Abstract
The peroxisome proliferator-activated receptors (PPARs) are members of the nuclear hormone receptor superfamily. These receptors are also ligand-dependent transcription factors responsible for the regulation of cellular events that range from glucose and lipid homeostases to cell differentiation and apoptosis. The importance of these receptors in lipid homeostasis and energy balance is well established. In addition to these metabolic and anti-inflammatory properties, emerging evidence indicates that PPARs can function as either tumor suppressors or accelerators, suggesting that these receptors are potential candidates as drug targets for cancer prevention and treatment. However, conflicting results have emerged regarding the role of PPARs on colon carcinogenesis. Therefore, further investigation is warranted prior to considering modulation of PPARs as an efficacious therapy for colorectal cancer chemoprevention and treatment.
1. INTRODUCTION
Understanding the biology of intestinal epithelial cells may reveal the molecular pathogenesis of a number of digestive diseases. One such disease, colorectal cancer (CRC), leads to significant cancer-related morbidity and mortality in most industrialized countries. Initiation and progression of CRC are a complex process that results from the loss of the normal regulatory pathways that govern a balance between epithelial cell proliferation and death. For example, alterations in multiple pathways such as Wnt/APC, COX-2, and Ras are known to play major roles in CRC progression. The standard treatment for advanced malignancies has improved greatly over the past decade but is still not satisfactory. Therefore, significant effort has been exerted to identify novel drug targets for both the prevention and treatment of this disease. One group of compounds found to decrease the risk of colorectal cancer includes nonsteroidal anti-inflammatory drugs (NSAIDs), which target the cyclooxygenase enzymes (COX-1 and COX-2). However, prolonged use of high doses of these inhibitors (except for aspirin) is associated with unacceptable cardiovascular side effects [1–3]. Thus, it is now crucial to develop more effective chemopreventive agents with minimal toxicity and maximum benefit.
Dietary fat intake is an environmental factor that is associated with some human diseases such as diabetes, obesity, and dyslipidemias. Some nuclear hormone receptors play a central role in regulating nutrient metabolism and energy homeostasis. These nuclear receptors are activated by natural ligands, including fatty acids and cholesterol metabolites. Among these receptors, special attention has been focused on the members of the peroxisome proliferator-activated receptors (PPARs) family, which were initially identified as mediators of the peroxisome proliferators in the early 1990s [4]. PPARs play a central role in regulating the storage and catabolism of dietary fats via complex metabolic pathways, including fatty acid oxidation and lipogenesis [5]. To date, three mammalian PPARs have been identified and are referred to as PPARα (NR1C1), PPARδ/β (NR1C2), and PPARγ (NR1C3). Each PPAR isotype displays a tissue-selective expression pattern. PPARα and PPARγ are predominantly present in the liver and adipose tissue, respectively, while PPARδ expresses in diverse tissues [6]. In common with other members of the type II steroid hormone receptor superfamily, PPARs are ligand-dependent transcription factors and form heterodimers with another obligate nuclear receptors, such as retinoid X receptors (RXRs) [4, 7, 8]. Each PPAR-RXR heterodimer binds to the peroxisome proliferator responsive element (PPRE) located in the promoter region of responsive genes.
It is well established that modulation of PPAR activity maintains cellular and whole-body glucose and lipid homeostases. Hence, great efforts have been made to develop drugs targeting these receptors. For example, PPARγ synthetic agonists, rosiglitazone and pioglitazone, are antidiabetic agents which suppress insulin resistance in adipose tissue. The antiatherosclerotic and hypolipidemic agents including fenofibrate and gemfibrozil are PPARα synthetic agonists that induce hepatic lipid uptake and catabolism. Genetic and pharmacological studies have also revealed important roles of PPARδ in regulating lipid metabolism and energy homeostasis. Genetic studies indicate that overexpression of constitutively active PPARδ in mouse adipose tissue reduced hyperlipidemia, steatosis, and obesity induced by either genetics or a high-fat diet. In contrast, PPARδ null mice treated in similar fashion exhibited an obese phenotype [9]. Pharmacologic studies demonstrate that the PPARδ selective-agonist (GW501516) attenuated weight gain and insulin resistance in mice fed with high-fat diets [10] and increased HDL-C while lowering tryglyceride levels and insulin in obese rhesus monkeys [11]. Furthermore, preclinical studies revealed that PPARδ agonists diminished metabolic derangements and obesity through increasing lipid combustion in skeletal muscle [12]. These results suggest that PPARδ agonists are potential drugs for use in the treatment of dyslipidemias, obesity, and insulin resistance. Therefore, the PPARδ agonist (GW501516) is currently in phase III clinical trials to evaluate its use for treatment of patients with hyperlipidemias and obesity. However, recent studies showing that some agonists of PPARs promote carcinogenesis in animal models have raised concerns about using these agonists for the treatment of metabolic diseases. For example, long-term administration of a PPARα agonist induces the development of hepatocarcinomas in mice but not in PPARα null animals, conclusively demonstrating that PPARα mediates these effects in promoting liver cancer [13]. Furthermore, the PPARδ agonist (GW501516) accelerates intestinal polyp growth in ApcMin/+ mice [14, 15]. These results raise concerns for developing this class of agents for human use and support the rationale for developing PPARδ antagonists as chemopreventive agents.
