Abstract
GH is an important anabolic hormone. We previously demonstrated in cell culture that the cell surface GH receptor (GHR) is susceptible to inducible metalloproteolytic cleavage that yields the shed receptor extracellular domain (called GH binding protein) and renders the cells desensitized to subsequent GH stimulation. Sepsis and inflammatory states are associated with hepatic desensitization to GH, although disparate mechanisms have been postulated in various animal models. Using C3H/HeJ mice, we now demonstrate that administration of lipopolysaccharide (LPS) causes marked hepatic desensitization to GH, assessed by monitoring signal transducer and activator of transcription 5 tyrosine phosphorylation and nuclear accumulation and with a novel noninvasive bioluminescence imaging system to track in vivo hepatic GH signaling serially in individual mice. This endotoxin-induced desensitization was accompanied by marked loss of hepatic GHR, which was not explained by changes in GHR mRNA abundance. Furthermore, we observe that LPS causes GH-binding protein shedding of a hepatically expressed wild-type GHR but not a GHR with a mutation in the metalloprotease cleavage site. These data suggest that in this model system, LPS-induced desensitization to GH is associated with proteolytic GHR cleavage. These data are the first to demonstrate inducible in vivo GHR proteolysis and suggest this is a mechanism to regulate GH sensitivity and its anabolic effects during sepsis or inflammation.
GH IS A PITUITARY factor conserved through vertebrate evolution as both a promoter of longitudinal growth and an important regulator of metabolism (1). GH’s effects are complex but in the short term are largely anabolic. The GH receptor (GHR) is a widely expressed cell surface receptor enriched in liver, muscle, and fat that binds GH in the extracellular domain (ECD) (2,3). GH binding to GHR triggers intracellular activation of Janus kinase 2 (JAK2) and subsequent tyrosine phosphorylation and nuclear translocation of signal transducer and activator of transcription 5 (STAT5), which promotes transcription of target genes driven by a STAT5-binding GH response element (GHRE) (2,4,5,6,7).
Receptor abundance is in general a key parameter for hormone responsiveness. In prior in vitro studies, we observed that human, rabbit, and murine GHRs are targets for inducible metalloprotease-mediated cleavage in the membrane-proximal ECD stem region (8,9,10,11,12,13,14,15,16). In several cell lines, this proteolysis is rapidly induced in serum-free conditions by treatment with phorbol ester, platelet-derived growth factor, or serum and acutely results in reduced GHR abundance, shedding of the receptor ECD, and diminished cellular response to GH (8,9,10,12,17). To date, in vivo stimuli that promote GHR metalloproteolysis have yet to be identified.
GH resistance is well appreciated in acute inflammatory states in both clinical and experimental settings; experimentally, administration of endotoxin in rats and several mouse strains has been reported to cause diminished hepatic GH signaling, and a diverse set of mechanisms have been implicated in this phenomenon (18,19,20,21,22,23,24,25,26). We now report that treatment of C3H/HeJ mice with endotoxin results in diminished hepatic GH-induced STAT5 signaling and concomitant reduction in hepatic GHR abundance unaccompanied by changes in hepatic GHR mRNA abundance. Furthermore, we demonstrate for the first time that endotoxin causes rapid proteolytic shedding of hepatically expressed GHR ECD. These findings strongly suggest that modulation of hepatic GHR abundance by proteolytic cleavage underlies at least in part endotoxin-induced hepatic desensitization to GH.
RESULTS
LPS Pretreatment Blunts Hepatic STAT5-Mediated Signaling in Response to Subsequent GH Stimulation
For these studies, we used C3H/HeJ mice, because preliminary data indicated that hepatic GHR and acute GH-induced hepatic STAT5 signaling were well detected in this model (Ref. 27 and unpublished data). The C3H/HeJ mouse (referred to below as C3H) is known to harbor a defect in the Toll-like-4 receptor (TLR4), which renders it less sensitive to the endotoxin lipopolysaccharide (LPS) (28). We first tested whether we could detect responsiveness to LPS by measuring IL-6 production in these mice. Intraperitoneal LPS administration yielded an appreciable increase in the liver tissue IL-6 level from 1.9 pg/ml (control) to 12.5 pg/ml and 50 pg/mg at 60 and 180 min after LPS, respectively, confirming response to LPS in this system.
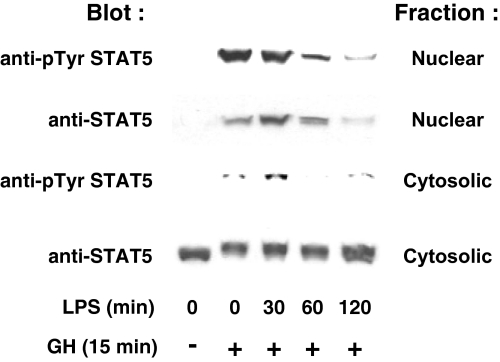
We tested the effect of LPS pretreatment on GH-induced hepatic signaling using two distinct approaches. First, we biochemically assessed acute liver STAT5 activation in response to systemic GH administration (Fig. 1). After an overnight fast, mice were treated with saline or LPS (10 μg/g ip) for the indicated durations. They were then administered saline or GH (3 μg/g ip) and killed 15 min later. Livers harvested at killing were homogenized and separated into cytosolic and nuclear fractions. As expected, GH acutely promoted appearance of tyrosine-phosphorylated STAT5 in the nuclear fraction without a dramatic change in cytosolic STAT5 abundance (the shift in migration of cytosolic STAT5 in response to GH is consistent with its tyrosine phosphorylation in that fraction as well. Notably, LPS pretreatment progressively lessened subsequent GH-induced nuclear accumulation of tyrosine-phosphorylated STAT5 (reduced by roughly 75% after 120 min of LPS pretreatment compared with no pretreatment; see figure legend for quantitation). Likewise, total STAT5 accumulated in the nucleus was reduced by approximately 70% after 120 min of LPS pretreatment compared with no pretreatment, and the level of tyrosine-phosphorylated STAT5 in the cytoplasmic fraction, when normalized for total cytosolic STAT5 abundance, was reduced by over 50% at the same time point. These findings suggest that LPS rapidly renders the liver less sensitive to GH-stimulated STAT5 activation.
Figure 1.
LPS Pretreatment Blunts Subsequent GH-Induced STAT5 Tyrosine Phosphorylation and Nuclear Accumulation in C3H/HeJ Mice
As detailed in Materials and Methods, mice were pretreated with LPS or PBS for the indicated durations and then with GH or PBS for 15 min. Livers were homogenized and separated into nuclear and cytoplasmic fractions, and these were immunoblotted to detect tyrosine-phosphorylated and total nuclear STAT5 and total and tyrosine-phosphorylated cytoplasmic STAT5. Densitometric analysis revealed the following relative abundances of tyrosine-phosphorylated nuclear STAT5 (expressed as a percentage of maximum): 0 min GH, 0%; 15 min GH, 100%; 15 min GH with 30 min LPS pretreatment, 85.3%; 15 min GH with 60 min LPS pretreatment, 38.3%; and 15 min GH with 120 min LPS, 25.2%.
