Abstract
Alternate interactions between the H19 imprinting control region (ICR) and one of the two Igf2 differentially methylated regions has been proposed as a model regulating the reciprocal imprinting of Igf2 and H19. To study the conformation of this imprint switch, we performed a systematic structural analysis across the 140 kb of the mouse Igf2-H19 region, which includes enhancers located both between the two genes as well as downstream of H19, by using a scanning chromosome conformation capture (3C) technique. Our results suggest that on the active paternal Igf2 allele, the various enhancers have direct access to the Igf2 promoters, whereas the imprinted silent maternal Igf2 allele assumes a complex three-dimensional knotted loop that keeps the enhancers away from the Igf2 promoters and allows them to interact with the H19 promoter. This complex DNA looping of the maternal allele is formed by interactions involving differentially methylated region 1, the ICR, and enhancers. Binding of CTC-binding factor to the maternal, unmethylated ICR in conjunction with the presence of multicomplex components including interchromosomal interactions, create a barrier blocking the access of all enhancers to Igf2, thereby silencing the maternal Igf2. This silencing configuration exists in newborn liver, mouse embryonic fibroblast, and embryonic stem cells and persists during mitosis, conferring a mechanism for epigenetic memory.
THE GENE ENCODING IGF-II (Igf2) and H19 gene are located about 80 kb apart on mouse chromosome 7 and are reciprocally imprinted, leading to paternal expression of Igf2 and maternal expression of H19 in most tissues (1,2,3,4). Parental allelic expression is determined by an imprinting control region (ICR) located between the two genes, upstream of the H19 promoter. The current model for the mechanism underlying this reciprocal imprinting proposes a critical role for CTC-binding factor (CTCF), a zinc finger DNA-binding protein that binds to four binding sites on the unmethylated maternal ICR (5,6,7,8). It has been proposed that the CTCF-ICR complex interacts with one of the Igf2 differentially methylated regions (DMR1) to form a maternal silencing loop, whereas the activating chromosome structure of the paternal Igf2 allele is formed by the interaction of the ICR with DMR2 (9). A recent modification of the maternal chromosome model posits a tighter Igf2 silencing loop of about 25 kb formed by DMR1 and a matrix attachment region (MAR3) downstream of Igf2, whereas the active maternal H19 is embedded in an active chromosomal hub (ACH) (10).
Each of these models is derived from studies using one type of tissue (embryonic or newborn liver) and is based on limited data from relatively few selected regions of the DMRs and ICR (9). The chromosome conformation capture (3C) analysis across the Igf2/H19 domain was limited to scanning from two locations, the ICR and the enhancer downstream of H19 (10). However, in addition to the downstream enhancers, there are intergenic enhancers that are located upstream of the ICR and are active in both imprinted and nonimprinted tissues (11) (12), suggesting that a simple looping model cannot adequately explain the imprinting of the Igf2 allele.
To obtain a more comprehensive view of the chromatin architecture in this region, we have performed systematic 3C scanning across the entire Igf2/H19 region from multiple target points in normal liver, mouse embryonic fibroblasts (MEF), and embryonic stem (ES) cells. On the basis of this detailed examination, we propose a three-dimensional, multiple-loop model organized by the CTCF-ICR complex that separates the maternal Igf2 promoters from all Igf2 proximal and distal enhancers. The maternal loop, consisting of a DMR1-ICR-enhancer knot, persists as a maternal epigenetic memory in various cell types. The paternal Igf2/H19 chromatin is embedded in an ACH where direct access of various enhancers to the Igf2 promoters activates the Igf2 gene, whereas H19 is rendered inactive by promoter methylation.
RESULTS
Expression of Igf2 and H19 in Livers, MEF, and SF1-G
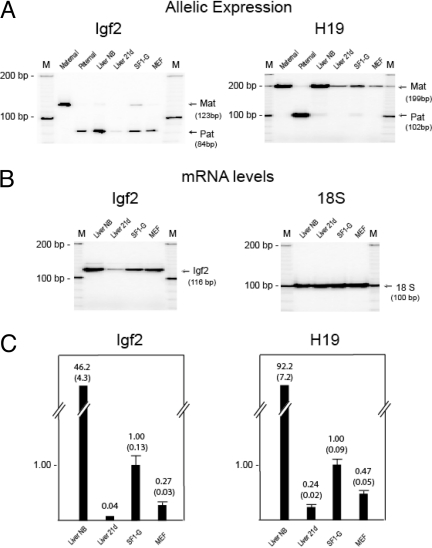
We chose to study three types of cells (liver, MEF, and ES cells) that were derived from Mus musculus × Mus spretus F1 interspecific mice. We first verified the reciprocal imprinting Igf2/H19 in these cells. As shown in Fig. 1A in livers (newborn and 21 d after birth), SF1-G, and MEF, Igf2 is expressed predominantly from the paternal allele (M. spretus), whereas H19 is expressed from the maternal allele (M. musculus). A minor maternal Igf2 band in SF1-G may indicate leaky or incomplete establishment of Igf2 imprinting in ES cells. Robust expression of Igf2 was seen in newborn livers (liver NB). Livers from 3-wk-old mice (liver 21d) showed much lower levels of Igf2 expression (Fig. 1B). Igf2 was expressed in SF1-G and MEF at levels higher than those of 21-d liver. H19 showed a similar pattern of expression with high expression levels in newborn livers and lower levels in 21-d liver, SF1-G, and MEF (Fig. 1A). The steady-state mRNA levels of Igf2 and H19 were measured by real-time quantitative (Q)-PCR (Fig. 1C). Levels of mRNA in livers, SF1-G, and MEF were calculated relative to SF1-G, which was set at 1.0. The standard RT-PCR results in Fig. 1, A and B, are consistent with the Q-PCR results shown in Fig. 1C.
Figure 1.
Expression of Igf2 and H19
A, Allelic expression in newborn liver (Liver NB), liver 21 d after birth (Liver 21d), SF1-G, and MEF. Igf2 is expressed from the paternal allele (Pat, 84 bp), whereas H19 is expressed from the maternal allele (Mat, 199bp). Controls (Maternal, Paternal) were from genomic DNAs. B, Igf2 expression by standard RT-PCR. M, 100- and 10-bp markers. C, Relative mRNA levels by real-time Q-PCR. The steady-state mRNA levels were calculated with reference to β-actin control, relative to ES cells (SF1-G), which were set at 1.0. Error bars represent sem.
Strategy for 3C Analysis in the Igf2-H19 Region
The 3C technique has been used to detect the physical proximity of specific sites on native chromosomes by religating DNA segments after the digestion of formalin-fixed nuclei (13). Identification of these nearby DNA regions depends on the presence of specific enzyme restriction sites; the propinquity of DNA regions devoid of sequences recognized by a given restriction enzyme cannot be determined (13). Various long-range, intra- and interchromosomal interactions have recently been discovered using this technique (9,14,15,16,17).
We analyzed short-range and long-range interactions of DNA segments located in the Igf2-H19 domain that contained EcoRI (RI) sites (Fig. 2A). To design 3C primers common for both mouse strains, we sequenced M. spretus Igf2-H19 genomic DNA crossing 32 RI sites and aligned them with the M. musculus sequence (supplemental Notes N1 and N2, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). We noted various informative single-nucleotide polymorphisms (SNPs), two polymorphic RI sites, and three large insertion/deletion (22-bp insertion in M. spretus, 22-bp deletion in M. spretus, and 44-bp deletion in M. spretus) polymorphisms.