2. PPARs AND COLORECTAL CANCER
Significant effort has been concentrated on deducing the role of PPARs in CRC and other cancers. A large body of evidence indicates that PPARγ serves as a tumor suppressor. Contradictory evidences suggest that PPARδ can act as either a tumor suppressor or tumor promoter. A few evidences support a role of PPARα in CRC.
2.1. PPARα
Although the tumor-promoting effects of PPARα in hepatocarcinomas are clear, less is known about the role of PPARα in human tumors. Generally, activation of PPARα by exogenous agonists causes inhibition of tumor cell growth in cell lines derived from CRC, melanoma, and glial brain tumors [16–18]. There is no evidence showing that PPARα expression is elevated in human cancers.
2.2. PPARγ
The prominent role of PPARγ in regulating cellular differentiation prompted a great effort to investigate the function of PPARγ in cancer field. While PPARγ is elevated in CRC [19], suggesting that this receptor may contribute to tumor biology, studies of PPARγ mutation in CRC from humans, animals, and cultured cells produced controversial results. One study showed that 8% of primary human colorectal tumors had a loss of function mutation in one allele of the PPARγ gene [20]. Recent data revealed that a Pro12Ala (P12A) polymorphism in the PPARγ gene is associated with increased risk of CRC [21, 22]. These results suggest a putative role for this receptor as a tumor suppressor. In contrast, another study showed that mutant PPARγ gene has not been detected in human colon tumor samples and CRC cell lines, suggesting that PPARγ mutations in human CRC is a rare event [23].
In vitro studies show that activation of PPARγ results in growth arrest of colon carcinoma cells through induction of cell-cycle arrest or/and apoptosis. Several potential downstream targets of PPARγ for mediating antitumor effects of PPARγ have been identified in various cancer cell types. Activation of PPARγ negatively regulates cell cycle progression by modulating a number of cell cycle regulators: (1) inhibiting E2F activity in transformed adipogenic cells [24], (2) Rb hyperphosphorylation in vascular smooth muscle cells and pituitary adenoma cells [25, 26], (3) cyclin D1 expression in Ras-transformed intestinal epithelial cells, pancreatic, or breast cancer cells [27–29], and (4) inducing CDK inhibitor expression such as p18, p21, and p27 in hepatoma cells [30]. Activation of PPARγ has also been reported to inhibit tumor cell growth by upregulation of the transcriptional repressor TSC22 in colon cancer cells [31] and GADD153 in nonsmall-cell lung carcinoma cells [32]. PPARγ agonists induce apoptosis by induction of PTEN expression in pancreatic, breast, and colon cancer cells [33] and inhibition of NFκB and Bcl-2 expression in colon cancer cells [34]. Moreover, PPARγ exhibits antiangiogenic effects by inhibiting VEGF expression in tumor cells and VEGF receptors in endothelial cells [35, 36]. It has also been reported that PPARγ agonists suppress tumor cell invasion in colon and breast cancer cells by downregulation of matrix metalloproteinase-7 (MMP-7) and induction of MMP inhibitors [37, 38]. In addition, the ability of PPARγ to suppress tumor growth is also through inhibiting APC/β-catenin and COX-2/PGE2 signaling pathways, which are pivotally involved in colon carcinogenesis [39–42].
However, the role of PPARγ in colorectal cancer progression is controversial because there are conflicting results in mouse models of colon cancer. Although PPARγ agonists inhibit colorectal carcinogenesis in xenograft models and in the azoxymethane (AOM)-induced colon cancer model [43, 44], these drugs are reported to have both tumor-promoting and tumor-inhibiting effects in a mouse model for familial adenomatous polyposis, the ApcMin/+ mouse. It has been reported that administration of PPARγ agonists significantly increases the number of colon adenomas in the ApcMin/+ mice [45–47] and even in wild-type C57BL/6 mice [48]. However, other studies show that treatment of 2 different Apc-mutant models (ApcMin/+ and ApcΔ1309) with the PPARγ agonist pioglitazone resulted in reduction in the number of both small and large intestinal polyps in a dose-dependent manner [49, 50]. These paradoxical observations appear to have been resolved by genetic studies showing that the heterozygous disruption of PPARγ is sufficient to increase tumor number in AOM-treated mice and that intestinal-specific PPARγ knockout promotes tumor growth in ApcMin/+ mice [39, 51]. These genetic evidences support the hypothesis that PPARγ serves as tumor suppressor in colorectal cancer. One possible explanation for the differences in phenotype caused by pharmaceutical versus genetic manipulation of PPARγ in mouse models may be due to the PPARγ-independent effect of the agonist drugs, drug doses used, and animal models employed. This controversial extends beyond CRC. For example, data are conflicting from different animal models of breast cancer as well. PPARγ agonist suppresses NMU-induced mammary carcinomas [52]. However, overexpression of a constitutively active form of PPARγ accelerates mammary gland tumor development in MMTV-PyV transgenic mice [53].