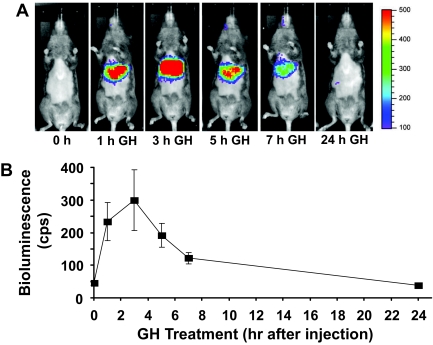
In the second approach, we used our previously characterized bioluminescence detection methods to noninvasively track in vivo hepatic GH-induced STAT5-dependent signaling (27). We recently demonstrated in nude mice that systemic administration of an adenovirus, Ad-GHRE-Luc, which encodes the firefly luciferase gene driven by eight repeats of the STAT5-dependent GHRE from the Spi2.1 gene cloned into the 5′-untranslated region allows via liver-specific infection the detection of GH-induced hepatic bioluminescence. In those studies, the maximal liver bioluminescence was achieved 3 h after a single GH injection. To test the applicability of this technique to the C3H strain, mice (n =7) were injected via tail vein with 1 × 109 plaque-forming units (pfu) Ad-GHRE-Luc (Fig. 2, A and B). Several days later, the mice were fasted overnight before obtaining baseline bioluminescence images (time 0) and were then administered GH (3 μg/g iv) and subjected to serial imaging over the next 24 h. Serial images from a representative mouse are displayed in Fig. 2A, and liver bioluminescence signals (mean ± se) from seven mice are graphically represented in Fig. 2B. As seen previously with nude mice (27), very little bioluminescence signal was detected in the liver or any other region before GH injection. In response to GH administration, robust specific hepatic bioluminescence was rapidly and transiently observed; as with nude mice, the signal in C3H mice was maximal 3 h after GH treatment, fell substantially by 7 h, and was essentially undetectable after 24 h. These data confirm the utility of our serial noninvasive system to track GH-induced hepatic STAT5-mediated signaling in vivo by extending it to C3H mice. Furthermore, we note that the response to GH presently seen in C3H mice appears more robust than that seen previously in nude mice. This may be consistent with our previous observation that immunoblottable liver GHR levels were greater in C3H than in nude mice (27).
Figure 2.
In Vivo GH Signaling, as Assessed Noninvasively by Bioluminescence Imaging in C3H/HeJ Mice
See Materials and Methods for details of Ad-GHRE-Luc injection into C3H mice. Mice were injected via tail vein with 1 × 109 pfu Ad-GHRE-Luc (n = 7 per condition). A, A representative mouse is shown with imaging at baseline (0 h) and 1, 3, 5, 7, and 24 h after administration of GH (3 μg/g iv) in the morning after 16 h food deprivation. Imaging was obtained with the IVIS system (Xenogen Corp., Alameda, CA) while under isoflurane anesthesia at 37 C with images collected on mice 10 min after ip injection of 2.5 mg luciferin. Image acquisition times were 300 sec, and data acquisition software insured no pixels were saturated during image collection. Liver light emission was measured using Xenogen software. In these images, light emission intensity is represented with pseudocolor scaling of bioluminescent images, which are overlaid on black and white photographs of mice collected at the same time. B, Quantitation of in vivo GH signaling. The light photons were measured in the liver using software provided by the vendor (Xenogen); data are expressed as means with error bars representing the se.
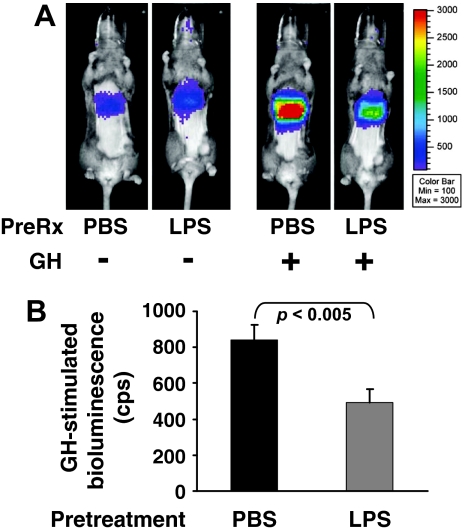
Using this noninvasive approach, we tested whether in C3H mice GH-responsive bioluminescence was affected by pretreatment with LPS (Fig. 3, A and B). Overnight fasted mice (n = 7 per condition) were treated with saline or LPS and 3 h later were imaged (considered the baseline measurement) just before receiving saline or GH, as in Materials and Methods. Guided by the data in Fig. 2, A and B, we limited subsequent imaging to 3 h after GH treatment (peak response) and compared this signal with that obtained at baseline. (In data not shown, LPS treatment itself promoted no significant change in hepatic bioluminescence in mice injected with Ad-GHRE-luc.) The responses of individual representative mice are shown as images in Fig. 3A, whereas the group means following analyses are graphically displayed in Fig. 3B. As expected, in mice not pretreated with LPS, GH (3 μg/g) induced substantial luciferase expression compared with baseline. Pretreatment with LPS, however, significantly blunted the GH response, reducing it by 42%, on average. The same LPS effect was found in additional groups of mice treated with either 0.25 or 1 μg/g GH (data not shown). Collectively, the data in Figs. 1–3 indicate that pretreatment with LPS rapidly reduces hepatic GH-induced STAT5 phosphorylation and STAT5-dependent gene transactivation.
Figure 3.
LPS-Induced Hepatic Desensitization to GH, as Assessed Noninvasively by Bioluminescence Imaging in C3H/HeJ Mice
A and B, Effect of LPS pretreatment. See Materials and Methods for details. Mice (n = 7 per condition) infected with Ad-GHRE-luc, as in Fig. 2, were treated with LPS (10 μg/g ip) or saline 3 h before being administered GH (3 μg/g) and imaged 3 h thereafter. A, Two representative mice are compared. One received PBS pretreatment, and the other received LPS, as indicated. Each was then administered GH (3 μg/g). Images of each are compared. B, Regions of interest analyses of liver luciferase expression for two groups presented in A. LPS treatment led to a statistically significant (P < 0.05) fall in GH-induced hepatic bioluminescence. Data in 3B are expressed as means ± sem. Mean basal (pre-GH treatment) bioluminescence was similar between the two groups (119 counts/sec and 140 counts/sec for PBS- and LPS-treated mice, respectively).