Figure 2.
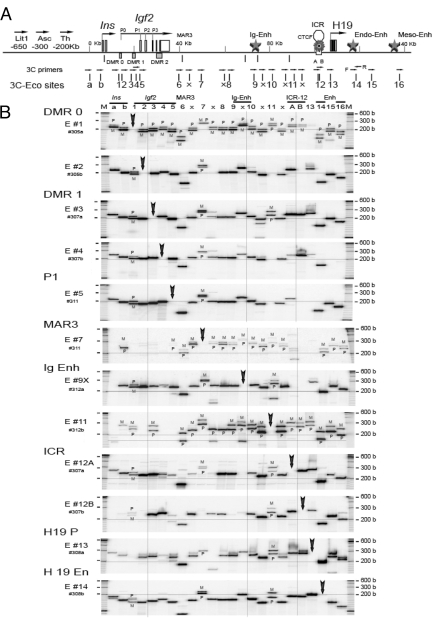
3C Analysis of Intrachromosomal Interactions across 140-kb Igf2/H19 Region in Mitotic MEF Cells
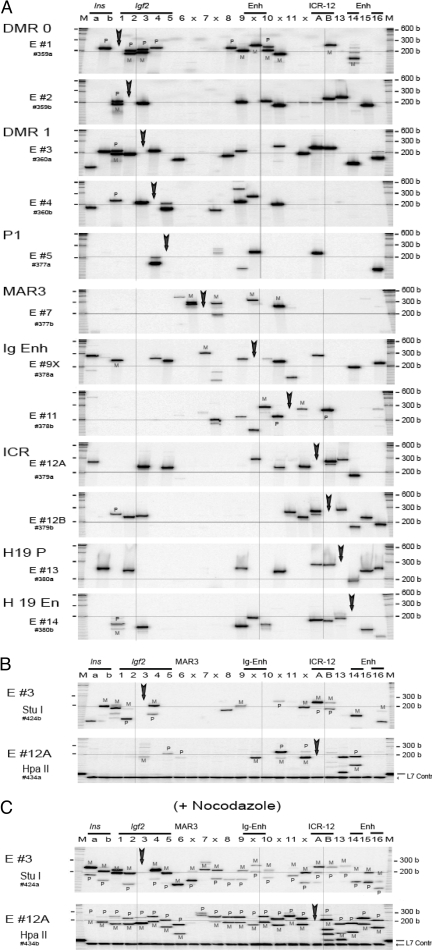
A, Map of Igf2/H19 region showing location of 24 target RI sites (a, b, 1–16, 6x, 7x, 9x, 10, and 11x) and the relative position of DMRs, MAR3, ICR, and intergenic (Ig), endoderm (Endo), and mesoderm (Meso) enhancers (Enh). One-directional, forward primers (arrows) that were identical in both M. musculus and M. spretus mouse strains were used in the 3C analysis. Each target primer (E #1 through E #16) was analyzed against one of the 24 forward primer sets. B, 3C interactions in mitotic MEF. Cells were treated with 1 mm nocodazole for 14 h. Each panel from top (E #1) to bottom (E #14) depicts 3C products across the 24 RI locations (columns a, b, and 1–16). In each column, two primers consisting of a target primer (vertical arrow) and a specified primer (marked by the column number) were used to amplify 3C cross-linked products. 3C products were sized on a polyacrylamide-urea gel. The horizontal line across the panel signifies 200 bp. The vertical line indicates alignment at DMR1, intergenic enhancer, and ICR. M at the top indicates size marker. M, Maternal; P, paternal.
We verified that the forward and reverse primer sets used in the 3C assay amplified both M. musculus and M. spretus genomic DNAs, and we used the forward primers for 3C analysis (supplemental Note N3). Because we employed single-orientation 3C primers (forward primers), only the 3C products that are derived from two RI fragments in opposite orientations can be amplified after 3C cross-ligation. Genomic DNAs and religated DNAs of the original orientation cannot be amplified in this reaction. Each 3C product is composed of two RI fragments of unique size (supplemental Note N3) that can be identified on a urea-polyacrylamide DNA sequencing gel. The amplified 3C products showed specific bands of predicted size with about 98% accuracy. We further confirmed the identity of all of the 3C products by restriction mapping and DNA sequencing.
Local and Long-Range Interactions of Igf2/H19 Locus
During mitosis, chromosomes assume an extremely compact conformation, and it would be expected that numerous short- and long-range interactions among DNA segments would be detected by 3C cross-ligation at this point in the cell cycle. If the 3C protocol and PCR amplification were accurate, we would find multiple, specific 3C products of predicted sizes. It is a common practice to use bacterial artificial chromosome clones of the gene of interest and random ligation of the digested fragments from the clone DNA as positive controls for 3C analysis. This approach provides all random combinations of DNA fragments and a standard calibration system for the PCR efficiency of the 3C primer sets. However, contaminated, artifactual cross-linked DNAs may be mistaken as genuine 3C cross-linked products. We suggest that cultured cells arrested in mitosis can be used to provide local intrachromosomal 3C positive controls.
As shown in Fig. 2B, MEF cells that were arrested in mitosis by treatment with nocodazole (1 mm for 14 h) showed numerous clear, distinct robust bands corresponding to 3C products of predicted size. Virtually identical results were obtained using ES cells (SF1-G) that were treated with nocodazole (data not shown). We confirmed the identity of the 3C products by determining their specific sizes on a short sequencing gel. The three insertion/deletion polymorphisms allowed direct allelic analysis without PCR bias. During mitosis, interactions involving the paternal and maternal alleles were observed as mostly doublet bands across the 140-kb Igf2/H19 region, indicating essentially equal parental allelic 3C interactions, thereby confirming that both parental alleles were amplified in our 3C analysis (Fig. 2B, panels E #1, #7, and #11). Furthermore, no single 3C product was present at each of the target sites (marked by a bold arrow in each panel) when a single target primer was used, a demonstration of a robust negative control in our assay.
In contrast to the extensive multifocal and nearly universal 3C interactions that were present across the Igf2/H19 domain in mitotic cells, normally cycling MEF (Fig. 3A), newborn liver (Fig. 4A), and ES (Fig. 5A) cells demonstrated limited and highly specific 3C interactions that were concentrated in four discrete domains: Igf2, intergenic enhancer, ICR, and H19 downstream enhancers. There were specific local short-range interactions within each domain and distinct long-range interaction among these four domains. The long-range domain interaction from the ICR was maintained in mitotic cells (compare Fig. 2B with 3A, panels E #12A and #12B), which may reflect the maintenance of the epigenetic marks in mitosis.
Figure 3.