2.3. PPARδ
PPARδ has been shown to play an important role in embryo implantation [54], atherogenic inflammation [55], regulating cell survival in the kidney following hypertonic stress [56], and skin following wound injury [57, 58]. The role of PPARδ in colorectal carcinogenesis is more controversial than that of PPARγ. The first evidence linking the PPARδ to carcinogenesis actually emerged from studies on gastrointestinal cancer. PPARδ is elevated in most human colorectal cancers and in tumors arising in the ApcMin/+ mice, and AOM-treated rats [59, 60]. Importantly, the PPARδ proteins are accumulated only in human CRC cells with highly malignant morphology [61]. Downregulation of PPARδ is correlated with antitumor effects of dietary fish oil/pectin in rats treated with radiation and AOM [62]. PPARδ was identified as a direct transcriptional target of APC/β-catenin/Tcf pathway and as a repression target of NSAIDs [59, 63]. A case-control study in a large population showed that the protective effect of NSAIDs against colorectal adenomas was reported to be modulated by a polymorphism in the PPARδ gene [64]. PPARδ expression and activity are also induced by oncogenic K-ras [65]. In addition, COX-2-derived PGl2 directly transactivates PPARδ [60], and COX-2-derived PGE2 indirectly induces PPARδ activation in CRC, hepatocellular carcinoma, and cholangiocarcinoma cells [66–68]. These studies indicate that PPARδ is a focal point of cross-talk between these signaling pathways.
In a murine xenograft cancer model, the disruption of both PPARδ alleles in human HCT-116 colon carcinoma cells decreased tumorigenicity, suggesting that activation of PPARδ promotes tumor growth [69]. However, PPARδ has been reported to have both tumor-promoting and tumor-inhibiting effects based on conflicting data obtained from mouse models of colon cancer. For example, activation of PPARδ by a selective synthetic PPARδ agonist (GW501516) or a PPARδ endogenous activator (PGE2) accelerates intestinal adenoma growth in ApcMin/+ mice by promoting tumor cell survival [14, 66]. A subsequent genetic study showed that deletion of PPARδ attenuates both small and large intestinal adenoma growth, and PPARδ is required for the tumor-promoting effects of PPARδ ligand (GW501516) and PGE2 in ApcMin/+ mice [15, 66]. Another study showed that loss of PPARδ in ApcMin/+ mice significantly reduced growth of tumors larger than a diameter of 2 mm, even though PPARδ deficiency did not affect overall tumor incidence [70]. In contrast to these reports suggesting that PPARδ serves as tumor accelerator, recent conflicting reports show that PPARδ deficiency enhances polyp growth in ApcMin/+ and AOM-treated mice in the absence of exogenous PPARδ stimulation [71, 72]. Moreover, a PPARδ ligand (GW0742) inhibits colon carcinogenesis in AOM-treated mice but promotes small intestinal polyp growth in ApcMin/+ mice [73].
One explanation for these disparate results may be due to differences in the genetic background of ApcMin/+ mice, animal breeding, or possibly to differences in the specific targeting strategy employed to delete PPARδ. For example, the average number of polyps in 13-week old ApcMin/+ mice on a C57BL/6 genetic background is about 50, while the polyp number in ApcMin/+ mice on a mixed-genetic-background (C57BL/6 × 129/SV) is about 120. Our results also show that the breeding strategy affects the number and size of polyps in mice even on the same genetic background. Mice generated by breeding female PPARδ −/−/ApcMin/+ with male PPARδ −/−/Apc+/+ exhibit increased adenoma number with a larger average size than those obtained by breeding female PPARδ −/−/Apc+/+ with male PPARδ −/−/ApcMin/+. Finally, the PPARδ null mice we studied were obtained from Beatrice Desvergne in Switzerland. These mice were generated by deleting exons 4 and 5 encoding the DNA binding domain [74], while Peters group generated the PPARδ knockout mice by inserting a neomycin resistance cassette into the last exon (exon 8) [75]. It has been suggested that the strategy employed to disrupt PPARδ by the Peters group might have led to a hypomorphic allele, which retains some aporeceptor function, thus making it difficult to correctly interpret their results. Indeed, conflicting results in the context of embryonic lethality have also been observed from these two PPARδ mutant mouse strains [74, 75]. To further clarify the role of PPARδ in colorectal tumorigenesis, it is important to investigate the role of PPARδ in animal models that are dependent on activation of other oncogenes or disruption of other tumor suppressors to verify our conclusions that activation of PPARδ is proneoplastic.
