Abstract
In Escherichia coli, 1-deoxy-d-xylulose (or its 5-phosphate, DXP) is the biosynthetic precursor to isopentenyl diphosphate [Broers, S. T. J. (1994) Dissertation (Eidgenössische Technische Hochschule, Zürich)], thiamin, and pyridoxol [Himmeldirk, K., Kennedy, I. A., Hill, R. E., Sayer, B. G. & Spenser, I. D. (1996) Chem. Commun. 1187–1188]. Here we show that an open reading frame at 9 min on the chromosomal map of E. coli encodes an enzyme (deoxyxylulose-5-phosphate synthase, DXP synthase) that catalyzes a thiamin diphosphate-dependent acyloin condensation reaction between C atoms 2 and 3 of pyruvate and glyceraldehyde 3-phosphate to yield DXP. We have cloned and overexpressed the gene (dxs), and the enzyme was purified 17-fold to a specific activity of 0.85 unit/mg of protein. The reaction catalyzed by DXP synthase yielded exclusively DXP, which was characterized by 1H and 31P NMR spectroscopy. Although DXP synthase of E. coli shows sequence similarity to both transketolases and the E1 subunit of pyruvate dehydrogenase, it is a member of a distinct protein family, and putative DXP synthase sequences appear to be widespread in bacteria and plant chloroplasts.
Isoprenoid-containing compounds constitute a large class of natural products, including a variety of essential compounds such as the carotenoids, the prenylquinones, and the chlorophylls (1, 2). The biosynthesis of isopentenyl diphosphate, the five-carbon building block of all isoprenoids, has long been assumed to proceed exclusively via the acetate/mevalonate pathway (3, 4). More recently, however, 13C incorporation into various isoprenic compounds in bacteria, algae, and plants has manifested the operation of an alternate pathway to isopentenyl diphosphate in these organisms (5–9). With mutants of Escherichia coli deficient in enzymes of triose phosphate metabolism, incorporation of 13C-labeled glycerol or pyruvate into ubiquinone Q8 revealed the metabolic precursors of the pathway as pyruvate and glyceraldehyde 3-phosphate (6). By feeding 2H-labeled 1-deoxy-d-xylulose to E. coli, Arigoni and co-workers demonstrated that this pentulose is the precursor of the five-carbon skeleton of isopentenyl diphosphate (ref. 10; D. Arigoni, personal communication).
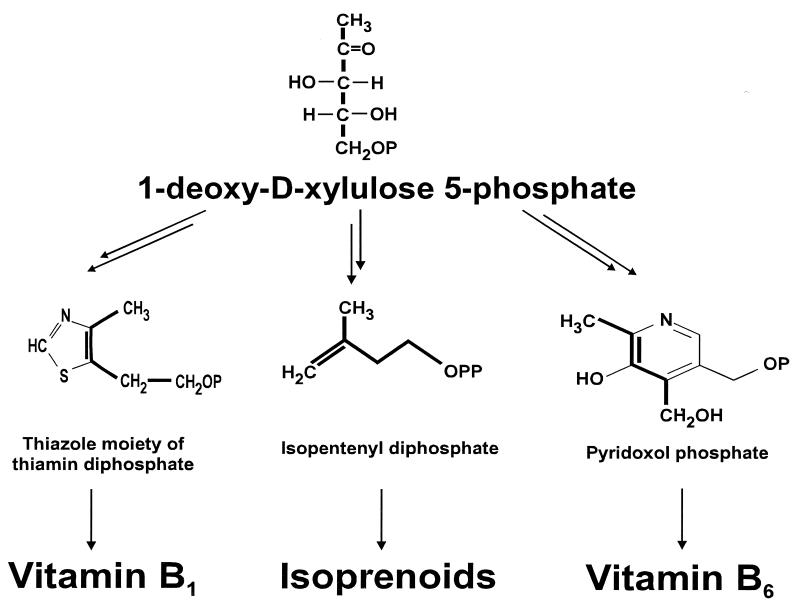
1-Deoxy-d-xylulose 5-phosphate (DXP) or the corresponding nonphosphorylated sugar not only is an intermediate on this pathway to isopentenyl diphosphate but also is involved in the biosyntheses of thiamin (vitamin B1) and of pyridoxol (vitamin B6) in E. coli (11–13) (see Fig. 1). Synthesis of DXP from pyruvate and d-glyceraldehyde 3-phosphate requires an acyloin condensation reaction whereby pyruvate is decarboxylated. Catalysis of this type of reaction is well documented as a secondary activity of thiamin diphosphate (ThDP)-dependent, pyruvate decarboxylating enzymes such as the E1 component (EC 1.2.4.1) of the pyruvate dehydrogenase complex (PDHC) or of pyruvate decarboxylase (EC 4.1.1.1) (14–17). Likewise, ThDP-dependent transketolases could also catalyze the transfer of an activated acetaldehyde group from pyruvate to glyceraldehyde 3-phosphate, although this reaction does not occur with wild-type transketolases (U.S., C. Wikner, S. Thorell, G.A.S., and