Abstract
Background:
Robotic-assisted laparoscopic radical prostatectomy (RALRP) has gained popularity in the United States due to claims of its superior 3-dimensional magnified vision and improved manual dexterity for surgeons that shorten the learning curve and facilitate the transition from standard open radical prostatectomy to laparoscopic prostatectomy as a minimally invasive procedure. The Canadian health care system, however, faces unique challenges when dealing with the introduction of new technologies. We report the initial experience with the use of the da Vinci robot for RALRP at the University of Western Ontario.
Methods:
We retrospectively reviewed the records of the initial 30 cases of RALRP with a minimum of 6 months follow-up. Data included the surgical times of various operative segments from cases 1–15 and 16–30, perioperative complications, early oncology and early functional results.
Results:
The lack of dedicated resources initially led to sporadic and infrequent cases. Nevertheless, there was improvement in surgical proficiency with significant difference in operative times between cases 1–15 and 16–30. Perioperative complications, though significant, were commensurate with reported early experiences from other centres worldwide, which reflects the learning curve with RALRP.
Conclusion:
Initiating a new surgical program that involves significant capital and maintenance costs, such as an RALRP program, within the Canadian health care system poses unique challenges for the surgical team. Nevertheless, our initial experience has encouraged us to proceed with the next phase of evaluation for the urological and oncological application of the technology.
Laparoscopic radical prostatectomy, first described in the early 1990s,1 was popularized by several European groups,2–4 with the main impetus being to decrease operative morbidity while upholding principles of oncological therapy and theoretically minimizing long-term morbidity, such as incontinence and erectile dysfunction.
Surgical robotics have improved laparoscopic surgical proficiency by:
providing superior 3-dimensional “up close” magnification and an unparalleled view of the deep pelvis and retropubic space;
facilitating surgical manipulation with superior dexterity via “wristed motions” and increased degrees of surgical freedom; and
improving precision of dissection by tremor filtration and movement scaling.5
The pioneering efforts of some US centres using the da Vinci surgical robot (Intuitive Surgical, Sunnyvale, Calif.) have popularized robotic-assisted laparoscopic radical prostatectomy (RALRP),5,6 reporting a significantly shortened learning curve for laparoscopic radical prostatectomy and facilitating the “transition” from open radical prostatectomy to RALRP.7
Although several Canadian centres are routinely performing laparoscopic prostatectomy, with our health care budgetary constraints, only 2 centres in Canada are currently equipped with a da Vinci robot capable of performing RALRP. In contrast, with the US health care system and the marketing initiatives of various centres, there has been a proliferation of da Vinci robots in the United States, with an exponential increase in the number of RALRPs performed in the past few years. Despite the numerous reports suggesting the superiority of RALRP over open approaches, there has not been high-level evidence to support this contention. Our objective was to establish a RALRP program at the University of Western Ontario, with the initial step of conducting a technical feasibility study of 30 cases, possibly as a lead-in to a Phase III randomized study between RALRP and standard open radical retropubic prostatectomy for clinical localized prostate cancer. Herein, we report the first short-term Canadian experience with RALRP, and we examine the challenges of implementing this technology in Canada.
Methods and Materials
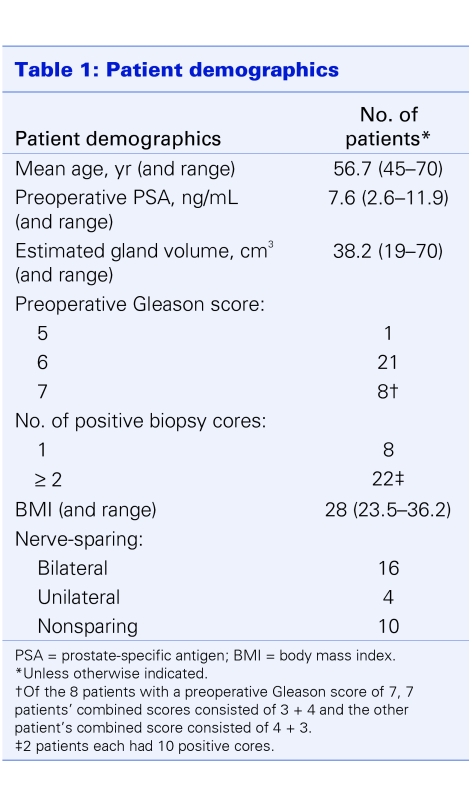
We established a feasibility study protocol and received local institutional review board approval. We obtained patient consent according to the research protocol. The robotic surgical team members (both “console” and “bedside laparoscopic” surgeons and nursing personnel) underwent formal approved training in the use of the da Vinci robot as well as the RALRP surgical procedure at the University of California at Irvine. The 2 console surgeons have fellowship training in urological oncology; one is primarily an open surgeon with 20 years of experience and a large oncology practice, and the other surgeon has significant laparoscopic experience. We prospectively collected data for the first 30 RALRP cases with a minimum of 6 months follow-up. Patient demographics are listed in Table 1.