Studies in other types of cancer also support the hypothesis that PPARδ serves as a tumor accelerator. A selective PPARδ agonist (GW501516) has been shown to stimulate proliferation of human breast, prostate, and hepatocellular carcinoma cells [68, 76, 77]. In a xenograft model, blocking PPARδ activation reduced ovarian tumor growth [78]. PPARδ knockout mice exhibited significant impaired angiogenesis and tumor growth after these mice were injected s.c. with mouse Lewis lung carcinoma and melanoma cells [79]. In a mouse mammary tumor model, treatment with the PPARδ agonist (GW501516) accelerated tumor formation, while a PPARγ agonist (GW7845) delayed tumor growth [80]. Taken together, the role of PPARδ in cancer biology remains unclear.
3. SUMMARY
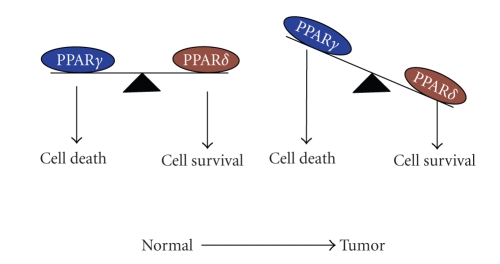
Despite extensive research on both PPARγ and PPARδ in CRC, the role of these receptors remains highly controversial in this disease. Emerging evidence demonstrates that cooperative interactions between Wnt, COX-2, and PPARs signaling pathways can initiate cellular transformation and promote progression of colorectal cancer. These studies provide support for evaluating the efficacy of PPARδ antagonists for cancer prevention and/or treatment. We propose a potential working model as a useful starting point for future studies (see Figure 1).
Figure 1.
A potential model for PPARs regulating colorectal tumor growth.
ACKNOWLEDGMENTS
This work is supported, in part, by the National Institutes of Health Grants RO1DK 62112, P01-CA-77839, R37-DK47297, and P30 CA068485 (RND). RND (R37-DK47297) is recipient of an NIH MERIT award. The authors also thank the National Colorectal Cancer Research Alliance (NCCRA) for generous support (RND).
References
- 1.Solomon SD, McMurray JJV, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. New England Journal of Medicine. 2005;352(11):1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 2.Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. New England Journal of Medicine. 2005;352(11):1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 3.Nussmeier NA, Whelton AA, Brown MT, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. New England Journal of Medicine. 2005;352(11):1081–1091. doi: 10.1056/NEJMoa050330. [DOI] [PubMed] [Google Scholar]
- 4.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347(6294):645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 5.Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends in Pharmacological Sciences. 2005;26(5):244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nature Reviews Cancer. 2004;4(1):61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- 7.Kliewer SA, Forman BM, Blumberg B, et al. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(15):7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83(6):841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y-X, Lee C-H, Tiep S, et al. Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell. 2003;113(2):159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka T, Yamamoto J, Iwasaki S, et al. Activation of peroxisome proliferator-activated receptor δ induces fatty acid β-oxidation in skeletal muscle and attenuates metabolic syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(26):15924–15929. doi: 10.1073/pnas.0306981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliver WR, Jr., Shenk JL, Snaith MR, et al. A selective peroxisome proliferator-activated receptor δ agonist promotes reverse cholesterol transport. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(9):5306–5311. doi: 10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans RM, Barish GD, Wang Y-X. PPARs and the complex journey to obesity. Nature Medicine. 2004;10(4):355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 13.Peters JM, Cattley RC, Gonzalez FJ. Role of PPARα in the mechanism of action of the nongenotoxic carcinogen and peroxisome proliferator Wy-14,643. Carcinogenesis. 1997;18(11):2029–2033. doi: 10.1093/carcin/18.11.2029. [DOI] [PubMed] [Google Scholar]
- 14.Gupta RA, Wang D, Katkuri S, Wang H, Dey SK, DuBois RN. Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-δ accelerates intestinal adenoma growth. Nature Medicine. 2004;10(3):245–247. doi: 10.1038/nm993. [DOI] [PubMed] [Google Scholar]
