Abstract
Numerous studies have shown that neuronal plasticity in the hippocampus and neocortex is regulated by estrogen and that aromatase, the key enzyme for estrogen biosynthesis, is present in cerebral cortex. Although the expression pattern of aromatase mRNA has been described in the monkey brain, its precise cellular distribution has not been determined. In addition, the degree to which neuronal aromatase is affected by gonadal estrogen has not been investigated. In this study, we examined the immunohistochemical distribution of aromatase in young ovariectomized female rhesus monkeys with or without long-term cyclic estradiol treatment. Both experimental groups showed that aromatase is localized in a large population of CA1-3 pyramidal cells, in granule cells of the dentate gyrus and in some interneurons in which it was co-expressed with the calcium binding proteins calbindin, calretinin, and parvalbumin. Moreover, numerous pyramidal cells were immunoreactive for aromatase in the neocortex, whereas only small subpopulations of neocortical interneurons were immunoreactive for aromatase. The widespread expression of the protein in a large neuronal population suggests that local intraneuroral estrogen synthesis may contribute to estrogen-induced synaptic plasticity in monkey hippocampus and neocortex of female rhesus monkeys. In addition, the apparent absence of obvious differences in aromatase distribution between the two experimental groups suggests that these localization patterns are not dependent on plasma estradiol levels.
Keywords: aromatase, estrogen, hippocampus, interneuron, neocortex, pyramidal cells
The brain is an important target for gonadal hormones, including estradiol (DeVoogd and Nottebohm, 1981; Arnold and Gorski, 1984; Gould et al., 1990; Galea et al., 2006; Parducz et al., 2006). The brain also expresses several steroidogenic enzymes (Stoffel-Wagner, 2001), such as aromatase, which catalyzes the conversion of androgens into estrogens, the last step in estrogen biosynthesis (Stoffel-Wagner, 2001). Following the pioneering work of Naftolin and collaborators (Naftolin et al., 1971), who demonstrated aromatase activity in the human fetal brain, numerous studies have characterized the expression and distribution of the enzyme in the developing and adult central nervous system of several vertebrates species (Ryan et al., 1972; Flores et al., 1973; Naftolin et al., 1975; Roselli et al., 1985; Schumacher and Balthazart, 1987; Jakab et al., 1993; Shinoda et al., 1994).
Circulating estradiol exerts multiple effects in the neocortex and the hippocampus, including morphologic and functional changes in cortical circuits (Gould et al., 1990; McEwen et al., 1995; Woolley, 1998; Foy et al., 1999; Adams et al., 2001; McEwen, 2002; Hao et al., 2003; Amin et al., 2005; Frye et al., 2005; Hao et al., 2006; Hao et al., 2007), regulation of adult hippocampal neurogenesis (Ormerod and Galea, 2001; Galea et al., 2006; Suzuki et al., 2007) and induction of neuroprotection from stroke and certain neurodegenerative processes (Azcoitia et al., 1998; Wise et al., 2000; Garcia-Segura et al., 2001; Kugaya et al., 2003; Rau et al., 2003; Yang et al., 2003). Recent findings indicate that local estradiol synthesis by aromatase may also affect cortical development, synaptic plasticity, and synaptic function (Sakamoto et al., 2003; Kretz et al., 2004; Leranth et al., 2004; Hojo et al, 2004; Murakami et al., 2006) and may contribute to endogenous neuroprotective mechanisms (Azcoitia et al., 2001; Azcoitia et al., 2003; Garcia-Segura et al., 2003; Sierra et al., 2003; Veiga et al., 2003; Wynne and Saldanha, 2004).
Both local formation of estradiol and aromatase expression have been reported in the hippocampus of several mammalian species, including mice (Garcia-Segura et al., 1999; Ivanova and Beyer, 2000), rats (Sanghera et al., 1991; Garcia-Segura et al., 1999; Wehrenberg et al., 2001; Hojo et al., 2004; Rune and Frotscher, 2005) and humans (Sasano et al. 1998; Stoffel-Wagner et al., 1999). Aromatase activity and mRNA expression using in situ hybridization have been analyzed in the monkey hippocampus (MacLusky et al., 1986; Yamada-Mouri et al., 1995; Wehrenberg et al., 2001). In addition, we have recently analyzed the expression of aromatase in the human temporal cortex by RT-PCR and immunohistochemistry (Yague et al., 2006). These findings suggest that the enzyme is present in a high number of neurons, especially in pyramidal neurons, and subpopulations of astrocytes (Yague et al., 2006). However, there is no data on the precise distribution of aromatase in the different populations of hippocampal and neocortical cells in the monkey cerebral cortex.
Although estradiol may show neuroprotective functions and regulates synaptic plasticity (Gould et al., 1990; Woolley, 1998; Azcoitia et al., 1999; Foy et al., 1999; Veiga et al., 2004), postmenopausal alterations in affective and cognitive behaviors are highly variable in women despite a marked drop in circulating estradiol. This suggests in some cases that local estradiol synthesis in the brain may compensate for the hormonal loss in circulation. Also, previous studies of the rat diencephalon showed that the treatment of ovariectomized (OVX) female rats with estradiol provoked a decrease in the aromatase mRNA expression, whereas the treatment of OVX rats with testosterone increased the aromatase mRNA expression in this brain region (Yamada et al., 1993). Thus, we assessed the cellular pattern of aromatase expression in the temporal neocortex and the hippocampus of OVX female rhesus monkeys that were submitted to a cyclic estradiol treatment to determine whether long-term cyclic changes in circulating estradiol may modify aromatase expression in these brain areas in females.
RESULTS
Aromatase in the hippocampus
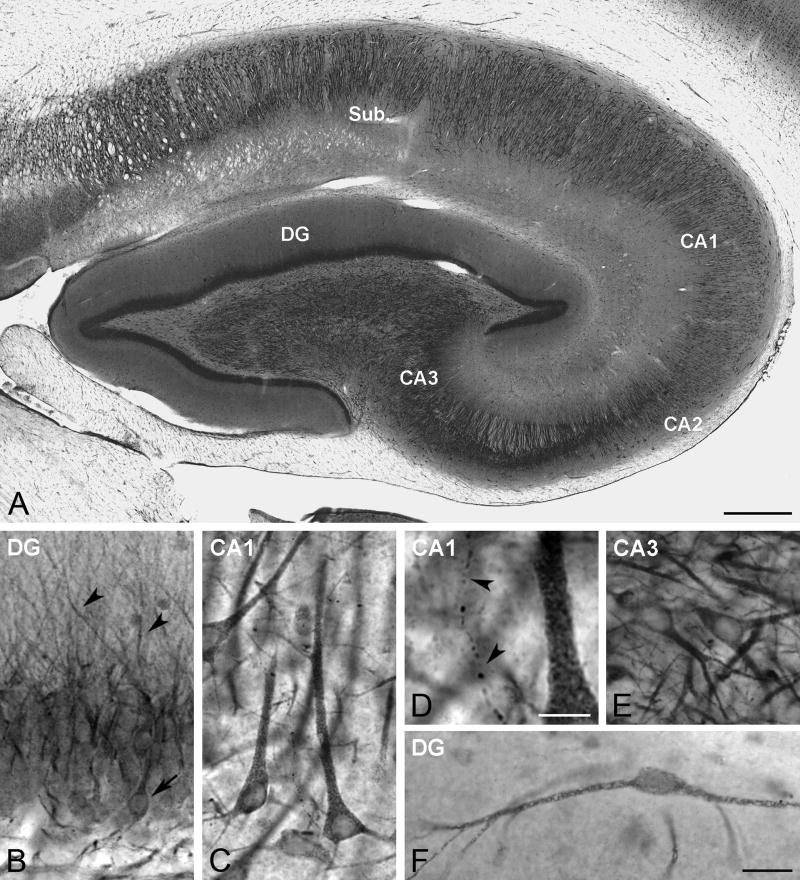
While we did not carry out detailed quantitative analyses of levels of immunoreactivity or number of labeled neurons, the pattern, extent, and intensity of aromatase immunostaining in the hippocampus was similar in all animals studied, regardless of treatment, suggesting that the presence or absence of circulating estradiol does not have obvious effects on aromatases expression or location. Aromatase-immunoreactive neurons were detected in different hippocampal regions, including the dentate gyrus and the stratum pyramidale of CA1-3 (Fig. 1). Neuronal cell nuclei were never immunostained (Figs. 1–3). Granule cells in the dentate gyrus (DG) showed aromatase immunoreactivity distributed mostly along the apical dendrites that reached the molecular layer (Figs. 1B, 2A). Only a few granule cells showed a well defined immunoreactive perikaryon (Fig. 1B). This compartimentalization of aromatase immunoreactivity in granule cells was clearly visualized after double immunostaining of aromatase and the neuronal marker NeuN (Fig. 2A).
