Abstract
Mechanisms controlling body weight involve gene regulation through the activation of signal transduction pathways. The Janus kinase/signal transducer and activator of transcription (STAT) signal transduction pathway is the mechanism primarily used by leptin in the hypothalamus. The transcription factor nescient helix-loop-helix 2 (Nhlh2) is a downstream target of leptin signaling and is expressed in proopiomelanocortin arcuate neurons. Proopiomelanocortin is cleaved by prohormone convertase 1/3 (PC1/3) to produce peptides that regulate the body’s response to energy availability. Previous studies show that the PC1/3 promoter contains STAT3 sites mediating leptin-induced PC1/3 expression, and that Nhlh2 is required for hypothalamic PC1/3 expression because Nhlh2 knockout mice have reduced PC1/3 mRNA levels. Studies herein reveal that leptin-induced PC1/3 gene expression is abrogated in N2KO mice, and that in a hypothalamic cell line both STAT3 and Nhlh2 are required for the full transcriptional response of a PC1/3 reporter gene after leptin stimulation. Furthermore, it is shown that Nhlh2 binds to E-box motifs found adjacent to STAT3 sites in the PC1/3 promoter both in vitro and in chromatin immunoprecipitation assays. Finally, two different protein-protein interaction assays confirm the presence of a STAT3:Nhlh2 heterodimer on the PC1/3 promoter. The Nhlh2:STAT3 heterodimer may be an important transcriptional regulator of other hypothalamic genes in the leptin signaling pathway. These data confirm Nhlh2 as an integral element of the Janus kinase/STAT signaling pathway and are the first to demonstrate coordinated control of PC1/3 transcription by Nhlh2 and STAT3 after leptin stimulation.
THE HYPOTHALAMUS processes signals related to metabolic state and energy storage and shifts energy balance in either a positive or negative direction, principally by acting on signaling pathways that affect appetite and energy expenditure. One of the primary signals mediating this response is the adipocyte cytokine leptin (1,2,3). Leptin binds to its receptor on hypothalamic neurons causing the phosphorylation of cellular proteins, changes in gene transcription, secretion of neuropeptides, and ultimately regulation of body weight. The primary leptin signal transduction pathway in the hypothalamus involves phosphorylation of Janus kinase 2 (Jak2), which results in phosphorylation of the signal transducer and activator of transcription 3 (STAT3) transcription factor. STAT3 then dimerizes and translocates to the nucleus to regulate gene transcription (4,5). Within the arcuate nucleus (ARC) of the hypothalamus, two genes, proopiomelanocortin (POMC) and prohormone convertase 1/3 (PC1/3) are coordinately regulated by leptin via the Jak/STAT signaling pathway (6,7,8).
Nescient helix-loop-helix 2 (Nhlh2) is a member of the large family of basic helix-loop-helix (bHLH) transcription factors (9). The bHLH transcription factors bind DNA through their basic domain at an E-box sequence denoted as CANNTG and interact with other transcription factors by forming heterodimers and homodimers through their HLH domains. Nhlh2 is expressed in the developing nervous system as well as the adult ARC of the hypothalamus (10). The role of Nhlh2 in the neuronal control of energy balance and the potential gene targets for this transcription factor became evident after the phenotype of Nhlh2 knockout mice (N2KO) was examined. N2KO mice develop energy balance problems with obesity beginning at approximately 12 wk of age. This classifies these animals as a model of adult-onset obesity. Unexpectedly, obesity in these animals is not accompanied by hyperphagia, but by reduced voluntary activity (11). N2KO mice were originally characterized as one of the first models of adult-onset obesity and the first model caused by the deletion of a neuronal transcription factor (12).
Nhlh2 is coexpressed with POMC in the ARC but there is no direct effect of Nhlh2 deletion on POMC mRNA levels. Rather, N2KO mice have a POMC-processing defect due to a 40–60% reduction in PC1/3 levels (10). Reduced PC1/3 levels lead to reduced levels of fully processed POMC-derived neuropeptides, such as αMSH, and increased levels of partially processed forms of POMC. These data support the hypothesis that Nhlh2 is necessary to maintain high levels of PC1/3 protein in hypothalamic neurons.
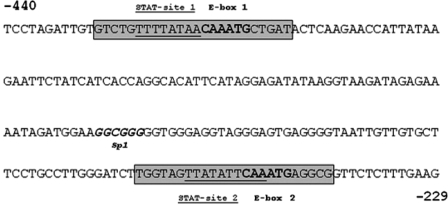
Analysis of the human and mouse PC1/3 promoter reveals two putative STAT3 and E-box motifs (13,14). The human PC1/3 promoter contains an additional characterized leptin-responsive STAT3 binding site that does not exist in the mouse promoter (6). Previous work has implicated these STAT sites in leptin-mediated expression of PC1/3. The E-box motifs have not previously been characterized. One of these E-boxes matches exactly to the E-box required for necdin gene regulation by Nhlh2 (15). Fox et al. compared the sequence and spacing of putative STAT3 and E-box motifs on the PC1/3 promoter. They found two putative sites for each transcription factor that were very well conserved in sequence although slightly different in spacing between the two sites (439 bp in human compared with 153 bp in mouse). The most striking aspect of the mouse and human PC1/3 promoters is the close proximity of the putative binding sites for STAT3 to the E-box motifs in each of these regions (Fig. 1) (14).
Figure 1.
The PC1/3 Proximal Promoter Region
A 212-bp fragment of the PC1/3 promoter was cloned into the pGL3 basic plasmid and used for luciferase reporter assays. Two putative STAT3 binding sites (underlined) and E-box motifs (bold) are indicated as site 1 and site 2. Gray boxes indicate primers used in the ChIP and EMSA assays.
Recent work from our laboratory demonstrated that leptin stimulates an increase in Nhlh2 mRNA levels (16). POMC and PC1/3 mRNA and peptide levels also fluctuate with leptin (6,16,17,18,19,20). Thus, it seems likely that the coordinated regulation of Nhlh2, PC1/3, and POMC by leptin leads to production of fully processed neuropeptides necessary to mediate downstream modulation of energy intake and usage (8). Given these data, we hypothesized that the Nhlh2 transactivation function is necessary for high levels of PC1/3 gene expression after leptin stimulation. We further hypothesized that Nhlh2 and STAT3 coordinately regulate PC1/3 gene expression through the proximal E-box/STAT3 motifs on the PC1/3 promoter.