- 15.Wang D, Wang H, Guo Y, et al. Crosstalk between peroxisome proliferator-activated receptor δ and VEGF stimulates cancer progression. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(50):19069–19074. doi: 10.1073/pnas.0607948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grabacka M, Plonka PM, Urbanska K, Reiss K. Peroxisome proliferator-activated receptor α activation decreases metastatic potential of melanoma cells in vitro via down-regulation of Akt. Clinical Cancer Research. 2006;12(10):3028–3036. doi: 10.1158/1078-0432.CCR-05-2556. [DOI] [PubMed] [Google Scholar]
- 17.Strakova N, Ehrmann J, Bartos J, Malikova J, Dolezel J, Kolar Z. Peroxisome proliferator-activated receptors (PPAR) agonists affect cell viability, apoptosis and expression of cell cycle related proteins in cell lines of glial brain tumors. Neoplasma. 2005;52(2):126–136. [PubMed] [Google Scholar]
- 18.Grau R, Punzón C, Fresno M, Iñiguez MA. Peroxisome-proliferator-activated receptor α agonists inhibit cyclo-oxygenase 2 and vascular endothelial growth factor transcriptional activation in human colorectal carcinoma cells via inhibition of activator protein-1. Biochemical Journal. 2006;395(1):81–88. doi: 10.1042/BJ20050964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DuBois RN, Gupta R, Brockman J, Reddy BS, Krakow SL, Lazar MA. The nuclear eicosanoid receptor, PPARγ, is aberrantly expressed in colonic cancers. Carcinogenesis. 1998;19(1):49–53. doi: 10.1093/carcin/19.1.49. [DOI] [PubMed] [Google Scholar]
- 20.Sarraf P, Mueller E, Smith WM, et al. Loss-of-function mutations in PPARγ associated with human colon cancer. Molecular Cell. 1999;3(6):799–804. doi: 10.1016/s1097-2765(01)80012-5. [DOI] [PubMed] [Google Scholar]
- 21.Slattery ML, Curtin K, Wolff R, et al. PPARγ and colon and rectal cancer: associations with specific tumor mutations, aspirin, ibuprofen and insulin-related genes (United States) Cancer Causes and Control. 2006;17(3):239–249. doi: 10.1007/s10552-005-0411-6. [DOI] [PubMed] [Google Scholar]
- 22.Landi S, Moreno V, Gioia-Patricola L, et al. Association of common polymorphisms in inflammatory genes interleukin (IL)6, IL8, tumor necrosis factor α, NFKB1, and peroxisome proliferator-activated receptor γ with colorectal cancer. Cancer Research. 2003;63(13):3560–3566. [PubMed] [Google Scholar]
- 23.Ikezoe T, Miller CW, Kawano S, et al. Mutational analysis of the peroxisome proliferator-activated receptor γ gene in human malignancies. Cancer Research. 2001;61(13):5307–5310. [PubMed] [Google Scholar]
- 24.Altiok S, Xu M, Spiegelman BM. PPARγ induces cell cycle withdrawal: inhibition of E2f/DP DNA-binding activity via down-regulation of PP2A. Genes and Development. 1997;11(15):1987–1998. doi: 10.1101/gad.11.15.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakino S, Kintscher U, Kim S, Yin F, Hsueh WA, Law RE. Peroxisome proliferator-activated receptor γ ligands inhibit retinoblastoma phosphorylation and G1→S transition in vascular smooth muscle cells. Journal of Biological Chemistry. 2000;275(29):22435–22441. doi: 10.1074/jbc.M910452199. [DOI] [PubMed] [Google Scholar]
- 26.Heaney AP, Fernando M, Melmed S. PPAR-γ receptor ligands: novel therapy for pituitary adenomas. The Journal of Clinical Investigation. 2003;111(9):1381–1388. doi: 10.1172/JCI16575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitamura S, Miyazaki Y, Hiraoka S, et al. PPARγ agonists inhibit cell growth and suppress the expression of cyclin D1 and EGF-like growth factors in ras-transformed rat intestinal epithelial cells. International Journal of Cancer. 2001;94(3):335–342. doi: 10.1002/ijc.1470. [DOI] [PubMed] [Google Scholar]
- 28.Qin C, Burghardt R, Smith R, Wormke M, Stewart J, Safe S. Peroxisome proliferator-activated receptor γ agonists induce proteasome-dependent degradation of cyclin D1 and estrogen receptor α in MCF-7 breast cancer cells. Cancer Research. 2003;63(5):958–964. [PubMed] [Google Scholar]
- 29.Toyota M, Miyazaki Y, Kitamura S, et al. Peroxisome proliferator-activated receptor γ reduces the growth rate of pancreatic cancer cells through the reduction of cyclin D1. Life Sciences. 2002;70(13):1565–1575. doi: 10.1016/s0024-3205(01)01524-7. [DOI] [PubMed] [Google Scholar]
- 30.Koga H, Sakisaka S, Harada M, et al. Involvement of p21WAF1/Cip1, p27Kip1, and p18INK4c in troglitazone-induced cell-cycle arrest in human hepatoma cell lines. Hepatology. 2001;33(5):1087–1097. doi: 10.1053/jhep.2001.24024. [DOI] [PubMed] [Google Scholar]