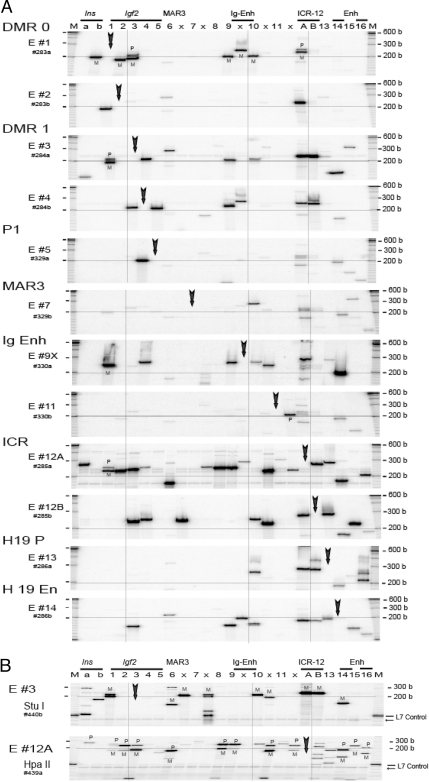
Local and Long-Range Interaction in Interphase MEF Cells
A, 3C interaction across the140-kb Igf2/H19 region in MEF cells. Each panel depicts 3C products across the 24 RI locations. In each column, a target primer (vertical arrow) and a specified primer (column number) were used to amplify 3C products. Further details are described in Fig. 1B. B, Parental allele-specific pattern of 3C interaction in MEF between DMR1 RI #3 (panel E #3, StuI digestion) and the 24 RI sites (top panel) and the interaction between ICR Eco #12A (panel E #12A, HpaII digestion) and the 24 sites (bottom panel). PCR products were labeled at the last PCR cycle (hot-stop protocol). A control L7 template added before digestion indicates completion of the restriction digest (arrow). M at the top indicates size marker. M, Maternal; P, paternal.
Figure 4.
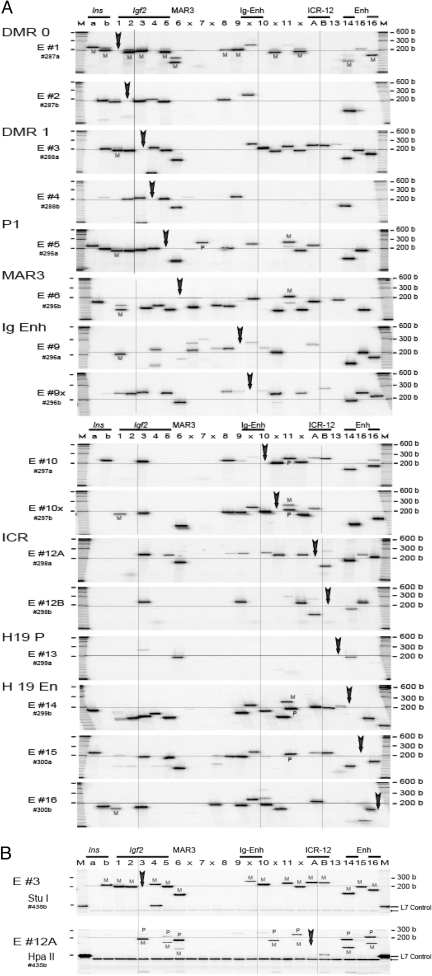
Local and Long-Range Interaction in Newborn Liver
A, 3C interaction across the140-kb Igf2/H19 region in newborn liver. Details are in Fig. 2A. B, Parental allele-specific pattern of 3C interaction in newborn liver between DMR1 RI #3 (panel E #3, StuI digestion) and the 24 RI sites (top panel) and the interaction between ICR Eco #12A (panel E #12A, HpaII digestion) and the 24 sites (bottom panel). PCR products were labeled at the last PCR cycle (hot-stop protocol). A control L7 template indicates complete digestion (arrow). M at the top indicates size marker. M, Maternal; P, paternal.
Figure 5.
Local and Long-Range Interaction in ES Cells
A, 3C analysis across 140-kb Igf2/H19 region in ES cells (SF1-G). Details are in Fig. 2A. B, Parental allele-specific pattern of 3C interaction in normal SF1-G between DMR1 RI #3 (E #3, StuI digestion) and the 24 RI sites (top panel) and the interaction between ICR Eco #12A (HpaII digestion) across the 140-kb region (bottom panel). Hot-stop PCR was employed. A control L7 template added before digestion indicates completion of the restriction digest. Note that the intensity of the 3C product in Fig. 5B, E #3 (column 3, lower panel) was weaker than expected. This may be attributable to variation in the handling/loading of this sample. See explanation in supplemental Note N7. C, Parental allele-specific pattern of 3C interaction in mitotic SF1-G. The ES cells were treated with 1 mm nocodazole for 14 h as shown in Fig. 2B. Analysis of allele-specific 3C interaction is detailed in Fig. 4B. A control L7 template indicates complete digestion. M at the top indicates size marker. M, Maternal; P, paternal.
DNA segments located between the four discrete domains were involved in only limited and primarily local interactions. Interactions among segments outside the four domains were not observed in newborn liver (data not shown) and were very weak in MEF cells (Fig. 3A, panels E #7 and #11). The interactions were mainly local and were primarily monoallelic (Fig. 5A). On the other hand, there were a number of long-range interactions originating from the Igf2-DMR0 (E #1) that were predominantly derived from the maternal allele in MEF (Fig. 3A), newborn liver (Fig. 4A), and ES (Fig. 5A) cells, suggesting that the maternal Igf2 region assumed a more compact conformation. This compact conformation was also evident when we determined interactions originating from Igf2-DMR1 (panel E #3), where virtually all observed 3C interactions were predominantly from the maternal DMR1 allele in MEF (Fig. 3B), in liver (Fig. 4B), and in ES (Fig. 5B) cells. Low levels of random interactions within the paternal allele in the vicinity of the Igf2 E #3 target could be detected in some fetal livers with greater input of 3C DNA and/or a greater number of PCR cycles (see supplemental Note N4).
The allelic, long-range interactions with the ICR play a pivotal role in the switch model. We noted that there are two adjacent RI sites, RI #12A and #12B, present in both M. musculus and M. spretus, which span a 136-bp fragment containing CTCF binding site #1 (supplemental Note N1). The 3C analysis from the two adjacent sites yielded similar but not identical patterns of domain interaction in MEF, liver, and ES cells (Figs. 2B, 3A, 4A, and 5A; panels E #12A and E #12B). Allelic analysis using the polymorphic HpaII site present in M. musculus (Figs. 3B, 4B, 5B; panel E #12A Hpa II) and BstNI present in M. spretus (data not shown) near the RI #12A site indicates a DMR1-ICR interaction that is predominantly on the maternal allele. The maternal DMR1-maternal ICR (RI 3–12A) 3C product was further verified by direct DNA sequencing (supplemental Fig. S1).
In contrast to a recent finding (10), we noted that in both MEF and liver, interaction between the site (RI #6) near the MAR3 and the ICR (RI #12A) was derived predominantly from the paternal RI #12A allele (Figs. 3B and 4B; panel E #12A, column 6). This finding is not consistent with the proposed maternal-specific MAR 3-ICR interaction (10). Intriguingly, in all three systems that we studied, the interactions between the DMR1 and the intergenic and H19 enhancers that would bring the enhancers closer to the Igf2 promoter region were derived primarily from the maternal and not the paternal DMR1 (Figs. 3B, 4B, and 5B; panel E #3). The proximity of the enhancers to the silenced maternal Igf2 promoter region challenges a model of maternal Igf2 silencing by a single chromosome loop. Our data suggest that it is necessary to form multiple chromosome loops in which the CTCF-ICR complex on the maternal unmethylated ICR can sequester all of the enhancers, including the intergenic enhancer, and thereby prevent direct activation of the Igf2 promoters.