Fig. 1.
Aromatase DAB immunoreactivity in the rhesus monkey hippocampus. (A) Panoramic view of aromatase distribution in the hippocampus (subject 29357). Sub, Subiculum; CA1-CA3 cornu Ammonis subfields 1–3; DG, Dentate gyrus. (B) Aromatase expression in the DG. The image shows aromatase immunostaining both in the perikaryon of some granule cells (arrow) as well as in dendrites that reach the molecular layer (arrowheads) (subject 27697). (C) Aromatase expression in CA1. The image shows several aromatase immunoreactive pyramidal cells (subject 29357). (D) Detail a high magnification of panel C showing an aromatase-immunoreactive fiber (arrowheads). (E) Aromatase expression in CA3. The image shows several neurons expressing aromatase (subject 30691). (F) Aromatase-immunoreactive neuron located in the molecular layer of the dentate gyrus (subject 28816). Scale bars in A: 500 μm; D: 10 μm; F: 25 μm (for B, C, E and F).
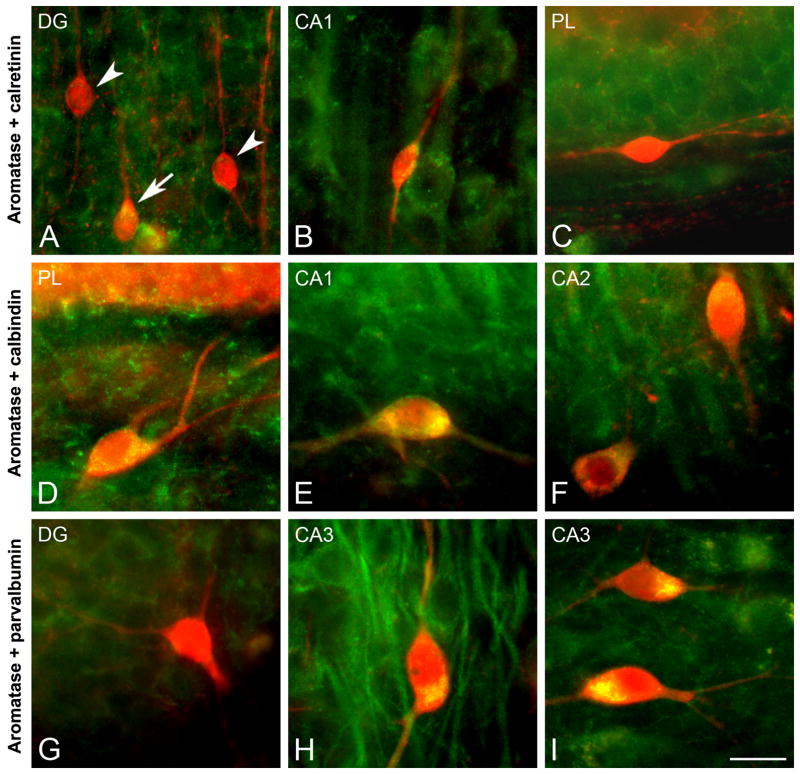
Fig. 3.
CLSM images demonstrating colocalization of aromatase (green) and calcium-binding proteins (red) CR, CB, and PV in the rhesus monkey hippocampus. (A–C) Colocalization of aromatase and CR in the hippocampus (subjects 26326, 27697, and 29357, respectively). (D–F) Colocalization of aromatase and CB in the hippocampus (subjects 30691, 28816, and 28816, respectively). (G–I) Colocalization of aromatase and PV in the hippocampus (subjects 30691, 28816, and 29628, respectively). (A) CR-immunoreactive neuron co-expresing aromatase (arrow) and two CR-immunoreactive neurons that do not co-express aromatase (arrowheads) in DG. (B) CR-immunoreactive neuron co-expressing aromatase in CA1. (C) Polymorphic layer (PL) of the dentate gyrus showing a CR-immunoreactive neuron that does not co-express aromatase. (D) Polymorphic layer of dentate gyrus showing a CB-immunoreactive neuron co-expressing aromatase. (E) CB-immunoreactive neuron co-expressing aromatase in the alveus of CA1. (F) Two CB-immunoreactive neurons co-expressing aromatase in CA2. (G) PV-immunoreactive neuron that does not co-express aromatase in the DG. (H,I) PV-immunoreactive neurons co-expressing aromatase in CA3. Scale bar in I: 25 μm.
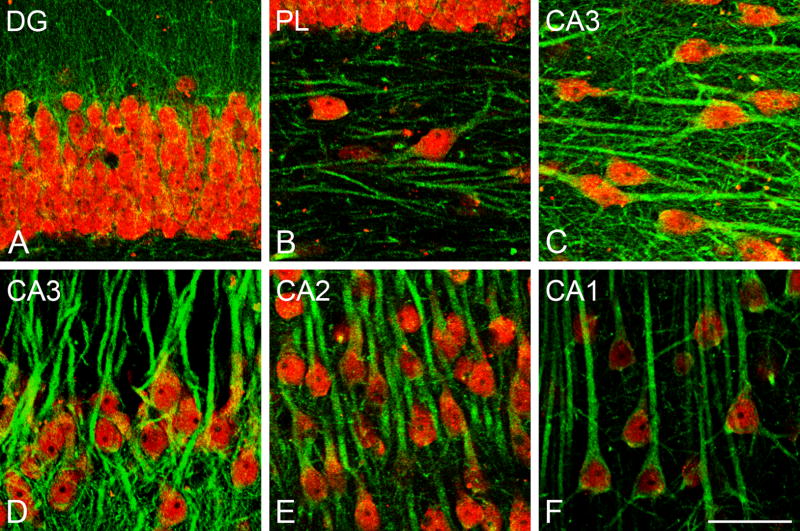
Fig. 2.
Confocal laser scanning microscope (CLSM) images demonstrating colocalization of aromatase (green) and NeuN (red) in the rhesus monkey hippocampus (subject 28816). (A) Colocalization of aromatase and NeuN in the granular cell layer of the DG. (B) Colocalization of aromatase and NeuN in the polymorphic layer (PL) of DG (C,D) Colocalization of aromatase and NeuN in CA3. (E) Colocalization of aromatase and NeuN in CA2. (F) Colocalization of aromatase and NeuN in CA1. Scale bar in F: 50 μm.