Table 1.
Surgical technique
The patients were placed in Trendelenburg position after anesthesia induction. All potential pressure points were protected with foam padding. The robot was parked between the abducted, slightly flexed legs supported by stirrups. Pneumoperitoneum was maintained throughout the procedure (15 mmHg). The camera port (12 mm), 2 robotic arm ports (8 mm) and 2 additional working ports (12 mm, for retraction, suction, and introduction and cutting of sutures) were carefully planned according to the geometry of the anatomic landmarks as per the group at the University of California at Irvine.8 We employed the transperitoneal antegrade approach with early division of the bladder neck. For the purpose of documenting our initial experience, the surgical procedure was divided into several segments. Bipolar cautery grasping forceps, monopolar spatula, “hot and cold” scissors with monopolar cautery capability and harmonic scalpel (Intuitive Surgical, Sunnyvale Calif.) were used for dissection. The dorsal venous complex was typically controlled with a 1 or 2-0 Vicryl suture. The vascular pedicle was controlled either with Hem-O-Loc clips (Pilling Weck Canada Ltd., Markham, Ont.) or the harmonic scalpel. The urethral–bladder neck anastomosis was completed with 2, 3.0 Monocryl absorbable sutures (one dyed and one undyed, tied together onto a small pledget of surgicel [Johnson and Johnson Canada Ltd., Markham, Ont.] to form an easily identifiable “double-ended” suture) as a running end-to-end anastomosis. After satisfactory completion of the anastomosis, the en bloc prostate and seminal vesicles specimen, which was placed in a laparoscopic entrapment bag, was removed through the slightly extended periumbilical port incision. A pelvic drain was left in all cases and typically removed on postoperative day 2, if there was no evidence of anastamotic leak.
Results
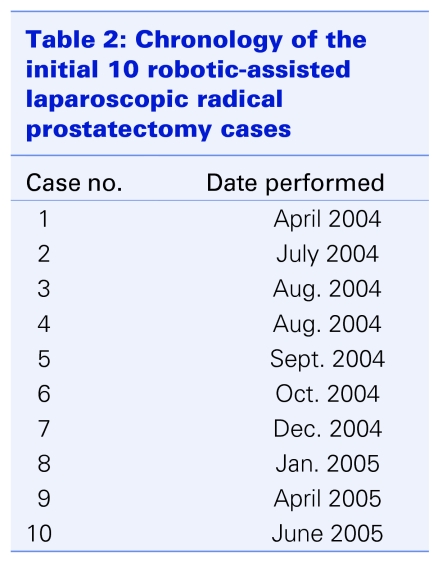
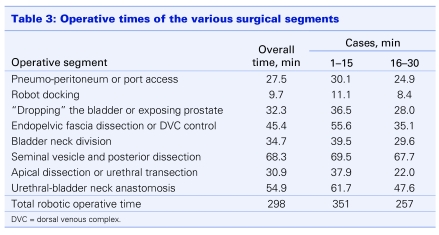
Owing to local logistic and resource limitations, especially in the early stages, cases were performed infrequently. To illustrate the frustratingly sporadic nature of the initial cases, the chronology of the first 10 cases is listed in Table 2. The mean operative times for various segments of the procedure are listed in Table 3, separately reported as “overall,” for all 30 cases“; cases 1–15”; and “cases the 16–30.” There were 3 open conversions, all owing to failure to progress. After the first 4 cases, we set a policy for open conversion if there was obvious failure to progress. The maximum time limit for open conversion was set at 5 hours. The 3 cases were converted at 175, 230 and 301 minutes, respectively. One of the cases was also complicated by the onset of hypercarbia, leading to an earlier conversion. Excluding the 3 converted cases from the calculations of robotic operative times, the mean surgery times improved 351 minutes for cases 1–15, 257 minutes for cases 16–30, with an average mean of 298 minutes (range 203–710 min). The corresponding median operative times for cases 1–15 and cases 16–30 were 310 minutes and 295 minutes, respectively.