LPS Reduces Hepatic GHR Abundance in C3H Mice in a Posttranscriptional Fashion
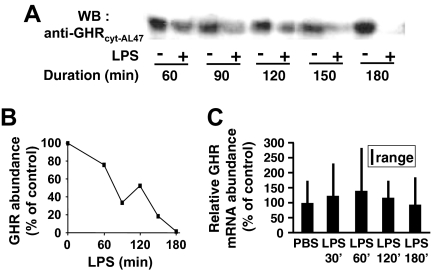
To probe the mechanism(s) whereby LPS causes hepatic desensitization to GH signaling, we first examined its effects on hepatic GHR abundance (Fig. 4, A and B). C3H mice were administered saline or LPS for the indicated durations, after which they were killed and livers were harvested and extracted. Equal amounts of liver extract were resolved by SDS-PAGE and immunoblotted with anti-GHRcyt-AL47, a serum raised against human GHR that also recognizes mouse GHR (11,18,27,29). Quantitation of receptor abundance in each LPS-treated sample vs. control samples demonstrated progressive loss of hepatic GHR over the 3-h period after LPS administration.
Figure 4.
LPS Induces Loss of Hepatic GHR Protein without Change in Levels of GHR L2 mRNA in C3H/HeJ Mice
A, As detailed in Materials and Methods, mice were pretreated with LPS or PBS for the indicated durations. After killing, livers were homogenized and equal aliquots of cytoplasmic extract were immunoblotted to detect GHR. B, Densitometric analysis of GHR abundance from the blot in A. Data are plotted as the percentage of GHR remaining after LPS treatment at each time point relative to the control (mice treated with PBS for the same duration as the LPS treatment). Data are representative of three independent experiments. C, GHR L2 mRNA abundance. Mice treated with LPS for the indicated durations were killed, and liver GHR mRNA levels were determined by RT-PCR, as in Materials and Methods. Expression of the housekeeping gene GAPDH was used as an internal control to normalize results (n = 3–6; mean and range) depicted relative to the expression in control (PBS treated) animals.
We next considered whether this LPS-induced reduction in C3H GHR abundance was related to effects on GHR mRNA abundance. Mice were treated with LPS for varying periods up to 3 h, after which livers were harvested and total RNA was extracted. RT-PCR was carried out on these RNA samples using primers that specifically amplify the major mouse liver GHR gene transcript (Fig. 4C). In contrast to the immunoblotting findings, this analysis indicated that LPS caused no change in GHR mRNA abundance in the livers of treated mice. These data indicate that in this system, LPS decreases hepatic GHR abundance and that the time course of this receptor loss is similar to that seen for LPS-induced desensitization to GH. Furthermore, this LPS-induced decline in GHR abundance cannot be explained by changes in GHR mRNA abundance and therefore most likely occurs at a posttranscriptional level.
LPS Promotes Hepatic GHR Proteolysis and GH Binding Protein (GHBP) Shedding in Vivo
We sought to further explore mechanisms for the LPS-induced posttranscriptional reduction in hepatic GHR, in particular asking whether it was the result of proteolytic processing of the receptor. Indeed, we have previously demonstrated acute phorbol ester- and growth factor-induced metalloproteolytic GHR loss of the mouse GHR both when endogenously expressed in mouse cells and when heterologously expressed in HEK-293 cells (10,13). Notably, the induced proteolysis of the mouse GHR in vitro was associated with dramatic desensitization to subsequent GH-induced signaling that was reversed upon blockade of the proteolysis (10).
We first measured the level of GHBP (corresponding to the receptor ECD) in the serum of LPS-treated C3H mice and found no significant difference in comparison with saline-treated mice (data not shown). Previous studies in rodents have shown that the vast majority of GHBP in the serum arises from alternative splicing of the GHR mRNA to yield a secreted form of GHR that encodes only the ECD joined to a hydrophilic peptide that replaces the transmembrane and intracellular domains (30,31,32). (This is in contrast to the situation in humans, rabbits, and other species in which no such alternatively spliced GHR mRNAs exist, and GHBP is believed to be heavily derived by proteolysis.) Thus, we hypothesized that in the mouse, acutely induced proteolysis that reduces liver GHR substantially and thereby results in ECD shedding might not yield a major change in the large circulating mouse GHBP pool.
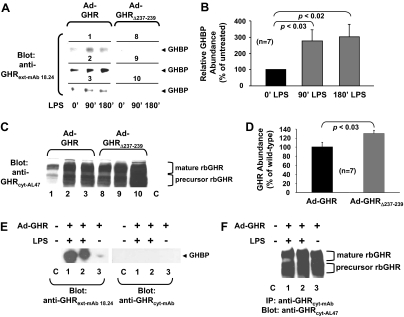
Thus, we addressed the question of whether LPS induced in vivo GHR proteolysis in C3H mice by monitoring the effect of LPS treatment on the fate of hepatically expressed rabbit GHR. For this, we constructed adenoviruses that direct the expression of either the wild-type rabbit GHR (Ad-GHR) or a rabbit GHR mutant, Ad-GHRΔ237–239. We previously demonstrated that GHRΔ237–239, which has a deletion of three residues in the juxtamembrane ECD stem region, is normally expressed at the cell surface and responds normally to GH but is completely refractory to inducible metalloproteolysis; furthermore, cells that harbor GHRΔ237–239, in contrast to those that harbor wild-type rabbit GHR, were not rendered insensitive to GH by stimuli that trigger receptor metalloproteolysis (12). We also exploited a new monoclonal antibody directed at the receptor ECD, anti-GHRext-mAb18.24, which detects rabbit, but not rodent, GHR.
C3H mice (n = 7 per group) were infected with either Ad-GHR or Ad-GHRΔ237–239 and 3 d later were administered LPS after an overnight fast. Blood samples were obtained just before LPS (0 min) and 90 and 180 min thereafter. Precleared serum was resolved by SDS-PAGE and immunoblotted with anti-GHRext-mAb18.24 to detect the shed rabbit GHR ECD (GHBP). Representative blots from three mice are shown in Fig. 5A, and densitometrically quantitated data from all mice in each group are presented in Fig. 5B. GHBP was detected at baseline in Ad-GHR-infected mice, and LPS caused a significant nearly 3-fold increase in circulating GHBP in these mice. Notably, no GHBP was detected basally or in response to LPS in mice infected with Ad-GHRΔ237–239. These data suggest that LPS enhanced in vivo hepatic GHR proteolysis.
Figure 5.