RESULTS
PC1/3 mRNA Levels in the ARC Respond Normally to Signals of Energy Availability in Wild-Type (WT) Mice But Not in N2KO Mice
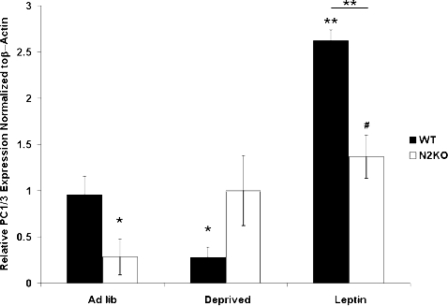
PC1/3 mRNA levels and POMC peptides are reduced by fasting in the ARC (22), but there is no information to date on leptin-mediated regulation of PC1/3 in the ARC, or whole hypothalamic levels of PC1/3 in different energy availability conditions. Analysis of global changes in PC1/3 expression levels using quantitative RT-PCR (qRT-PCR) was performed using whole hypothalamus. cDNA was synthesized from RNA isolated from whole hypothalamus of normal (WT) and N2KO mice in three conditions: ad libitum (ad lib) fed, subjected to a 24-h fast, or subjected to a 24-h fast with a 2-h leptin treatment. In WT mice PC1/3 mRNA levels showed a 75% reduction (P ≤ 0.05) with food deprivation and exceeded ad lib-fed levels after leptin treatment (P < 0.01) (Fig. 2). Hypothalamic PC1/3 mRNA levels in N2KO mice showed no significant variation between the deprived condition and ad lib feeding. Leptin-treated N2KO mice have significantly higher PC1/3 mRNA levels (P ≤ 0.05) than ad lib-fed N2KO mice, and significantly lower PC1/3 mRNA levels (P < 0.05) than leptin-treated WT mice (Fig. 2).
Figure 2.
PC1/3 mRNA Levels in the Hypothalamus Respond Normally to Signals of Energy Availability in WT Mice But Not in N2KO Mice
Whole hypothalamic RNA was isolated from WT and N2KO animals that were given ad libitum access to food (Ad lib), food deprived for 24 h (Deprived), or food deprived for 24 h and exposed to leptin for 2 h (Leptin). Relative expression levels of PC1/3 RNA, which were measured using qRT-PCR and normalized to β-actin, are shown. The results are expressed as mean ± se; *, P < 0.05; **, P < 0.01 to WT ad lib except where indicated; the bar indicates significance between genotypes; #, P < 0.05 to N2KO ad lib (n = 6 animals per condition).
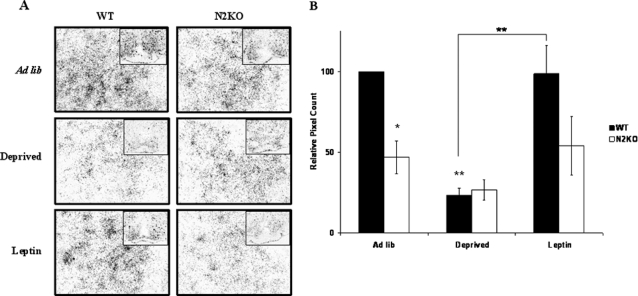
PC1/3 levels were then examined using in situ hybridization to look specifically at the ARC hypothalamus from WT and N2KO mice. Confirming our previous findings (10), ARC PC1/3 levels were significantly lower in ad lib-fed N2KO mice compared with WT mice (P ≤ 0.05; Fig. 3, A and B). Similarly to whole hypothalamus, PC1/3 levels in the ARC of WT mice showed a significant drop (P < 0.01) of 4-fold after food deprivation. These levels returned to normal after 2 h of leptin treatment. In N2KO mice, PC1/3 levels did not show any significant fluctuations (P > 0.8) in the three conditions (Fig. 3, A and B).
Figure 3.
PC1/3 mRNA Levels in the ARC Respond Normally to Signals of Energy Availability in WT Mice But Not in N2KO Mice
A, A 33P-labeled cRNA probe to PC1/3 was used to label hypothalamic sections of brains from WT and N2KO mice taken from animals under differing states of energy availability. WT and N2KO animals that were given ad libitum access to food (Ad lib), food deprived for 24 h (Deprived), or food deprived for 24 h and exposed to leptin for 2 h (Leptin) were used. Pictures are shown at magnification ×40. Inset, ×10 magnification. B, PC1/3 expression displayed as pixel count relative to WT ad lib. The results are expressed as mean ± se; *, P < 0.05; **, P < 0.01 to WT ad lib except where indicated (n = 6 animals per condition).
Leptin Stimulation of the PC1/3 Promoter Is Regulated by Nhlh2 and STAT3 in Vitro
The PC1/3 promoter is known to be regulated by the STAT3 transcription factor as part of the leptin Jak2/STAT3 signaling pathway (6). Two of the STAT3 sites within this promoter region are adjacent to or overlapping with E-box motifs that could potentially be binding sites for Nhlh2 (Fig. 1) (14). Therefore, whether Nhlh2 could transactivate the PC1/3 promoter in a hypothalamic cell line was investigated.
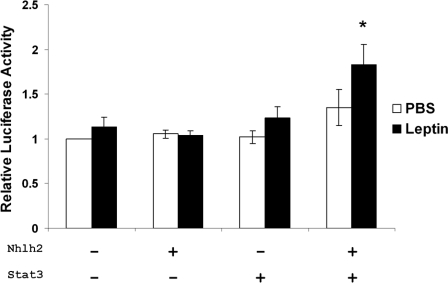
N29/2 cells are a POMC-like arcuate cell line used as a model of the POMC neuron (23). To test whether Nhlh2 transactivates the PC1/3 promoter, a 212-bp fragment (−229 to −440) of the murine PC1/3 promoter spanning the two putative E-box motifs was subcloned into the pGL3 luciferase reporter plasmid (Fig. 1). This construct is expressed in N29/2 cells at basal levels (data not shown). There was no induction of promoter activity when either Nhlh2 or STAT3 alone was cotransfected into the cells with leptin receptor, either in the presence or absence of leptin. When both Nhlh2 and STAT3 were transfected together with leptin stimulation, there was a 2-fold induction of PC1/3 promoter activity compared with basal levels (Fig. 4). These results suggest that both Nhlh2 and STAT3 are required for leptin-induced activation of the PC1/3 promoter.
Figure 4.
Leptin Stimulation of the PC1/3 Promoter Requires Nhlh2 and STAT3
Activity of the WT PC1/3-luc reporter transfected in N29–2 cells in the absence (white bars) or presence (black bars) of leptin. Cells were transfected with PC1/3-luc reporter alone, or in combination with Nhlh2 alone, STAT3 alone, or with both Nhlh2 and STAT3 as indicated. Luciferase activity was measured and normalized to the expression of β-gal-encoding plasmid. Normalized activity is presented relative to the values obtained in cells transfected with PC1/3-luc alone ± se; *, P < 0.05.