Table 2.
Table 3.
The complication rate was fairly significant 3 patients suffered transient brachial neurapraxia due to the exaggerated Trendelenburg position and prolonged procedure in the earlier patients; 1 patient developed a pelvic hematoma (10-cm diameter that spontaneously resolved); 2 patients had transient renal dysfunction with acute tubular necrosis; 2 demonstrated a transient anastomotic leak; 1 patient had a catheter blockage problem and underwent open exploration and suprapubic cystostomy in the immediate postoperative period; and upon removal of the pelvic drain, 1 patient developed an omental hernia that required immediate repair.
The mean estimated blood loss was 385 mL The mean postoperative hemoglobin level was 108 g/L (range 84–139 g/L). No patients received tranfusions. The median hospital stay was 3.5 (range 2–6) days. There was no deliberate effort toward early catheter removal and thus the mean duration of indwelling catheter was 12 days. Postoperative cystogram was not routinely performed. We did not employ a clinical care pathway specific for robotic prostatectomy in terms of analgesia management or diet progression.
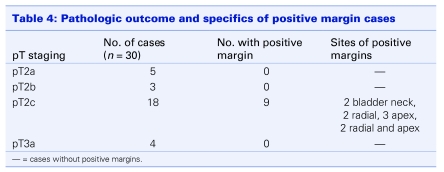
The pathological outcomes are listed in Table 4. A positive surgical resection margin was seen in 9 of the 30 patients (2 bladder neck, 2 radial, 3 apex and, 2 radial and apex). All 9 cases were in patients with pT2 disease. Two patients with pT3a disease and 2 patients with pT2c disease underwent adjuvant radiotherapy. With a short median follow-up of only 9 months, all patients had an undetectable postoperative serum prostate specific antigen level (< 0.05 ng/dL).
Table 4.
In terms of functional results, 3 patients had moderate stress incontinence (1 patient requiring 3–4 pads daily, 2 patients requiring 2–3 pads daily) with strenuous activities at 18 months postsurgery. Twenty-one patients did not need pads, and 6 patients used 1 pad or fewer daily. International Index of Erectile Function follow-up data scores are incomplete for erectile function analysis and will be the subject of a follow-up report.
Discussion
Although the use of the da Vinci robot has been advocated as a means to shorten the time and to the decrease the number of cases required to achieve functionality and surgical proficiency in laparoscopic prostatectomy, there is still a significant learning curve with RALRP.7,9 Patel and colleagues estimated that 20–25 cases were required to achieve technical proficiency with RALRP.10 Herrell and Smith, who have vast experience with open radical retropubic prostatectomy and, more recently, RALRP procedures, define achieving a “comfort” level at 150 cases and an “expert” level at 250 cases.11,12 Ahlering and colleagues observed their RALRP operative times continually declining until case 19 and then they were essentially maintained at approximately that nadir level.7
By virtue of our geographic proximity to the United States and the ubiquity of Internet information, Canadian patients are especially aware of new surgical technologies such as the RALRP. However, in spite of the urological literature being replete with opinions and reports of results from leaders and champions of robotic surgery in the United States, we have to evaluate the feasibility of the implementation of such a new technology in a very different Canadian health care environment. Patients are often less aware of the fact that our health care system, with restricted budgetary and resource allocation by the government, poses significant challenges for health care providers hoping to introduce expensive new technologies, such as robotic surgery, to the system. For the neophyte surgeon, the inevitably longer surgical times with the initial RALRP procedures pose an additional problem for his or her clinical practice long wait-times for surgery and long waiting lists will be exacerbated. The limited resources meant only a small number of cases could be treated over a relatively lengthy period with a sporadic schedule (Table 2). This severely hindered improvement in technical skills and accumulation of clinical experience. The hospital resource issue common to all Canadian health care institutions has yet to be resolved, although we have been able to perform more cases in the past year and now have experience in 80 cases.