LPS Promotes Shedding of GHBP from Adenovirally Expressed GHR in C3H/HeJ and Athymic Nude Mice
A and B, GHBP shedding. Mice (n = 7 per group) were infected with either Ad-GHR or Ad-GHRΔ237–239, as indicated, both of which are rabbit GHRs. As detailed in Materials and Methods, mice were treated with LPS, and serum obtained from each mouse was precleared with protein G-Sepharose and blotted with a monoclonal antibody that recognizes the rabbit, but not mouse, GHR ECD. Panel A, Blots from three representative mice from each group are shown; panel B, data from the entire Ad-GHR group were densitometrically analyzed and are displayed as mean ± sem. Panels C and D, GHR abundance. Livers from mice analyzed in panels A and B were homogenized, and equal aliquots of cytoplasmic extract were resolved by SDS-PAGE and immunoblotted for GHR. Panel C, Samples from the same three mice per group as were evaluated in A are displayed. The positions of mature and precursor GHR are noted. C is liver cytoplasmic extract from a mouse not infected with either Ad-GHR or Ad-GHRΔ237–239. (Note that at this exposure, endogenous GHR is not detected.) Panel D, Densitometric analysis of the blots in panel C. Data are displayed as mean ± sem (n = 7) relative to the GHR level in Ad-GHR-infected mice. Panels E and F, GHBP shedding and GHR abundance in nude mice. Panel E, As detailed in Materials and Methods, nude mice were treated as in panel A to detect shed GHBP after infection with Ad-GHR or control, as indicated. Serum samples were blotted with anti-GHRext-mAb18.24 and, as a negative control, anti-GHRcyt-mAb. Note LPS-induced appearance of specifically detected GHBP in serum. Panel F, Livers from the same mice in panel E were extracted, as in panel C, and immunoprecipitated with anti-GHRcyt-mAb. Precipitated proteins were resolved by SDS-PAGE and immunoblotted for GHR. Note similar expression levels of adenovirally expressed GHR and its absence in the control mouse liver.
To verify that both the wild-type GHR and GHRΔ237–239 were expressed, mice were killed after the final blood sample was taken, and livers were homogenized and analyzed by immunoblotting with anti-GHRcyt-AL47 (Fig. 5C). This revealed that both receptors were indeed expressed. Notably, densitometric analysis of the results from all mice in each group (Fig. 5D) indicated that in fact hepatic receptor abundance was greater in C3H mice that expressed the mutant receptor, perhaps reflecting its inability to be cleaved in response to LPS.
Of note, we also tested whether GHBP shedding could be observed in another mouse strain in addition to C3H. Nude mice infected with Ad-GHR were treated with LPS for 2 h, after which serum was resolved by reducing SDS-PAGE (Fig. 5E). Duplicate gels were blotted with anti-GHRext-mAb18.24 or anti-GHRcyt-mAb (reactive with GHR intracellular domain and thus negative control), allowing specific detection of LPS-induced GHBP. Blotting of liver extracts (Fig. 5F) verified similar expression of adenovirally driven GHR in each mouse. These data suggest that the ability of LPS to promote GHR proteolysis and GHBP shedding is detectable in another mouse strain as well as in C3H mice.
LPS-Induced Reduction in Hepatic GHR Is Not Accompanied by Changes in the Level of Liver TNFα Converting Enzyme (TACE)
We previously implicated TACE as a metalloprotease that is critical in mediating GHR proteolysis in two separate tissue culture model systems (9,15). TACE has two forms. The pro-TACE form is a 120-kDa transmembrane protein with a negative regulatory region, the so-called pro-domain, at the N terminus of the ECD (33,34,35,36). Mature TACE (110 kDa) results from cleavage of the pro-domain after biosynthesis and constitutes the active, cell-surface form of the enzyme (37,38).
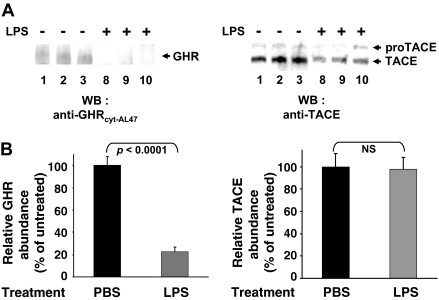
We explored whether the LPS-induced increase in GHR proteolysis might relate to changes in TACE abundance or the ratio between pro-TACE and TACE (Fig. 6). C3H mice (n = 7 per condition) were treated with PBS or LPS for 6 h, and livers were evaluated by immunoblotting for GHR (Fig. 6, A and B, left panels) and TACE (Fig. 6, A and B, right panels) abundance. As anticipated, hepatic GHR abundance was dramatically reduced in LPS-treated mice compared with those exposed to PBS. In contrast, blotting of the same liver extracts revealed no appreciable changes in either pro-TACE or TACE levels. Thus, LPS did not exert its effects on GHR processing by either increasing TACE or enhancing its rate of maturation from the pro form to the mature form.
Figure 6.
LPS-Induced GHR Loss Is Not Accompanied by Change in TACE Abundance in Livers of C3H/HeJ Mice
A, Mice (n = 7 per group) were treated with LPS or saline, as indicated, for 6 h, after which they were killed. Livers were homogenized, and equal aliquots of cytoplasmic extract were immunoblotted for GHR (left) or TACE (right). Data from three representative mice from each group are shown. The positions of pro-TACE and TACE are shown. B, Blots such as in A from seven representative mice from each group were densitometrically analyzed. GHR and TACE abundance are displayed as mean ± sem relative to the GHR level in PBS-treated mice.
DISCUSSION
In this manuscript, we address the effects of endotoxin administration on hepatic GH sensitivity. A variety of studies in humans and animals indicate that inflammatory states including sepsis or endotoxinemia lead to a state of hepatic GH resistance (18,19,20,21,22,23,24,25,26). In some instances, this reduced GH sensitivity was accompanied by decreased hepatic GHR mRNA levels (18,20,22,39), which may lead to reduced GHR protein and thus GH resistance. Endotoxin-induced increases in suppressors of cytokine signaling (SOCS) proteins have also been detected and have been implicated in GH insensitivity (18,19,24,40). In vitro studies have also examined these issues. In H4-II-E rat hepatoma cells, SOCS-3 expression was implicated in the GH resistance promoted by IL-1β (41). In a separate recent study using both endogenous and reconstitution systems, LPS was shown to suppress GHR mRNA expression via cytokine-independent pathways (42). Thus, it appears that multiple mediators and mechanisms may contribute to hepatic GH insensitivity in the face of these inflammatory stimuli.
We found that in C3H mice, LPS caused substantial hepatic GH resistance. We measured this resistance both by direct assessment of GH-induced STAT5 tyrosine phosphorylation and nuclear accumulation and by use of a novel noninvasive bioluminescence imaging system that allows serial monitoring of GH-induced STAT5-dependent transcriptional activation (more below). This LPS-induced desensitization is phenomenologically consistent with the findings of others mentioned above in different model systems. However, our data differ from others in several important ways. First, we noted a dramatic LPS-induced loss of hepatic GHR abundance, the time course of which generally correlated with the time course of desensitization to hepatic GH signaling. Second, this GHR loss in response to LPS was unaccompanied by decreased liver GHR mRNA levels, suggesting that it was governed by a posttranscriptional mechanism. To our knowledge, this is the first report of such a mechanism related to hepatic GH desensitization. We pursued this further by determining whether LPS could cause in vivo GHR proteolysis and found that when expressed in the liver, a wild-type receptor, but not one that is mutated in the metalloprotease cleavage site, yielded constitutive and LPS-augmented circulation of the GHBP (which is comprised of the receptor ECD). GHBP shedding was also seen when wild-type GHR was adenovirally expressed in the livers of nude mice. These findings suggest that hepatic GHR is susceptible in vivo to metalloproteolytic processing that reduces receptor abundance; this is reflected by corresponding GHBP shedding. Although it is believed that metalloproteolytic GHBP shedding occurs in humans and other species, in vivo inducers of this GHR processing have heretofore not been reported. We hypothesize from our findings that inducible GHR loss, rather than the concomitantly generated GHBP per se, underlies the desensitization of the liver to subsequent GH stimulation.
