Abstract
Steroidogenic factor 1 (SF-1) plays key roles in adrenal and gonadal development, expression of pituitary gonadotropins, and development of the ventromedial hypothalamic nucleus (VMH). If kept alive by adrenal transplants, global knockout (KO) mice lacking SF-1 exhibit delayed-onset obesity and decreased locomotor activity. To define specific roles of SF-1 in the VMH, we used the Cre-loxP system to inactivate SF-1 in a central nervous system (CNS)-specific manner. These mice largely recapitulated the VMH structural defect seen in mice lacking SF-1 in all tissues. In multiple behavioral tests, mice with CNS-specific KO of SF-1 had significantly more anxiety-like behavior than wild-type littermates. The CNS-specific SF-1 KO mice had diminished expression or altered distribution in the mediobasal hypothalamus of several genes whose expression has been linked to stress and anxiety-like behavior, including brain-derived neurotrophic factor, the type 2 receptor for CRH (Crhr2), and Ucn 3. Moreover, transfection and EMSAs support a direct role of SF-1 in Crhr2 regulation. These findings reveal important roles of SF-1 in the hypothalamic expression of key regulators of anxiety-like behavior, providing a plausible molecular basis for the behavioral effect of CNS-specific KO of this nuclear receptor.
STEROIDOGENIC FACTOR 1 (SF-1, officially designated NR5A1) is a nuclear receptor that is expressed in the gonads, adrenal cortex, anterior pituitary, hypothalamus, and spleen (1). A number of target genes by which SF-1 mediates development and function of the gonads, adrenal cortex, and pituitary gonadotropes have been identified. However, the roles of SF-1 in the VMH, and its potential hypothalamic target genes remain less well understood.
Within the central nervous system (CNS), SF-1 is first expressed at approximately embryonic d 9 (E9) in the region of the ventral neural tube that forms the hypothalamic primordium (2) and subsequently localizes to the VMH. The VMH, which is linked to neurophysiological and behavioral effects that include body weight regulation, spontaneous locomotor activity, anxiety-like behavior, and female reproductive behavior, is characterized chemoarchitecturally by the positions of cells expressing specific proteins within and surrounding the nucleus (3,4). Consistent with its early expression during VMH development, SF-1 knockout (KO) mice exhibit marked structural perturbation of the VMH (5,6), and the absence of SF-1 markedly affects the expression and location of many neurochemical markers in the mediobasal hypothalamus (7,8,9). Consistent with a link of the VMH to energy homeostasis, adrenal-transplanted SF-1 KO mice exhibited late-onset obesity and decreased locomotor activity (10).
To evaluate SF-1 function in the VMH and to ensure that the effect of the SF-1 KO reflected direct CNS effects rather than secondary effects of gonadal, adrenal, or pituitary insufficiency, we used the Cre-loxP strategy to inactivate SF-1 specifically in the CNS. CNS-specific KO of SF-1 dramatically altered the locations of cells in and around the VMH and was accompanied by significant changes in the expression of several genes, including estrogen receptor α (ERα), brain-derived neurotrophic factor (BDNF), and the type 2 receptor for CRH (Crhr2). Consistent with the decreased expression of BDNF and Crhr2, mice with CNS-specific KO of SF-1 had increased anxiety-like behavior in a number of behavioral tests. These CNS-specific SF-1 KO mice provide a novel genetic model for defining the roles of SF-1 in VMH development and function.
RESULTS
The Conditional Deletion Strategy Inactivates SF-1 Expression Selectively in the Hypothalamus
The conditional (F) SF-1 allele was generated as described (11); mice homozygous for the F allele showed no apparent phenotype and were fertile. To define specific roles of SF-1 in the brain, we combined the F allele and the original null (N) allele (12) with a nestin-Cre transgene that directs Cre expression to the CNS (13).
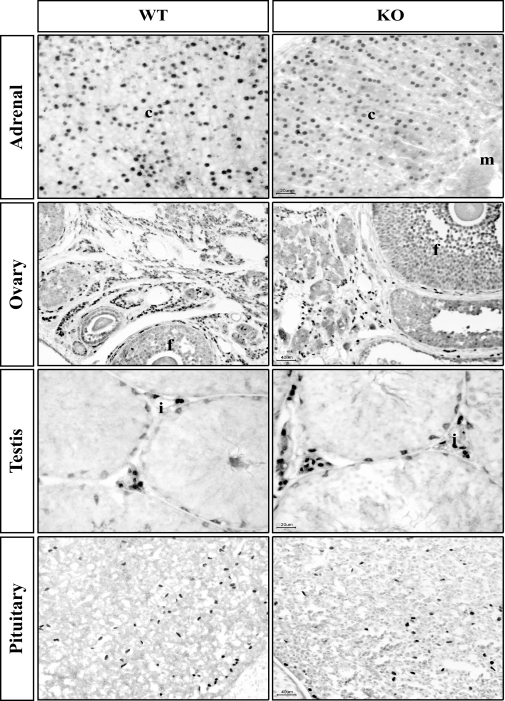
We first examined SF-1 expression in different tissues of adult mice with various genotypes. SF-1 immunoreactivity in adult mice with the F/N, nestin-Cre positive genotype (designated CNS-specific SF-1 KO) was normal in the adrenal cortex, gonads (ovary and testis), and the anterior pituitary (Fig. 1). Thus, Cre-mediated recombination directed by the nestin-Cre transgene does not affect SF-1 expression in sites outside of the brain.
Figure 1.
Effect of the Nestin-Cre Transgene on SF-1 Expression in Other Sites of SF-1 Expression
Sections from the indicated tissues were analyzed by immunohistochemistry for SF-1 expression as described in Materials and Methods. c, Cortex; m, medulla; f, follicle; i, interstial cells.
In addition to the structural integrity of the adrenal cortex and gonads, hormonal analyses of the nestin-Cre SF-1 KO mice established that these organs also were functionally intact. Thus, as shown in Table 1, no differences were seen in plasma levels of corticosterone (peak or trough), testosterone (males), or estradiol (females).
Table 1.
Plasma Hormone Levels in WT and CNS-Specific SF-1 KO Mice
| Hormone | Genotype | Level |
|---|---|---|
| Corticosterone | ||
| Peaka | WT | 161.2 ± 15.5 (n = 19) |
| KO | 165.9 ± 19.4 (n = 21) | |
| Trougha | WT | 31.5 ± 7.8 (n = 10) |
| KO | 28.7 ± 5.3 (n = 6) | |
| Testosterone in males (ng/ml) | WT | 1.1 ± 0.45 (n = 8) |
| KO | 1.3 ± 0.33 (n = 14) | |
| Estradiol in females (pg/ml) | WT | 6.3 ± 4.33 (n = 9) |
| KO | 5.7 ± 2.09 (n = 18) |
All differences as measured by Student’s t test were not significant.