Chromatin Immunoprecipitation (ChIP) and EMSA Reveal that Nhlh2 Binds to Both E-Box Motifs on the PC1/3 Promoter
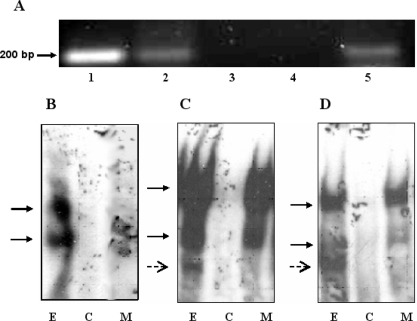
Although the transactivation studies demonstrate a requirement for Nhlh2, they do not reveal whether Nhlh2 acts directly on the PC1/3 promoter or if Nhlh2 acts in trans on a secondary gene that then effects PC1/3 promoter activity. To determine whether Nhlh2 can bind to the PC1/3 promoter and therefore directly effect PC1/3 expression, a ChIP assay was performed on cells expressing myc-tagged Nhlh2. Chromatin from N29/2 cells were immunoprecipitated using an anti-myc antibody to pull down all regions bound to the N2-myc fusion protein. Primers to the PC1/3 promoter region containing the putative E-box motifs were then used to amplify the immunoprecipitated chromatin. As shown in Fig. 5A (lane 5), the PC1/3 promoter containing the putative E-box motifs was pulled down by the antibody to c-myc, indicating that N2-myc was occupying the endogenous PC1/3 promoter. PCR for the PC1/3 promoter was also performed on a PC1/3 promoter plasmid and the ChIP input material as positive controls (Fig. 5A, lanes 1 and 2). A ChIP of cells not transfected with Nhlh2-myc as well as a no-antibody control were included as negative controls (Fig. 5A, lanes 3 and 4).
Figure 5.
ChIP and EMSA Reveal Nhlh2 Binds Both E-Box Motifs on the PC1/3 Promoter
A, ChIP assay demonstrating binding of Nhlh2-myc to the PC1/3 promoter. Cross-linked chromatin from N29–2 hypothalamic cells stimulated with leptin for 15 min were incubated with antibodies against c-myc. Immunoprecipitates were analyzed by PCR using primers specific for the PC1/3 promoter region containing E-box and STAT3 binding sites. A PCR control using the PC1/3 promoter plasmid was included (lane 1). The input (lane 2) included in the PCR represents 10% of the total chromatin. A ChIP of cells not transfected with Nhlh2-myc was included as a negative control (lane 3) as well as a beads only (no antibody) control (lane 4). Lane 5 shows the PC1/3 promoter immunoprecipitated with the Nhlh2-myc chromatin complex. B–D, EMSA experiments demonstrating binding of Nhlh2 and both E-box motifs. B, Site 1 corresponds with the E-box motif at −425. C, Site 2 corresponds with the E-box motif at −259. D, The control used for Nhlh2 binding, from the necdin promoter, has previously been shown to bind Nhlh2 (25). Extracts from N29/2 cells transfected with Nhlh2 were incubated with labeled oligonucleotide (lane E). Specificity of binding is demonstrated by cold competitor eliminating binding (lane C), whereas cold mutant did not eliminate binding (lane M). Specific binding is indicated with solid arrows; nonspecific binding is indicated with dashed arrows.
The nature of the ChIP assay is such that motifs found on fragments within approximately 200 bp will immunoprecipiate together. The two E-box motifs are too near to each other to distinguish which E-box motif Nhlh2 was bound to in the ChIP assay. Therefore, EMSAs were performed to determine which of the two E-box motifs was capable of binding the Nhlh2 transcription factor. Two different oligonucleotides spanning the STAT3/E-box promoter regions tested in the luciferase assays were designed. As an additional positive control, oligonucleotides were designed to the E-box motif on the necdin promoter, because this motif has already been shown to bind Nhlh2 (15). Nuclear extracts from N29/2 cells transfected with murine Nhlh2 showed that the necdin and both PC1/3 E-boxes were bound (Fig. 5, B–D, lanes E). Competition analysis with excess cold oligonucleotide confirmed that this binding was specific (Fig. 5, B–D, lanes C). However, a mutated necdin E-box oligonucleotide in excess was unable to compete away binding of the nuclear extract for any of the oligonucleotides (Fig. 5, B–D, lanes M).
Leptin Stimulation of the PC1/3 Promoter Requires STAT3 Sites and Both E-Box Motifs
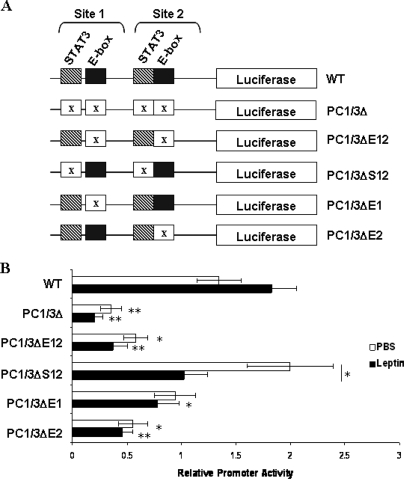
To determine whether leptin stimulation of the PC1/3 promoter requires both of the E-boxes and both of the STAT motifs on the PC1/3 promoter, various substitution mutants of the two STAT3 sites and the two E-box motifs were created (Fig. 6A). As before, luciferase assays were performed in N29/2 cells transiently transfected with both Nhlh2 and STAT3 in either the presence or absence of leptin stimulation. When compared with expression levels of the WT promoter (WT), mutating all four binding sites (PC1/3Δ) significantly decreased PC1/3 promoter activity by more than 60% (P < 0.01). A 50% reduction is also observed when both E-boxes are mutated, even though the STAT3 sites remained intact (PC1/3ΔE12, P < 0.05). Mutation of both STAT3 sites (PC1/3ΔS12) does not affect basal PC1/3 promoter activity (P > 0.3), but leptin stimulation is lost (P < 0.05) (Fig. 6B). Together these data suggest that the E-boxes are required for basal expression levels of PC1 and the STAT binding sites are required for leptin stimulation of the promoter.
Figure 6.
Leptin Stimulation of the PC1/3 Promoter Requires STAT3 and Both E-Box Motifs
A, Substitution mutants in all possible combinations for the two STAT3 sites with either one or both of the E-box motifs were made. The PC1/3Δ construct includes mutations in all four binding sites. PC1/3ΔE12 and PC1/3ΔS12 have either both E-box motifs or both STAT3 binding sites mutated, respectively. PC1/3ΔE1 and PC1/3ΔE2 included mutation in either E-box site 1 or B-box site 2, respectively. B, Activity of the WT PC1/3-luc reporter (WT) transfected in N29–2 cells in the presence (black bars) or absence (white bars) of leptin. Cells were transfected with the indicated PC1/3-luc reporter, Nhlh2, and STAT3. The luciferase activity was measured and normalized to the expression of β-gal-encoding plasmid. Activity is presented relative to the values obtained in cells transfected with PC1/3-luc alone ± se. *, P < 0.05; **, P < 0.01 to basal WT expression, except where indicated.
Individual mutations of each of the E-box motifs were tested to determine whether one or both of the motifs are required for Nhlh2 regulation of the PC1/3 promoter. Mutating the E-box furthest from the start site (E-box 1, PC1/3ΔΕ1) only had a significant effect on PC1/3 promoter activity under leptin stimulation, whereas mutating the E-box closest to the start site (E-box 2, PC1/3ΔΕ2) had a more pronounced effect on luciferase expression with a loss of approximately 50% PC1/3 promoter activity levels in both leptin-stimulated and unstimulated cells (P < 0.01; P ≤ 0.05, respectively) (Fig. 6B). Thus, in a hypothalamic arcuate-like cell line, both Nhlh2 and STAT3 are responsible for regulating PC1/3 gene expression, with STAT3 responsible for leptin induction and Nhlh2 required for both its basal expression and response of the PC1/3 promoter to leptin stimulation.