In the subiculum and in CA1-3, the vast majority of aromatase-immunoreactive neurons had the typical morphology of pyramidal cells (Fig. 1C, E), showing a reticular pattern of aromatase immunostaining both in the perikaryon and in the initial segment of the dendrites (Fig. 1C, D). The colocalization of aromatase and NeuN shows that the majority of neurons in the stratum pyramidale express aromatase (Fig. 2C–F). Although the majority of aromatase-immunoreactive neurons in the hippocampus corresponded to neurons in CA1-3 and the granule cell layer of the DG, some neurons in other hippocampal regions, such as the molecular and polymorphic layers of the DG were immunoreactive for aromatase. Figure 1F illustrates an example of an aromatase-immunoreactive neuron in the molecular layer of the DG. Figure 2B shows colocalization of aromatase and NeuN in the polymorphic layer of the DG. A few NeuN-immunoreactive neurons expressed aromatase in these hippocampal regions (Fig. 2B). In addition, some structures with typical axonal morphology showed aromatase immunoreactivity (Fig. 1D).
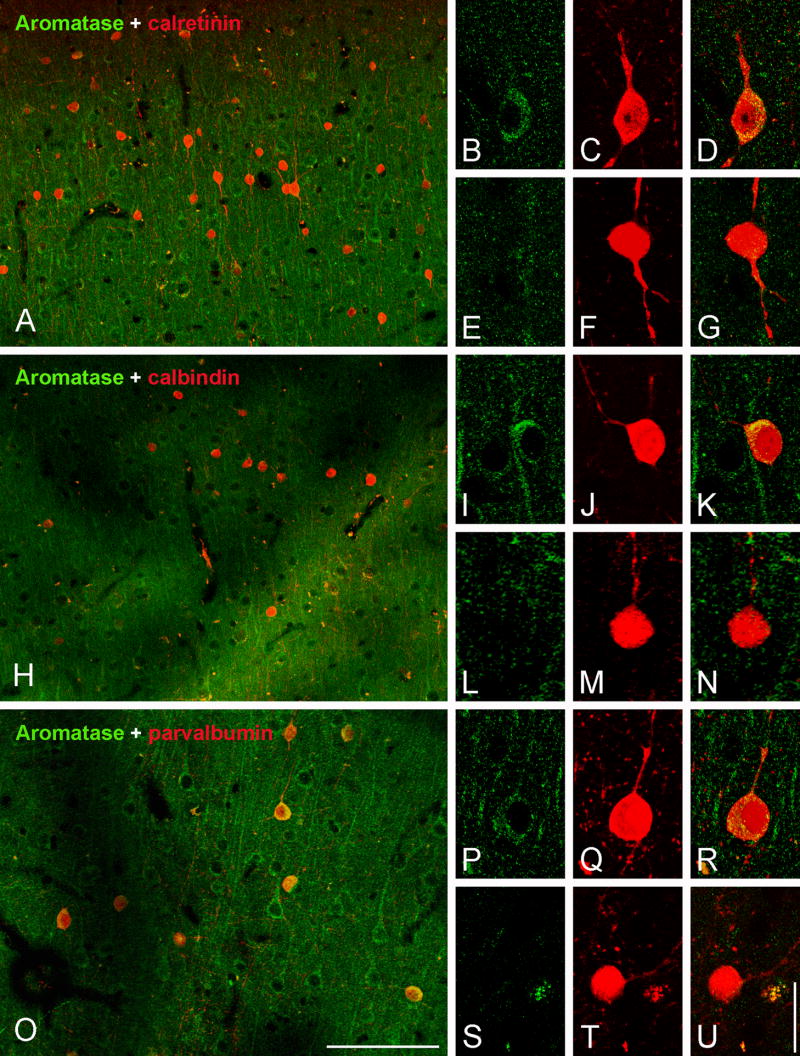
To characterize the specific subpopulations of neurons that express aromatase we carried out an analysis of colocalization of aromatase and calcium-binding proteins in specific hippocampal regions (Fig. 3). The double staining of aromatase with calretinin (CR) revealed neurons that expressed both proteins in regions such as the DG and CA1 ( Fig. 3A, B), whereas other CR-immunoractive neurons did not co-express aromatase (Fig. 3A, C). In contrast, all calbindin (CB) immunoreactive neurons observed were also immunoreactive for aromatase across all hippocampal fields (Fig. 3D–F). Finally, the analysis of colocalization of aromatase and parvalbumin (PV) showed that most but not all PV-immunoreactive cells co-express aromatase (Fig. 3G–I). Double immunostaining of aromatase and GFAP did not reveal colocalization in the hippocampus (Fig. 4A–C), suggesting that astrocytes do not express aromatase in hippocampus.
Fig. 4.
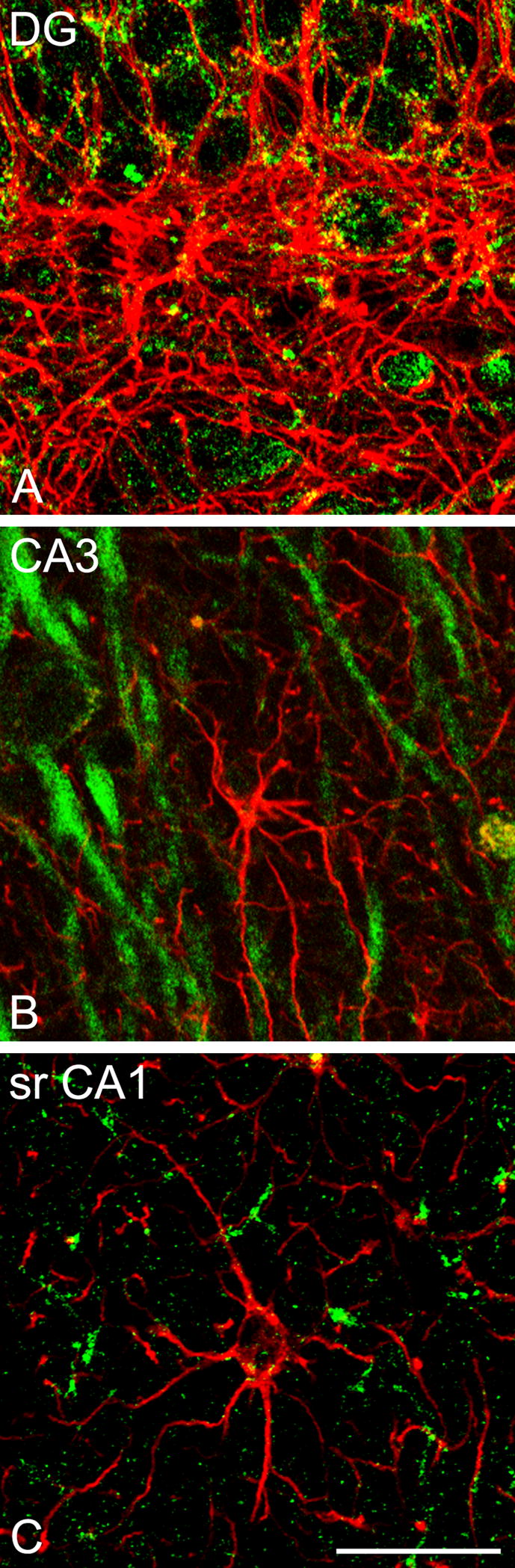
CLSM images demonstrating colocalization of aromatase (green) and GFAP (red) in the rhesus monkey hippocampus (subject 28816). (A) Astrocytes that do not co-express aromatase in the DG. (B) Detail of CA3 demonstrating an astrocyte that does not co-express aromatase. (C) Detail of CA1 stratum radiatum (srCA1) demonstrating an astrocyte that does not co-express aromatase. Scale bar: 25 μm.
Aromatase in the temporal neocortex
As observed for the hippocampus, the pattern and extent of aromatase immunostaining in the temporal neocortex was similar in both treatment groups; demonstrating no obvious effect of estradiol treatment. Many neurons in the neocortex showed aromatase immunoreactivity in the perikaryon and proximal processes (Fig. 5). However, the intensity of immunostaining in neocortex was lower than in the hippocampus (Figs. 5, 6). As in the hippocampus, neuronal cell nuclei in the neocortex were never immunostained (Figs. 5, 6). Moreover, some immunostained processes could be identified as axons by their small varicosities in the neocortex (Fig. 5E).