Mice were bled from 1700 to 1830 h for peak and 0730 to 0900 h for trough.
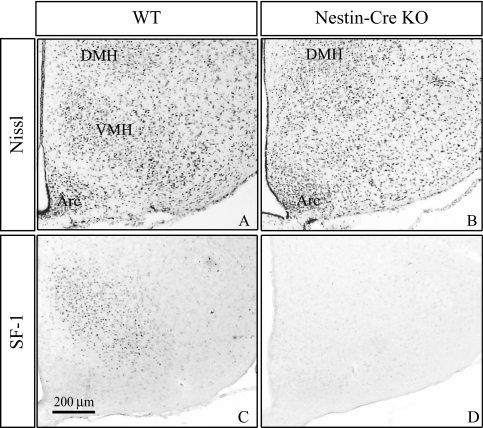
We next determined the effect of nestin-Cre-mediated SF-1 recombination on VMH structure and SF-1 expression. In coronal sections at this level (Fig. 2), the mediobasal hypothalamus in wild-type (WT) mice contains three major groups of cells: the VMH, the dorsomedial hypothalamic nucleus (DMH), and the arcuate nucleus (ARC). As revealed by Nissl staining (Fig. 2A), the WT VMH has a characteristic oval structure and expresses immunoreactive SF-1 (Fig. 2C) with a gradient from dorsomedial (highest) to ventrolateral (lowest). A striking effect was seen when the nestin-Cre transgene disrupted SF-1; the VMH nuclear organization was not discernible (Fig. 2B), closely resembling the global SF-1 KO mice (5,6). Moreover, there was complete loss of SF-1 immunoreactivity in the mediobasal hypothalamus (Fig. 2D).
Figure 2.
Effect of CNS-Specific SF-1 Inactivation on VMH Structure and SF-1 Immunoreactivity
As described in Materials and Methods, brains from adult mice of the indicated genotypes were prepared and sections from the mid-region of the VMH were processed for cresyl violet Nissl staining (A and B) or immunohistochemistry for SF-1 protein (C and D). Arc, ARC.
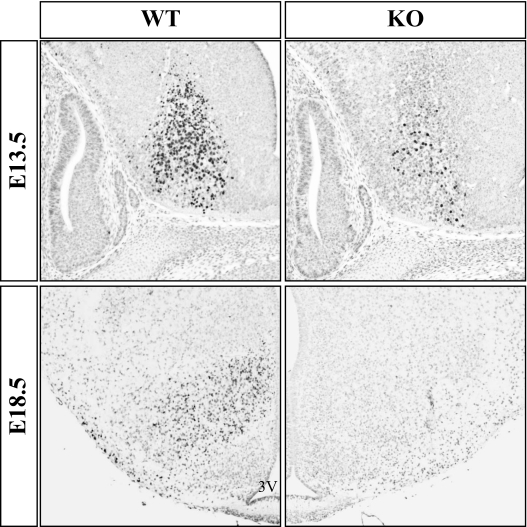
Although the mouse VMH first emerges as a distinct nucleus at E15–E17, SF-1 is expressed by neural precursors at E9, and the birthdates of VMH neurons range from E10–E14. To determine when the nestin-Cre transgene inactivated SF-1, we examined SF-1 expression beginning at E13.5 (Fig. 3, top panels). Cells containing immunoreactive SF-1 in the WT brain clustered in the ventral diencephalon; the developing pituitary also expressed SF-1 (data not shown). In the CNS-specific SF-1 KO section, the number of SF-1-expressing cells was considerably diminished at E13.5. Similar decreases in hypothalamic expression were observed in serial sections throughout the region of the presumptive VMH (data not shown). In analyses of SF-1 immunoreactivity at E18.5 (Fig. 3, bottom panels), we observed complete loss of SF-1 immunoreactivity throughout the region of the developing VMH. Collectively, these studies indicate that SF-1 inactivation by the nestin-Cre transgene predominantly has occurred by E13.5, with complete silencing by E18.5.
Figure 3.
The Nestin-Cre Transgene Inactivates the Conditional SF-1 Allele in Utero
Mouse embryos were harvested from timed-pregnant dams at E13.5 or E18.5, and embryos were processed and genotypes determined. SF-1 immunoreactivity was determined in sections from comparable regions of the developing hypothalamus of WT and CNS-specific SF-1 KO embryos. Top, Sagittal sections of embryos harvested at E13.5. Bottom, Coronal sections of embryos harvested at E18.5.
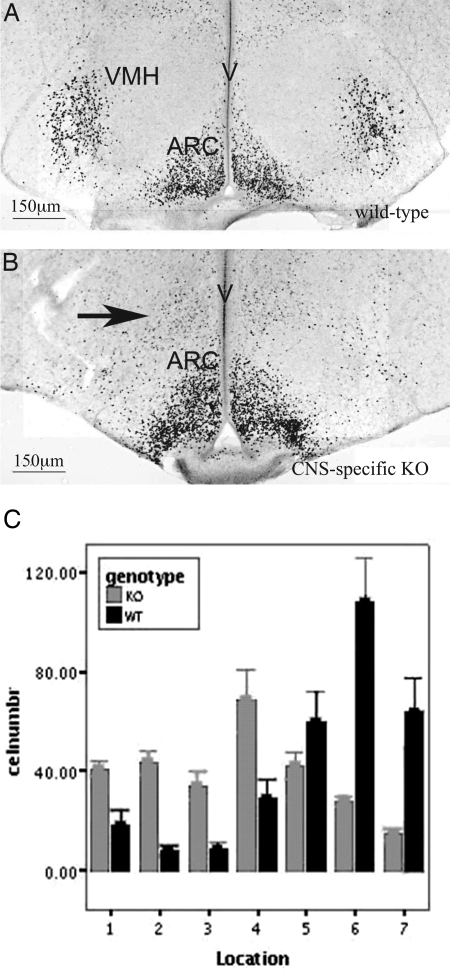
The nestin-Cre-mediated inactivation of SF-1 also caused a marked redistribution of cells containing immunoreactive ERα in the mediobasal hypothalamus. In brain sections from WT mice, ERα-containing cells were tightly localized to the ventrolateral region of the VMH and the ARC (Fig. 4, A and C). Although unchanged in number, these ERα-positive cells in sections from the CNS-specific SF-1 KO mice were displaced medially (Fig. 4, B and C), closely resembling the global SF-1 KO mice (8,10). Thus, these mice reveal striking effects of SF-1 disruption on both VMH cytoarchitecture and SF-1 expression in a manner highly reminiscent of global SF-1 KO mice.