The Nhlh2 and STAT3 Transcription Factors Heterodimerize and Interact on the PC1/3 Promoter
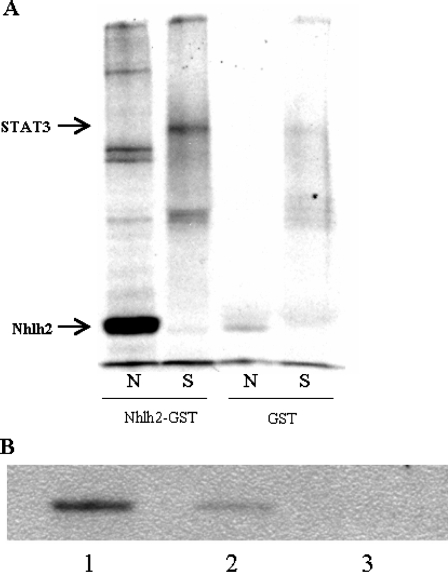
The bHLH transcription factors, like Nhlh2, can interact with other transcription factors by forming heterodimers and homodimers through their HLH domains. STAT3 homodimerizes and can also interact with bHLH proteins (24). The close proximity of Nhlh2 and STAT3 binding sites in the PC1/3 promoter, and the results of the transactivation assays, led us to hypothesize that Nhlh2 and STAT3 can interact in a protein-protein heterodimer. To test this, glutathione-S-transferase (GST) pull-down assays were performed with bacterially produced GST-Nhlh2 (fusion proteins containing the Nhlh2 protein linked with GST) and in vitro-translated 35S-labeled STAT3. Like Nhlh1 (25), Nhlh2 can homodimerize (Fig. 7A). Nhlh2 can also heterodimerize with STAT3, as shown by its ability to pull down 35S-labeled STAT3 in the experiment (Fig. 7A).
Figure 7.
Nhlh2 and STAT3 Interact on the PC1/3 Promoter
A, Bacterially synthesized GST-Nhlh2 and GST proteins were incubated with 35S-labeled in vitro-translated Nhlh2 (N) and STAT3 (S) proteins. Glutathione agarose beads were used to pull the complexes out of solution, and the proteins were then analyzed on polyacrylamide gels. GST protein alone was used to control for background. GST-Nhlh2 protein forms a homodimer with Nhlh2, and a STAT3:gst-Nhlh2 heterodimer is shown. B, A ChIP assay was performed on leptin-stimulated cells, and STAT3 immunoprecipitation in the Nhlh2 complex was analyzed by Western blot. Lane 1 shows STAT3 immunoprecipitation in the Nhlh2-myc-chromatin complex. Cells that were not transfected with Nhlh2-myc and a no-antibody control were included (lanes 2 and 3, respectively).
To ask whether Nhlh2 can form a complex on the endogenous PC1/3 promoter in N29/2 cells, a modified ChIP assay was performed. In this assay, N2-myc was used to immunoprecipitate chromatin complexes, which were then subjected to Western analysis using a STAT3 antibody. As shown in Fig. 7B, STAT3 protein is present in the immunoprecipitated complex with Nhlh2. Together, these experiments reveal Nhlh2 and STAT3 interaction as a mechanism for leptin-induction of PC1/3 gene expression.
DISCUSSION
Cell-based studies and in vivo experiments over the last 11 yr have led to a relatively detailed understanding of regulation of intracellular signaling by the leptin receptor. This study describes how Nhlh2 is involved in this leptin signaling pathway in hypothalamic neurons. Nhlh2 in combination with STAT3 can induce PC1/3 expression after leptin stimulation. The results place Nhlh2 as a critical downstream regulator of leptin signaling, specifically working with STAT3 to regulate a known target gene PC1/3.
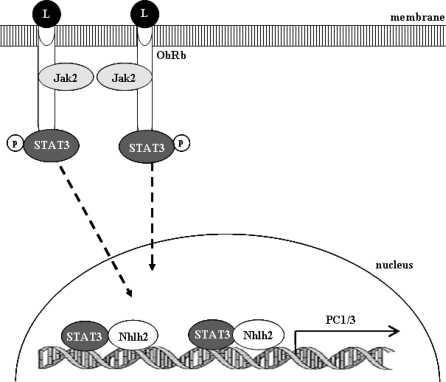
Several studies have shown that PC1/3 and POMC are coordinately regulated by leptin via the Jak/STAT signaling pathway (6,17,19,26,27). We describe here a novel mechanism for the transcriptional regulation of PC1/3 within neurons of the ARC (Fig. 8). STAT3 and Nhlh2 mediate a coordinated effort, involving heterodimer formation resulting in high levels of PC1/3 gene transcription. The characterization of this mechanism is relevant to understanding the neural intracellular signaling pathways and ultimately biological processes.
Figure 8.
Schematic Representation of Leptin-Induced STAT3 and Nhlh2-Dependent Transactivation of the PC1/3 Gene Promoter in Arcuate Neurons
Leptin (L) binds to the leptin receptor (ObRb) on the membrane of ARC neurons triggering an intracellular signaling cascade, which radiates from activation of Jak2 and STAT3. Phosphorylated (p) STAT3 translocates to the nucleus where it binds with Nhlh2 at two putative E-box/STAT3 motifs. STAT3 and Nhlh2 coordinately regulate transcription of PC1/3 through both binding sites.
STAT3 was previously shown to induce expression of PC1/3 after leptin stimulation (6). That study, performed in human embryonic kidney cell line, 293T, showed a 7-fold activation in the PC1/3 promoter, which is much higher than demonstrated in this study. Several factors could be contributing to this difference. First, N29/2 cells endogenously express PC1/3 (data not shown), whereas 293T cells, a human embryonic kidney cell line, do not (6). Therefore, the PC1/3 luciferase construct is already expressed to a certain level in these cells giving the appearance of a lower stimulation. Second, the 7-fold increase was seen using the human promoter containing an additional STAT3 binding site not present in the mouse promoter. Although the other two E-box and STAT3 binding sites are well conserved, expression levels in mice compared with humans can vary greatly (14,28). Lastly, the minimal promoter used in the studies herein was designed to specifically target the E-box and STAT3 overlapping motifs within the PC1/3 promoter, and as such is a much smaller fragment (∼200 bp) of the PC1/3 promoter than used in the previous study (971 bp) (6). In fact, our minimal promoter fragment does not include one other potential STAT3 binding site, or other putative binding sites for SP1, AP1 complex, cAMP response element, and nuclear factor-κB, all of which may further up-regulate PC1/3 in response to energy availability signals.