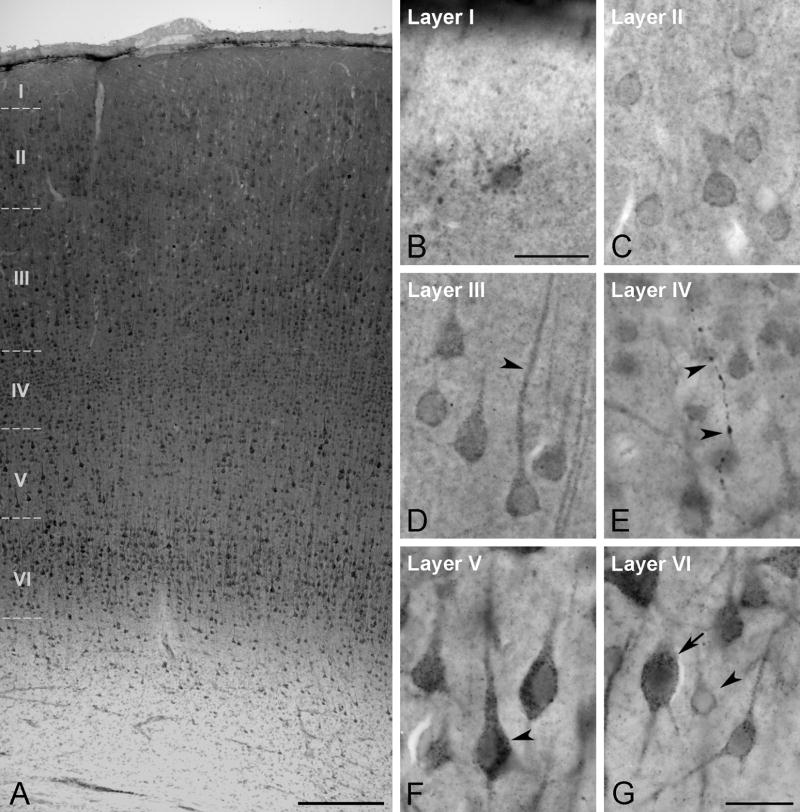
Fig. 5.
Aromatase DAB immunoreactivity in the rhesus monkey temporal neocortex. (A) Low magnification overview of aromatase distribution in the temporal neocortex (subject 29357). (B–G) Details of the aromatase distribution in the different layers of the temporal neocortex (subject 29357). (B) Aromatase immunoreactive cell in layer I. (C) Detail of several neurons expressing aromatase in layer II. (D) Aromatase immunoreactivity in pyramidal cells of layer III. The aromatase immunostaining is localized both in the perikarion and in main dendrites (arrowhead). (E) Detail of an aromatase immunoreactive fiber in layer IV ( arrowheads). (F) Layer V showing pyramidal cells intensely immunoreactive for aromatase specially in the perikaryon (arrowhead). (G) Detail of aromatase immunoreactive neurons in layer VI. Some neurons show high level of aromatase immunostaining (arrow), whereas other are slightly immunoreactive (arrowhead). Scale bars in A: 250 μm; B: 25 μm; G: 25 μm (for C–G).
Fig. 6.
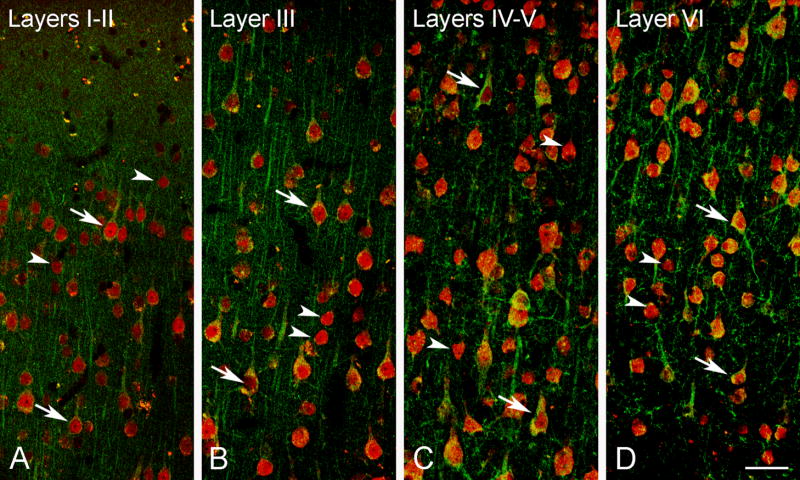
CLSM images demonstrating colocalization of aromatase (green) and NeuN (red) in the rhesus monkey temporal neocortex (subject 30691). Colocalization of aromatase and NeuN in layers I–II (A), layer III (B), layers IV–V (C) and layer VI (D). While some NeuN immunoreactive neurons co-express aromatase (arrows), other NeuN immunoreactive were immunonegative for aromatase (arrowheads). Scale bar in D: 50 μm.
Aromatase-immunoreactive neurons were abundant in layers II through VI (Fig. 5C–G). In general, neurons in layers III, V and VI showed the highest staining intensity, with a particularly prominent staining in layers V and VI (Fig. 5F, G). Double labeling experiments with NeuN revealed that not all neurons were immunoreactive for aromatase, even in those cortical layers where aromatase-labeled cells were more abundant (Fig. 6).
Most aromatase-immunoreactive neocortical neurons had the typical morphology of pyramidal cells (Figs 5, 6). Additionally, a small number of aromatase-immunoreactive neurons showed immunoreactivity for markers expressed by interneurons (Fig. 7). Finally, as in the hippocampus, colocalization of aromatase and the glial marker GFAP was not detected in the neocortex (Fig. 8).
Fig. 7.
CLSM images demonstrating colocalization of aromatase (green) and calcium-binding proteins (red) in layers II–III of the rhesus monkey temporal neocortex. (A–G) Colocalization of aromatase and CR. (H–N) Co-localization of aromatase and CB. (O–U) Colocalization of aromatase and PV. (A) Overview of layer II (subject 30691). (B–D) CR-immunoreactive neuron co-expressing aromatase (subject 26326). (E–G) CR-immunoreactive neuron that does not co-express aromatase (subject 26326). (H) Overview of layer II (subject 30691). (I–K) CB-immunoreactive neuron co-expressing aromatase (subject 29357). (L–N) CB-immunoreactive neuron that does not co-express aromatase (subject 27697). (O) Overview of layer II–III (subject 30691). (P–R) PV-immunoreactive neuron co-expressing aromatase (subject 29357). (S-U) PV-immunoreactive neuron that does not co-express aromatase (subject 28816). Scale bars in O: 100 μm (A,H,O); U: 20 μm (for all high magnification images).
Fig. 8.