G. Schneider, unpublished data). It was therefore our working hypothesis that a DXP synthase might share sequence motifs with the E1 subunit of PDHC and with transketolases. With the advent of full genomic sequence information for the metabolically best studied bacterium, E. coli (18), it became possible to screen its genome for such genes encoding products similar to transketolase and E1. We show here that an ORF at 9 min of the E. coli chromosome encodes an enzyme that synthesizes DXP from pyruvate and glyceraldehyde 3-phosphate. Homologous genes were identified in a range of other bacteria and cyanobacteria, as well as in higher plants such as Arabidopsis thaliana. We propose that this enzyme be called 1-deoxy-d-xylulose-5-phosphate synthase (DXP synthase).
Figure 1.
1-Deoxy-d-xylulose 5-phosphate as a common precursor of thiamin, pyridoxol, and isoprenoids.
MATERIALS AND METHODS
Materials.
A sample of chemically synthesized 1-deoxy-d-xylulose, used as a standard substance for HPLC peak identification, was given to us by D. Arigoni (Eidgenössische Technische Hochschule, Zürich). [2-14C]Pyruvate was obtained from DuPont/NEN. Other chemicals were from commercial origin.
Bacterial Strains, Plasmids, and Culture Conditions.
E. coli LJ110 wild-type strain [W3110 fnr+; prototrophic; obtained from J. Lengeler and K. Jahreis, Osnabrück, Germany] was used as donor for chromosomal DNA and was grown in 2 YT medium (16 g/liter tryptone, 10 g/liter yeast extract, and 10 g/liter NaCl). For cloning and expression purposes, E. coli DH5α (supE44 ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1) (19) and E. coli JM 109 (recA1 supE44 endA1 hsdR17 gyrA96 relA1 thi Δ(lac–proAB) F′ [traD36 proAB+ lacIq lacZΔM15]) (20) were grown in Luria–Bertani (LB) medium (21). Plasmids used for cloning and expression of dxs in E. coli were derivatives of pUCBM20 (Boehringer Mannheim). LB agar plates (21) were supplemented with ampicillin (100 mg/liter) for strains harboring plasmids. 5-Bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) was used at 40 mg/liter.
Assay of DXP Synthase.
The assay system for DXP synthase consisted of 200 mM sodium citrate buffer at pH 6.0, 10 mM pyruvate, 30 mM dl-glyceraldehyde 3-phosphate, 20 mM MgCl2, 1.5 mM ThDP, 1 mM dithiothreitol, 0.4 mM EDTA, 1 μCi (37 kBq) of [2-14C]pyruvate, and enzyme sample, in a total volume of 50 μl. After incubation for 1–4 h at 30°C the reaction was stopped by perchloric acid precipitation of proteins. The supernatant was neutralized with K2CO3. An aliquot of the supernatant was treated with alkaline phosphatase (15 units; Boehringer Mannheim) for 30 min at 30°C. Controls were lacking pyruvate or glyceraldehyde 3-phosphate. Phosphorylated and dephosphorylated products were analyzed by HPLC with an Aminex HPX-87H (300 × 7.8 cm) HPLC column (Bio-Rad), eluted with 6 mM H2SO4 at 65°C. For peak detection a UV monitor at 185 nm and a radioactivity monitor (Berthold LB506C) connected in series were used. Concentrations of 1-deoxy-d-xylulose were estimated by using a standard curve. One unit of enzyme activity was defined as the formation of 1 μmol of DXP per min under the conditions described above.
Purification of DXP Synthase from Recombinant E. coli.