Nhlh2 likely regulates the transcription of many genes and may do so using different mechanisms. We have shown that on the PC1/3 promoter STAT3 and Nhlh2 interact as a heterodimer to mediate leptin-stimulated PC1/3 expression. This is a novel mechanism for Nhlh2, which has previously been shown to interact with LMO2 or LMO3 at the E-box motif CAGCTG (29,30). Our results identify a new binding partner for Nhlh2 as well as a new E-box binding site of interaction (CAAATG). It is notable that there is a faint band in the ChIP assay in cells not transfected with Nhlh2-myc (Fig. 5A, lane 2). Although there is always some background binding in experiments of this type, the possibility remains that full-length endogenous myc, which contains bHLH domains, could be bringing down some STAT3. Further studies will be needed to examine the possibility of a myc:STAT3 heterodimer.
Precise regulation of the PC1/3 gene is crucial to the regulation of energy balance, because PC1/3 enzymatic function is necessary for neuropeptide processing within the hypothalamus, pancreas, and other tissues (21,31). Both mice and humans with mutations in the PC1/3 gene display obesity (32,33). Likewise, N2KO mice have a 40–60% reduction in PC1/3 expression in the ARC of the hypothalamus, leading to adult-onset obesity (10,12). PC1/3 heterozygous null mice tend to be mildly obese starting at approximately 10 wk of age, characterizing this as an adult-onset form of obesity similar to N2KO mice (34). This is particularly interesting because both PC1/3 heterozygotes and N2KO mice have a similar level of PC1/3 reduction accompanied by a similar delay in the onset of obesity (10,11). It would be expected that PC1/3 heterozygous mice would still regulate PC1/3 levels after leptin stimulation, although to our knowledge this has not been tested.
In the present study, N2KO mice failed to modulate PC1/3 levels in response to either energy deprivation (food deprivation) or energy availability (leptin and ad lib feeding). Although there is a significant increase in PC1 mRNA levels in leptin-treated N2KO mice compared with ad lib N2KO mice in the qRT-PCR, this increase is less robust than in WT mice and not significant compared with deprived N2KO mice. Thus, Nhlh2 transcriptional activity is necessary for both basal and induced expression of PC1/3.
It is interesting to note the differences between the qRT-PCR and the in situ data. We believe the variance that is seen in the PC1/3 expression is due to the whole hypothalamic RNA used in the qRT-PCR. Nhlh2 is not expressed in all cells in the hypothalamus, and it is possible that differences are present between different hypothalamic nuclei. This is why in situ hybridization analysis was used to look specifically at the ARC.
Establishment of the signaling pathways regulating gene expression in response to energy availability is crucial for our full understanding of normal and altered states of body weight control. As shown by these data, the bHLH transcription factor Nhlh2 plays an important role in mediating basal and induced responses of the PC1/3 gene, ultimately controlling the levels of fully processed neuropeptides that are secreted in response to peripheral signals. Nhlh2 control of PC1/3 has now been shown to be part of the well-characterized lepin-Jak/STAT pathway in the hypothalamus. Further work to identify other factors that may be present in the STAT3:Nhlh2 complex, as well of other targets of this complex, will shed light on the functional signals mediating body weight control in animals and humans.
MATERIALS AND METHODS
Experimental Animals
All animal protocols were approved by the respective Institutional Animal Care and Use Committees at both the University of Massachusetts, Amherst, and the Virginia Polytechnic Institute and State University. Animal colony maintenance, breeding, and genotyping have been described previously (10). N2KO and normal mice were maintained in 12-h light, 12-h dark conditions with ad libitum access to food (4.5% crude fat). Only male mice were used for all experiments, to eliminate the need for estrous cycle analysis in female mice. WT and N2KO mice were randomly assigned to one of three treatment groups (n = 6 per group): [ad lib fed (ad lib), food deprived for 24 h (deprived), food deprived for 24 h + 2 h leptin (3 mg/kg body weight in PBS) injection (deprived + leptin)]. All mice were euthanized by CO2 asphyxiation at 1300 h to standardize hormone and steroid levels that fluctuate hourly. Brains were isolated by dissection, and a hypothalamic tissue block was made and fresh frozen on dry ice and sectioned on a cryostat at 12 μm.
In Situ Hybridization
Sections containing the ARC were identified based on the position of a dense group of cells in the region of the median eminence of the hypothalamus. The cRNA probes (riboprobes) were prepared using linearized plasmid according to the manufacturer’s directions using the Promega T3/T7 Riboprobe kit (Promega Corp., Madison, WI). The cRNA probe for mouse PC1/3 was obtained from Nabil Seidah and has been described previously. The methods for prehybridization and hybridization of the slides have also been reported previously (21). Approximately 1 × 106 cpm of 33P-labeled probe was diluted into 25 μl of hybridization buffer and added to the center of each tissue section affixed to slides. The slides were hybridized for 16–18 h at 52 C in the Boekel Slide Moat (Feasterville, PA). After several posthybridization washes to remove unbound probes, the slides were exposed to phosphorimager screens overnight. The intensity of the signal was used to determine the length of exposure to emulsion. The slides then were dipped in Hypercoat LM-1 liquid emulsion (Amersham Biosciences, Buckinghamshire, UK) and developed using Kodak products (Eastman Kodak, Rochester, NY). The ×40 magnification in situ hybridization images were grayscale digitalized and quantified using ImageJ (Public Domain, Developed at the National Institute of Mental Health, Bethesda, MD) where the same threshold was used for comparison sets. The signal to noise ratio was adjusted, and the total number of positive pixels per unit area was calculated for each brain region. Six mice were tested under each energy availability condition in the ARC of the hypothalamus.
qRT-PCR from Whole Hypothalamus to Detect PC1/3 Gene Expression
WT and N2KO mice were tested under differing states of energy availability [ad lib, deprived, deprived + leptin (3 mg/kg body weight, in PBS)]. Brains were isolated by dissection. A hypothalamic block was isolated by cutting the center millimeter of brain in a 2-mm Mouse Brain Matrix (Zivic Laboratories Inc., Pittsburgh, PA). The brain segment was put into 4 m guanidine isothiocyanate buffer and homogenized. Samples were layered over 5.7 m cesium chloride buffer and spun for 18 h at 120,000 × g at 20 C. The supernatant was discarded, and RNA was resuspended in diethylpyrocarbonate water. RNA was then DNAse treated. RNA samples were submitted to the Virginia Bioinformatics Institute (VBI) (Virginia Polytechnic Institute and State University, Blacksburg, VA). All RNA samples were assayed on the Agilent 2100 BioAnalyzer (Agilent Technologies, Santa Clara, CA) for a rapid quantitative and qualitative assessment. All samples passed this initial quality control check and then were processed.
cDNA was created using reverse transcriptase in a magnesium buffer (Promega Corp.) for 1 h at 42 C. qRT-PCR for PC1/3 expression in the hypothalamus was performed using mouse PC1/3 primers (Table 1) and mouse β-actin (catalog no. PPM02945A; Superarray Bioscience Corp., Frederick, MD) and the iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA). mRNA levels of PC1/3 were normalized against β-actin. β-Actin levels remain stable during changes in energy availability in WT mice and in N2KO mice, and are constant between WT and N2KO animals (data not shown). Normalized levels of mRNA were measured in triplicate per individual mouse from which sample means were calculated for each mouse. Five mice per experimental group were averaged, and data are reported as the fold-difference (in log) from the WT ad lib-fed experimental group. For each mRNA amplified, melting-curve analysis was done to confirm the presence of a single amplicon. A reaction from WT ad lib animals was used to subclone and sequence duplicate amplicons and confirm correct amplification of the target mRNA.