Our studies were largely performed in C3H/HeJ mice. This mouse strain is relatively hyporesponsive to endotoxin by virtue of a missense mutation it carries in the gene encoding TLR4 (28). TLR4 is a cell surface transmembrane protein receptor for endotoxin, and the mutation in C3H/HeJ cells impairs signaling by at least two pathways that promote cytokine responses (43,44). Consistent with previous reports (45,46), we found that these mice responded to LPS with liver cytokine response, albeit with a markedly blunted and delayed pattern compared with that reported for other strains (47,48). As a practical matter, we found this mouse strain attractive for these studies for several reasons, including the relatively high abundance of liver GHR (27), the robustness of the in vivo signaling responsiveness (Figs. 2 and 3), and the ability to dose the mice with LPS and not encounter the morbidity/mortality often seen with such treatment in other strains
As noted above, our recent data in cell culture and reconstitution systems indicate that LPS directly suppresses GHR gene expression in a TLR4-dependent fashion (42). In comparing our current data with previous studies, we consider the possibility that the relatively blunted TLR4-mediated LPS response in C3H/HeJ mice might result in less robust GHR transcriptional effects and therefore relatively more detectable proteolytic GHR down-modulation. We note that in previous studies of proteolytic shedding in these mice by Carpenter et al. (49), substantial shedding of cell surface TNF receptors was observed in response both to bacterial infection and LPS in C3H/HeJ mice, even though the mice were quite hyporesponsive in terms of cytokine production. Recent data suggest that LPS may activate intracellular targets including the cryopyrin inflammasome independent of TLR4 signaling, thereby dissociating caspase-1 activation from IL-1β secretion (50). Thus, the LPS-induced GHR loss and generation of shed GHBP that we observed in C3H/HeJ mice has a precedent. Deciphering of the mechanisms involved in LPS-induced desensitization to GH will require better understanding of how the GHR proteolytic response is initiated. TACE inactivation in myeloid cells has recently been shown to prevent lethality after LPS administration, confirming that TACE is a critical in vivo target for LPS signaling (51). Along these lines, we consider it important that an LPS-induced change in TACE abundance was not detected in our studies.
Future studies of proteolytic mechanisms of LPS-induced hepatic GH desensitization should be fostered by use of our noninvasive in vivo bioluminescence imaging assay, first described in nude mice (27). In this report, we demonstrated that this assay allowed robust, reliable, and serial assessment of liver GH signaling within individual mice and among groups of similarly treated mice. Importantly, using this noninvasive method, we were able to readily detect an LPS-induced decrease in GH signaling that paralleled that observed when mice were killed and the livers biochemically analyzed for STAT5 phosphorylation and nuclear accumulation. Thus, by using this serial noninvasive system, we will be able to more readily probe the effects of genetic or other manipulations of the metalloproteolytic machinery to ask mechanistic questions about how endotoxinemia affects GH sensitivity within individual mice. Although we observed LPS-induced GHBP shedding in nude mice as well as in C3H mice, we will in future studies determine the degree of generalizability of our findings by examining other mouse strains in more detail.
The role of the GH axis in the clinical settings of sepsis and critical illness has captured significant attention. GH treatment has long been seen as potentially useful to prevent catabolic consequences (52,53), and the degree of GH resistance in the septic state has been postulated to be prognostic of poor survival (54). Yet, a large study of critically ill adults suggested that treatment with high doses of GH increased mortality (55). We believe our current studies strongly suggest that enhanced GHR metalloproteolysis may at least in part underlie the hepatic GH resistance seen in endotoxemic states. However, we realize that proteolysis may be only one of several contributing factors and that further mechanistic studies are warranted.
MATERIALS AND METHODS
Materials
Routine reagents were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise noted. Restriction endonucleases were obtained from New England Biolabs (Beverly, MA). Recombinant human GH was kindly provided by Eli Lilly Co. (Indianapolis, IN). LPS Escherichia coli 026:B6 (L3755) was obtained from Sigma. An ELISA kit for determination of tissue IL-6 abundance was obtained from R&D Systems (Minneapolis, MN).
IL-6 ELISA
Cytoplasmic proteins were prepared from mouse liver using the NE-PER kit as per manufacturer’s recommendations (Pierce Chemical Co., Rockford, IL). Liver tissue IL-6 abundance was determined by using cytoplasmic protein extracts and an ELISA kit as per manufacturer’s recommendations (R&D Systems).
Antibodies
Monoclonal anti-STAT5 (W-17) was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-phospho-STAT5 affinity-purified rabbit polyclonal antibody [recognizing the tyrosine-phosphorylated form (Tyr-694) of STAT5a and STAT5brsqb] was purchased from Zymed Laboratories Inc. (San Francisco, CA). The rabbit polyclonal antiserum, anti-GHRcyt-AL47, raised against a bacterially expressed N-terminally His-tagged fusion protein incorporating human GHR residues 271–620 (the entire cytoplasmic domain) (56), has been previously described (11). Anti-GHRext-mAb18.24 is a mouse monoclonal antibody (IgG1κ) that was a sister clone of our previously described anti-GHRext-mAb; both were raised against a bacterially expressed glutathione-S-transferase fusion protein incorporating rabbit GHR residues 1–246 (8,14,57,58). This monoclonal anti-GHR antibody was purified from hybridoma supernatant using protein G-Sepharose (at the University of Alabama at Birmingham Multipurpose Arthritis Center Hybridoma Core facility). Anti-GHRcyt-mAb, a mouse monoclonal antibody directed toward the cytoplasmic domain of the rabbit GHR, has been described previously (16,57,59). Anti-TACE (AL45) rabbit polyclonal antiserum has been described previously (9).
Adenoviruses
Construction, production, and purification of Ad-GHR, Ad-GHRΔ237–239, and Ad-GHRE-Luc have been described previously (15,27).
Animal Experiments
Animal protocols followed all regulations and were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. C3H/HeJ mice (males and females, as specified below) were purchased from Jackson Laboratories (Bar Harbor, ME). Female athymic nude mice were obtained from the National Cancer Institute Frederick Research Laboratory (Frederick, MD). Mice were induced and maintained with isoflurane gas anesthesia for all injections and imaging studies and monitored continuously to allow the lowest dose (typically 1–1.5%) to prevent movement. For imaging experiments, the mice were fed a nutritionally complete casein-based diet (formula 89222; Harlan Teklad, Madison, WI) beginning 4 d before imaging to reduce background phosphorescence from the abdominal region.