Figure 4.
CNS-Specific KO of SF-1 Alters the Distribution of ERα-Positive Neurons in the Mediobasal Hypothalamus
Immunohistochemistry was performed and ERα-positive cells were counted in 80-μm wide columns as described in Materials and Methods. In WT mice, most immunoreactive cells were located laterally (columns 5–7, 400–560 μm from the third ventricle). In CNS-specific SF-1 KO mice, ERα-immunoreactive cells were located significantly more medially (P < 0.01, Genotype × Column ANOVA).
CNS-Specific SF-1 KO Mice Have Increased Anxiety-Like Behavior
Based on observations made by an observer blinded to genotype, CNS-specific SF-1 KO mice repeatedly tried to escape when attempts were made to handle them or their cages. To assess anxiety-like behavior systematically, we used several different test paradigms (14,15). Similar results were obtained when statistical analyses were limited to subgroups of the same sex (data not shown), suggesting that the behavioral effects of the CNS-specific SF-1 KO were not sexually dimorphic.
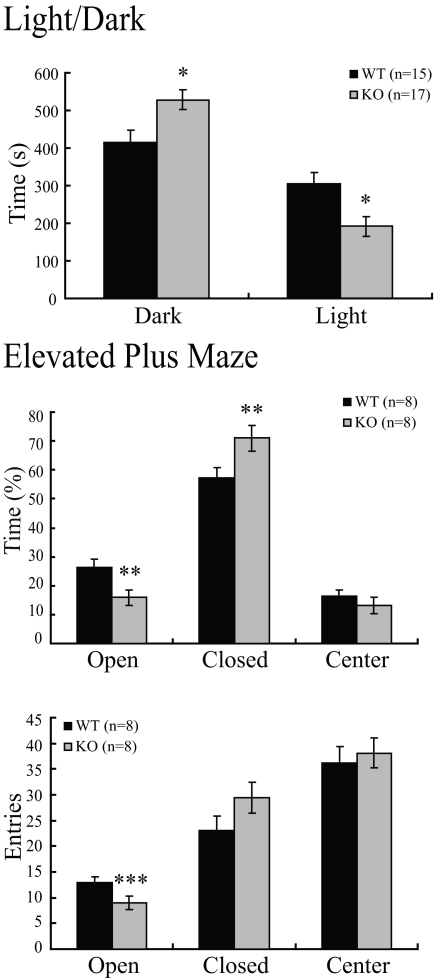
First, we performed a light/dark exploration test, as described in Materials and Methods. CNS-specific SF-1 KO mice exhibited significantly reduced exploratory behavior in the light chamber and preferentially stayed in the dark chamber (Fig. 5, top panel). Similarly, studies with the elevated plus maze showed that CNS-specific SF-1 KO mice spent significantly more time in the closed arms and less time in the open arms than did WT controls (Fig. 5, middle panel). Overall activity, as measured by total arm entries, did not differ significantly between CNS-specific SF-1 KO and WT mice; open-arm entries, however, differed significantly between the two genotypes (Fig. 5, bottom panel).
Figure 5.
CNS-Specific SF-1 KO Mice Have Increased Anxiety-Like Behavior in the Light/Dark and Elevated Plus Maze
The light/dark test and elevated plus maze were performed as described in Materials and Methods. In the light/dark test, CNS-specific SF-1 KO mice spent significantly more time in the dark chamber and significantly less time in the light chamber than WT controls (*, P < 0.01). In the elevated plus maze, time spent in each compartment (top) and the total numbers of entries into each compartment (bottom) are shown. The CNS-specific SF-1 KO mice spent significantly less time in the open compartment, significantly more time in the closed compartment (**, P < 0.01), and had significantly fewer entries into the open compartment (***, P < 0.05).
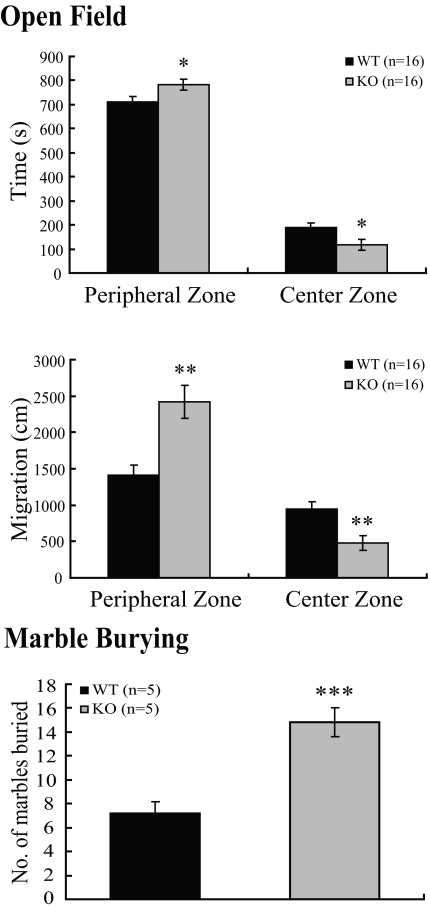
In the open field test, CNS-specific SF-1 KO mice spent significantly less time in the center zone (Fig. 6, top panel) and traveled less distance in this zone (Fig. 6, middle panel) relative to WT mice. In the marble-burying test (15), the CNS-specific SF-1 KO mice buried a significantly greater number of marbles during the observation period (Fig. 6, bottom panel), again consistent with increased anxiety-like behavior.
Figure 6.
CNS-Specific SF-1 KO Mice Exhibit Increased Anxiety-Like Behavior in Open-Field and Marble-Burying Tests
In the open field test, the times spent in the peripheral and central zones and migration in each zone were measured. The CNS-specific SF-1 KO mice spent significantly more time in the peripheral zone and significantly less time in the center zone (*, P < 0.05) and migrated significantly more distance in peripheral zone and less in center zone (**, P < 0.01). Increased anxiety-like behavior of CNS-specific SF-1 KO mice in the marble-burying test. The marble-burying test was performed as described in Materials and Methods. CNS-specific SF-1 KO mice buried significantly more marbles during the test interval than WT controls (***, P < 0.001).
Collectively, these multiple tests consistently demonstrated increased anxiety-like behavior in CNS-specific SF-1 KO mice, at least as assessed by standard tests of mouse behavior.