Table 1.
Oligonucleotides Used in Quantitative PCR, Subcloning, Mutagenesis, ChIP, and EMSA Experiments
| Oligonucleotide Sequence (5′–3′) | Purpose |
|---|---|
| AGTTGGAGGCATAAGGCTG | qRT-PCR, murine PC1/3 mRNA, forward |
| GCCTTCTGGGCTAGTCTG | qRT-PCR, murine PC1/3 mRNA, reverse |
| AACAGATCTCCTAGATTGTGTCTGTTTTATAACAAATGC | Subcloning, murine PC1/3 promoter, forward |
| TCCAAGCTTCAAAGAGAACCGCCTCATTTGAATATAACT | Subcloning, murine PC1/3 promoter, reverse |
| GTCTGTTTTATAACAAATGCTGAT | ChIP assay, murine PC1/3 promoter, forward |
| CGCCTCATTTGAATATAACTACCA | ChIP assay, murine PC1/3 promoter, reverse |
| GTCTGTTTTATAACAAATGCTGAT | EMSA, murine PC1/3 site 1, sense |
| ATCAGCATTTCTTATAAAACAGAC | EMSA, murine PC1/3 site 1, antisense |
| TGGTAGTTATTCAAATGAGGC | EMSA, murine PC1/3 site 2, sense |
| CGCCTCATTTGAATATAACTACCA | EMSA, murine PC1/3 site 2, antisense |
| GGGCCCTCATTTTCATGTGGGGCC | EMSA, murine necdin, sense |
| CCCCCAGGCCCCACATGAAAATGA | EMSA, murine necdin, antisense |
| GGATGGGTGCGTGGGGCC | EMSA, mutant necdin, sense |
| GGCCCCACGCACCCATCC | EMSA, mutant necdin, antisense |
| GATCTCCTAGATTGTGTCTGTTTTATAATGAATGCTGATACTCAAGAACCAT | Murine PC1/3ΔE1, forward |
| ATGGTTCTTGAGTATCAGCATTCATTATAAAACAGACACAATCTAGGAGATC | Murine PC1/3ΔE1, reverse |
| GCCTTGGGATCTTGGTAGTTATATTCAACCGAGGCGGTTCTCTTT | Murine PC1/3ΔE2, forward |
| AAAGAGAACCGCCTCGGTTGAATATAACTACCAAGATCCCAAGGC | Murine PC1/3ΔE2, reverse |
| CTCGAGATCTCCTAGATTGTGTCTGTGCCATAACAAATGCTGATACTCAAGAACC | Murine PC1/3ΔS12, forward |
| GGTTCTTGAGTATCAGCATTTGTTATGGCACAGACACAATCTAGGAGATCTCGAG | Murine PC1/3ΔS12, reverse |
| TTCCTGCCTTGGGATCTTGGTATCCATATTCAAATGAGGCGGTTCTC | Murine PC1/3ΔS12, forward |
| GAGAACCGCCTCATTTGAATATGGATACCAAGATCCCAAGGCAGGAA | Murine PC1/3ΔS12, reverse |
| CGGGCTCGAGATCTCCTAGATTGTGTCTGTGCCATAATGAATGCTGATACTCAAGAACCATTATAAGAA | Murine PC1/3Δ, using PC1/3ΔE12, forward |
| TTCTTATAATGGTTCTTGAGTATCAGCATTCATTATGGCACAGACACAATCTAGGAGATCTCGAGCCCG | Murine PC1/3Δ, using PC1/3ΔE12, reverse |
| CTTGGGATCTTGGTATCCATATTCAACCGAGGCGGTTCTCTT | Murine PC1/3Δ, using PC1/3ΔE12, forward |
| AAGAGAACCGCCTCGGTTGAATATGGATACCAAGATCCCAAG | Murine PC1/3Δ, using PC1/3ΔE12, reverse |
PC1/3 Promoter Construct
The PC1/3 promoter was amplified from the murine PC1/3-βgal promoter (a generous gift from Dr. Nabil Seidah, Clinical Research Institute of Montreal, Montreal, Quebec, Canada) using PCR. Primers that amplify from −440 to −229 were used. BglII and HindIII restriction sites were introduced for subsequent cloning into the pGL3 basic luciferase reporter vector (Promega). Primer sequences are shown in Table 1. Annealing of primers was performed at 55 C for 30 sec followed by elongation at 68 C for 1 min using Platinum Pfx DNA Polymerase (Invitrogen, Carlsbad, CA). The PCR product was analyzed by agarose gel electrophoresis, and the expected product was extracted and purified using the DNA Gel Extraction Kit (Millipore Corp., Billerica, MA). Purified product was cloned into pGL3 basic vector to yield the WT PC1/3 promoter construct. The correct size and orientation of the cloned insert were analyzed by sequencing the plasmid DNA at VBI (Virginia Polytechnic Institute and State University, Blacksburg, VA).
Cell Culture and Transfections
The hypothalamic N29/2 cell line (Cellutions Biosystems, Toronto, Ontario, Canada) was maintained in DMEM supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 10 μg/ml streptomycin (HyClone Laboratories, Inc., Logan, UT) at 37 C in 5% CO2. Cells were transfected using Effectene transfection reagent (QIAGEN, Valencia, CA) according to the recommendations by the manufacturer. Briefly, cells were plated into 12-well plates 24 h before their transfection and were transfected with 435 ng of DNA per well (200 ng reporter; 35 ng of cytomegalovirus-β-gal plasmid (a generous gift from Dr. D. Joseph Jerry, University of Massachusetts, Amherst, MA); and 200 ng of the appropriate combination of Nhlh2, STAT3 [a generous gift from Dr. James Darnell, The Rockefeller University, New York, NY) and/or empty vector (pcDNA-zeo)]. The cytomegalovirus-β-gal plasmid was used as the internal control to check the transfection efficiency. Transfections were performed in triplicate. In all transfections, total input DNA was kept constant and controlled by adding the empty vector where appropriate.
Luciferase and β-Galactosidase Assays
N29/2 cells were serum starved overnight 24 h after transfection. Cells then were treated with either 100 mm leptin or vehicle (PBS) for 6 h in serum-free media. Cells were lysed 6 h after stimulation, in 200 μl Reporter Lysis Buffer (Promega) according to the manufacturer’s recommendations. Aliquots (20 μl) were used for the luciferase (luciferase assay system) and β-galactosidase assays (Promega). For each assay, the basal WT promoter total luciferase activity normalized against β-galactosidase activity was taken to be 1, and results were expressed as fold activation over the control value. The data presented are means ± se of three or more independent experiments.