Cells of E. coli JM109 carrying plasmid pUCBM20dxs were grown at 37°C to an OD of 0.8 at 600 nm and induced with isopropyl β-d-thiogalactoside (IPTG) (0.4 mM) for at least 4 h. Cells were harvested by centrifugation, washed with buffer A (50 mM Tris⋅HCl/1 mM dithiothreitol/0.5 mM thiamin diphosphate/5 mM MgCl2, pH 7.5), resuspended in the same buffer (1 g cell wet weight per 2.5 ml), and sonified in a Branson Sonifier (10 30-sec pulses at 40-W output, duty cycle 50%) with cooling in an ethanol/ice bath. After centrifugation (1 h at 38,000 × g) the supernatant was used as the cell-free extract. Ammonium sulfate (22.5 g/100 ml, 40% saturation) was added at 4°C. The precipitate was resuspended in buffer A, the ammonium sulfate was removed by repeated ultrafiltration, and the resulting solution was centrifuged (30 min, 38,000 × g). The sample was then applied to a 100-ml bed volume Q-Sepharose HP anion-exchange column (Pharmacia Biotech), washed with buffer A, and eluted with an increasing salt gradient (0–1 M NaCl in buffer A). DXP synthase eluted at 0.1 to 0.2 M NaCl. In a second anion-exchange chromatography, a DEAE-650S tentacle column (Merck, Darmstadt, Germany) was used with the same buffer and salt regime.
In vitro Synthesis of DXP for NMR Structural Analysis.
DXP was enzymatically synthesized and analyzed by NMR spectroscopy. The NMR spectra of the enzymatic product was identical to the spectra of a chemically synthesized DXP sample [1H NMR (2H2O, 400-MHz) δ 4.57 (d, J = 1.9 Hz, 1H), 4.38 (td, J = 6.5 and 1.9 Hz, 1H) 3.9 (dd, J = 6.5 and 7.3 Hz, 2H), 2.34 (s, 3H); 31P NMR (2H2O, 162-MHz): δ 5.2 (JPH = 7.3)] (S.V.T., L. D. Vu, T.P.B., U.S., G.A.S., S.B.-M., and H.S., unpublished data).
DNA Techniques.
Standard techniques for cloning (21), transformation (19), and PCR amplification of DNA (22) were applied. The E. coli dxs gene was amplified by PCR using primers DXSECO5 (5′-CCGAATTCAGGCCCCTGATGAGTTTTGAT-3′) and DXSECO3 (5′-TTGCATGCAGGAGTGGAGTAGGGATTATG-3′) corresponding to base pairs 19,636–19,616 (5′ end), and 17,747–17,769 (3′ end), respectively, of the sequence deposited in GenBank (18); the underlined sequences denote the engineered restriction sites for EcoRI and SphI, respectively. A 100-pmol portion of each primer was used with template chromosomal DNA (1.6 ng) from E. coli K-12 wild-type strain LJ 110 (W3110). The resulting 1.9-kb PCR fragment was purified, cleaved with EcoRI plus SphI, and ligated with pUCBM20 (Boehringer Mannheim) that had been opened with the same enzymes. After transformation into strain JM109, the integrity of the plasmid was checked by restriction analyses and DNA sequencing using an automatic nonradioactive system (Pharmacia).
RESULTS
Identification of the dxs Gene from E. coli.
The complete genome sequence of Escherichia coli has become available recently (18). At 9 min of the chromosome map (nucleotides 17,765–19,627) we detected an open reading frame (ORF) that displayed a high percentage of amino acid residues identical or similar to those in transketolases from E. coli K-12 (23, 24) (see Fig. 2) and from other organisms, as well as to those in the E1 subunit of PDHC. As it was the only ORF from the total genome of E. coli with such a prominent similarity, it appeared to be a promising candidate for a putative DXP synthase. Its location just 24 bp downstream of the stop codon of the ispA gene, encoding farnesylpyrophosphate synthase (25), pointed to a putative operon encompassing these two genes. Our suggestion about the nature of this ORF was enhanced by independent evidence that it is, indeed, involved in isoprenoid biosynthesis of E. coli (M. Rohmer, personal communication).
Figure 2.
Alignment of DXP synthase sequence from E. coli, CLA1 protein of A. thaliana, and transketolase A of E. coli. ECODXS, E. coli DXP synthase (ref. 18, this paper); CLA1AT, A. thaliana CLA1 protein, underlined is the N-terminal putative chloroplast transit peptide (26); ECOTKT1, transketolase A of E. coli (23). The shaded sequences denote the putative ThDP-binding site. The boldface characters denote amino acid residues that are highly conserved in all putative DXP synthase sequences, and asterisks below the sequence denote residues that are also identical in transketolase A from E. coli. # denotes amino acid residues that form the substrate channel of transketolase (27).