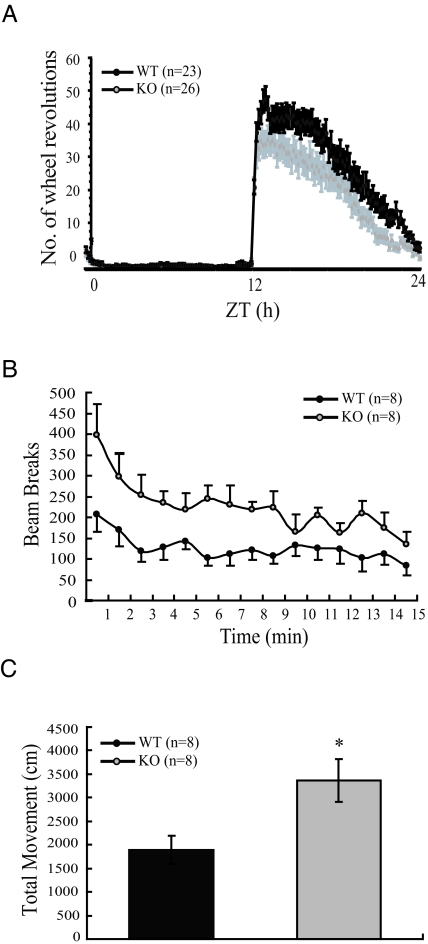
In global SF-1 KO mice given adrenal transplants, spontaneous locomotor activity was diminished significantly relative to WT controls (10). We therefore also examined the effect of the CNS-specific SF-1 KO on locomotor activity in adult mice. Consistent with the anxiety-like behavior described above, locomotor activity was significantly increased in CNS-specific SF-1 KO mice when the mice were subjected to the stress of transfer into a new cage (Fig. 7, B and C). By contrast, and consistent with previous results (10), wheel-running activity was significantly decreased in CNS-specific SF-1 KO mice after the mice were acclimated to their cages. As shown in Fig. 7A, the pattern of activity throughout the 24 h did not differ appreciably in the WT and CNS-specific SF-1 KO mice. However, daily activity was decreased considerably in CNS-specific SF-1 KO mice relative to WT littermates. These data, consistent with those obtained with the global SF-1 KO mice (10), suggest that the previously observed chronic decrease in locomotor activity reflects inactivation of SF-1 specifically in VMH neurons.
Figure 7.
Locomotor Activity of WT and CNS-Specific SF-1 KO Mice in Habitual and Novel Settings
A, Locomotor activity in a habitual setting was measured with a running wheel as described in Materials and Methods for adult WT and CNS-specific SF-1 KO mice. The 0–12 on the x-axis indicates daytime (light) and 12–24 indicates nighttime (dark). Zeitgeber time (ZT) is plotted on the x-axis, and the average number of wheel turns is plotted on the y-axis. Vertical bars indicate sem. Wheel-running activity was significantly lower in CNS-specific SF-1 KO mice than in WT controls. B, Locomotor activity of WT and CNS-specific SF-1 KO mice also measured in a novel environment. CNS-specific SF-1 KO mice showed significantly higher numbers of beam breaks/min over a 15-min period. The data (A and B) were expressed as mean ± sem and analyzed by repeated measures two-way ANOVA followed by Bonferroni’s post hoc correction. (P < 0.05, genotype × time). C, Total movement of WT and CNS-specific SF-1 KO mice situated in a novel environment is shown as total distance (cm) of movement vs. time. CNS-specific SF-1 KO mice showed significantly higher movement than WT controls (*, P < 0.01).
VMH Expression of BDNF and Crhr2 Is Decreased in CNS-Specific SF-1 KO Mice
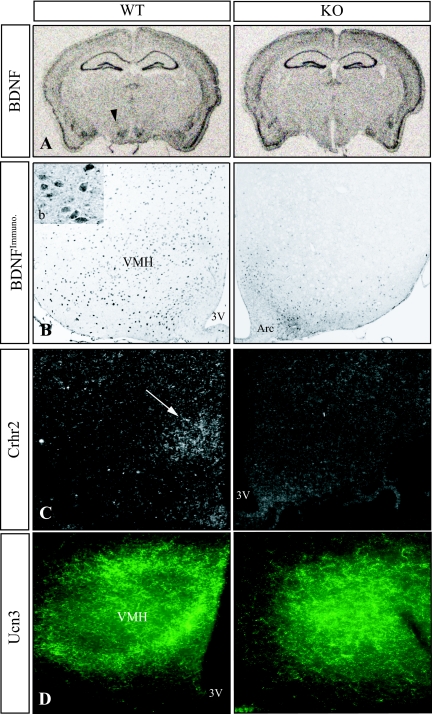
The phenotype of the CNS-specific SF-1 KO mice resembles in several respects that described in mice with heterozygous inactivation or conditional KO of BDNF (16,17,18), and BDNF expression reportedly is decreased in the mediobasal hypothalamus of global SF-1 KO mice (7). In addition, high BDNF expression in the neonatal VMH has been described (19), and SF-1 is expressed at high levels specifically in the embryonic and perinatal VMH (5,20). In situ hybridization studies showed that BDNF transcripts were decreased considerably in the region of the VMH in adult CNS-specific SF-1 KO mice (n = 4; Fig. 8A), consistent with studies that used a β-galactosidase knock-in allele to assess BDNF expression in neonatal, global SF-1 KO mice (7). In contrast, levels of BDNF transcripts in the ARC, hippocampus, and other brain regions examined were comparable in WT and CNS-specific SF-1 KO mice (Fig. 8A). To confirm the decreased VMH expression of BDNF mRNA at the level of protein in CNS-specific KO mice, we used immunohistochemical analyses. BDNF immunoreactivity was preserved in the ARC but was decreased considerably in the region of the VMH in the CNS-specific SF-1 KO section (Fig. 8B). These studies point to an effect of the CNS-specific KO of SF-1 on BDNF expression and are consistent with the model that decreased levels of BDNF contribute to the behavioral phenotype in these mice.
Figure 8.
Gene Expression in the Hypothalamus of CNS-Specific SF-1 KO Mice
Brain sections of adult mice were prepared and gene expression was analyzed by in situ hybridization and immunohistochemistry. Sections shown represent a low-power view (A) and high-power views (B–D) of the middle of the VMH in its rostral-caudal extent. A, BDNF expression. The arrowhead shows the region of the VMH. B, Immunohistochemistry for BDNFprotein. The inset (b) shows the cytoplasmic staining of the anti-BDNF antibody. 3V, Third ventricle; Arc, ARC. C, Expression of Crhr2 transcripts as determined by in situ hybridization. The arrow indicates the region of the VMH. D, Immunohistochemical detection of Ucn 3-positive fibers in mediobasal hypothalamus.