Maintenance of Maternal-Specific DMR1-ICR Interaction in ES Cells during Mitosis
Normally growing ES cells (Fig. 5A) demonstrate four domains of local and long-range interaction patterns similar to those of MEF (Fig. 3A) and newborn liver (Fig. 4A). In normal ES cells, interactions from DMR1 (RI #3) were predominantly from the maternal allele (Fig. 5B). During mitosis, when there is a compact chromosome conformation that generates multiple biparental 3C interactions across the region, the interaction between DMR1 (RI #3 site) and the ICR (RI #12A site) was predominately from the maternal DMR1 allele (Fig. 5C, panel E #3, column ICR-12A). Reciprocal scanning from the RI #12A site demonstrated that the DMR1-ICR interaction was from the maternal ICR-12A allele (Fig. 5C, panel E #12A, column RI #3). The DMR1-ICR interaction was predominantly from the maternal allele (90–95% by PhosphorImager scanning), and this interaction may represent an epigenetic mark on the maternal allele during normal cell cycling and during mitosis. This conclusion is consistent with the notion that CTCF binding and higher-order chromatin structure of the H19 gene are maintained in mitotic chromatin (18).
Interaction of Enhancers and Igf2 Promoter on the Paternal Allele in Newborn Liver
To analyze allelic interaction from the H19 downstream enhancer site, we used a polymorphic restriction site (KpnI or MboI) downstream of the RI #14 location. For these studies, we performed standard 3C assays using a reverse primer (RI 14R) and one of the 32 forward primers that include the 24 sites of the Igf2/H19 region and four upstream and four downstream sites (Fig. 6A). Digestion with KpnI, an enzyme that digests the paternal (M. spretus) allele, indicated strong enhancer interactions across the region reaching to the Igf2 promoter (Fig. 6B, columns RI #4, #5) on the paternal allele. The maternal allele showed much weaker interactions further upstream (up to RI #b), which might reflect some background interactions. The 3C interaction between the DMR1 E #3 and the endoderm enhancer E #14 reverse primer was low and not detectable in both parental alleles (Fig. 6, A and B, column #3) whereas the E #14 forward primer revealed detectable DMR1-enhancer interaction on the maternal allele (Fig. 4B). These differences may be attributable to the choice of primer orientation (see supplemental Note N5).
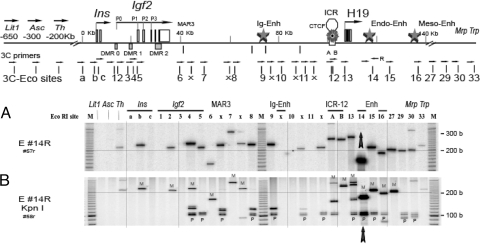
Figure 6.
Interaction of Enhancers and Igf2 Promoter in Newborn Liver
A, 3C analysis was performed using a reverse primer (Eco 14R) and one of each of 32 forward primers across the Igf2/H19 region. Note that the 32 sites include the 24 sites of the Igf2/H19 region in Figs. 1–4 and as well as four upstream and four downstream sites. Cross-linked liver DNA (50 ng) was amplified for 36 cycles. Major 3C products of correct size were observed in the 140-kb region (columns a through 30). A correct PCR product from Eco 14 forward/reverse primers was observed at column 14. B, Digestion with KpnI was used to identify the two parental alleles. Paternal allele (M. spretus) has the KpnI restriction site. All 3C products derived from the paternal chromosome contain a common KpnI fragment of 105 and 101 bases (two strands of DNA, seen as a doublet). M at the top indicates size marker. M, Maternal; P, paternal.
The enhancer interactions with locations within the Igf2 promoter-ICR region were predominantly paternal. The interactions were derived exclusively from the paternal allele at the Igf2 promoter locus (Fig. 6B, column RI #5) and near the intergenic enhancers (Fig. 6B column RI #8 and #9), which is consistent with 3C data from other research groups (10) and is consistent with the stringent imprinting of Igf2 in newborn livers. The presence of a strong domain interaction between Eco #14 enhancer, intergenic enhancer, and Igf2 promoter region on the paternal allele (shown in Fig. 6B) was also reflected in 3C analysis using the RI #14 forward primer in MEF (Fig. 3A, panel E #14), newborn liver (Fig. 4A, panel E #14), and ES (Fig. 5A, panel E #14) cells.
Local interactions from the RI #14 enhancer locus to RI #12A, #13, #15, and #16) were from both parental RI #14 alleles (Fig. 6B), which may simply reflect the nature of frequent local interactions within the relatively short span of about 20 kb of DNA. In contrast to the biallelic RI #14-#12A interaction, at the ICR locus, the interaction RI #14-#12B was exclusively from the maternal allele that includes maternal RI #14 fragment (Fig. 6B, column # RI 12B) and maternal RI #12B fragment (data not shown). Because the RI #12B fragment (136 b) harbors CTCF binding site #1, it is possible that CTCF binding at this location on the maternal allele may direct the ICR enhancer RI #14 interaction, suggesting a direct role of CTCF in organizing ICR-enhancer interaction on the maternal allele.
DISCUSSION
To examine the Igf2/H19 chromosome conformation in detail, we have analyzed 3C interactions by scanning 24 RI sites across the Igf2/H19 region using 12 (MEF and ES cells) or 16 (newborn liver) common target sites. Some of our 24 RI sites coincided and therefore were directly comparable with the RI sites in a recent publication (10). To avoid any allelic bias, we sequenced the M. spretus genomic DNA before designing specific M. musculus/M. spretus 3C primers, noting the presence of two polymorphic RI sites in the 140-kb Igf2/H19 region (supplemental Note N2).
It is important to point out that 3C products that are formed by religation of digested nuclei reflect only the proximity of various sites on the chromosome and do not necessarily provide evidence of direct physical contact in vivo. Strong interactions among neighboring DNA fragments and low levels of random collisions across about 100 kb of DNA may be detected (19), which is consistent with our observation of local interactions within the four domains across the Igf2/H19. Even within each chromosome domain, the intensity and the presence/absence of a specific 3C product varied substantially, reflecting details of local topology of the chromosome conformation.
On the paternal chromosome, the activation of Igf2 was associated with the paternal allele-specific promoter-enhancer association (Fig. 6B). The mechanism underlying the promoter-enhancer association is unclear, but it is probably not organized by the methylated paternal ICR that does not bind CTCF. The H19 enhancer (RI #14) interacts across the ICR-Igf2 region exclusively from the paternal allele, bringing the ICR close to Igf2 DMR2 and MAR3. The observed paternal-specific interactions ICR-DMR2 (9) and ICR-MAR3 (Fig. 3B, panel E #12A, column RI #6) may be the consequence (rather than the cause) of the organizing paternal enhancer interactions. As opposed to the maternal allele, our data suggest that the chromatin surrounding the paternal Igf2/H19 region is more relaxed and is embedded in an ACH as a default state in which Igf2 is active, whereas H19 is rendered inactive by promoter methylation.