ChIP Assay
ChIP assays were performed using the ChIP kit (Upstate Biotechnology, Inc., Charlottesville, VA) according to the manufacturer’s recommendations. Cells were transfected with Nhlh2-myc (a generous gift from Dr. Thomas Braun, University of Halle-Wittenberg, Germany) and STAT3 and treated with leptin for 15 min. Proteins bound to DNA were cross linked using formaldehyde at a final concentration of 1% for 15 min at 37 C. Protein-DNA complexes were immunoprecipitated using 1 mg myc-tag mouse monoclonal primary antibody (9B11) (Cell Signaling Technology, Danvers, MA). Nhlh2-myc promoter complexes were measured by PCR. The primers used to amplify the mouse PC1/3 promoter are given in Table 1. The samples were electrophoresed using a 1% agarose gel and visualized by ethidium bromide staining.
EMSA
Oligonucleotides were annealed and labeled with T4 polynucleotide kinase (Promega) and [γ-32P] deoxy (d)-ATP (PerkinElmer, Waltham, MA; 3000 Ci/mmol). Oligonucleotides used for EMSA analysis are shown in Table 1. Labeled oligonucleotides were used as probes or remained unlabeled as competitors. A total of 5 μg protein was incubated with 35 fmol of [γ-32P] dATP-labeled probe in binding buffer (Promega) for 10 min at room temperature. DNA-protein complexes were separated from free DNA by electrophoresis on a 4% nondenaturing polyacrylamide gel. All gels were pre-run in 0.5× Tris-borate-EDTA buffer for 30 min before electrophoresis at 250V for 1–2 h. Gels were dried under vacuum and exposed to film (Eastman Kodak). For competition experiments, 10-fold molar excess of unlabeled oligonucleotide was added to the binding reaction.
Site-Directed Mutagenesis
The PC1/3 mutant constructs were generated using the Platinum Pfx DNA Polymerase (Invitrogen) using the WT PC1/3 promoter construct as a template, this time with a 5-min elongation. Substitution mutations at both E-box sites at −416 bp (PC1/3ΔE1) and −252 bp (PC1/3ΔE2) upstream from the start site of transcription were created with primers shown in Table 1. Substitution mutations at E-box site 2 were created using the same primers for PC1/3ΔE2 and the PC1/3ΔE1 plasmid as a template to create PC1ΔE12. Serial substitution mutations at both STAT sites at −424 and −259 bp (PC1/3ΔS12) upstream from the start site of transcription were created using primers shown in Table 1. Serial substitution mutations at both sites 1 and 2 at −424 and −259 bp upstream from the start site of transcription were created using PC1/3ΔE12 as a template to create PC1/3Δ with the primers listed on Table 1. All mutations were confirmed by DNA sequencing at VBI.
TNT and GST Pull-Down Assays
The TNT T7 Couple Reticulocyte Lysate System (Promega) was used to make in vitro-translated 35S-Met-radiolabeled Nhlh2 and STAT3 proteins according to the manufacturer’s recommendations. 35S-Met-radiolabeled translation products were separated by SDS-PAGE and exposed to autoradiographic film. Protein-protein interactions were performed with 5 ml of in vitro-translated 35S-Met-radiolabeled Nhlh2 or STAT3 proteins, and the fusion protein Nhlh2-GST or GST alone as a control. The bound protein were analyzed by SDS-PAGE in a 10% polyacrylamide gel and identified by autoradiography.
ChIP Coimmunoprecipitation and Western Blot Analysis
For coimmunoprecipitations, N29/2 cells were used and the same procedure used as for the ChIP assay. Cells were transfected with Nhlh2-myc and STAT3 and treated with leptin for 15 min. Proteins bound to DNA were cross linked and protein-DNA complexes were immunoprecipitated using a myc-tag mouse monoclonal primary antibody (9B11) (Cell Signaling Technology). Immunocomplexes were collected by adding Protein A-agarose beads. Nhlh2-STAT3-DNA complexes were measured by Western blot analysis. Samples were boiled in SDS-PAGE loading buffer for 5 min. Proteins were separated in a 10% acrylamide SDS-PAGE gel. Proteins were transferred onto nitrocellulose membranes. Blots were probed with anti-STAT3 (Millipore Corp., Billerica, MA) to investigate the status of Nhlh2-STAT3 complexes. Immunoreactive proteins were blotted with a secondary enhanced chemiluminescence antimouse IgG, horseradish peroxidase-linked F(ab′)2 fragment from sheep (GE Healthcare Life Sciences, Piscataway, NJ) and visualized using ECL Plus reagents (GE Healthcare Life Sciences). Images were then exposed to x-ray film and developed.
Statistical Analysis
Statistical analysis was performed using MiniTab software (MiniTab, Inc., State College, PA). Values reported in all analyses were expressed as the mean ± se. Data were analyzed using one-way ANOVA followed by Tukey’s multiple comparison post hoc test. Statistical significance was accepted at P ≤ 0.05. Photographs of gels and blots are representative experiments that were reproduced at least three times with similar results.
Acknowledgments
We thank Dr. Nabil Seidah, Dr. Thomas Braun, Dr. James Darnell, and Dr. Joseph Jerry for generous gifts of plasmid DNA; Amy S. Burnside for helpful discussions and training on transfection and EMSAs; and Ila Joshi and Jeremy Samon for training and support with the ChIP assay. Mouse recombinant leptin was purchased from the National Hormone and Pituitary Program and the National Institutes of Diabetes and Digestive and Kidney Diseases, and Dr. A. F. Parlow. Mr. Christopher Coyle and Ms. Alison Bardwell provided excellent technical support. We also thank Franc-Eric Wiedmer for careful reading of the manuscript and the Fox family for their love and support.
Footnotes
This work was supported by National Institutes of Health Grant DK059903 (to D.J.G).
Disclosure Statement: The authors have no disclosures of conflict of interest.
First Published Online March 20, 2008
Abbreviations: ARC, Arcuate nucleus; bHLH, basic helix-loop-helix; ChIP, chromatin immunoprecipitation; GST, glutathione-S-transferase; Jak, Janus kinase; Nhlh2, nescient helix-loop-helix 2; N2KO, Nhlh2 knockout mice; PC1/3; prohormone convertase 1/3; POMC, proopiomelanocortin; qRT-PCR, quantitative RT-PCR; STAT, signal transducer and activator of transcription; WT, wild type.