Targeted disruption of the gene encoding the type 2 receptor for CRH, designated Crhr2, is also associated with increased anxiety-like behavior (21,22,23). Of note, Crhr2 is highly expressed in cells of the VMH, where its expression is stimulated by leptin (24). We therefore used in situ hybridization to examine expression of Crhr2 transcripts. In sections from CNS-specific KO brains, expression in most brain regions was comparable with that in sections from WT brains (data not shown). However, Crhr2 transcripts in the VMH were selectively decreased in the CNS-specific SF-1 KO brain (Fig. 8C). Consistent with this, immunohistochemical analyses also revealed decreased expression in the VMH but not the ARC in the CNS-specific SF-1 KO mice (data not shown). These findings suggest that the decreased Crhr2 expression also results from impaired SF-1 function in the VMH.
Although CRH can activate Crhr2, the physiologic ligand(s) may be related peptides termed the urocortins. In particular, urocortin 3 (Ucn 3) has been implicated in appetite regulation, locomotor activity, and anxiety-like behavior, including direct actions in the VMH (25,26). In sections from WT mice, immunoreactive Ucn 3-positive fibers clustered densely at the VMH periphery, with only a few fibers extending into the interior of the nucleus (Fig. 8D) (27). In CNS-specific SF-1 KO mice (Fig. 8D), this fiber organization was markedly disrupted, and Ucn 3 fibers extended throughout the region where the VMH normally resides.
SF-1 Directly Regulates Crhr2 Promoter Activity
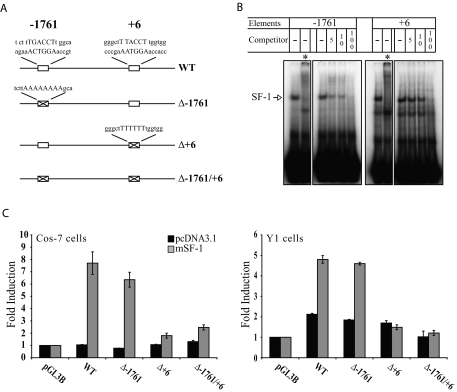
Of the anxiety-related genes studied here, previous studies demonstrated that BDNF is a direct target gene of SF-1 (28). We next performed similar experiments to explore whether Crhr2 is also a direct SF-1 target. Inspection of the sequence around the transcription site of Crhr2 β, the isoform that is expressed in the VMH, revealed two sequences matching the binding requirements for SF-1 (Fig. 9A). As shown in Fig. 9B, oligonucleotides containing each sequence formed a specific complex in EMSAs; these complexes were supershifted by the addition of an anti-SF-1 antiserum and were inhibited in a dose-dependent manner by unlabeled competitors, confirming that they contained SF-1 and were specific.
Figure 9.
SF-1 Directly Regulates Crhr2 Expression
A, Schematic of the Crhr2 promoter and location of putative SF-1-responsive elements. The consensus SF-1 binding sites are the reverse complement of the sequences shown. Also shown are specific mutations in putative SF-1-responsive elements as described in Materials and Methods. B, SF-1 specifically binds the putative elements in EMSAs. Electrophoretic mobility shift experiments using Y1 adrenocortical nuclear extracts and duplex oligonucleotide probes containing the putative SF-1-responsive elements were performed as described in Materials and Methods. *, Reactions that included a specific anti-SF-1 antiserum. C, SF-1 stimulates Crhr2 promoter activity in transient cotransfection experiments in a manner that requires the putative SF-1-responsive element at +6. Transient transfection experiments were performed in Cos-7 and Y1 cells as described in Materials and Methods. Mutation of the putative SF-1-responsive element at +6 abolished SF-1 stimulation in transient cotransfection assays. The data were expressed as mean ± sem and analyzed by two-way ANOVA followed by Bonferroni’s post hoc correction. (P < 0.0001, SF-1 transfection × Crhr2 promoter constructs).
To determine whether SF-1 could stimulate Crhr2 promoter activity, we performed transient cotransfection experiments as described in Materials and Methods. As shown in Fig. 9C, SF-1 stimulated promoter activity of Crhr2 5′-flanking sequences in COS-7 cells; a lower, but still significant 2.5-fold-induction was seen in Y1 adrenocortical cells, which endogenously express SF-1. Mutation of the putative SF-1-responsive element at −1761 did not affect promoter activity or SF-1 induction; in contrast, mutation of the element at +6 diminished both basal promoter activity and the response to SF-1 (Fig. 9C). Collectively, these studies argue strongly that Crhr2 is a direct target of SF-1.
DISCUSSION
In this study, we generated mice with selective inactivation of SF-1 in the CNS, confirming the direct role of CNS expression of this nuclear receptor in VMH development and directly linking SF-1 and the VMH with complex traits such as anxiety-like behavior and locomotor activity.
Although many SF-1 target genes have been identified in the gonads, adrenal cortex, and pituitary gonadotropes (1), our understanding of SF-1 target genes in VMH neurons is more limited. Both the N-methyl-D-aspartic acid (NMDA) subunit (29) and BDNF (28) have been reported as direct SF-1 target genes. In a separate approach, laser-capture microdissection was used to identify genes that were expressed selectively in the VMH relative to the DMH and ARC, including the neuropeptide cerebellin and the membrane receptor tenM/2 (30). Recently, Ingraham and colleagues (31) used microdissection to examine VMH-enriched gene expression in samples from neonatal mice. Potential direct target genes for SF-1 identified in their studies include the transcription factors Fezf1 and Nkx2.2, Nptx2, and A2bp1. Although Crhr2 did not reach their threshold for VMH-specific expression, it was expressed in the neonatal VMH; the use of newborn pups likely preferentially highlighted developmental genes and excluded some genes that are not highly expressed until later in life.
Studies described here have defined direct roles of SF-1 in the CNS in the establishment of the VMH. Whether these effects involve terminal differentiation, migration of SF-1 neurons into the proper location, or both remains to be established. In accord with prior results (8,10), cells containing immunoreactive ERα in this study were found in more medial positions compared with sections from WT mice. In addition, the distribution of Ucn 3-immunoreactive fibers in the mediobasal hypothalami differed considerably between WT and CNS-specific SF-1 KO mice. These data are consistent with the model that the absence of SF-1 markedly perturbs the cellular elements in the region of the VMH and possibly causes a redistribution of afferents to aberrantly positioned cells (9).
A consistent finding in the current study was that CNS-specific SF-1 KO mice have increased anxiety-like behavior as assessed in multiple independent paradigms, including the light/dark test, elevated plus maze, open field test, locomotor activity in a novel environment, and marble burying. Despite our previous finding of obesity in global SF-1 KO mice rescued by adrenal transplantation, body weights of the WT and CNS-specific SF-1 KO mice studied here did not differ significantly (data not shown). Thus, the increased anxiety-like behavior and decreased wheel running in habitual surroundings of the CNS-specific SF-1 KO mice cannot be attributed to obesity.