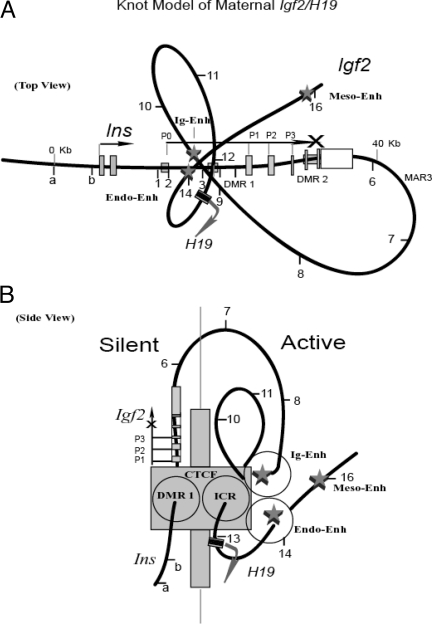
The use of multiple data points across this imprinted region indicates that there is a close interaction between the four domains on the maternal Igf2/H19 chromosome, suggesting a knot model formed by the four domains: DMR1, intergenic enhancer, ICR, and H19 enhancer (Fig. 7, top view). In this three-dimensional model, the Igf2 promoter region and the enhancers are located on the opposite sites of the ICR. A loop formed by the DMR1-ICR interaction superimposed on a fold out loop formed by ICR-intergenic enhancers would position the intergenic enhancer on the opposite side of Igf2 (Fig. 7, side view). The observed strong 3C DMR1-ICR interaction signifies close contact, whereas the weaker interaction between DMR1- enhancers may reflect propinquity rather than direct contact.
Figure 7.
Knot Model of the Inactive Igf2 Allele
A, A complex looping configuration organized by a CTCF complex binding at the unmethylated ICR. Two internal loops formed by three-point interaction (ICR-intergenic enhancer-H19 enhancer) are superimposed on a larger loop that brings the ICR-CTCF-enhancers complex close to the Igf2 DMR1 region. Relative location of Insulin and Igf2 promoters (P0, P1–P3), and location of the RI sites (a, b, and 1–16) are shown. The intergenic enhancer and H19 downstream enhancers (endoderm and mesoderm) are marked. Active H19 transcription is shown as an arrow. B, Side view of the knot model. The triple loops bring all enhancers (intergenic, endoderm, and mesoderm) to one side and the Igf2 promoter region to the other side of the ICR-CTCF complex. Further modification, interacting with other protein complexes (such as CHD8, EZH2, and SUZ12; see text) and other interchromosomal regions, may create a huge multicomplex (shown as a blocking wall) that covers the entire Igf2 12-kb promoter region, which sequesters all the enhancers preventing direct Igf2 activation. Endo, Endoderm; Ig, intergenic; Meso, mesoderm.
The binding of CTCF to the maternal, unmethylated ICR has been proposed as a mechanism to block the access of H19 downstream enhancers to the Igf2 promoters, leading to the inactivation of the maternal Igf2 (6,7,20,21). However, the mechanism by which CTCF can act as an insulator to block the activity of downstream enhancers is not known.
In a recent publication, Yoon et al. (22) analyzed several ICR insertion mutations using 3C and chromatin immunoprecipitation. The authors have proposed that the organization of chromosome looping is a general model of CTCF insulator function. The chromosome loops are formed by association of CTCF with downstream enhancers and Igf2 promoters. The interaction of CTCF with the enhancers allows CTCF to isolate downstream enhancers and to prevent them from direct association with upstream promoters. This enhancer-trapping activity was position dependent, and the authors have suggested a functional tracking mechanism from the enhancer (22). The model of functional tracking from the enhancer fails to explain the silencing of the intergenic enhancers, because intergenic enhancers are upstream of the maternal ICR-CTCF and therefore should not be affected by CTCF-blocking activity in their looping model.
In our model, by knotting and folding out, all enhancers, including the intergenic enhancers, are positioned close to the maternal ICR-CTCF complex but on the opposite side of Igf2 (Fig. 7). We propose a three-dimensional structural model whereby CTCF binding at the maternal ICR organizes multiple chromosome loops and serves as a blocking wall separating the inactive domain (Igf2) from the active domain (enhancers and H19). Our knot looping model showing juxtaposition of DMR1-ICR-intergenic enhancer-endo-enhancer on the maternal allele (Fig. 7) differs substantially from the Kurukuti et al. model (10) that depicts a close vicinity of DMR1-MAR3-ICR (for further discussion, see supplemental Note N6).
In addition to positing an extra loop to bring the intergenic enhancers into the maternal ICR-CTCF complex, our model proposes an interaction between the maternal ICR and the Igf2 DMR1 that acts as a repressor element (23). The maternal ICR-DMR1 interaction is in agreement with previous reports (9,10) but conflicts with the ICR-promoter interaction model (22). Although the discrepancy can be explained by the use of different restriction sites selected for the 3C assays [Yoon et al. (22) used BamHI/BglII; we used RI], our 3C scanning of all RI sites across the Igf2 promoters/DMR region suggests a dominant interaction at RI #3 site near DMR1 rather than RI #4 and RI #5 sites near the P1 promoter. Because DMR1 is located between Igf2 P0 and P1 promoters (∼4 kb away from each site), and the other promoters are also quite close (P2 and P3, 2 and 4 kb downstream of P1, respectively), the complete 12 kb of the Igf2 promoter region (P0–P3) may be brought close to, and therefore controlled by, the maternal ICR-CTCF.
The Igf2 P0 transcript is imprinted and expressed exclusively in the labyrinthine trophoblast of the placenta from the paternal allele (24). The silencing of the maternal Igf2 P0 in placenta is consistent with our model whereby the Igf2 promoter P0 is blocked by the maternal ICR-CTCF complex.
How can the CTCF complex binding to the unmethylated ICR block a direct contact between Igf2 promoters and the enhancers? We propose that the size of this very large and complex ICR-CTCF module may lead to steric interference. CTCF blocking activity is regulated by poly(ADP-ribosyl)ation (25). Active CTCF also interacts with chromodomain helicase DNA-binding protein 8 (CHD8), a sucrose nonfermenting 2 (SNF2)-like chromodomain helicase protein; the CTCF-CHD8 complex regulates DNA methylation and histone acetylation around the CTCF binding sites (26). We have recently found that CTCF also interacts with suppressor of zeste 12 homolog (SUZ12), a member of the polycomb group (PcG) repressive protein complex, to coordinate ICR-CTCF insulation (Li, T., J. F. Hu, X. Qiu, J. Q. Ling, H. L. Chen, S. K. Wang, A. J. Hou, T. H. Vu, and A. R. Hoffman, unpublished). By interacting with the enhancers, the ICR complex would integrate other enhancer-binding proteins such as FoxA and Zac1 proteins that bind to the endodermal H19 enhancers (27,28). Furthermore, the CTCF complex also mediates interchromosomal interactions, bringing the paternal Wsb1/Nf1 locus on chromosome 11 (17) as well as other imprinting regions (29) to the maternal Igf2/H19 ICR on chromosome 7. The huge multicomplex ICR-CTCF may form a blocking wall covering the Igf2 12-kb promoter region, thereby preventing direct contact and activation of the maternal Igf2 promoter by the enhancers. The silencing knot of the DMR1-ICR-enhancers is found in newborn liver, MEF, and ES cells, under normal growing conditions and during mitosis, suggesting a persistent, maternal epigenetic memory in various cell types and in ES cells. Although we believe that the basic maternal knot model is a plausible theoretical structure, our analysis was based on a population of the cells, and thus, what we observe is the sum total of all interactions in the cell population, not the interactions on a single chromosome template. The provisional nature of our model could be tested when methods to analyze the interactions on a single chromosome template become available.