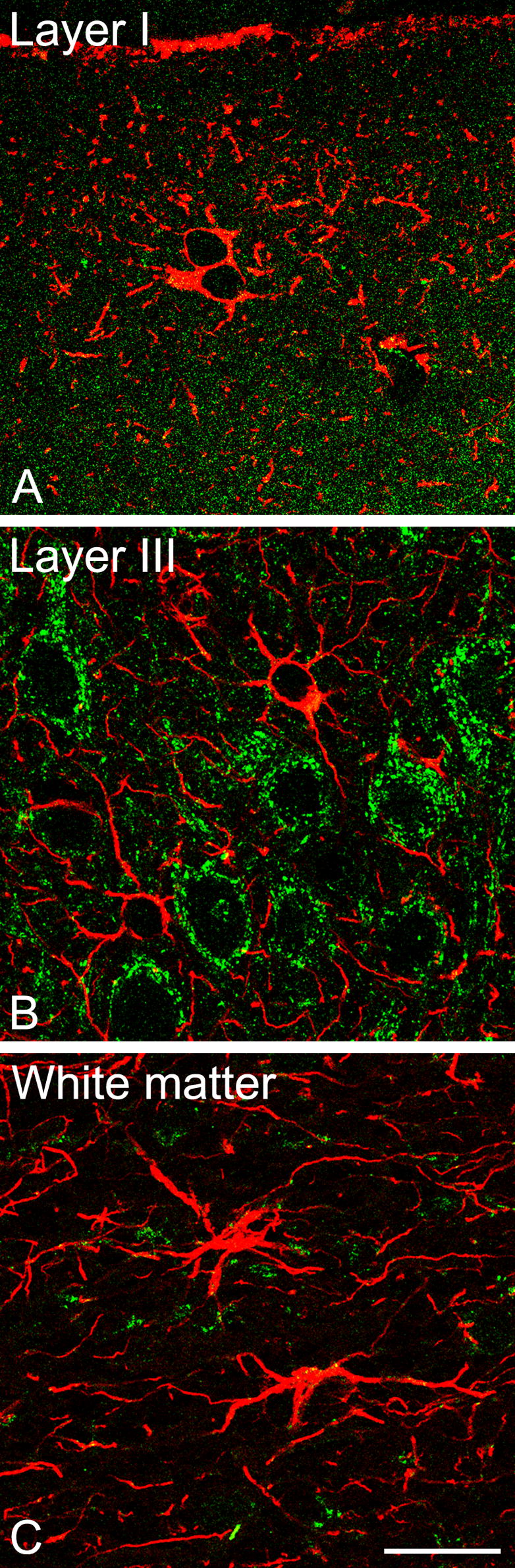
CLSM colocalization demonstrating aromatase (green) and GFAP (red) in the rhesus monkey temporal neocortex. (A) Layer I of temporal neocortex showing several astrocytes that do not co-express aromatase (subject: 29357). (B) Layer III of the temporal neocortex showing two astrocytes that do not co-express aromatase (subject: 29357). (C) White matter of the temporal neocortex showing two astrocytes that do not co-express aromatase (subject: 30691). Scale bar: 20 μm.
DISCUSSION
Aromatase immunoreactivity in the hippocampus
The aromatase expression patterns in the hippocampus and temporal neocortex of the female rhesus monkey, extends data from previous studies in several brain structures of different vertebrates (Sanghera et al., 1991; Shen et al., 1994; Yamada-Mouri et al., 1995; Saldanha and Schlinger, 1997; Sasano et al., 1998; Garcia-Segura et al., 1999; Stoffel-Wagner et al., 1999; Ivanova and Beyer, 2000; Saldanha et al., 2000; Wehrenberg et al., 2001; Hojo et al., 2004; Rune and Frotscher, 2005; Yague et al., 2006). Expression of aromatase in the hippocampus of the rhesus monkey is in agreement with the previous work of Wehrenberg and collaborators (2001) who showed aromatase mRNA by in situ hybridization, in pyramidal neurons and granule cells in the hippocampus of marmoset monkeys (Callithrix jacchus). According to our findings, aromatase is localized in the perikaryon, dendrites and some axonal processes in select neuronal populations. We detected aromatase immunoreactivity in a large population of cells in the monkey hippocampus, which consisted mostly of excitatory neurons, including pyramidal cells in CA1-3 and granule cells in the DG. Certain GABAergic interneurons, identified by the expression of the calcium-binding proteins CR, CB, and PV, also showed immunoreactivity for aromatase. Double immunostaining of aromatase and CR, CB, or PV revealed that only some CR and PV interneurons also express aromatase. On the contrary, all the CB interneurons found expressed aromatase. This pattern of immunostaining coincides with that described for aromatase immunoreactivity in neuronal somata and neuronal processes in the human brain (Naftolin et al., 1996; Ishunina et al., 2005; Yague et al., 2006).
Aromatase immunoreactivity in the neocortex
In temporal neocortical regions, pyramidal cells expressing aromatase were found across layers II–VI, although the most intense immunostaining was present in pyramidal cells located in cortical layers V and VI. This matched our previous findings in the human temporal neocortex (Yague et al., 2006). As in the hippocampus, localization patterns were similar across the treatment groups. The distribution of aromatase in pyramidal cells is important when considering the implications of the different types of pyramidal cells in cortical circuits. Those pyramidal cells projecting to the subcortical nuclei are located in layers V–VI, whereas those projecting to other ipsi- or contralateral cortical areas are found mainly in layers II–III (for reviews see Jones, 1981; Jones, 1984; White, 1989; Felleman and Van Essen, 1991; DeFelipe and Fariñas, 1992; Lund et al., 1994; Rockland, 1997; Morrison et al., 1998). The expression of aromatase in many pyramidal neurons located through layers II–VI, suggests that local estradiol formation mediated by aromatase, may affect a wide variety of extrinsic and intrinsic excitatory circuits.
Immunocytochemical studies in the primate neocortex indicate that the majority of interneurons are GABAergic (reviewed in Houser et al., 1984; Jones, 1993), and that specific subpopulations of GABAergic neurons are immunoreactive for CB, CR, or PV. Each of these three calcium-binding proteins labels distinct types of interneurons, and there is minimal colocalization (reviewed in Andressen et al., 1993; DeFelipe, 1993; DeFelipe, 1997). For example, double bouquet cells can be labeled for CB or CR, but never for PV, whereas chandelier cells contain PV although a subpopulation in layers V–VI contains CB. However, chandelier terminals are never labeled with CR. The finding that a significant population of GABAergic interneurons does not express aromatase is in agreement with our previous observations in the human temporal neocortex (Yague et al., 2006). The restriction of aromatase immunostaining to discrete subpopulations of interneurons indicates a highly selective expression of the enzyme in the monkey temporal cortex. The impact of the lack of aromatase in certain pyramidal and GABAergic neurons should be explored in future physiological and pathological studies of excitatory and inhibitory cortical circuits.
Absence of aromatase immunoreactivity in astrocytes
Interestingly, neither hippocampal nor neocortical astrocytes showed aromatase expression. This finding is in contrast with our previous observations in the human temporal neocortex (Yague et al., 2006) where we have detected that a subpopulation of astrocytes expresses aromatase. However, the findings of the present study are in agreement with previous results obtained in rodents and birds where glial cells usually do not express aromatase under normal conditions, but do under different paradigms of cerebral lesion (Garcia-Segura et al., 1999; Azcoitia et al., 2003; Peterson et al., 2004). Because the aromatase gene (cyp19 gene) is under a complex transcriptional regulation, with multiple promoters controlling the aromatase expression in different species (Harada et al., 1993; Bulun et al., 2003; Golovine et al., 2003), the existing differences between the cyp19 gene promoter region among rodents, monkeys and humans could account for these differences in the glial aromatase expression among different species.
Effects of estrogen therapy
Several factors, including androgens, have been shown to regulate aromatase expression in the brain (Balthazart et al., 1992; Harada et al., 1992; Honda et al., 1994). The results of the present study do not corroborate the hypothesis that chronic cyclic changes of estrogen levels in plasma may affect aromatase immunoreactivity in the brain, as the observed aromatase cellular distribution in the hippocampus and temporal neocortex is similar across treatment groups. Due to the qualitative nature of our analysis, we can not exclude that ovariectomy or estrogen therapy may affect aromatase activity or the levels of aromatase expression per cell, however our findings do suggest that there is not a marked change in the cellular pattern of expression of the enzyme.