In Vivo Hepatic GH Signaling via Adenovirally Expressed GHRE-Luc Reporter
Female C3H/HeJ mice (6–8 wk old) were used. In the experiment shown in Fig. 2, A and B, mice (n = 7) were injected iv with Ad-GHRE-Luc (1 × 109 pfu) and subjected to imaging studies 3 d later. After an overnight fast, a baseline (0-h) bioluminescence image was obtained, and GH (3 μg/g iv) was injected. Imaging studies were repeated 1, 3, 5, 7, and 24 h after the GH injection. For imaging, mice were injected with luciferin ip (2.5 mg) and imaged after 10 min with the IVIS-100 Imaging System, as detailed in the figure legend. Images were collected on anesthetized mice oriented in the same position on a heated shelf (37 C). Image acquisition time was 300 sec, and the camera binning was 8. Data acquisition software insured that no pixels were saturated. Light emission from the liver regions (relative photons per second) was measured by region of interest analyses with software provided by the vendor. The intensity of light emission was represented with a pseudocolor scaling of the bioluminescent images. The bioluminescent images were overlaid on black and white photographs of the mice. In the experiment shown in Fig. 3, A and B, the same adenoviral infection protocol was followed. LPS was administered 3 h before GH, and images were obtained before and 3 h after GH, as indicated in the figure legend.
Assessment of Liver STAT5 Activation and GHR Protein and mRNA Abundance
Male C3H/HeJ mice (6–8 wk old) were used in the experiments shown in Figs. 2 and 4. Mice were pretreated with LPS (10 μg/g ip) or PBS for the indicated durations before administration of GH (3 μg/g ip) or saline, as indicated. Fifteen minutes later, mice were killed and livers harvested. For evaluation of liver GHR STAT5 and tyrosine-phosphorylated STAT5 levels, frozen livers were extracted with the NE-PER system (Nuclear and Cytoplasmic Extraction Kit from Pierce). Cytoplasmic and nuclear extracts were resolved by SDS-PAGE and immunoblotted, as previously described (9,12,27,60). Immunoblotting detection reagents (SuperSignal West Pico chemiluminescent substrate) were from Pierce. Stripping and reprobing of blots was accomplished according to the manufacturer’s suggestions. In the experiments in Fig. 5, C and F, in which hepatic GHR levels were determined, livers were extracted as above, and proteins were either resolved and immunoblotted or subjected to immunoprecipitation, as indicated, with anti-GHRcyt-mAb (as in Ref. 14), after which immunoblotting was performed.
GHR L2 mRNA levels were determined by real-time PCR analysis. Total RNA was extracted using TRI reagent (Molecular Research Center). Real-time quantitative RT-PCR using the ABI Prism 7000 sequence detection system (PE Applied Biosystems, Foster City, CA) was performed and analyzed following protocols described previously (39). The primers used for detecting mouse L2 transcript have been described previously (39). Normalization of expression of GHR transcripts was carried out by concomitant measurement of the steady-state abundance of the housekeeping gene GAPDH.
GHBP Detection
Female C3H/HeJ or nude mice (6–8 wk old) were used, as indicated in the figure legends. In the experiment shown in Fig. 5, A and B, C3H/HeJ mice (n = 7 per group) were injected with Ad-GHR or Ad-GHRΔ237–239 (1 × 109 pfu) and studied 3 d later. After overnight fast, a baseline (0-h) blood sample was obtained, which was followed by LPS administration (10 μg/g ip). Thereafter, blood was obtained after 90 and 180 min. Serum was separated by centrifugation and then precleared by incubation with protein G-Sepharose beads. Equal aliquots of precleared serum were subjected to SDS-PAGE and immunoblotted, as above, with anti-GHRext-mAb18.24. The same procedures were used in the experiment in Fig. 5E, but female nude mice were used, and mice either untreated or treated as indicated with LPS for 2 h were compared with each other. In this experiment, equal aliquots of serum were separated by SDS-PAGE and immunoblotted with anti-GHRext-mAb18.24 and, as a negative control, anti-GHRcyt-mAb, which reacts with the receptor cytoplasmic domain and therefore would not react with GHBP.
Statistical Analysis
For bioluminescence imaging data, one and two-way ANOVA was carried out using SAS, version 8.2 (SAS Institute Inc., Cary, NC). Densitometric quantitation of immunoblots was performed using a high-resolution scanner and the ImageJ 1.30 program (developed by W. S. Rasband, Research Services Branch, National Institute of Mental Health, National Institutes of Health, Bethesda, MD). Pooled data from several experiments are displayed as mean ± se. The significance (P value) of differences of pooled densitometric results was estimated by t tests.
Acknowledgments
We acknowledge the expert assistance of Synethia Kidd, and we thank Dr. Roy Black for his critical review of the manuscript.
Footnotes
Present address for X.W.: Institute of Cell Biology, Shandong University, School of Medicine, and Department of Endocrinology, Shandong Provincial Hospital of Shandong University, Jinan, China 250021
This work was supported by National Institutes of Health (NIH) Grant DK58259 and a VA Merit Review Award (both to S.J.F.), NIH Grant P30 CA-13148-34, NIH Grant DK02700 (to L.A.D.), and DK49845 (to R.K.M.).
Parts of this work were presented at the 86th and 89th Annual Meetings of The Endocrine Society in New Orleans, LA (2004), and Toronto, Ontario, Canada (2007), respectively.
Disclosure Summary: X.W., J.J., J.W., G.B., Y.G., R.K.M., L.A.D., K.R.Z., and S.J.F. have nothing to declare.
First Published Online March 6, 2008
Abbreviations: ECD, Extracellular domain; GHBP, GH binding protein; GHR, GH receptor; GHRE, GH response element; LPS, lipopolysaccharide; pfu, plaque-forming units; STAT5, signal transducer and activator of transcription 5; TACE, TNFα converting enzyme; TLR4, Toll-like-4 receptor.