Human IGF-II/H19 is imprinted in a fashion similar to that seen in mouse. Aberrant IGF-II imprinting or loss of imprinting that results in biallelic IGF-II expression has been observed in a number of neoplasms (30,31,32). The molecular mechanisms underlying the imprinting and loss of imprinting of IGF-II/H19 have not been fully elucidated. Imprinting of IGF-II/H19 is also associated with differential methylation and CTCF binding in the ICR (33,34,35). We speculate that in the human gene, a huge CTCF complex also forms a blocking wall covering the IGF-II promoter region, preventing direct access of enhancer(s) to the IGF-II promoter. It is possible that when this architecture is disrupted, imprinting is lost, and biallelic expression of IGF-II ensues, leading to a neoplastic diathesis.
MATERIALS AND METHODS
Breeding Interspecific Mice
Animal breeding and all animal procedures were performed according to protocols approved by the Institutional Care and Use Committee at the Veterans Affairs Palo Alto Health Care System. M. spretus (Spretus) male and M. musculus (C57BL6J) female mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mating between normal domestic mice (Musculus) with the wild-derived Spretus mice was usually much less successful than normal breeding within strains. We have improved the fecundity of this interspecific mating frequency by providing the mice with a piece of delicious home-grown apple (Fuji) at the onset of mating. Our observation indicates that communication and courtship were much improved as the mice shared the snack. Pregnancy ensued in three of the nine (33%) breeding pairs, a 10-fold improvement in our previous pregnancy rates.
Newborn F1 Liver, MEF, and ES Cells
Livers from newborn F1 mice (1–3 d) were freshly dissected and used for the 3C experiment, and 17-d embryos were used to prepare MEF by a standard protocol. MEF was cultured in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin and grown at 37 C with 5% CO2. The mouse ES cell line, SF1-G (kindly provided by Dr. R. Feil) was derived from normal fertilization of Musculus female and Spretus male (36,37). The SF1-G (∼20 passages) that was cultured on gelatin-coated plates in DMEM supplemented with leukemia inhibitory factor, LIF (1000 U/ml) maintained normal Igf2-H19 imprinting (38).
Expression of Igf2 and H19 by RT-PCR
RT was carried out using both random hexamers and oligo d(T)17 primers. RT-PCR was performed using the following sets of primers: Igf2-116 primers (T-2658 forward, 5′-tgg ccc tcc tgg aga cat act gtg c-3′, and T-2659 reverse, 5′ ttt gaa gaa ctt gcc cac ggg gta tc-3′), Igf2-123 primers (M-84 forward, 5′-CTT GTG CTG GAT CGC TGC TGC TTA CG-3′, and M-219 reverse, 5′-CTG CGA CGG TTG GCA CGG CTT GA-3′), and H19-199 primers (p4025 forward, 5′-TAA GTC GAT TGC ACT GGT TTG GAG T-3′, and p4026 reverse, 5′-TGA TGG AAC TGC TTC CAG ACT AGG-3′). PCR conditions were 97 C for 2 min, 32 cycles of 97 C for 20 sec, 1 min at 62 C, and 1 min at 72 C, followed by a final extension at 72 C for 5 min. The PCR products (5 μl) were labeled with [α-32P]dCTP, and digested with 1 U DpnII (Igf2-123) or Fok I (H19-199) in a 20-μl reaction at 37 C for 2 h. Control 18S-100 (T-181 forward, 5′-GAG CGA CCA AAG GAA CCA TA-3′, and T-182 reverse, 5′-CTT GTC TCA AAG ATT AAG CCA TGC-3′) was amplified at 23 cycles (97 C for 20 sec, 1 min at 62 C, and 1 min at 72 C). PCR products were analyzed on a 5% polyacrylamide-urea gel and visualized with a PhosphorImager.
Relative mRNA Levels by Q-PCR
Q-PCR assays were run in quadruplicate on a 384-well plate using an ABI Prism 7900HT, SYBR Green, and the ABI protocol. The Igf2-123 primers (M-84/M-219) and H19-120 primers (T-402 forward, 5′-TTG GAG TCC CGG AGA TAG CTT TGA G-3′, and T-403 reverse, 5′-CAG TTG CCC TCA GAC GGA GAT GG-3′) were used in the Q-PCR assays. Relative levels were determined by a ΔCt and ΔΔCt with reference to mouse β-actin-71 controls (T-2151 forward, 5′-TCC AGC AGA TGT GGA TCA GCA-3′, and T-2171 reverse, 5′-GAA GCA CTT GCG GTG CAC GAT-3′).
Treatment of Cells with Nocodazole
Nocodazole (Sigma Chemical Co., St. Louis, MO) has been used for mitotic synchronization (18). To define proper treatment conditions, in a pilot experiment, we treated MEF and SF1-G cells with various concentrations of nocodazole (0.05–2 μm) for 12–24 h. We obtained a recovery of detached mitotic cells (∼50%) without evidence of toxicity at 0.5–1 μm for 12–16 h. In subsequent experiments, MEF and SF1-G (at ∼70% confluency) were incubated in fresh medium containing 1 μm nocodazole for 14 h. Mitotic cells were collected by manually shaking the dishes (shake-off) and then by centrifugation (500 × g for 10 min). Collected cells were resuspended in PBS and immediately fixed by formaldehyde for 3C analysis.
3C Assay
Tissues were dissected from newborn F1 mice, rinsed with PBS, minced, and sieved through a 70-μm nylon cell strainer (BD Biosciences, Billerica, MA) into PBS. The cell suspensions were fixed immediately with formaldehyde in PBS at room temperature for 10 min. Control growing MEF and SG1-G cells that attached to the culture plates were rinsed with PBS and directly fixed with 2% formaldehyde in PBS (5 ml for 10-cm plates) at room temperature for 10 min. Mitotic MEF and SF1-G cells were fixed under the same conditions. The 3C assays were carried out essentially as described (www.epigenome-noe.net/researchtools/protocol) with some modifications. Briefly, cells were fixed in 2% formaldehyde, quenched with 0.125 m glycine, and then lysed with 0.2% Nonidet P-40 on ice for 2 h with stirring. Normal MEF and SF1-G cells that were fixed and quenched on culture plates were collected on ice in Nonidet P-40 lysis buffer using a flat blade. Nuclei were collected by centrifugation, resuspended in NEB EcoRI buffer (New England Biolabs, Beverly, MA) containing 0.3% SDS, treated at 37 C for 1 h, and quenched with 1.8% Triton X-100 at 37 C for 1 h. Treated nuclei (∼10 million) were digested with RI (1000 U) or triple enzymes (RI, BamHI, and HindIII; 1000 U each) in 500 μl NEB EcoRI buffer at 37 C for about 18 h. After enzyme deactivation (1% SDS, 65 C for 20 min) and quenching (2.6% Triton X-100, 37 C for 1 h) aliquots of digested nuclei (∼3 μg DNA) were ligated with T4 DNA ligase (2000 U T4 ligase in 1.2 ml ligation buffer) at 16 C for 4 h. Cross-linked DNAs were treated with proteinase K (100 μg, 65 C overnight) and RNase A (1 μg, 37 C for 30 min) and concentrated in a speedVac (Savant, NY) to reduce to approximately one fifth volume before phenol-chloroform extraction. Control samples (before/after digestion and before/after ligation) were also treated with proteinase K and RNase A before phenol-chloroform extraction. DNAs were precipitated with ammonium acetate (2 m) and 2-propanol (1.5 vol.). The pellet was washed with cold 75% ethanol three times and then dissolved in low Tris-EDTA buffer (1 mm Tris, 0.1 mm EDTA, pH 8.0).