Functional implications of aromatase expression in the hippocampus and neocortex
The expression of aromatase in hippocampal and neocortical neurons of monkeys is of particular interest given that estrogen, the product of aromatase activity, promotes plastic changes in synapses in the rat and monkey hippocampus (Gould et al., 1990; Choi et al., 2003; Hao et al., 2003). In rodents, there is evidence to support that estradiol-mediated synaptic plasticity in the hippocampus is mediated not only by gonadal estrogens, but also by estrogens synthesized locally in the hippocampus (Adams et al., 2001; Kretz et al., 2004; Leranth et al., 2004; Rune and Frotscher, 2005; Murakami et al., 2006). In vitro studies have shown that the pharmacological inhibition of aromatase activity in hippocampal slices decreases the number of dendritic spines and the number of dendritic spine synapses on CA1 pyramidal neurons (Kretz et al., 2004; Rune and Frotscher, 2005; Prange-Kiel and Rune, 2006; Prange-Kiel et al., 2006). In addition, aromatase inhibition within the brain in vivo prevents the increase in the number of CA1 spine synapses induced by testosterone and dehydroepiandrosterone administration to female rats (Hajszan et al., 2004; Leranth et al., 2004; MacLusky et al., 2004, 2006). This, along with our present findings that show abundant expression of aromatase in the soma and dendrites of hippocampal and neocortical pyramidal neurons of monkey, suggests that in primates, local aromatase activity may play a role in the modulation of cortical synaptic plasticity. In addition, the expression of aromatase by different subpopulations of interneurons is of interest, since the enzyme increases GABA synthesis in hippocampal cultures (Zhou et al., 2007). In addition, GABAA receptor inhibition decreases both the number of dendritic spines and the synthesis of estradiol in hippocampal slices (Zhou et al., 2007), suggesting that aromatase may play an important role in cortical function by linking the activity of specific subsets of GABAergic interneurons with synaptic plasticity in pyramidal cells.
In summary, our findings indicate that the enzyme aromatase is widely expressed in the hippocampus and temporal neocortex of the female rhesus monkey. The enzyme is predominantly expressed in pyramidal cells of the hippocampus and neocortex, suggesting that local estrogen formation in the brain may affect the function of excitatory cortical cells. In addition a subpopulation of interneurons, identified by the expression of calcium-binding proteins, also expresses aromatase. These findings suggest that local estrogen formation may be relevant for cortical function in primates.
MATERIALS AND METHODS
Experimental groups and treatment
The study was carried out in young OVX female rhesus monkey (Macaca mulatta) that received estradiol or vehicle (see Table 1). All experiments were conducted in compliance with the National Institutes of Health Guidelines for the Care and Use of Experimental Animals approved by the Institutional Animal Care and Use Committee at the University of California-Davis.
Table 1.
Animal experimental groups used in the immunohistochemical study. Animals were separated into two groups: ovariectomized and treated with estradiol (OVX+estradiol) and a control ovariectomized treated with vehicle (OVX+vehicle).
| ANIMAL CODE | AGE | POST OVARIECTOMY | DURATION OF TREATMENT | |
|---|---|---|---|---|
| years-months | years-months | years-months | ||
| CONTROL GROUP
(OVX+vehicle) |
26908 | 9–11 | 2–9 | 2-0 |
| 28816 | 7-5 | 3-0 | 2–6 | |
| 29357 | 6–9 | 2–3 | 1–3 | |
| 30691 | 4–9 | 2–8 | 1–10 | |
| ESTRADIOL REPLACEMENT GROUP
(OVX+estradiol) |
26326 | 10-10 | 2-3 | 1–11 |
| 27697 | 9-0 | 2–11 | 2–7 | |
| 27723 | 8–10 | 2–6 | 2-0 | |
| 29628 | 5–9 | 2–3 | 1–11 |
Eight young female rhesus monkeys (mean age ± SEM, 10 years ± 3 months) were used in this study. The monkeys were bilaterally OVX and were assigned to age-matched OVX+vehicle and OVX+estradiol treatment groups. Following an average post- ovariectomy interval of 29 weeks, OVX+estradiol monkeys received estradiol cypionate (100 μg/ml of sterile peanut oil, i.m.; Pharmacia, Peapack, NJ) in a single injection every three weeks. OVX+vehicle monkeys were provided an equivalent volume of vehicle injection according to the same schedule. Treatment extended over approximately 2 years (Table 1). Estradiol and vehicle injections were coded and administered in a blind fashion until all experiments were completed. Serum estradiol values were measured at perfusion, 24 hours after the final injection. (See Hao et al., 2007 for details).
Immunohistochemistry
Single-labeling procedure
Peroxidase immunohistochemistry was performed on vibratome sections (50 μm thick). Sections were treated for 45 minutes with a solution of ethanol (50%) and hydrogen peroxide (5%) in phosphate buffer (PB) to quench endogenous peroxidase activity. Sections were then incubated for 48 hours at 4°C with a rabbit polyclonal antibody (Garcia-Segura et al., 1999; Yague et al., 2006) generated from a 15-amino acid peptide corresponding to residues 488-502 of mouse aromatase, a region homologous to human and monkey aromatase (Beyer et al., 1994). This antibody has been shown to specifically recognize aromatase in the human brain (Yague et al., 2006). The primary antibody was diluted 1:2,000 in PB with 0.3% Triton X-100 (PBT), 0.3% bovine serum albumin and 5% normal goat serum. After incubation with the primary antibody, sections were washed in PB and incubated, for 1 hour at room temperature, in biotinylated goat anti-rabbit IgG (Pierce Meridian, IL; diluted 1:1,000 in PBT). Sections were then processed using the Vectastain ABC immunoperoxidase kit (Vector, Burlingame, CA) and the antibody distribution was detected histochemically with 0.05% 3,3′-diaminobenzidine tetrahydrochloride as a chromogen (DAB; Sigma, St. Louis, MO) and 0.01% hydrogen peroxide. The sections were mounted, dehydrated, cleared with xylene and coverslipped. Immunostaining was absent when the aromatase antibody omitted. In addition, incubation of the antibody with the immunogenic peptide resulted in a dose-dependent reduction of the staining (see also Yague et al., 2006).
Double-labeling procedures
Double immunohistochemical staining was performed to colocalize aromatase with neuronal and glial markers. Tissue sections were incubated for 48 hours at 4°C with the primary antibodies in PBT containing 5% normal goat serum. The aromatase antibody described above was combined with a mouse monoclonal anti-glial fibrillary acidic protein (GFAP) antibody (Sigma, G-3893; diluted 1:1,000), or with a mouse monoclonal antibody against the neuronal marker NeuN (MAB 337, Chemicon, Temecula, CA, diluted 1:2,000). Other sections were incubated with the anti-aromatase antibody in combination with mouse monoclonal antibodies against the calcium-binding proteins CB, CR and PV in order to show co-expression in subpopulations of interneurons (Swant, Bellinzona, Switzerland, catalogue codes 300, 6B3, and 235, respectively, all diluted 1:2,000). Tissue sections were subsequently washed in PB and incubated for 1 hour at room temperature with goat anti-rabbit IgG conjugated with green Alexa (488 nm) and goat anti-mouse IgG conjugated with red Alexa (594 nm) (Molecular Probes, Eugene, OR; diluted 1:1,000 in PBT). Selected areas in brightfield or in fluorescence were photographed using a digital camera adapted to a fluorescence microscope (Zeiss Axiphot, Germany) and using a Zeiss LSM 510 microscope equipped with Zeiss 10x (Plan-Neofluar, 0.3 NA) and 20x (Plan-Apochromat, 0.8 NA) objective lenses. Immunostaining was absent when the first antibodies were omitted.
Acknowledgments
We thank Anne Canfield, Mary Roberts, Deborah Kent, Sona Santos, and Heather McKay for technical assistance, and Dr. Don Canfield for veterinary care. Additionally, we thank Dr. Peter Rapp and Dr. Jiandong Hao for helpful discussion. This work was supported in part by NIA Grant AG016765.