Likewise, the increased anxiety-like behavior was not sexually dimorphic because both male and female CNS-specific SF-1 KO mice showed highly significant increases in anxiety-like behavior relative to WT mice of the same sex. Finally, although we have not performed the full battery of behavioral tests, we have not observed any obvious increase in anxiety-like behavior in male parents that carry the nestin-Cre transgene but are heterozygous for SF-1 function. Although we cannot completely exclude effects of the nestin-Cre transgene itself (32), we believe that the changes in VMH structure and gene expression and the increased anxiety-like behavior largely reflect the absence of SF-1 in VMH neurons.
The VMH has been characterized as part of a medial hypothalamic defensive system that plays a key role in response to potential predators (33,34). This defensive system may also be important when considering anxiety-like behavior because predators may be considered as anxiety provoking in animal models (35). In agreement with this, CNS-specific SF-1 KO mice in the current study had decreased expression or altered distribution in the mediobasal hypothalamus of several genes implicated in anxiety-like behavior, including BDNF, Crhr2, and Ucn 3. These findings link SF-1 expression in mouse VMH neurons with the neural circuitry associated with anxiety-like behavior and raise the possibility that SF-1 may play similar roles in human beings.
We anticipate that ongoing efforts will identify other genes implicated in anxiety-like behavior that are expressed both in the VMH and in other sites, such that the previously described SF-1/Cre transgene (36) can be used to define specific roles in the VMH in the setting of normal function in other sites. At least for BDNF and Crhr2, the available data suggest direct induction by SF-1 in cells that contain both proteins (Ref. 28 and this study). Further analyses such as chromatin immunoprecipitation assays will be needed to establish that SF-1 actually occupies the putative SF-1-responsive elements of these genes to regulate their expression.
In summary, CNS-specific SF-1 KO mice have decreased locomotor activity and increased anxiety-like behavior. Although the mechanisms underlying these effects remain to be defined, analyses of gene expression revealed alterations in VMH expression of key mediators of anxiety-like behavior, including BDNF and Crhr2, both of which can be directly regulated by SF-1. Future studies that define how SF-1 modulates the expression of these and other genes in VMH neurons will undoubtedly provide new insights into the complex pathways by which the VMH and other hypothalamic nuclei regulate key physiological functions.
MATERIALS AND METHODS
Generation and Analysis of CNS-Specific SF-1 KO Mice
All animal experiments were approved by the Institutional Animal Care Research Advisory Committee at University of Texas Southwestern. Mice homozygous for the floxed (F) SF-1 allele (11) were crossed with mice heterozygous for the null (N) SF-1 allele (12) carrying the nestin-Cre transgene [The Jackson Laboratory, Bar Harbor, ME; strain B6.Cg (SJL)-TgN (Nes-Cre)Kln (13)]. The mice have been backcrossed on the C57BL/6 background for over five generations, thereby minimizing potential confounding effects of genetic background on the behavioral studies. Mice designated WT included those with all genotypes except SF-1 F/N, Cre-positive, because no CNS structural abnormalities or behavioral phenotypes were seen in subgroup analyses of mice carrying the WT SF-1 allele or in the F/N mice in the absence of the Cre transgene.
All mice were maintained on a 12-h light, 12-h dark cycle and fed ad libitum on regular mouse chow (4% fat by weight). During behavioral studies, mice were analyzed individually as described below.
Histology, Immunohistochemistry, and in Situ Hybridization
Mice were anesthetized and perfused with 4% paraformaldehyde in PBS, pH 7.4. Brains were postfixed with 4% paraformaldehyde in PBS at 4 C overnight, cryoprotected in 30% sucrose for 24 h, embedded in OCT compound (Sakura Finetek, Torrance, CA), and sectioned at 25 μm on a cryostat. Sections for ERα immunohistochemistry were cut at 60 μm using a vibrating microtome (Leica VT1000S; Leica Microsystems, Deerfield, IL) and processed as described (8,9). Sections were stained for Nissl substance with cresyl violet using standard procedures. Immunohistochemical procedures were performed using mounted or free-floating sections through the rostral-caudal extent of the hypothalamus. Paraffin sections (7 μm) were used for SF-1 immunohistochemistry with tissues from embryos at E13.5 and E18.5. Sections were preincubated with 0.3% H2O2 for 15 min and then permeabilized with 0.5% Triton X-100 in PBS for 20 min. To reduce nonspecific reactions, sections were treated with 1.5% normal serum from the species used to generate the secondary antibody. Every other section through the hypothalamus was incubated overnight at 4 C with one of the following antibodies: rabbit anti-SF-1 (1:1500; a generous gift of Dr. Ken Morohashi, Kyushu, Japan), rabbit anti-Ucn 3 (1:19,000; a generous gift of Dr. Wylie Vale, The Salk Institute, La Jolla, CA), rabbit anti-ERα (1:5000 dilution of C1355; Upstate Biotechnology, Inc., Lake Placid, NY), rabbit anti-BDNF (1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), or goat anti-Crhr2 (1:1500; Santa Cruz Biotechnology, Inc.). After washes in PBS, the sections were incubated with fluorescein-conjugated or biotinylated secondary antibody for 1 h at room temperature and developed using the Vectastain ABC detection system (Vector Laboratories, Inc., Burlingame, CA). Finally, sections were rinsed three times in PBS for 10 min and mounted. In situ hybridization was performed on every fourth serial section from four brains each from WT and CNS-specific SF-1 KO mice. Probes included BDNF (rat BDNF, 350-bp antisense probe that recognizes all BDNF transcripts), and Crhr2 (β form of mouse Crhr2, 1.1 kb from 47–1056, accession no.: NM_009953) labeled with [35S] uridine triphosphate. Digital images from tissue sections processed for bright-field or epifluorescence microscopy were captured and imported into Photoshop.
Behavioral Studies
CNS-specific SF-1 KO and WT mice were matched for sex and age, and littermates were housed together. The mice were subjected to a standard battery of behavioral tests as described (14,15). Mice at 12–16 wk of age were analyzed in behavioral studies. Statistical significance was determined using Student’s t test, two-way ANOVA, and repeated measures two-way ANOVA and values represent means ± sem.
In the light/dark exploration test, mice were tested in plexiglass activity chambers (45 × 52 × 52 cm, height × width × length, ENV-520; Med Associates, St. Albans, VT) that were divided into two parts (light:dark = 1:1) by a wall containing a door (13 × 5 cm) at the level of floor. The test mice were always placed in the light chamber with the door closed. After a brief interval, the door was opened to allow mice to explore the chambers.