Mouse Igf2/H19 Polymorphisms, 3C Primers, and DNA Sequencing
The Musculus × Spretus interspecific F1 has abundant informative SNPs (about one SNP per 100 bases in the Igf2/H19 region). We searched the restriction map of RI, HindIII and BamHI in the approximately180-kb Musculus Igf2-H19 region and other upstream regions of the Ins/Igf2 locus (tyrosine hydroxylase, TH, −200 kb; achaete-scute complex homolog-like 2, Ascl-2 or Mash2, −300 kb; and antisense Lit1, −650 kb) by a University of California, Santa Cruz, program (http://genome.ucsc.edu/) and chose RI as 3C restriction sites. The upstream locations (−200 to −650 kb) served as 3C long-range interaction controls. Based on the M. musculus genome sequence (University of California, Santa Cruz, Assembly February 2006), we designed primers to amplify M. spretus genomic DNA (∼500 bases), crossing these RI sites using a primer design software (http://biotools.umassmed.edu/bioapps/primer3). Thirty-three Spretus fragments (of 37 Musculus amplicons) could be amplified. We sequenced the DNA fragments in both orientations and located the informative SNPs and insertions/deletions within about 200 bases upstream/downstream of the 32 RI sites. Forward and reverse 3C primers (melting temperature ∼74 C) were designed in the identical Musculus/Spretus sequence regions (supplemental Note N1). In our initial studies, single and robust 3C products within the 140-kb Igf2/H19 region were obtained using about 50 ng of 3C-ligated DNAs. Primers 200 kb upstream (Th, Asc2, and Lit1) and 150 kb downstream (primer Eco #33) yielded no significant bands of correct size, suggesting 3C interactions were limited within the 140-kb region. Twenty-four forward primers within the 140-kb region were used for subsequent 3C assays. Selected cross-linked 3C products identified on urea-polyacrylamide gel were directly sequenced in both orientations by PCR sequencing using the corresponding 3C primers.
PCR Amplification and Parental Allele Differentiation
We used a standard hot-start PCR protocol (6 μl volume under liquid wax, 0.1 μm appropriate primers, 50 μm dNTPs, 0.4 U KlenTaq I polymerase, and 50 ng cross-linked DNAs) for 3C amplification. PCR conditions were 97 C for 120 sec followed by 36 cycles of 97 C for 20 sec and 68 C for 120 sec and finally 72 C for 10 min. To facilitate the 3C scanning from multiple target points, we employed a two-step PCR. The first amplification was performed in a 40-μl PCR containing 0.5–1.0 μg cross-linked DNA and a set of 32 forward 3C primers (97 C for 120 sec followed by 36 cycles of 97 C for 20 sec and 68 C for 120 sec). The PCR products were diluted (1000-fold in a final reaction) and labeled in a 6-μl PCR (22 cycles of 97 C for 20 sec and 68 C for 120 sec) containing the selected two 3C primers and 0.2 μCi [32P]dCTP. We used a hot-stop protocol to analyze the relative levels of the two parental alleles. Labeled nucleotide ([32P]dCTP) or 32P end-labeled primers were added in 1× PCR mixture for the last 22 cycles. Further labeling conditions were 97 C for 3 min followed by 68 C for 5 min and 72 C for 10 min. Labeled PCR products were digested with appropriate enzymes (New England Biolabs; 1 U) in a total volume of 10 μl at 37 C for 12 h under liquid wax. To control the restriction digestion, we added a 32P-labeled L7 template that contains the restriction site to the PCR product before digestion. The digested products were separated on 5% polyacrylamide-urea gel, visualized, and quantified by a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Supplementary Material
Acknowledgments
We thank R. Feil, W. Reik, W. Dean, and A. Wagschal for the gift of the SF1-G cell line.
Footnotes
Present address for X.Q.: The Third Xiangya Hospital, Central South University, People’s Republic of China 410000.
Present address for X.Q. and Q.L.: The People’s Hospital, Liuyang City, People’s Republic of China 410300.
Present address for H.L.C.: Department of Endocrinology, Xiangya Hospital, Central South University, Hunan Province, People’s Republic of China.
Present address for J.F.H.: GMR Epigenetics Corp., Palo Alto, California 94304.
This work was supported by National Institutes of Health Grant DK 36054 and by the Research Service of the Department of Veterans Affairs.
Disclosure Statement: The authors have nothing to disclose.
First Published Online March 20, 2008
Abbreviations: ACH, Active chromosomal hub; 3C, chromosome conformation capture; CHD8, chromodomain helicase DNA-binding protein 8; CTCF, CTC-binding factor; DMR, differentially methylated region; ES, embryonic stem; ICR, imprinting control region; MAR, matrix attachment region; MEF, mouse embryonic fibroblast; Q, quantitative; RI, EcoRI; SNP, single-nucleotide polymorphism.
References
- DeChiara TM, Robertson EJ, Efstratiadis A 1991 Parental imprinting of the mouse insulin-like growth factor II gene. Cell 64:849–859 [DOI] [PubMed] [Google Scholar]
- Bartolomei MS, Zemel S, Tilghman SM 1991 Parental imprinting of the mouse H19 gene. Nature 351:153–155 [DOI] [PubMed] [Google Scholar]
- Zemel S, Bartolomei MS, Tilghman SM 1992 Physical linkage of two mammalian imprinted genes, H19 and insulin-like growth factor 2. Nat Genet 2:61–65 [DOI] [PubMed] [Google Scholar]
- Leighton PA, Saam JR, Ingram RS, Stewart CL, Tilghman SM 1995 An enhancer deletion affects both H19 and Igf2 expression. Genes Dev 9:2079–2089 [DOI] [PubMed] [Google Scholar]
- Bell AC, Felsenfeld G 2000 Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature 405:482–485 [DOI] [PubMed] [Google Scholar]
- Hark AT, Schoenherr CJ, Katz DJ, Ingram RS, Levorse JM, Tilghman SM 2000 CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature 405:486–489 [DOI] [PubMed] [Google Scholar]
- Kanduri C, Pant V, Loukinov D, Pugacheva E, Qi CF, Wolffe A, Ohlsson R, Lobanenkov VV 2000 Functional association of CTCF with the insulator upstream of the H19 gene is parent of origin-specific and methylation-sensitive. Curr Biol 10:853–856 [DOI] [PubMed] [Google Scholar]
- Szabo P, Tang SH, Rentsendorj A, Pfeifer GP, Mann JR 2000 Maternal-specific footprints at putative CTCF sites in the H19 imprinting control region give evidence for insulator function. Curr Biol 10:607–610 [DOI] [PubMed] [Google Scholar]
- Murrell A, Heeson S, Reik W 2004 Interaction between differentially methylated regions partitions the imprinted genes Igf2 and H19 into parent-specific chromatin loops. Nat Genet 36:889–893 [DOI] [PubMed] [Google Scholar]