- 31.Gupta RA, Sarraf P, Brockman JA, et al. Peroxisome proliferator-activated receptor γ and transforming growth factor-β pathways inhibit intestinal epithelial cell growth by regulating levels of TSC-22. Journal of Biological Chemistry. 2003;278(9):7431–7438. doi: 10.1074/jbc.M208076200. [DOI] [PubMed] [Google Scholar]
- 32.Satoh T, Toyoda M, Hoshino H, et al. Activation of peroxisome proliferator-activated receptor-γ stimulates the growth arrest and DNA-damage inducible 153 gene in non-small cell lung carcinoma cells. Oncogene. 2002;21(14):2171–2180. doi: 10.1038/sj.onc.1205279. [DOI] [PubMed] [Google Scholar]
- 33.Farrow B, Evers BM. Activation of PPARγ increases PTEN expression in pancreatic cancer cells. Biochemical and Biophysical Research Communications. 2003;301(1):50–53. doi: 10.1016/s0006-291x(02)02983-2. [DOI] [PubMed] [Google Scholar]
- 34.Chen GG, Lee JFY, Wang SH, Chan UPF, Ip PC, Lau WY. Apoptosis induced by activation of peroxisome-proliferator activated receptor-gamma is associated with Bcl-2 and Nf-κB in human colon cancer. Life Sciences. 2002;70(22):2631–2646. doi: 10.1016/s0024-3205(02)01510-2. [DOI] [PubMed] [Google Scholar]
- 35.Xin X, Yang S, Kowalski J, Gerritsen ME. Peroxisome proliferator-activated receptor γ ligands are potent inhibitors of angiogenesis in vitro and in vivo. Journal of Biological Chemistry. 1999;274(13):9116–9121. doi: 10.1074/jbc.274.13.9116. [DOI] [PubMed] [Google Scholar]
- 36.Panigrahy D, Singer S, Shen LQ, et al. PPARγ ligands inhibit primary tumor growth and metastasis by inhibiting angiogenesis. The Journal of Clinical Investigation. 2002;110(7):923–932. doi: 10.1172/JCI15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu H, Zang C, Fenner MH, Possinger K, Elstner E. PPARγ ligands and ATRA inhibit the invasion of human breast cancer cells in vitro. Breast Cancer Research and Treatment. 2003;79(1):63–74. doi: 10.1023/a:1023366117157. [DOI] [PubMed] [Google Scholar]
- 38.Shen D, Deng C, Zhang M. Peroxisome proliferator-activated receptor γ agonists inhibit the proliferation and invasion of human colon cancer cells. Postgraduate Medical Journal. 2007;83(980):414–419. doi: 10.1136/pmj.2006.052761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Girnun GD, Smith WM, Drori S, et al. APC-dependent suppression of colon carcinogenesis by PPARγ . Proceedings of the National Academy of Sciences of the United States of America. 2002;99(21):13771–13776. doi: 10.1073/pnas.162480299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inoue H, Tanabe T, Umesono K. Feedback control of cyclooxygenase-2 expression through PPARγ . Journal of Biological Chemistry. 2000;275(36):28028–28032. doi: 10.1074/jbc.M001387200. [DOI] [PubMed] [Google Scholar]
- 41.Quraishi O, Mancini JA, Riendeau D. Inhibition of inducible prostaglandin E2 synthase by 15-deoxy-Δ12,14-prostaglandin J2 and polyunsaturated fatty acids. Biochemical Pharmacology. 2002;63(6):1183–1189. doi: 10.1016/s0006-2952(02)00844-4. [DOI] [PubMed] [Google Scholar]
- 42.Schröder O, Yudina Y, Sabirsh A, Zahn N, Haeggström JZ, Stein J. 15-deoxy-Δ12,14-prostaglandin J2 inhibits the expression of microsomal prostaglandin E synthase type 2 in colon cancer cells. Journal of Lipid Research. 2006;47(5):1071–1080. doi: 10.1194/jlr.M600008-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Sarraf P, Mueller E, Jones D, et al. Differentiation and reversal of malignant changes in colon cancer through PPARγ . Nature Medicine. 1998;4(9):1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 44.Osawa E, Nakajima A, Wada K, et al. Peroxisome proliferator-activated receptor γ ligands suppress colon carcinogenesis induced by azoxymethane in mice. Gastroenterology. 2003;124(2):361–367. doi: 10.1053/gast.2003.50067. [DOI] [PubMed] [Google Scholar]
- 45.Lefebvre A-M, Chen I, Desreumaux P, et al. Activation of the peroxisome proliferator-activated receptor γ promotes the development of colon tumors in C57BL/6J-APCMin/+ mice. Nature Medicine. 1998;4(9):1053–1057. doi: 10.1038/2036. [DOI] [PubMed] [Google Scholar]
- 46.Saez E, Tontonoz P, Nelson MC, et al. Activators of the nuclear receptor PPARγ enhance colon polyp formation. Nature Medicine. 1998;4(9):1058–1061. doi: 10.1038/2042. [DOI] [PubMed] [Google Scholar]
- 47.Pino MV, Kelley MF, Jayyosi Z. Promotion of colon tumors in C57BL/6J-APCmin/+ mice by thiazolidinedione PPARγ agonists and a structurally Unrelated PPARγ agonist. Toxicologic Pathology. 2004;32(1):58–63. doi: 10.1080/01926230490261320. [DOI] [PubMed] [Google Scholar]