A commercially available apparatus (San Diego Instruments, San Diego, CA) was used for the elevated plus maze. Mice were placed in the center zone of the maze for 2 min and then allowed to explore the maze for 15 min. Parameters measured included the frequencies of total-, open- and closed-arm entries, as well as the time spent in each zone of the maze.
In the open-field paradigm and to measure locomotor activity in a novel environment, Versamax activity monitors (AccuScan Instruments, Columbus, OH) were used in 40- × 40-cm open field chambers enclosed in soundproof boxes. Movement was measured with infrared beams. The time spent in peripheral vs. that in the central zone and the migration in each zone were measured during a 15-min testing period.
To measure spontaneous locomotor activity in habitual surroundings, mice were maintained for 7 d on a standard light/dark cycle with standard food in cages containing individual running wheels, and wheel-running activity was measured across the 24-h cycle. For each mouse, wheel-running activity was accumulated in 10-min bins for 7 d and then averaged to yield one 24-h profile. Data were then averaged and plotted with respect to Zeitgeber time.
In the marble-burying paradigm (15), 20 round glass marbles (diameter of ∼1 cm) were evenly placed in a Perspex cage (21 × 38 × 14 cm) in five rows of four marbles on a 5-cm layer of sawdust bedding that was lightly compressed to form a flat surface. After 15-min exposure to the marbles, the mouse was removed from the cage, and the buried marbles were counted. Marbles that were at least two-thirds covered by sawdust were scored as “buried.”
Cell Transfection Analyses
Cells were cultured at 37 C in a humidified incubator containing 5% CO2. Cos-7 monkey kidney cells were maintained in DMEM supplemented with 10% fetal bovine serum and 100 U/ml penicillin-streptomycin (Invitrogen, Carlsbad, CA). Mouse Y1 adrenocortical tumor cells were cultured using Ham’s F10 medium (Mediatech, Inc., Herndon, VA) supplemented with 15% horse serum, 5% fetal bovine serum, and 100 U/ml penicillin-streptomycin. Cells were plated in 12-well plates at a density of 3 × 105 cells/well) and then cultured for 24 h. They then were transfected with Fugene 6 (Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s instructions. Approximately 24 h after transfection, cells were lysed and luciferase activities were determined using the Dual Luciferase Reporter Assay System (Promega, Madison, WI) with the FLUOstar OPTIMA luminometer (BMG Labtech, Durham, NC). All transfections were done in triplicate and repeated in three independent experiments.
The 5′-flanking region of the β isoform of Crhr2 (−2000 to +109) was amplified by PCR and cloned upstream of a luciferase reporter gene in pGL3-basic. To examine the role of two potential SF-1-responsive elements, site-directed mutagenesis was used to mutate sequences corresponding to potential SF-1-responsive elements, all in the context of the −2000 promoter construct. The PCR primers were 5′-CTGAAGATGTCCTCTTAAAAAAAAGCACCCACTGTGAGACAG-3′ for the −1761 mutant and 5′-TGCCCTGGTGCCCAGGGCTTTTTTTTGGTGGGTAGGTCGGGCAGG-3′ for the +6 mutant.
EMSAs
Duplex oligonucleotides containing the sequences of potential SF-1-responsive elements were labeled with 32P by fill-in with Klenow fragment and used as probes for EMSAs as described (37). Nuclear extracts from mouse Y1 adrenocortical tumor cells were used as a source of SF-1. Labeled probes were incubated with nuclear extracts (5–10 mg) at room temperature for 30 min in 10 nm HEPES (pH 7.9), 0.1 mm EDTA, 0.25 mm l-dithiothreitol, 0.01% Nonidet P-40, and 10% glycerol. In some reactions, a rabbit antiserum raised against full-length SF-1 (1 ml) was included in the binding reaction. Unlabeled duplex oligonucleotides were added as specific competitors at 5-, 10-, and 100-fold molar excesses. The reactions were resolved by electrophoresis on a 6% polyacrylamide gel in 0.25× Tris-buffered EDTA at 120 V for 3 h, and the dried gels were then analyzed by Phosphorimager scanning (Molecular Dynamics, Sunnyvale, CA).
Acknowledgments
We thank Drs. Kristy McClellan of Colorado State University (Fort Collins, CO); Nancy Stallings, Robert Hammer, Steve McKnight, Jeffrey Long, Hiromasa Funato, and Yun-Hee Choi of UT-Southwestern (Dallas, TX); Wylie Vale of The Salk Institute (La Jolla, CA); and Roger Cone of the Vollum Institute (Portland, OR) for helpful discussions; Yelena Krimkevich for technical assistance; Carole Dudley, Sandy Estill, and Claudia Erbel-Seile of UT-Southwestern (Dallas, TX) for assistance in performing the behavioral assays; and Luis Parada of UT-Southwestern (Dallas, TX) for the BDNF probe.
Footnotes
This work was supported by National Institutes of Health Grants DK54480 (to K.L.P.), P20RR20691 (to K.L.P.), and MH082679 (to S.A.T.) and by the Wilson Center for Endocrine Research.
Disclosure Statement: The authors have nothing to disclose.
First Published Online March 27, 2008
Abbreviations: ARC, Arcuate nucleus; BDNF, brain-derived neurotrophic factor; CNS, central nervous system; Crhr2, CRH receptor type 2; DMH, dorsomedial hypothalamic nucleus; E, embryonic day; ER, estrogen receptor; KO, knockout; SF-1, steroidogenic factor 1; VMH, ventromedial hypothalamic nucleus; WT, wild type.