References
- Isaksson OG, Eden S, Jansson JO 1985 Mode of action of pituitary growth hormone on target cells. Annu Rev Physiol 47:483–499 [DOI] [PubMed] [Google Scholar]
- Frank SJ, Messina JL 2002 Growth hormone receptor. In: Oppenheim JJ, Feldman M, eds. Cytokine reference on-line. London, UK: Academic Press, Harcourt; 1–21 [Google Scholar]
- de Vos AM, Ultsch M, Kossiakoff AA 1992 Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science 255:306–312 [DOI] [PubMed] [Google Scholar]
- Carter Su C, Schwartz J, Smit LS 1996 Molecular mechanism of growth hormone action. Annu Rev Physiol 58:187–207 [DOI] [PubMed] [Google Scholar]
- Argetsinger LS, Campbell GS, Yang X, Witthuhn BA, Silvennoinen O, Ihle JN, Carter-Su C 1993 Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74:237–244 [DOI] [PubMed] [Google Scholar]
- Waxman DJ, Frank SJ 2000 Growth hormone action: signaling via a JAK/STAT-coupled receptor. In: Conn PM, Means A, eds. Principles of molecular regulation: Totowa, NJ: Humana Press; 55–83 [Google Scholar]
- Yoon JB, Berry SA, Seelig S, Towle HC 1990 An inducible nuclear factor binds to a growth hormone-regulated gene. J Biol Chem 265:19947–19954 [PubMed] [Google Scholar]
- Alele J, Jiang J, Goldsmith JF, Yang X, Maheshwari HG, Black RA, Baumann G, Frank SJ 1998 Blockade of growth hormone receptor shedding by a metalloprotease inhibitor. Endocrinology 139:1927–1935 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jiang J, Black RA, Baumann G, Frank SJ 2000 TACE is a growth hormone binding protein sheddase: the metalloprotease TACE/ADAM-17 is critical for (PMA-induced) growth hormone receptor proteolysis and GHBP generation. Endocrinology 141:4324–4348 [DOI] [PubMed] [Google Scholar]
- Guan R, Zhang Y, Jiang J, Baumann CA, Black RA, Baumann G, Frank SJ 2001 Phorbol ester- and growth factor-induced growth hormone (GH) receptor proteolysis and GH-binding protein shedding: relationship to GH receptor down-regulation. Endocrinology 142:1137–1147 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Guan R, Jiang J, Kopchick JJ, Black RA, Baumann G, Frank SJ 2001 Growth hormone (GH)-induced dimerization inhibits phorbol ester-stimulated GH receptor proteolysis. J Biol Chem 276:24565–24573 [DOI] [PubMed] [Google Scholar]
- Wang X, He K, Gerhart M, Huang Y, Jiang J, Paxton RJ, Yang S, Lu C, Menon RK, Black RA, Baumann G, Frank SJ 2002 Metalloprotease-mediated GH receptor proteolysis and GHBP shedding. Determination of extracellular domain stem region cleavage site. J Biol Chem 277:50510–50519 [DOI] [PubMed] [Google Scholar]
- Wang X, He K, Gerhart M, Jiang J, Paxton RJ, Menon RK, Black RA, Baumann G, Frank SJ 2003 Reduced proteolysis of rabbit growth hormone (GH) receptor substituted with mouse GH receptor cleavage site. Mol Endocrinol 17:1931–1943 [DOI] [PubMed] [Google Scholar]
- Jiang J, Wang X, He K, Li X, Chen C, Sayeski PP, Waters MJ, Frank SJ 2004 A conformationally sensitive GHR [growth hormone (GH) receptor] antibody: impact on GH Signaling and GHR Proteolysis. Mol Endocrinol 18:2981–2996 [DOI] [PubMed] [Google Scholar]
- Loesch K, Deng L, Cowan JW, Wang X, He K, Jiang J, Black RA, Frank SJ 2006 JAK2 influences growth hormone receptor metalloproteolysis. Endocrinology 147:2839–2849 [DOI] [PubMed] [Google Scholar]
- Yang N, Wang X, Jiang J, Frank SJ 2007 Role of the growth hormone (GH) receptor transmembrane domain in receptor predimerization and GH-induced activation. Mol Endocrinol 21:1642–1655 [DOI] [PubMed] [Google Scholar]
- Baumann G, Frank SJ 2002 Metalloproteinases and the modulation of GH signaling. J Endocrinol 174:361–368 [DOI] [PubMed] [Google Scholar]
- Denson LA, Held MA, Menon RK, Frank SJ, Parlow AF, Arnold DL 2003 Interleukin-6 inhibits hepatic growth hormone signaling via upregulation of Cis and Socs-3. Am J Physiol Gastrointest Liver Physiol 284:G646–G654 [DOI] [PubMed] [Google Scholar]
- Mao Y, Ling PR, Fitzgibbons TP, McCowen KC, Frick GP, Bistrian BR, Smith RJ 1999 Endotoxin-induced inhibition of growth hormone receptor signaling in rat liver in vivo. Endocrinology 140:5505–5515 [DOI] [PubMed] [Google Scholar]
- Defalque D, Brandt N, Ketelslegers JM, Thissen JP 1999 GH insensitivity induced by endotoxin injection is associated with decreased liver GH receptors. Am J Physiol 276:E565–E572 [DOI] [PubMed] [Google Scholar]
- Bergad PL, Schwarzenberg SJ, Humbert JT, Morrison M, Amarasinghe S, Towle HC, Berry SA 2000 Inhibition of growth hormone action in models of inflammation. Am J Physiol Cell Physiol 279:C1906–C1917 [DOI] [PubMed] [Google Scholar]
- Wang P, Li N, Li JS, Li WQ 2002 The role of endotoxin, TNF-α, and IL-6 in inducing the state of growth hormone insensitivity. World J Gastroenterol 8:531–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong-Brown LQ, Brown CR, Cooney RN, Frost RA, Lang CH 2003 Sepsis-induced muscle growth hormone resistance occurs independently of STAT5 phosphorylation. Am J Physiol Endocrinol Metab 285:E63–E72 [DOI] [PubMed] [Google Scholar]
- Chen Y, Sun D, Krishnamurthy VM, Rabkin R 2007 Endotoxin attenuates growth hormone-induced hepatic insulin-like growth factor I expression by inhibiting JAK2/STAT5 signal transduction and STAT5b DNA binding. Am J Physiol Endocrinol Metab 292:E1856–E1862 [DOI] [PubMed] [Google Scholar]
- Dahn MS, Lange MP 1998 Systemic and splanchnic metabolic response to exogenous human growth hormone. Surgery 123:528–538 [DOI] [PubMed] [Google Scholar]
- Lang CH, Pollard V, Fan J, Traber LD, Traber DL, Frost RA, Gelato MC, Prough DS 1997 Acute alterations in growth hormone-insulin-like growth factor axis in humans injected with endotoxin. Am J Physiol 273:R371–R378 [DOI] [PubMed] [Google Scholar]
- Frank SJ, Wang X, He K, Yang N, Fang P, Rosenfeld RG, Hwa V, Chaudhuri TR, Deng L, Zinn KR 2006 In vivo imaging of hepatic growth hormone signaling. Mol Endocrinol 20:2819–2830 [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B 1998 Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085–2088 [DOI] [PubMed] [Google Scholar]
- Reddy GR, Pushpanathan MJ, Ransom RF, Holzman LB, Brosius 3rd FC, Diakonova M, Mathieson P, Saleem MA, List EO, Kopchick JJ, Frank SJ, Menon RK 2007 Identification of the glomerular podocyte as a target for growth hormone action. Endocrinology 148:2045–2055 [DOI] [PubMed] [Google Scholar]
- Baumbach WR, Horner DL, Logan JS 1989 The growth hormone-binding protein in rat serum is an alternatively spliced form of the rat growth hormone receptor. Genes Dev 3:1199–1205 [DOI] [PubMed] [Google Scholar]