- 48.Yang K, Fan K-H, Lamprecht SA, et al. Peroxisome proliferator-activated receptor γ agonist troglitazone induces colon tumors in normal C57BL/6J mice and enhances colonic carcinogenesis in APC1638N/+ M l h1+/− double mutant mice. International Journal of Cancer. 2005;116(4):495–499. doi: 10.1002/ijc.21018. [DOI] [PubMed] [Google Scholar]
- 49.Niho N, Takahashi M, Kitamura T, et al. Concomitant suppression of hyperlipidemia and intestinal polyp formation in Apc-deficient mice by peroxisome proliferator-activated receptor ligands. Cancer Research. 2003;63(18):6090–6095. [PubMed] [Google Scholar]
- 50.Niho N, Takahashi M, Shoji Y, et al. Dose-dependent suppression of hyperlipidemia and intestinal polyp formation in Min mice by pioglitazone, a PPARγ ligand. Cancer Science. 2003;94(11):960–964. doi: 10.1111/j.1349-7006.2003.tb01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McAlpine CA, Barak Y, Matise I, Cormier RT. Intestinal-specific PPARγ deficiency enhances tumorigenesis in APMin/+ mice. International Journal of Cancer. 2006;119(10):2339–2346. doi: 10.1002/ijc.22115. [DOI] [PubMed] [Google Scholar]
- 52.Suh N, Wang Y, Williams CR, et al. A new ligand for the peroxisome proliferator-activated receptor-γ (PPAR-γ), GW7845, inhibits rat mammary carcinogenesis. Cancer Research. 1999;59(22):5671–5673. [PubMed] [Google Scholar]
- 53.Saez E, Rosenfeld J, Livolsi A, et al. PPARγ signaling exacerbates mammary gland tumor development. Genes and Development. 2004;18(5):528–540. doi: 10.1101/gad.1167804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lim H, Gupta RA, Ma W-G, et al. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARδ . Genes and Development. 1999;13(12):1561–1574. doi: 10.1101/gad.13.12.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee C-H, Chawla A, Urbiztondo N, Liao D, Boisvert WA, Evans RM. Transcriptional repression of atherogenic inflammation: modulation by PPARδ . Science. 2003;302(5644):453–457. doi: 10.1126/science.1087344. [DOI] [PubMed] [Google Scholar]
- 56.Hao C-M, Redha R, Morrow J, Breyer MD. Peroxisome proliferator-activated receptor δ activation promotes cell survival following hypertonic stress. Journal of Biological Chemistry. 2002;277(24):21341–21345. doi: 10.1074/jbc.M200695200. [DOI] [PubMed] [Google Scholar]
- 57.Di-Poï N, Tan NS, Michalik L, Wahli W, Desvergne B. Antiapoptotic role of PPARβ in keratinocytes via transcriptional control of the Akt1 signaling pathway. Molecular Cell. 2002;10(4):721–733. doi: 10.1016/s1097-2765(02)00646-9. [DOI] [PubMed] [Google Scholar]
- 58.Di-Poï N, Michalik L, Tan NS, Desvergne B, Wahli W. The anti-apoptotic role of PPARβ contributes to efficient skin wound healing. Journal of Steroid Biochemistry and Molecular Biology. 2003;85(2-5):257–265. doi: 10.1016/s0960-0760(03)00215-2. [DOI] [PubMed] [Google Scholar]
- 59.He T-C, Chan TA, Vogelstein B, Kinzler KW. PPARδ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99(3):335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gupta RA, Tan J, Krause WF, et al. Prostacyclin-mediated activation of peroxisome proliferator-activated receptor δ in colorectal cancer. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(24):13275–13280. doi: 10.1073/pnas.97.24.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takayama O, Yamamoto H, Damdinsuren B, et al. Expression of PPARδ in multistage carcinogenesis of the colorectum: implications of malignant cancer morphology. British Journal of Cancer. 2006;95(7):889–895. doi: 10.1038/sj.bjc.6603343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vanamala J, Glagolenko A, Yang P, et al. Dietary fish oil and pectin enhance colonocyte apoptosis in part through suppression of PPARδ/PGE2 and elevation of PGE3. Carcinogenesis. 2008;29(4):790–796. doi: 10.1093/carcin/bgm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ouyang N, Williams JL, Rigas B. NO-donating aspirin isomers downregulate peroxisome proliferator-activated receptor (PPAR)δ expression in APCmin/+ mice proportionally to their tumor inhibitory effect: implications for the role of PPARδ in carcinogenesis. Carcinogenesis. 2006;27(2):232–239. doi: 10.1093/carcin/bgi221. [DOI] [PubMed] [Google Scholar]
- 64.Siezen CLE, Tijhuis MJ, Kram NR, et al. Protective effect of nonsteroidal anti-inflammatory drugs on colorectal adenomas is modified by a polymorphism in peroxisome proliferator-activated receptor δ . Pharmacogenetics and Genomics. 2006;16(1):43–50. doi: 10.1097/01.fpc.0000182778.03180.f3. [DOI] [PubMed] [Google Scholar]