Grant sponsor: National Institutes of Health; Grant number: AG16765 (JHM);
Grant sponsors: Ministerio de Educación y Ciencia, Spain; Grant number: SAF 2005-00272. European Union; Grant number: LSHM-CT-2005-518245.
LIST OF ABBREVIATIONS
- CA
cornu Ammonis
- CB
calbindin
- CR
calretinin
- CLSM
confocal laser scanning microscope
- DAB
diaminobenzidine tetrahydrochloride
- DG
dentate gyrus
- GFAP
glial fibrillary acidic protein
- NeuN
neuron-specific nuclear protein
- OVX
ovariectomized
- PV
parvalbumin
- PB
phosphate buffer
- PBT
phosphate buffer with Triton X-100
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Adams MM, Shah RA, Janssen WG, Morrison JH. Different modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proc Natl Acad Sci USA. 2001;98:8071–8076. doi: 10.1073/pnas.141215898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;41:43–58. doi: 10.1177/1534582305277152. [DOI] [PubMed] [Google Scholar]
- Andressen C, Blümcke I, Celio MR. Calcium-binding proteins selective markers of nerve cells. Cell Tissue Res. 1993;271:181–208. doi: 10.1007/BF00318606. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Garcia-Segura LM. Estradiol prevents kainic acid-induced neuronal loss in the rat dentate gyrus. NeuroReport. 1998;14:3075–3079. doi: 10.1097/00001756-199809140-00029. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Fernandez-Galaz C, Sierra A, Garcia-Segura LM. Gonadal hormones affect neuronal vulnerability to excitotoxin-induced degeneration. J Neurocytol. 1999;28:699–710. doi: 10.1023/a:1007025219044. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Veiga S, Honda S, Harada N, Garcia-Segura LM. Brain aromatase is neuroprotective. J Neurobiol. 2001;47:318–329. doi: 10.1002/neu.1038. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Veiga S, Garcia-Segura LM. Aromatase expression by reactive astroglia is neuroprotective. Ann NY Acad Sci. 2003;1007:298–305. doi: 10.1196/annals.1286.028. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Surlemont C, Harada N, Naftolin F. Neuroanatomical specificity in the autoregulation of aromatase-immunoreactive neurons by androgens and estrogens: an immunocytochemical study. Brain Res. 1992;574:280–290. doi: 10.1016/0006-8993(92)90828-w. [DOI] [PubMed] [Google Scholar]
- Beyer C, Tramonte R, Hutchison RE, Sharp PJ, Barker PJ, Huskisson NS, Hutchison JB. Aromatase-immunoreactive neurons in the adult female chicken brain detected using a specific antibody. Brain Res Bull. 1994;33:583–588. doi: 10.1016/0361-9230(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Sebastian S, Takayama K, Suzuki T, Sasano H, Shozu M. The human CYP19 (aromatase P450) gene: update on physiologic roles and genomic organization of promoters. J Steroid Biochem Mol Biol. 2003;86:219–224. doi: 10.1016/s0960-0760(03)00359-5. [DOI] [PubMed] [Google Scholar]
- Choi JM, Romeo RD, Brake WG, Bethea CL, Rosenwaks Z, McEwen BS. Estradiol increases pre- and postsynaptic proteins in the CA1 region of the hippocampus in female rhesus macaques (Macaca mulatta) Endocrinology. 2003;144:4734–4738. doi: 10.1210/en.2003-0216. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Fariñas I. The pyramidal neuron of the cerebral cortex morphological and chemical characteristics of the synaptic inputs. Prog Neurobiol. 1992;39:563–607. doi: 10.1016/0301-0082(92)90015-7. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. Neocortical neuronal diversity chemical heterogeneity revealed by colocalization studies of classic neurotransmitters, neuropeptides, calcium-binding proteins and cell surface molecules. Cereb Cortex. 1993;3:273–289. doi: 10.1093/cercor/3.4.273. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat. 1997;14:1–19. doi: 10.1016/s0891-0618(97)10013-8. [DOI] [PubMed] [Google Scholar]
- DeVoogd T, Nottebohm F. Gonadal hormones induce dendritic growth in the adult avian brain. Science. 1981;214:202–204. doi: 10.1126/science.7280692. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Flores F, Naftolin F, Ryan KJ, White RJ. Estrogen formation by the isolated perfused rhesus monkey brain. Science. 1973;180:1074–1075. doi: 10.1126/science.180.4090.1074. [DOI] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17β-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Dudek B. Estradiol to aged female or male mice improves learning in inhibitory avoidance and water maze tasks. Brain Res. 2005;1036:101–108. doi: 10.1016/j.brainres.2004.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LAM, Spritzer MD, Barker JM, Pawluski JL. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16:225–232. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, Hutchison JB. Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience. 1999;89:567–578. doi: 10.1016/s0306-4522(98)00340-6. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Veiga S, Sierra A, Melcangi RC, Azcoitia I. Aromatase: a neuroprotective enzyme. Prog Neurobiol. 2003;71:31–41. doi: 10.1016/j.pneurobio.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Golovine K, Schwerin M, Vanselow J. Three different promoters control expression of the aromatase cytochrome p450 gene (cyp19) in mouse gonads and brain. Biol Reprod. 2003;68:978–984. doi: 10.1095/biolreprod.102.008037. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;104:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Leranth C. Dehydroepiandrosterone increases hippocampal spine synapse density in ovariectomized female rats. Endocrinology. 2004;145:1042–1045. doi: 10.1210/en.2003-1252. [DOI] [PubMed] [Google Scholar]
- Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Alen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J Comp Neurol. 2003;465:540–550. doi: 10.1002/cne.10837. [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WGM, Lou W, Lasley BL, Hof PR, Morrison JH. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci USA. 2007;104:11465–11470. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Yamada K, Foidart A, Balthazart J. Regulation of aromatase cytochrome P-450 (estrogen synthetase) transcripts in the quail brain by testosterone. Mol Brain Res. 1992;15:19–26. doi: 10.1016/0169-328x(92)90146-3. [DOI] [PubMed] [Google Scholar]
- Harada N, Utsumi T, Takagi Y. Tissue-specific expression of the human aromatase cytochrome P-450 gene by alternative use of multiple exons 1 and promoters, and switching of tissue-specific exons 1 in carcinogenesis. Proc Natl Acad Sci USA. 1993;90:11312–11316. doi: 10.1073/pnas.90.23.11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017β and P450 aromatase localized in neurons. Proc Natl Acad Sci USA. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S, Harada N, Takagi Y. Novel exon 1 of the aromatase gene specific for aromatase transcripts in human brain. Biochem Biophys Res Commun. 1994;198:1153–1160. doi: 10.1006/bbrc.1994.1163. [DOI] [PubMed] [Google Scholar]
- Houser CR, Vaughn JE, Hendry SHC, Jones EG, Peters A. GABA neurons in the cerebral cortex. In: Jones EG, Peters A, editors. Cerebral cortex. Vol. 2. Plenum Press; New York: 1984. pp. 63–89. [Google Scholar]