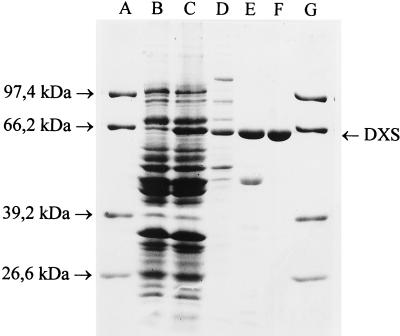
To analyze the biochemical function of this ORF, its DNA was amplified by PCR using chromosomal DNA from E. coli K-12 wild-type strain W3110. DNA sequence analyses confirmed the deposited sequence (GenBank accession no. U82664). The resulting 1.9-kb DNA fragment on vector pUCBM20 carried a minimal ribosome binding site (AGG) 7 bp upstream of the start codon, and expression in strain JM109 was driven by the resident lac promoter. In cell-free extracts of clones carrying the insert DNA, a new prominent band could be observed at an apparent molecular mass of 65 kDa on SDS/PAGE (Fig. 3), even without induction by IPTG. This size compared favorably with the deduced subunit size (620 amino acid residues, 67,617 Da).
Figure 3.
SDS/PAGE of different steps in DXP synthase purification. Lanes 1 and 7, Combithek size markers (Boehringer Mannheim). Lane 2, Cell-free extract of strain JM109/pBM20dxs without IPTG; lane 3, same plus IPTG; lane 4, after ammonium sulfate precipitation (40% saturation); lane 5, after anion-exchange chromatography on Q Sepharose HP; lane 6, after anion-exchange chromatography on DEAE-650S tentacle column. DXS denotes the protein band containing DXP synthase.
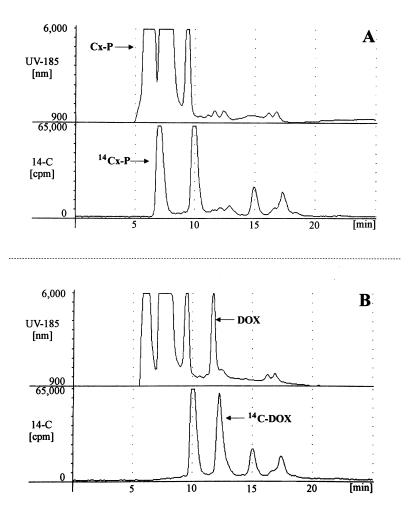
The cell-free extracts were analyzed for their ability to form DXP from pyruvate and glyceraldehyde 3-phosphate by HPLC analysis of the phosphorylated reaction products that eluted in the void volume of the column (Fig. 4A) or of the reaction products after dephosphorylation with alkaline phosphatase (Fig. 4B). Combined UV and radioactivity monitoring of eluates allowed the identification of 1-deoxy-D-xylulose after the dephosphorylation step, visible as a novel UV and 14C peak as compared with the run before dephosphorylation. Judging from the 120-fold increased activity found in these extracts as compared with those of E. coli wild-type cells, the ORF encoded an enzyme that synthesized DXP from pyruvate and glyceraldehyde 3-phosphate (Table 1).
Figure 4.
UV (A185) and 14C elution profiles of DXP synthase assay mixtures after HPLC on an Aminex HPX-87H column (Bio-Rad). The column eluate was directed through a UV (arbitrary units) and a radioactivity monitor connected in series. (A) UV and 14C elution profiles of the assay mixture with cell-free extract of E. coli JM109 pUCBM20dxs after 2 h of incubation at 30°C. Phosphorylated (Cx-P) and 14C-labeled reaction products (14Cx-P) appeared in the void volume. (B) Elution profiles of the assay mixture shown in A after treatment with alkaline phosphatase, showing a novel UV and 14C peak (DOX, 14C-DOX). Peaks at retention times of 9.3 min (UV) and 10.0 min (14C) represent pyruvate; other peaks stem from components of the assay mixture or other reaction products.
Table 1.