- 65.Shao J, Sheng H, DuBois RN. Peroxisome proliferator-activated receptors modulate K-Ras-mediated transformation of intestinal epithelial cells. Cancer Research. 2002;62(11):3282–3288. [PubMed] [Google Scholar]
- 66.Wang D, Wang H, Shi Q, et al. Prostaglandin E2 promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor δ . Cancer Cell. 2004;6(3):285–295. doi: 10.1016/j.ccr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 67.Xu L, Han C, Wu T. A novel positive feedback loop between peroxisome proliferator-activated receptor-δ and prostaglandin E2 signaling pathways for human cholangiocarcinoma cell growth. Journal of Biological Chemistry. 2006;281(45):33982–33996. doi: 10.1074/jbc.M600135200. [DOI] [PubMed] [Google Scholar]
- 68.Xu L, Han C, Lim K, Wu T. Cross-talk between peroxisome proliferator-activated receptor δ and cytosolic phospholipase A2 α/cyclooxygenase-2/prostaglandin E2 signaling pathways in human hepatocellular carcinoma cells. Cancer Research. 2006;66(24):11859–11868. doi: 10.1158/0008-5472.CAN-06-1445. [DOI] [PubMed] [Google Scholar]
- 69.Park BH, Vogelstein B, Kinzler KW. Genetic disruption of PPARδ decreases the tumorigenicity of human colon cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(5):2598–2603. doi: 10.1073/pnas.051630998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barak Y, Liao D, He W, et al. Effects of peroxisome proliferator-activated receptor δ on placentation, adiposity, and colorectal cancer. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(1):303–308. doi: 10.1073/pnas.012610299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harman FS, Nicol CJ, Marin HE, Ward JM, Gonzalez FJ, Peters JM. Peroxisome proliferator-activated receptor-δ attenuates colon carcinogenesis. Nature Medicine. 2004;10(5):481–483. doi: 10.1038/nm1026. [DOI] [PubMed] [Google Scholar]
- 72.Reed KR, Sansom OJ, Hayes AJ, et al. PPARδ status and Apc-mediated tumourigenesis in the mouse intestine. Oncogene. 2004;23(55):8992–8996. doi: 10.1038/sj.onc.1208143. [DOI] [PubMed] [Google Scholar]
- 73.Marin HE, Peraza MA, Billin AN, et al. Ligand activation of peroxisome proliferator-activated receptor β inhibits colon carcinogenesis. Cancer Research. 2006;66(8):4394–4401. doi: 10.1158/0008-5472.CAN-05-4277. [DOI] [PubMed] [Google Scholar]
- 74.Nadra K, Anghel SI, Joye E, et al. Differentiation of trophoblast giant cells and their metabolic functions are dependent on peroxisome proliferator-activated receptor β/δ . Molecular and Cellular Biology. 2006;26(8):3266–3281. doi: 10.1128/MCB.26.8.3266-3281.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peters JM, Lee SST, Li W, et al. Growths, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor β(δ) Molecular and Cellular Biology. 2000;20(14):5119–5128. doi: 10.1128/mcb.20.14.5119-5128.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stephen RL, Gustafsson MCU, Jarvis M, et al. Activation of peroxisome proliferator-activated receptor δ stimulates the proliferation of human breast and prostate cancer cell lines. Cancer Research. 2004;64(9):3162–3170. doi: 10.1158/0008-5472.can-03-2760. [DOI] [PubMed] [Google Scholar]
- 77.Glinghammar B, Skogsberg J, Hamsten A, Ehrenborg E. PPARδ activation induces COX-2 gene expression and cell proliferation in human hepatocellular carcinoma cells. Biochemical and Biophysical Research Communications. 2003;308(2):361–368. doi: 10.1016/s0006-291x(03)01384-6. [DOI] [PubMed] [Google Scholar]
- 78.Daikoku T, Tranguch S, Chakrabarty A, et al. Extracellular signal-regulated kinase is a target of cyclooxygenase-1-peroxisome proliferator-activated receptor-δ signaling in epithelial ovarian cancer. Cancer Research. 2007;67(11):5285–5292. doi: 10.1158/0008-5472.CAN-07-0828. [DOI] [PubMed] [Google Scholar]
- 79.Abdollahi A, Schwager C, Kleeff J, et al. Transcriptional network governing the angiogenic switch in human pancreatic cancer. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(31):12890–12895. doi: 10.1073/pnas.0705505104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yin Y, Russell RG, Dettin LE, et al. Peroxisome proliferator-activated receptor δ and γ agonists differentially alter tumor differentiation and progression during mammary carcinogenesis. Cancer Research. 2005;65(9):3950–3957. doi: 10.1158/0008-5472.CAN-04-3990. [DOI] [PubMed] [Google Scholar]