bacteriol. 1975 Apr;122(1):307–315. doi: 10.1128/jb.122.1.307-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGESWARARAO G., BLOBEL H. ANTIGENIC SUBSTANCES ISOLATED FROM FILTRATES OF VIBRIO FETUS BROTH CULTURES. Am J Vet Res. 1963 Nov;24:1313–1320. [PubMed] [Google Scholar]
- ROBERTSTAD G. W., MORRISON S. M. An improved method for the rapid cultivation of large yields of Vibrio fetus. Am J Vet Res. 1957 Jul;18(68):705–707. [PubMed] [Google Scholar]
- Simon P. C. A simple technique for mass cultivation of Campylobacter fetus. Can J Comp Med. 1976 Jul;40(3):318–319. [PMC free article] [PubMed] [Google Scholar]
- Skirrow M. B., Benjamin J. '1001' Campylobacters: cultural characteristics of intestinal campylobacters from man and animals. J Hyg (Lond) 1980 Dec;85(3):427–442. doi: 10.1017/s0022172400063506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirrow M. B., Benjamin J. Differentiation of enteropathogenic Campylobacter. J Clin Pathol. 1980 Nov;33(11):1122–1122. doi: 10.1136/jcp.33.11.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedhem A., Kaijser B. Campylobacter fetus subspecies jejuni: a common cause of diarrhea in Sweden. J Infect Dis. 1980 Sep;142(3):353–359. doi: 10.1093/infdis/142.3.353. [DOI] [PubMed] [Google Scholar]
- TYRRELL E. A., MACDONALD R. E., GERHARDT P. Biphasic system for growing bacteria in concentrated culture. J Bacteriol. 1958 Jan;75(1):1–4. doi: 10.1128/jb.75.1.1-4.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]