DXP synthase activities of cell-free extracts of E. coli
| Source of enzyme | Specific activity, nmol⋅min−1 per mg protein |
|---|---|
| E. coli LJ110 | 0.4 |
| E. coli JM109/pUCBM20dxs −IPTG | 12.2 |
| E. coli JM109/pUCBM20dxs +IPTG | 51.6 |
| DXP synthase (DEAE-650S fraction) | 850 |
Extracts were of wild-type E. coli LJ110, of recombinant strain JM109/pUCBM20dxs after growth of cells in the absence (−IPTG) or presence (+IPTG) of inducer, and of the enriched DXP synthase preparation after the final DEAE-650S tentacle anion-exchange chromatography.
Properties of DXP Synthase.
Using ammonium sulfate precipitation and two successive anion-exchange chromatographies (Q-Sepharose HP, DEAE-650S tentacle column), we purified DXP synthase to near homogeneity (>95%) from cell-free extracts of E. coli JM109/pUCBM20dxs (Table 1). The purified protein showed a prominent band on SDS/PAGE at the expected size of 65 kDa (Fig. 3). By using limited Edman degradation, the N-terminal residues were determined as FDIAKY, which matched the residues deduced from the sequence of dxs, with the exception of the first formylmethionine and the second residue, serine, which could not be read unambiguously. The enzyme lost its activity after dialysis against buffer without ThDP. The activity could be recovered to more than 50% when ThDP was added again, demonstrating that the enzyme is dependent on thiamin for its catalytic function (data not shown). The structure of the enzyme- catalyzed condensation product of pyruvate and glyceraldehyde 3-phosphate was verified by preparative synthesis of DXP with the enriched DXP synthase. The 1H and 31P NMR spectra of the isolated compound were identical with the spectra of a synthesized sample of DXP (S.V.T., L. D. Vu, T.P.B., U.S., G.A.S., S.B.-M. and H.S., unpublished results).
DXP Synthase Is a Member of a Distinct Protein Family in Bacteria and Plants.
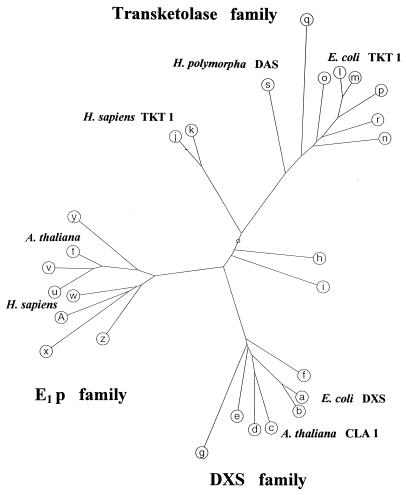
The DXP synthase sequence was compared with other known protein sequences in the data banks by using the BlastP and BlastX programs of the European Molecular Biology Laboratory Husar package (European Molecular Biology Laboratory, Heidelberg, Germany). It had been noted before that the ORF, which we now wish to term dxs, was very similar to a hypothetical protein from Haemophilus influenzae (Swiss-Prot P45205) and also that it was similar to both transketolase-like enzymes as well as to E1 proteins from PDHC of various organisms. Our new data bank search allowed us to group a series of related protein sequences into a family of (at least putative) proteins that are more closely related to each other than to the transketolases (EC 2.2.1.1) or to E1 of PDHC (EC 1.2.4.1). An unrooted tree was constructed and is shown in Fig. 5. All presumptive DXP synthase sequences are smaller than typical bacterial transketolases. They show a sequence motif that shares features with a typical binding site for the ThDP cofactor common to pyruvate decarboxylases, acetolactate synthases, and transketolases (32, 33). No function has been assigned yet to the ORFs from the Gram-positive bacterium Bacillus subtilis (Swiss-Prot P54523) or from the photosynthetic gene clusters of Rhodobacter capsulatus (P26242) and the cyanobacterium Synechocystis sp. PCC6803 (S75175). For the dicotyledonous plant A. thaliana (mouse-ear cress), a homologous gene (CLA1 or Def) that encodes a chloroplast protein had been described recently (26). Mutants in the gene (cla1–1) had been found to be achlorotic, as they were deficient in chloroplast formation and nearly devoid of carotenoids and chlorophyll a and b (less than 3% of wild-type content); however, the biochemical reason for this deficiency was not elucidated, although it had been noticed that CLA1 was very similar to transketolases and especially to ORF C2814 from the photosynthetic gene cluster of the bacterium R. capsulatus (26, 34).