Currently, operative time, both in pure laparoscopic and robotic-assisted procedures, has been used as an objective surrogate for the learning curve associated with the procedure7,10,11 and has consistently been shown to decrease as surgical experience is gained.10,13,14 Despite the small number of cases, improvement in mean operative times of the various segments of the procedure (including anesthesia time) have been noted. Sim and colleagues reported a comparable experience with their initial 17 cases performed in Singapore over a 10-month period.15 They have subsequently reported their 2-year experience with 100 patients, demonstrating acceleration of improvement in technical proficiency as well as surgical outcomes using a team approach.16 Our initial mean surgical times improved substantially between the first 15 and subsequent 15 cases. The median surgical times, avoiding large distortion by the extreme data points (e.g., one case lasted 720 min), still show the central tendency toward improvement in operative times, albeit less dramatic than the mean operative times.
An integral component of the learning curve for laparoscopic prostatectomy, with or without robotic assistance, is the complication rate. Based on the experience of others and on our own experience, in addition to ongoing appraisals and technical modifications, there are adjustments to patient selection criteria, to preoperative assessment and to patient positioning. We also recommend setting a reasonable upper time limit for open conversion (e.g., 5 h) if there is a definite lack of progression. The high margin positivity rate in these largely low-risk patients (all 9 cases were patients with pT2 disease) reflects our early lack of experience, which should improve with further experience and ongoing critical self-appraisal.4,17,18 Atug and colleagues reported significant improvement in surgical margin positivity from 45.4% to 11.7% for their first 33 cases, compared with cases 67 to 100.19 Although the da Vinci robot provides technical advantages in terms of manual dexterity and magnified 3-dimensional vision, it does not provide a novice with “instant” laparoscopic capabilities and proficiency in terms of tissue plane recognition; however, the learning curve should be abbreviated.7 For the Canadian centres embarking on an RALRP program, one can expect longer initial surgical times and higher complication rates. Recruiting a robotic fellowship trained individual would alleviate part of the problem, although there is still a significant learning curve for the nursing and anesthesia teams. Formal mentorship, and possibly telementoring, is another alternative strategy.20
By design, we were not aggressive with early discharge or catheter removal in this early group of patients. Many of the patients could have been discharged 1 or 2 days earlier and would have achieved some cost savings. With more experience and proficiency, earlier discharge should be routine. Nevertheless, in their cost analysis, Lotan and colleagues concluded that, even with a shorter hospital stay, RALRP did not compensate for the high initial capital costs and subsequent maintenance expenditures.21
Conclusion
There are significant obstacles to initiating a new surgical program with an expensive new technology, especially in the resource-restricted Canadian health care system. The early results and complication rates in the initial phase of the first Canadian RALRP program are commensurate with those at other centres, even with the additional challenge of sporadic patient scheduling and the lack of dedicated resources. Despite the obstacles within the context of the Canadian health care system, our early short-term results and patient satisfaction are sufficiently encouraging for us to persevere with RALRP, and we have now collectively acquired experience in over 100 cases.
Footnotes
This article has been peer reviewed.
Competing interests: None declared.