- Kurukuti S, Tiwari VK, Tavoosidana G, Pugacheva E, Murrell A, Zhao Z, Lobanenkov V, Reik W, Ohlsson R 2006 CTCF binding at the H19 imprinting control region mediates maternally inherited higher-order chromatin conformation to restrict enhancer access to Igf2. Proc Natl Acad Sci USA 103:10684–10689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewell RA, Arney KL, Arima T, Barton SC, Brenton JD, Surani MA 2002 Novel conserved elements upstream of the H19 gene are transcribed and act as mesodermal enhancers. Development 129:1205–1213 [DOI] [PubMed] [Google Scholar]
- Charalambous M, Menheniott TR, Bennett WR, Kelly SM, Dell G, Dandolo L, Ward A 2004 An enhancer element at the Igf2/H19 locus drives gene expression in both imprinted and non-imprinted tissues. Dev Biol 271:488–497 [DOI] [PubMed] [Google Scholar]
- Dekker J, Rippe K, Dekker M, Kleckner N 2002 Capturing chromosome conformation. Science 295:1306–1311 [DOI] [PubMed] [Google Scholar]
- Horike S, Cai S, Miyano M, Cheng JF, Kohwi-Shigematsu T 2005 Loss of silent-chromatin looping and impaired imprinting of DLX5 in Rett syndrome. Nat Genet 37:31–40 [DOI] [PubMed] [Google Scholar]
- Spilianakis C, Flavell RA 2004 Long-range intrachromosomal interactions in the T helper type 2 cytokine locus. Nature Immunol 5:1017–1027 [DOI] [PubMed] [Google Scholar]
- Spilianakis CG, Lalioti MD, Town T, Lee GR, Flavell RA 2005 Interchromosomal associations between alternatively expressed loci. Nature 435:637–645 [DOI] [PubMed] [Google Scholar]
- Ling JQ, Li T, Hu JF, Vu TH, Chen HL, Qiu XW, Cherry AM, Hoffman AR 2006 CTCF mediates interchromosomal colocalization between Igf2/H19 and Wsb1/Nf1. Science 312:269–272 [DOI] [PubMed] [Google Scholar]
- Burke LJ, Zhang R, Bartkuhn M, Tiwari VK, Tavoosidana G, Kurukuti S, Weth C, Leers J, Galjart N, Ohlsson R, Renkawitz R 2005 CTCF binding and higher order chromatin structure of the H19 locus are maintained in mitotic chromatin. EMBO J 24:3291–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J 2006 The three ‘C’ s of chromosome conformation capture: controls, controls, controls. Nat Methods 3:17–21 [DOI] [PubMed] [Google Scholar]
- Leighton PA, Ingram RS, Eggenschwiler J, Efstratiadis A, Tilghman SM 1995 Disruption of imprinting caused by deletion of the H19 gene region in mice. Nature 375:34–39 [DOI] [PubMed] [Google Scholar]
- Thorvaldsen JL, Duran KL, Bartolomei MS 1998 Deletion of the H19 differentially methylated domain results in loss of imprinted expression of H19 and Igf2. Genes Dev 12:3693–3702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon YS, Jeong S, Rong Q, Chung JH, Pfeifer K 2007 Analysis of the H19ICR Insulator. Mol Cell Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden S, Constancia M, Hashimshony T, Dean W, Goldstein B, Johnson AC, Keshet I, Reik W, Cedar H 2001 An upstream repressor element plays a role in Igf2 imprinting. EMBO J 20:3518–3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constancia M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C, Reik W 2002 Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature 417:945–948 [DOI] [PubMed] [Google Scholar]
- Yu W, Ginjala V, Pant V, Chernukhin I, Whitehead J, Docquier F, Farrar D, Tavoosidana G, Mukhopadhyay R, Kanduri C, Oshimura M, Feinberg AP, Lobanenkov V, Klenova E, Ohlsson R 2004 Poly(ADP-ribosyl)ation regulates CTCF-dependent chromatin insulation. Nat Genet 36:1105–1110 [DOI] [PubMed] [Google Scholar]
- Ishihara K, Oshimura M, Nakao M 2006 CTCF-dependent chromatin insulator is linked to epigenetic remodeling. Mol Cell 23:733–742 [DOI] [PubMed] [Google Scholar]
- Long L, Spear BT 2004 FoxA proteins regulate H19 endoderm enhancer E1 and exhibit developmental changes in enhancer binding in vivo. Mol Cell Biol 24:9601–9609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varrault A, Gueydan C, Delalbre A, Bellmann A, Houssami S, Aknin C, Severac D, Chotard L, Kahli M, Le Digarcher A, Pavlidis P, Journot L 2006 Zac1 regulates an imprinted gene network critically involved in the control of embryonic growth. Dev Cell 11:711–722 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Tavoosidana G, Sjolinder M, Gondor A, Mariano P, Wang S, Kanduri C, Lezcano M, Sandhu KS, Singh U, Pant V, Tiwari V, Kurukuti S, Ohlsson R 2006 Circular chromosome conformation capture (4C) uncovers extensive networks of epigenetically regulated intra- and interchromosomal interactions. Nat Genet 38:1341–1347 [DOI] [PubMed] [Google Scholar]
- Feinberg A, Cui H, Ohlsson R 2002 DNA methylation and genomic imprinting: insights from cancer into epigenetic mechanisms. Semin Cancer Biol 12:389 [DOI] [PubMed] [Google Scholar]
- Ogawa O, Eccles MR, Szeto J, McNoe LA, Yun K, Maw MA, Smith PJ, Reeve AE 1993 Relaxation of insulin-like growth factor II gene imprinting implicated in Wilms’ tumour. Nature 362:749–751 [DOI] [PubMed] [Google Scholar]
- Rainier S, Johnson LA, Dobry CJ, Ping AJ, Grundy PE, Feinberg AP 1993 Relaxation of imprinted genes in human cancer. Nature 362:747–749 [DOI] [PubMed] [Google Scholar]
- Ulaner GA, Vu TH, Li T, Hu JF, Yao XM, Yang Y, Gorlick R, Meyers P, Healey J, Ladanyi M, Hoffman AR 2003 Loss of imprinting of IGF2 and H19 in osteosarcoma is accompanied by reciprocal methylation changes of a CTCF-binding site. Hum Mol Genet 12:535–549 [DOI] [PubMed] [Google Scholar]
- Ulaner GA, Yang Y, Hu JF, Li T, Vu TH, Hoffman AR 2003 CTCF binding at the insulin-like growth factor-II (IGF2)/H19 imprinting control region is insufficient to regulate IGF2/H19 expression in human tissues. Endocrinology 144:4420–4426 [DOI] [PubMed] [Google Scholar]
- Cui H, Onyango P, Brandenburg S, Wu Y, Hsieh CL, Feinberg AP 2002 Loss of imprinting in colorectal cancer linked to hypomethylation of H19 and IGF2. Cancer Res 62:6442–6446 [PubMed] [Google Scholar]
- Dean W, Bowden L, Aitchison A, Klose J, Moore T, Meneses JJ, Reik W, Feil R 1998 Altered imprinted gene methylation and expression in completely ES cell-derived mouse fetuses: association with aberrant phenotypes. Development 125:2273–2282 [DOI] [PubMed] [Google Scholar]
- Khosla S, Aitchison A, Gregory R, Allen ND, Feil R 1999 Parental allele-specific chromatin configuration in a boundary-imprinting-control element upstream of the mouse H19 gene. Mol Cell Biol 19:2556–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Vu TH, Ulaner GA, Littman E, Ling JQ, Chen HL, Hu JF, Behr B, Giudice L, Hoffman AR 2005 IVF results in de novo DNA methylation and histone methylation at an Igf2–H19 imprinting epigenetic switch. Mol Hum Reprod 11:631–640 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.