Figure 5.
Unrooted tree of DXP synthases (DXS family), transketolases, E1 proteins, and related proteins. A multiple alignment of sequences was constructed by a computer program (PepPepSearch) with an algorithm of Smith and Waterman (28). The alignment was obtained by using a deletion scoring function (29) and a Dayhoff matrix (30). The tree was generated by using an algorithm of Gonnet (31). Swiss-Prot/GenBank accession numbers of sequences are given for further identification. Characters a–g indicate DXS family sequences (a = E. coli U82664; b = Haemophilus influenzae P45205; c = A. thaliana U27099; d = Rhodobacter capsulatus P26242; e = Synechocystis sp. D90903; f = Bacillus subtilis P54523; g = Mycobacterium leprae P46708), h and i are sequences of unknown function consisting of 316 amino acid residues (Methanococcus jannaschii G64384) and 345 residues (Rhizobium sp. P55573). The transketolase family consists of j and k representing two human transketolases (P29401 and P51854), l = E. coli transketolase 1, P27302; m = H. influenzae P43757; n = Saccharomyces cerevisiae P23254; o = B. subtilis P45694; p = Alcaligenes eutrophus P21725; q = Mycoplasma genitalium P47312; r = Solanum tuberosum S58083, and s is dihydroxyacetone synthase (DAS, formaldehyde transketolase) from Hansenula polymorpha P06834. The E1 p family consists of E1 protein sequences from PHDCs (t = A. thaliana P52901; u = human M24848; v = S. cerevisiae P32473; w = B. subtilis P21881; x = Mycoplasma capricolum MCU62057; y = Thiobacillus ferrooxidans TFU81808) and E1 proteins of oxoisovalerate dehydrogenase complexes (z = B. subtilis P37941; A = Pseudomonas putida P09061.
DISCUSSION
Here we provide evidence that in E. coli synthesis of DXP from the precursors pyruvate and glyceraldehyde 3-phosphate is performed by a thiamin diphosphate-dependent enzyme, DXP synthase. The enzyme DXP synthase was purified 17-fold from a recombinant overproducer of E. coli carrying the cloned dxs gene to a specific activity of 0.85 unit/mg of protein. We propose the name dxs for the gene encoding the DXP synthase. Previously, an enzyme-catalyzed synthesis of 1-deoxy-d-xylulose was reported in several bacteria (14). There, the underlying enzyme activity was identified as the E1 component of the PDHC, which catalyzed the decarboxylation of pyruvate and the condensation of the enzyme-bound hydroxyethyl-ThDP with glyceraldehyde (16). However, because glyceraldehyde in its nonphosphorylated form is not a usual intermediate of central metabolism and because mutants carrying deletions of genes encoding the PDHC in E. coli (ΔaceEF) were auxotrophic only for acetate and not for pyridoxol or other supplements (35), the physiological relevance of this catalytic activity remains obscure. Transketolases could theoretically also catalyze DXP formation. In fact, mutants of E. coli that lacked both transketolase A and B activities, besides being auxotrophic for various aromatic amino acids and vitamins, were also auxotrophic for pyridoxine. However, growth could be complemented by compounds that were not part of the deoxyxylulose branch of pyridoxine biosynthesis (36).
DXP synthase appears to be widespread in bacteria (Gram-positive, Gram-negative, and cyanobacteria) as well as in plant chloroplasts (A. thaliana, rice, pine).§ Interestingly, an evolutionary tree (Fig. 5) of all complete DXP synthase-like sequences linked the gene product of the A. thaliana CLA1 gene into a phylogenetic group together with the ORF C2814 of the photosynthetic bacterium R. capsulatus, but apart from the cyanobacterium Synechocystis. Partial expressed sequence tags for other plants are also deposited in data banks—e.g., Oryza (rice) (emest10:OSS11339 A and OSS11559 A) and Pinus taeda (emest10:PT224) that grouped together with A. thaliana (data not shown). Although CLA1 is a nuclear gene of A. thaliana, its gene product is imported into the chloroplasts (26). It can be envisioned that the CLA1 gene is another example of a gene of prokaryotic origin that entered the plant genome as the result of the endosymbiotic process that engulfed the predecessors of modern chloroplasts.