References
- 1.Schuessler WW, Schulam PG, Clayman RV, et al. Laparoscopic radical prostatectomy initial short-term experience. Urology 1997;50:854-7. [DOI] [PubMed] [Google Scholar]
- 2.Guillonneau B, Vallancien G. Laparoscopic radical prostatectomy the montsouris experience. J Urol 2000;163:418-22. [DOI] [PubMed] [Google Scholar]
- 3.Abbou CC, Salomon L, Hoznek A, et al. Laparoscopic radical prostatectomy preliminary results. Urology 2000;55:630-3. [DOI] [PubMed] [Google Scholar]
- 4.Rassweiler J, Sentker L, Seemann O, et al. Laparaoscopic radical prostatectomy with the Heilbronn technique an analysis of the first 180 cases. J Urol 2001;166:2101-5. [PubMed] [Google Scholar]
- 5.Menon M, Tewari A. Vattikuti Institute Prostatectomy Team Robotic radical prostatectomy and the Vattikuti Urology Institute Technique an interim analysis of results and technical points. Urology 2003;61:15-20. [DOI] [PubMed] [Google Scholar]
- 6.Menon M, Tewari A, Peabody JO, et al. Vattikuti Institute prostatectomy, a technique of robotic radical prostatectomy for management of localized carcinoma of the prostate experience of over 1100 cases. Urol Clin North Am 2004;31:701-17. [DOI] [PubMed] [Google Scholar]
- 7.Ahlering TE, Skarecky A, Lee D, et al. Successful transfer of open surgical skills to a laparoscopic environment using a robotic interface initial experience with laparoscopic radical prostatectomy. J Urol 2003;170:1738-41. [DOI] [PubMed] [Google Scholar]
- 8.Pick DL, Lee DL, Skarecky DW, et al. Anatomic guide for port placement for da Vinci robotic radical prostatectomy. J Endourol 2004;18:572-5. [DOI] [PubMed] [Google Scholar]
- 9.Ahlering TE, Woo D, Eichel L, et al. Robot-assisted versus open radical prostatectomy a comparison of one surgeon's outcomes. Urology 2004;63:819-23. [DOI] [PubMed] [Google Scholar]
- 10. Patel VR, Tully AS, Holmes R, et al. Robotic radical prostatectomy in the community setting – the learning curve and beyond initial 200 cases. J Urol 2005;174:269-72 [DOI] [PubMed] [Google Scholar]
- 11.Herrell SD, Smith JA Jr. Robotic-assisted laparoscopic prostatectomy What is the learning curve? Urology 2005;66:105-7. [DOI] [PubMed] [Google Scholar]
- 12.Smith JA Jr, Herrell SD. Robotic-assisted laparoscopic prostatectomy Do minimally invasive approaches offer significant advantages? J Clin. Oncol 2005;23:8170-5. [DOI] [PubMed] [Google Scholar]
- 13.Turk I, Deger S, Winkelamann B, et al. Laparoscopic radical prostatectomy technical aspects and experience with 125 cases. Eur Urol 2001;40:46-53. [DOI] [PubMed] [Google Scholar]
- 14.Dasgupta P, Jones A, Gill IS. Robotic urological surgery a perspective. BJU Int 2005;95:20-3. [DOI] [PubMed] [Google Scholar]
- 15.Sim HG, Yip SK, Lau WK, et al. Early experience with robotic assisted laparoscopic radical prostatectomy. Asian J Surg 2004;27:321-5. [DOI] [PubMed] [Google Scholar]
- 16.Sim HG, Yip SK, Lau WK, et al. Team-based approach reduces learning curve in robot-assisted laparoscopic radical prostatectomy. Int J Urol 2006;13:560-4. [DOI] [PubMed] [Google Scholar]
- 17.Touijer K, Kuroiwa K, Saranchuk JW, et al. Quality improvement in laparoscopic radical prostatectomy for pT2 prostate cancer impact of video documentation review on positive surgical margin. J Urol 2005;173:765-8. [DOI] [PubMed] [Google Scholar]
- 18.Ahlering TE, Eichel L, Edwards RA, et al. Robotic radical prostatectomy a technique to reduce pT2 positive margins. Urology 2004;64:1224-8. [DOI] [PubMed] [Google Scholar]
- 19.Atug F, Castle EP, Srivastav SK, et al. Positive surgical margins in robotic-assisted radical prostatectomy impact of learning curve on oncologic outcomes. [Discussion 871-2]. Eur Urol 2006;49:866-71. [DOI] [PubMed] [Google Scholar]
- 20.Costello AJ, Haxhimolla H, Crowe H, et al. Installation of telerobotic surgery and initial experience with telerobotic radical prostatectomy. BJU Int 2005;96:34-8. [DOI] [PubMed] [Google Scholar]
- 21.Lotan Y, Cadeddu JA, Gettman MT. The new economics of radical prostatectomy cost comparison of open, laparoscopic and robot assisted techniques. J Urol 2004;172143:1-5. [DOI] [PubMed] [Google Scholar]