- Smith WC, Kuniyoshi J, Talamantes F 1989 Mouse serum growth hormone (GH) binding protein has GH receptor extracellular and substituted transmembrane domains. Mol Endocrinol 3:984–990 [DOI] [PubMed] [Google Scholar]
- Sadeghi H, Wang BS, Lumanglas AL, Logan JS, Baumbach WR 1990 Identification of the origin of the growth hormone-binding protein in rat serum. Mol Endocrinol 4:1799–1805 [DOI] [PubMed] [Google Scholar]
- Black RA, White JM 1998 ADAMs: focus on the protease domain. Curr Opin Cell Biol 10:654–659 [DOI] [PubMed] [Google Scholar]
- Becherer JD, Blobel CP 2003 Biochemical properties and functions of membrane-anchored metalloprotease-disintegrin proteins (ADAMs). Curr Top Dev Biol 54:101–123 [DOI] [PubMed] [Google Scholar]
- Huovila AP, Turner AJ, Pelto-Huikko M, Karkkainen I, Ortiz RM 2005 Shedding light on ADAM metalloproteinases. Trends Biochem Sci 30:413–422 [DOI] [PubMed] [Google Scholar]
- Seals DF, Courtneidge SA 2003 The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev 17:7–30 [DOI] [PubMed] [Google Scholar]
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP 1997 A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature 385:729–733 [DOI] [PubMed] [Google Scholar]
- Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, Hoffman CR, Kost TA, Lambert MH, Leesnitzer MA, McCauley P, McGeehan G, Mitchell J, Moyer M, Pahel G, Rocque W, Overton LK, Schoenen F, Seaton T, Su JL, Warner J, Willard D, Becherer JD 1997 Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-α. Nature 385:733–736 [DOI] [PubMed] [Google Scholar]
- Denson LA, Menon RK, Shaufl A, Bajwa HS, Williams CR, Karpen SJ 2001 TNF-α downregulates murine hepatic growth hormone receptor expression by inhibiting Sp1 and Sp3 binding. J Clin Invest 107:1451–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson A, Le Cam A, Maiter D, Edery M, Thissen JP 2000 Potentiation of growth hormone-induced liver suppressors of cytokine signaling messenger ribonucleic acid by cytokines. Endocrinology 141:3687–3695 [DOI] [PubMed] [Google Scholar]
- Boisclair YR, Wang J, Shi J, Hurst KR, Ooi GT 2000 Role of the suppressor of cytokine signaling-3 in mediating the inhibitory effects of interleukin-1β on the growth hormone-dependent transcription of the acid-labile subunit gene in liver cells. J Biol Chem 275:3841–3847 [DOI] [PubMed] [Google Scholar]
- Dejkhamron P, Thimmarayappa J, Kotlyarevska K, Sun J, Lu C, Bonkowski EL, Denson LA, Menon RK 2007 Lipopolysaccharide (LPS) directly suppresses growth hormone receptor (GHR) expression through MyD88-dependent and -independent Toll-like receptor-4/MD2 complex signaling pathways. Mol Cell Endocrinol 274:35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Takeda K 2004 Toll-like receptor signalling. Nat Rev Immunol 4:499–511 [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S 2004 TLR signaling pathways. Semin Immunol 16:3–9 [DOI] [PubMed] [Google Scholar]
- Qureshi ST, Gros P, Malo D 1999 The Lps locus: genetic regulation of host responses to bacterial lipopolysaccharide. Inflamm Res 48:613–620 [DOI] [PubMed] [Google Scholar]
- Sultzer BM 1969 Genetic factors in leucocyte responses to endotoxin: further studies in mice. J Immunol 103:32–38 [PubMed] [Google Scholar]
- De Maio A, Mooney ML, Matesic LE, Paidas CN, Reeves RH 1998 Genetic component in the inflammatory response induced by bacterial lipopolysaccharide. Shock 10:319–323 [DOI] [PubMed] [Google Scholar]
- Suzuki K, Tateda K, Matsumoto T, Gondaira F, Tsujimoto S, Yamaguchi K 2000 Effects of interaction between Escherichia coli verotoxin and lipopolysaccharide on cytokine induction and lethality in mice. J Med Microbiol 49:905–910 [DOI] [PubMed] [Google Scholar]
- Carpenter A, Evans TJ, Buurman WA, Bemelmans MH, Moyes D, Cohen J 1995 Differences in the shedding of soluble TNF receptors between endotoxin-sensitive and endotoxin-resistant mice in response to lipopolysaccharide or live bacterial challenge. J Immunol 155:2005–2012 [PubMed] [Google Scholar]
- Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G 2007 Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 26:433–443 [DOI] [PubMed] [Google Scholar]
- Horiuchi K, Kimura T, Miyamoto T, Takaishi H, Okada Y, Toyama Y, Blobel CP 2007 Cutting edge: TNF-α-converting enzyme (TACE/ADAM17) inactivation in mouse myeloid cells prevents lethality from endotoxin shock. J Immunol 179:2686–2689 [DOI] [PubMed] [Google Scholar]
- Voerman HJ, van Schijndel RJ, Groeneveld AB, de Boer H, Nauta JP, van der Veen EA, Thijs LG 1992 Effects of recombinant human growth hormone in patients with severe sepsis. Ann Surg 216:648–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Chung T, Hinds CJ 2006 Treatment with GH and IGF-1 in critical illness. Crit Care Clin 22:29–40, vi [DOI] [PubMed] [Google Scholar]
- Onenli-Mungan N, Yildizdas D, Yapicioglu H, Topaloglu AK, Yuksel B, Ozer G 2004 Growth hormone and insulin-like growth factor 1 levels and their relation to survival in children with bacterial sepsis and septic shock. J Paediatr Child Health 40:221–226 [DOI] [PubMed] [Google Scholar]
- Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, Vundelinckx G, Hinds CJ 1999 Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med 341:785–792 [DOI] [PubMed] [Google Scholar]
- Leung DW, Spencer SA, Cachianes G, Hammonds RG, Collins C, Henzel WJ, Barnard R, Waters MJ, Wood WI 1987 Growth hormone receptor and serum binding protein: purification, cloning and expression. Nature 330:537–543 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jiang J, Kopchick JJ, Frank SJ 1999 Disulfide linkage of growth hormone (GH) receptors (GHR) reflects GH-induced GHR dimerization. Association of JAK2 with the GHR is enhanced by receptor dimerization. J Biol Chem 274:33072–33084 [DOI] [PubMed] [Google Scholar]
- Kim SO, Jiang J, Yi W, Feng GS, Frank SJ 1998 Involvement of the Src homology 2-containing tyrosine phosphatase SHP-2 in growth hormone signaling. J Biol Chem 273:2344–2354 [DOI] [PubMed] [Google Scholar]
- Loesch K, Deng L, Wang X, He K, Jiang J, Frank SJ 2007 Endoplasmic reticulum-associated degradation of growth hormone receptor in Janus kinase 2-deficient cells. Endocrinology 148:5955–5965 [DOI] [PubMed] [Google Scholar]
- Deng L, He K, Wang X, Yang N, Thangavel C, Jiang J, Fuchs SY, Frank SJ 2007 Determinants of growth hormone receptor down-regulation. Mol Endocrinol 21:1537–1551 [DOI] [PubMed] [Google Scholar]