Isoprenoid biosynthesis in plants has recently been revised on the basis of studies with labeled precursors. In feeding studies using Salvia miltiorrhiza, Arigoni and co-workers clearly demonstrated (10) that 2H-labeled 1-deoxy-d-xylulose was incorporated into ferruginol (90% incorporation of label) and into sitosterol (10%), underlining the precursor function of this deoxypentulose. In incorporation studies with 13C-labeled glucose, isoprenoids in the chloroplast (β-carotene, lutein, phytol side chains of chlorophylls, plastoquinone-9) showed a different 13C-isotopic incorporation compared with cytosolic isoprenoids such as sitosterol and stigmasterol (9). Whereas the labeling in the cytosolic sterols fit well with the classical mevalonate pathway as described for yeasts or mammalian liver tissue (3, 4), the labeling of the chloroplast isoprenoids could best be explained by the route via DXP (9). This alternative isoprenoid biosynthetic pathway had been previously described for some eubacteria (5, 6) and a green alga (8). The phenotype of the cla1–1 mutant of A. thaliana (26) as well as the sequence similarity between CLA1 and dxs of E. coli suggest that the alternative isoprenoid biosynthetic pathway is also used in the A. thaliana chloroplast. Experiments are needed to elucidate the biochemical function of CLA1.
Acknowledgments
We thank D. Arigoni (Zürich, Switzerland) for a gift of 1-deoxy-d-xylulose and for making us aware of the dissertation of S. Broers in his laboratory. We thank Lotti Birgel, Ursula Heibey, and Martha Chmielus for expert technical assistance, Joseph Lengeler and Knut Jahreis (Osnabrück, Germany) for certified strain LJ110, and Michel Rohmer (Strasbourg, France) for sharing results prior to publication. G.A.S. and U.S. were supported in part by the Deutsche Forschungsgemeinschaft through SFB380/B21. T.P.B. was supported by a grant from the National Institutes of Health (DK44083).
ABBREVIATIONS
- DXP
1-deoxy-d-xylulose 5-phosphate
- PDHC
pyruvate dehydrogenase complex
- ThDP
thiamin diphosphate
- IPTG
isopropyl β-d-thiogalactoside
Footnotes
Although data bank entries from genomic sequencing efforts for fugu fish (Fugu rubripes) or an insect (Onchocerca) might suggest that sequences closely related to DXP synthase also occur in animals, a critical inspection of the allegedly eukaryotic sequences by us revealed that these sequences shared up to 99% identical residues with the E. coli sequence; in both cases, E. coli cells had been used as hosts for construction of the gene banks and therefore a contamination with prokaryotic genes is reasonable.
References
- 1.Coolbear T, Threlfall D R. In: Biosynthesis of Terpenoid Lipids. Ratledge C, Wilkinson S G, editors. New York: Academic; 1989. pp. 115–254. [Google Scholar]
- 2.Bach T J. Lipids. 1995;30:191–202. doi: 10.1007/BF02537822. [DOI] [PubMed] [Google Scholar]
- 3.Banthorpe D V, Charlwood B V, Francis M J O. Chem Rev. 1972;72:115–155. doi: 10.1021/cr60276a002. [DOI] [PubMed] [Google Scholar]
- 4.Beyia E D, Porter J W. Annu Rev Biochem. 1976;45:113–142. doi: 10.1146/annurev.bi.45.070176.000553. [DOI] [PubMed] [Google Scholar]
- 5.Rohmer M, Knani M, Simonin P, Sutter B, Sahm H. Biochem J. 1993;295:517–524. doi: 10.1042/bj2950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohmer M, Seemann M, Horbach S, Bringer-Meyer S, Sahm H. J Am Chem Soc. 1996;118:2564–2566. [Google Scholar]
- 7.Eisenreich W, Menhard B, Hylands P J, Zenk M H, Bacher A. Proc Natl Acad Sci USA. 1996;93:6431–6436. doi: 10.1073/pnas.93.13.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwender J, Seemann M, Lichtenthaler H K, Rohmer M. Biochem J. 1996;316:73–80. doi: 10.1042/bj3160073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichtenthaler H K, Schwender J, Disch A, Rohmer M. FEBS Letters. 1997;400:271–274. doi: 10.1016/s0014-5793(96)01404-4. [DOI] [PubMed] [Google Scholar]
- 10.Broers, S. T. J. (1994) Dissertation no. 10978 (Eidgenössische Technische Hochschule, Zürich).