References
- Parker KL, Rice DA, Lala DS, Ikeda Y, Luo X, Wong M, Bakke M, Zhao L, Frigeri C, Hanley NA, Stallings N, Schimmer BP 2002 Steroidogenic factor 1: an essential mediator of endocrine development. Recent Prog Horm Res 57:19–36 [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Takeda Y, Shikayama T, Mukai T, Hisano S, Morohashi KI 2001 Comparative localization of Dax-1 and Ad4BP/SF-1 during development of the hypothalamic-pituitary-gonadal axis suggests their closely related and distinct functions. Dev Dyn 220:363–376 [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW 1994 Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol 348:41–79 [DOI] [PubMed] [Google Scholar]
- McClellan KM, Parker KL, Tobet S 2006 Development of the ventromedial nucleus of the hypothalamus. Front Neuroendocrinol 27:193–209 [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL 1995 The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol Endocrinol 9:478–486 [DOI] [PubMed] [Google Scholar]
- Shinoda K, Lei H, Yoshii H, Nomura M, Nagano M, Shiba H, Sasaki H, Osawa Y, Ninomiya Y, Niwa O, Morohashi K-i, Li E 1995 Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev Dyn 204:22–29 [DOI] [PubMed] [Google Scholar]
- Tran PV, Lee MB, Marin O, Xu B, Jones KR, Reichardt LF, Rubenstein JR, Ingraham HA 2003 Requirement of the orphan nuclear receptor SF-1 in terminal differentiation of ventromedial hypothalamic neurons. Mol Cell Neurosci 22:441–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellovade TL, Young M, Ross EP, Henderson R, Caron K, Parker K, Tobet SA 2000 Disruption of the gene encoding SF-1 alters the distribution of hypothalamic neuronal phenotypes. J Comp Neurol 423:579–589 [DOI] [PubMed] [Google Scholar]
- Davis AM, Seney ML, Stallings NR, Zhao L, Parker KL, Tobet SA 2004 Loss of steroidogenic factor 1 alters cellular topography in the mouse ventromedial nucleus of the hypothalamus. J Neurobiol 60:424–436 [DOI] [PubMed] [Google Scholar]
- Majdic G, Young M, Gomez-Sanchez E, Anderson P, Szczepaniak LS, Dobbins RL, McGarry JD, Parker KL 2002 Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology 143:607–614 [DOI] [PubMed] [Google Scholar]
- Zhao L, Bakke M, Krimkevich Y, Cushman LJ, Parlow AF, Camper SA, Parker KL 2001 Steroidogenic factor 1 (SF1) is essential for pituitary gonadotrope function. Development 128:147–154 [DOI] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker KL 1994 A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481–490 [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G 1999 Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 23:99–103 [DOI] [PubMed] [Google Scholar]
- Crawley JN 2001 What’s wrong with my mouse? New York: Wiley Liss [Google Scholar]
- Deacon RM 2006 Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat Protoc 1:122–124 [DOI] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L 1999 Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci USA 96:15239–15244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernie SG, Liebl DJ, Parada LF 2000 BDNF regulates eating behavior and locomotor activity in mice. EMBO J 19:1290–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan RM, Jaenisch R 2001 Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol 15:1748–1757 [DOI] [PubMed] [Google Scholar]
- Sugiyama N, Kanba S, Arita J 2003 Temporal changes in the expression of brain-derived neurotrophic factor mRNA in the ventromedial nucleus of the hypothalamus of the developing rat brain. Brain Res 115:69–77 [DOI] [PubMed] [Google Scholar]
- Roselli CE, Jorgensen EZ, Doyle MW, Ronnekleiv OK 1997 Expression of the orphan receptor steroidogenic factor-1 mRNA in the rat medial basal hypothalamus. Brain Res 44:66–72 [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, Hermanson O, Rosenfeld MG, Spiess J 2000 Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet 24:415–419 [DOI] [PubMed] [Google Scholar]
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF 2000 Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet 24:410–414 [DOI] [PubMed] [Google Scholar]
- Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP 2000 Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet 24:403–409 [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Makino S, Asaba K, Hashimoto K 1999 Leptin effects on the expression of type-2 CRH receptor mRNA in the ventromedial hypothalamus in the rat. J Neuroendocrinol 11:307–314 [DOI] [PubMed] [Google Scholar]
- Ohata H, Suzuki K, Oki Y, Shibasaki T 2000 Urocortin in the ventromedial hypothalamic nucleus acts as an inhibitor of feeding behavior in rats. Brain Res 861:1–7 [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Rivier J, Vale WW, Koob GF 2003 Locomotor suppressive and anxiolytic-like effects of urocortin 3, a highly selective type 2 corticotropin-releasing factor agonist. Brain Res 980:206–212 [DOI] [PubMed] [Google Scholar]
- Li C, Vaughan J, Sawchenko PE, Vale WW 2002 Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotrophin-releasing factor receptor expression. J Neurosci 22:991–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PV, Akana SF, Malkovska I, Dallman MF, Parada LF, Ingraham HA 2006 Diminished hypothalamic bdnf expression and impaired VMH function are associated with reduced SF-1 gene dosage. J Comp Neurol 498:637–648 [DOI] [PubMed] [Google Scholar]
- Pieri I, Klein M, Bayertz C, Gerspach J, van der Ploeg A, Pfizenmaier K, Eisel U 1999 Regulation of the murine NMDA-receptor-subunit NR2C promoter by Sp1 and fushi tarazu factor1 (FTZ-F1) homologues. Eur J Neurosci 11:2083–2092 [DOI] [PubMed] [Google Scholar]
- Segal JP, Stallings NR, Lee CE, Zhao L, Socci N, Viale A, Harris TM, Soares MB, Childs G, Elmquist JK, Parker KL, Friedman JM 2005 Use of laser-capture microdissection for the identification of marker genes for the ventromedial hypothalamic nucleus. J Neurosci 25:4181–4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurrasch DM, Cheung CC, Lee FY, Tran PV, Hata K, Ingraham HA 2007 The neonatal ventromedial hypothalamus transcriptome reveals novel markers with spatially distinct patterning. J Neurosci 27:13624–13634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni PE, Scuoppo C, Imayoshi I, Taulli R, Dastru W, Sala V, Betz UA, Muzzi P, Martinuzzi D, Vercelli AE, Kageyama R, Ponzetto C 2006 High levels of Cre expression in neuronal progenitors cause defects in brain development leading to microencephaly and hydrocephaly. J Neurosci 26:9593–9602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dielenberg RA, Hunt GE, McGregor IS 2001 “When a rat smells a cat”: the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience 104:1085–1097 [DOI] [PubMed] [Google Scholar]
- Canteras NS 2002 The medial hypothalamic defensive system: hodological organization and functional implications. Pharm Biochem Behav 71:481–491 [DOI] [PubMed] [Google Scholar]
- McGregor IS, Hargreaves GA, Apfelbach R, Hunt GE 2004 Neural correlates of cat odor-induced anxiety in rats: region-specific effects of the benzodiazepine midazolam. J Neurosci 24:4134–4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham NC, Verma-Kurvari S, Parada LF, Parker KL 2006 Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis 44:419–424 [DOI] [PubMed] [Google Scholar]
- Cho YM, Youn BS, Chung SS, Kim KW, Lee HK, Yu KY, Park HJ, Shin HD, Park KS 2004 Common genetic polymorphisms in the promoter of resistin gene are major determinants of plasma resistin concentrations in humans. Diabetologia 47:559–565 [DOI] [PubMed] [Google Scholar]