References
- Baskin DG, Blevins JE, Schwartz MW 2001 How the brain regulates food intake and body weight: the role of leptin. J Pediatr Endocrinol Metab 14(Suppl 6):1417–1429 [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F 1995 Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269:540–543 [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Thomas ME, Duncan JS, Rayner DV 1995 Effects of fasting and refeeding on ob gene expression in white adipose tissue of lean and obese (ob/ob) mice. FEBS Lett 368:488–490 [DOI] [PubMed] [Google Scholar]
- Hegyi K, Fulop K, Kovacs K, Toth S, Falus A 2004 Leptin-induced signal transduction pathways. Cell Biol Int 28:159–169 [DOI] [PubMed] [Google Scholar]
- Vaisse C, Halaas JL, Horvath CM, Darnell Jr JE, Stoffel M, Friedman JM 1996 Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet 14:95–97 [DOI] [PubMed] [Google Scholar]
- Sanchez VC, Goldstein J, Stuart RC, Hovanesian V, Huo L, Munzberg H, Friedman TC, Bjorbaek C, Nillni EA 2004 Regulation of hypothalamic prohormone convertases 1 and 2 and effects on processing of prothyrotropin-releasing hormone. J Clin Invest 114:357–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS 1999 Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev 20:68–100 [DOI] [PubMed] [Google Scholar]
- Nillni EA 2007 Regulation of prohormone convertases in hypothalamic neurons: implications for prothyrotropin-releasing hormone and proopiomelanocortin. Endocrinology 148:4191–4200 [DOI] [PubMed] [Google Scholar]
- Atchley WR, Fitch WM 1997 A natural classification of the basic helix-loop-helix class of transcription factors. Proc Natl Acad Sci USA 94:5172–5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing E, Nillni EA, Sanchez VC, Stuart RC, Good DJ 2004 Deletion of the Nhlh2 transcription factor decreases the levels of the anorexigenic peptides α melanocyte-stimulating hormone and thyrotropin-releasing hormone and implicates prohormone convertases I and II in obesity. Endocrinology 145:1503–1513 [DOI] [PubMed] [Google Scholar]
- Coyle CA, Jing E, Hosmer T, Powers JB, Wade G, Good DJ 2002 Reduced voluntary activity precedes adult-onset obesity in Nhlh2 knockout mice. Physiol Behav 77:387–402 [DOI] [PubMed] [Google Scholar]
- Good DJ, Porter FD, Mahon KA, Parlow AF, Westphal H, Kirsch IR 1997 Hypogonadism and obesity in mice with a targeted deletion of the Nhlh2 gene. Nat Genet 15:397–401 [DOI] [PubMed] [Google Scholar]
- Ftouhi N, Day R, Mbikay M, Chretien M, Seidah NG 1994 Gene organization of the mouse pro-hormone and pro-protein convertase PC1. DNA Cell Biol 13:395–407 [DOI] [PubMed] [Google Scholar]
- Fox DL, Vella KR, Good DJ 2007 Energy balance pathways converging on the Nhlh2 transcription factor. Front Biosci 12:3983–3993 [DOI] [PubMed] [Google Scholar]
- Kruger M, Ruschke K, Braun T 2004 NSCL-1 and NSCL-2 synergistically determine the fate of GnRH-1 neurons and control necdin gene expression. EMBO J 23:4353–4364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella KR, Burnside AS, Brennan KM, Good DJ 2007 Expression of the hypothalamic transcription factor Nhlh2 is dependent on energy availability. J Neuroendocrinol 19:499–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG 1997 Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes 46:2119–2123 [DOI] [PubMed] [Google Scholar]
- Li QL, Jansen E, Friedman TC 1999 Regulation of prohormone convertase 1 (PC1) by gp130-related cytokines. Mol Cell Endocrinol 158:143–152 [DOI] [PubMed] [Google Scholar]
- Nilaweera KN, Barrett P, Mercer JG, Morgan PJ 2003 Precursor-protein convertase 1 gene expression in the mouse hypothalamus: differential regulation by ob gene mutation, energy deficit and administration of leptin, and coexpression with prepro-orexin. Neuroscience 119:713–720 [DOI] [PubMed] [Google Scholar]
- McCowen KC, Chow JC, Smith RJ 1998 Leptin signaling in the hypothalamus of normal rats in vivo. Endocrinology 139:4442–4447 [DOI] [PubMed] [Google Scholar]
- Sharlin DS, Bansal R, Zoeller RT 2006 Polychlorinated biphenyls exert selective effects on cellular composition of white matter in a manner inconsistent with thyroid hormone insufficiency. Endocrinology 147:846–858 [DOI] [PubMed] [Google Scholar]
- Perello M, Stuart RC, Nillni EA 2007 Differential effects of fasting and leptin on proopiomelanocortin peptides in the arcuate nucleus and in the nucleus of the solitary tract. Am J Physiol Endocrinol Metab 292:E1348–E1357 [DOI] [PubMed] [Google Scholar]
- Belsham DD, Cai F, Cui H, Smukler SR, Salapatek AM, Shkreta L 2004 Generation of a phenotypic array of hypothalamic neuronal cell models to study complex neuroendocrine disorders. Endocrinology 145:393–400 [DOI] [PubMed] [Google Scholar]
- Ivanova AV, Ivanov SV, Zhang X, Ivanov VN, Timofeeva OA, Lerman MI 2004 STRA13 interacts with STAT3 and modulates transcription of STAT3-dependent targets. J Mol Biol 340:641–653 [DOI] [PubMed] [Google Scholar]
- Brown L, Espinosa III R, Le Beau MM, Siciliano MJ, Baer R 1992 HEN1 and HEN2: a subgroup of basic helix-loop-helix genes that are coexpressed in a human neuroblastoma. Proc Natl Acad Sci USA 89:8492–8496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CC, Clifton DK, Steiner RA 1997 Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology 138:4489–4492 [DOI] [PubMed] [Google Scholar]
- Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK 1999 Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron 23:775–786 [DOI] [PubMed] [Google Scholar]
- Yanai I, Graur D, Ophir R 2004 Incongruent expression profiles between human and mouse orthologous genes suggest widespread neutral evolution of transcription control. Omics 8:15–24 [DOI] [PubMed] [Google Scholar]
- Han C, Liu H, Liu J, Yin K, Xie Y, Shen X, Wang Y, Yuan J, Qiang B, Liu YJ, Peng X 2005 Human Bex2 interacts with LMO2 and regulates the transcriptional activity of a novel DNA-binding complex. Nucleic Acids Res 33:6555–6565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama M, Ozaki T, Inuzuka H, Tomotsune D, Hirato J, Okamoto Y, Tokita H, Ohira M, Nakagawara A 2005 LMO3 interacts with neuronal transcription factor, HEN2, and acts as an oncogene in neuroblastoma. Cancer Res 65:4587–4597 [DOI] [PubMed] [Google Scholar]
- Seidah NG, Chretien M 1999 Proprotein and prohormone convertases: a family of subtilases generating diverse bioactive polypeptides. Brain Res 848:45–62 [DOI] [PubMed] [Google Scholar]
- Lloyd DJ, Bohan S, Gekakis N 2006 Obesity, hyperphagia and increased metabolic efficiency in Pc1 mutant mice. Hum Mol Genet 15:1884–1893 [DOI] [PubMed] [Google Scholar]
- Jackson RS, Creemers JW, Ohagi S, Raffin-Sanson ML, Sanders L, Montague CT, Hutton JC, O'Rahilly S 1997 Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet 16:303–306 [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhou A, Dey A, Norrbom C, Carroll R, Zhang C, Laurent V, Lindberg I, Ugleholdt R, Holst JJ, Steiner DF 2002 Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proc Natl Acad Sci USA 99:10293–10298 [DOI] [PMC free article] [PubMed] [Google Scholar]