- 11.Hill R E, Sayer B G, Spenser I D. J Am Chem Soc. 1989;111:1916–1917. [Google Scholar]
- 12.Hill R E, Himmeldirk K, Kennedy I A, Pauloski R M, Sayer B G, Wolf E, Spenser I D. J Biol Chem. 1996;271:30426–30435. doi: 10.1074/jbc.271.48.30426. [DOI] [PubMed] [Google Scholar]
- 13.Himmeldirk, K., Kennedy, I. A., Hill, R. E., Sayer, B. G. & Spenser, I. D. (1996) Chem. Commun. 1187–1188. [DOI] [PubMed]
- 14.Yokota A, Sasajima K. Agric Biol Chem. 1984;48:149–158. [Google Scholar]
- 15.Yokota A, Sasajima K. Agric Biol Chem. 1984;48:1643–1645. [Google Scholar]
- 16.Yokota A, Sasajima K. Agric Biol Chem. 1986;50:2517–2524. [Google Scholar]
- 17.Bringer-Meyer S, Sahm H. Biocatalysis. 1988;1:321–331. [Google Scholar]
- 18.Roberts, D., Allen, E., Araujo, R., Aparicio, A., Chung, E., Davis, K., Duncan, M., Federspiel, N., Hyman, R., Kalman, S., Komp, C., Kurdi, O., Lew, H., Lin, D., Namath, A., Oefner, P., Schramm, S. & Davis, R. W. (1996) Sequence of Minutes 4–25 of Escherichia coli, GenBank database accession no. U82664.
- 19.Hanahan D. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 20.Yanisch-Perron C, Vieira J, Messing J. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 22.Mullis K B, Faloona F A. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- 23.Sprenger G A. Biochim Biophys Acta. 1993;1216:307–310. doi: 10.1016/0167-4781(93)90161-6. [DOI] [PubMed] [Google Scholar]
- 24.Iida A, Teshiba S, Mizobuchi K. J Bacteriol. 1993;175:5375–5383. doi: 10.1128/jb.175.17.5375-5383.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujisaki S, Hara H, Nishimura Y, Horiuchi K, Nishino T. J Biochem. 1990;108:995–1000. doi: 10.1093/oxfordjournals.jbchem.a123327. [DOI] [PubMed] [Google Scholar]
- 26.Mandel M A, Feldmann K A, Herrera-Estrella L, Rocha-Sosa M, León P. Plant J. 1996;9:649–658. doi: 10.1046/j.1365-313x.1996.9050649.x. [DOI] [PubMed] [Google Scholar]
- 27.Lindqvist Y, Schneider G, Ermler U, Sundström M. EMBO J. 1992;11:2373–2379. doi: 10.1002/j.1460-2075.1992.tb05301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith T F, Waterman M S. J Mol Biol. 1981;147:195–197. doi: 10.1016/0022-2836(81)90087-5. [DOI] [PubMed] [Google Scholar]
- 29.Benner S A, Cohen M A, Gerloff D L. J Mol Biol. 1993;229:205–305. doi: 10.1006/jmbi.1993.1035. [DOI] [PubMed] [Google Scholar]
- 30.Gonnet G H, Cohen M A, Benner S A. Science. 1992;256:1443–1445. doi: 10.1126/science.1604319. [DOI] [PubMed] [Google Scholar]
- 31.Gonnet G H. In: Computational Methods in Genome Research. Suhai S, editor. New York: Plenum; 1994. [Google Scholar]
- 32.Reynen M, Sahm H. J Bacteriol. 1988;170:3310–3313. doi: 10.1128/jb.170.7.3310-3313.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawkins C F, Borges A, Perham R N. FEBS Lett. 1989;255:77–82. doi: 10.1016/0014-5793(89)81064-6. [DOI] [PubMed] [Google Scholar]
- 34.Youvan D C, Bylina E J, Alberti M, Begusch H, Hearst J E. Cell. 1984;37:949–957. doi: 10.1016/0092-8674(84)90429-x. [DOI] [PubMed] [Google Scholar]
- 35.Chang Y-Y, Cronan J E. J Bacteriol. 1982;151:1279–1289. doi: 10.1128/jb.151.3.1279-1289.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao G, Winkler M E. J Bacteriol. 1994;176:6134–6138. doi: 10.1128/jb.176.19.6134-6138.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]