- Ishunina TA, van Beurden D, van der Meulen G, Unmehopa UA, Hol EM, Huitinga I, Swaab DF. Diminished aromatase immunoreactivity in the hypothalamus, but not in the basal forebrain nuclei in Alzheimer’s disease. Neurobiol Aging. 2005;26:173–194. doi: 10.1016/j.neurobiolaging.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Ivanova T, Beyer C. Ontogenetic expression and sex differences of aromatase and estrogen receptor-α/β mRNA in the mouse hippocampus. Cell Tissue Res. 2000;300:231–237. doi: 10.1007/s004410000199. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Horvath TL, Leranth C, Harada N, Naftolin F. Aromatase immunoreactivity in the rat brain: gonadectomy-sensitive hypothalamic neurons and an unresponsive “limbic ring” of the lateral septum-bed nucleus-amygdala complex. J Steroid Biochem Mol Biol. 1993;44:481–498. doi: 10.1016/0960-0760(93)90253-s. [DOI] [PubMed] [Google Scholar]
- Jones EG. Anatomy of cerebral cortex columnar input-output organization. In: Schmitt FO, Worden FG, Adelman G, Dennis M, editors. Cerebral cortex. MIT Press; Cambridge: 1981. pp. 199–235. [Google Scholar]
- Jones EG. Laminar distributions of cortical efferent cells. In: Peters A, Jones EG, editors. Cerebral cortex. Plenum Press; New York: 1984. pp. 521–552. [Google Scholar]
- Jones EG. GABAergic neurons and their role in cortical plasticity in primates. Cereb Cortex. 1993;3:361–372. doi: 10.1093/cercor/3.5.361-a. [DOI] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugaya A, Epperson CN, Zoghbi S, van Dyck CH, Hou Y, Fujita M, Staley JK, Garg PK, Seibyl JP, Innis RB. Increase in prefrontal cortex serotonin 2A receptors following estrogen treatment in postmenopausal women. Am J Psychiatry. 2003;160:1522–1524. doi: 10.1176/appi.ajp.160.8.1522. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci. 2004;24:495–499. doi: 10.1523/JNEUROSCI.4516-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund JS, Yoshioka T, Levitt JB. Substrates for interlaminar connections in area V1 of the macaque monkey cerebral cortex. In: Peters A, Rockland KS, editors. Cerebral cortex. Plenum; New York: 1994. pp. 37–60. [Google Scholar]
- McEwen BS, Gould E, Orchinik M, Weiland NG, Woolley CS. Oestrogens and the structural and functional plasticity of neurons implications for memory, aging and neurodegenerative processes. Ciba Found Symp. 1995;191:52–66. doi: 10.1002/9780470514757.ch4. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F, Goldman-Rakic PS. Estrogen formation and binding in the cerebral cortex of the developing rhesus monkey. Proc Natl Acad Sci USA. 1986;83:513–516. doi: 10.1073/pnas.83.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Leranth C. Effects of dehydroepiandrosterone and flutamide on hippocampal CA1 spine synapse density in male and female rats: implications for the role of androgens in maintenance of hippocampal structure. Endocrinology. 2004;145:4154–4161. doi: 10.1210/en.2004-0477. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Prange-Kiel J, Leranth C. Androgen modulation of hippocampal synaptic plasticity. Neuroscience. 2006;138:957–965. doi: 10.1016/j.neuroscience.2005.12.054. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR, Huntley GW. Neurochemical organization of the primate visual cortex. In: Bloom FE, Björklund A, Hökfelt T, editors. Handbook of chemical neuroanatomy. Elsevier; Amsterdam: 1998. pp. 299–433. [Google Scholar]
- Murakami G, Tsurugizawa T, Hatanaka Y, Komatsuzaki Y, Tanabe N, Mukai H, Hojo Y, Kominami S, Yamazaki T, Kimoto T, Kawato S. Comparison between basal and apical dendritic spines in estrogen-induced rapid spinogenesis of CA1 principal neurons in the adult hippocampus. Biochem Biophys Res Commun. 2006;351:553–558. doi: 10.1016/j.bbrc.2006.10.066. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Petro Z. Aromatization of androstenedione by limbic system tissue from human fetuses. J Endocrinol. 1971;51:795–796. doi: 10.1677/joe.0.0510795. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Davies IJ, Petro Z, Kuhn M. The formation and metabolism of estrogens in brain tissues. Adv Biosci. 1975;15:105–121. [PubMed] [Google Scholar]
- Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain. An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology. 1996;63:149–155. doi: 10.1159/000126951. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Galea LA. Reproductive status influences cell proliferation and cell survival in the dentate gyrus of adult female meadow voles: a possible regulatory role for estradiol. Neuroscience. 2001;102:369–379. doi: 10.1016/s0306-4522(00)00474-7. [DOI] [PubMed] [Google Scholar]
- Parducz A, Hajszan T, Maclusky NJ, Hoyk Z, Csakvari E, Kurunczi A, Prange-Kiel J, Leranth C. Synaptic remodeling induced by gonadal hormones: neuronal plasticity as a mediator of neuroendocrine and behavioral responses to steroids. Neuroscience. 2006;138:977–985. doi: 10.1016/j.neuroscience.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Lee DW, Fernando G, Schlinger BA. Radial glia express aromatase in the injured zebra finch brain. J Comp Neurol. 2004;475:261–269. doi: 10.1002/cne.20157. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Rune GM. Direct and indirect effects of estrogen on rat hippocampus. Neuroscience. 2006;138:765–772. doi: 10.1016/j.neuroscience.2005.05.061. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Fester L, Zhou L, Lauke H, Carretero J, Rune GM. Inhibition of hippocampal estrogen synthesis causes region-specific downregulation of synaptic protein expression in hippocampal neurons. Hippocampus. 2006;16:464–471. doi: 10.1002/hipo.20173. [DOI] [PubMed] [Google Scholar]
- Rau SW, Dubal DB, Bottner M, Gerhold LM, Wise PM. Estradiol attenuates programmed cell death after stroke-like injury. J Neurosci. 2003;10:11420–11426. doi: 10.1523/JNEUROSCI.23-36-11420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockland KS. Elements of cortical architecture hierarchy revisited. In: Rockland KS, Kaas JH, Peters A, editors. Cerebral cortex. Plenum Press; New York: 1997. pp. 243–294. [Google Scholar]
- Roselli CE, Horton LE, Resko JA. Distribution and regulation of aromatase activity in the rat hypothalamus and limbic system. Endocrinology. 1985;117:2471–2477. doi: 10.1210/endo-117-6-2471. [DOI] [PubMed] [Google Scholar]
- Rune GM, Frotscher M. Neurosteroid synthesis in the hippocampus: role in synaptic plasticity. Neuroscience. 2005;136:833–842. doi: 10.1016/j.neuroscience.2005.03.056. [DOI] [PubMed] [Google Scholar]
- Ryan KJ, Naftolin F, Reddy V, Flores F, Petro Z. Estrogen formation in the brain. Am J Obstet Gynecol. 1972;114:454–460. doi: 10.1016/0002-9378(72)90204-9. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Mezaki Y, Shikimi H, Ukena K, Tsutsui K. Dendritic growth and spine formation in response to estrogen in the developing Purkinje cell. Endocrinology. 2003;144:4466–4477. doi: 10.1210/en.2003-0307. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Schlinger BA. Estrogen synthesis and secretion in the brown-headed cowbird (Molothrus ater) Gen Comp Endocrinol. 1997;105:390–401. doi: 10.1006/gcen.1996.6841. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Sanghera MK, Simpson ER, McPhaul MJ, Kozlowski G, Conley AJ, Lephart ED. Immunocytochemical distribution of aromatase cytochrome P450 in the rat brain using peptide-generated polyclonal antibodies. Endocrinology. 1991;129:2834–2844. doi: 10.1210/endo-129-6-2834. [DOI] [PubMed] [Google Scholar]
- Sasano H, Takashashi K, Satoh F, Nagura H, Harada N. Aromatase in the human central nervous system. Clin Endocrinol. 1998;48:325–329. doi: 10.1046/j.1365-2265.1998.00390.x. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Balthazart J. Neuroanatomical distribution of testosterone-metabolizing enzymes in the Japanese quail. Brain Res. 1987;422:137–148. doi: 10.1016/0006-8993(87)90548-8. [DOI] [PubMed] [Google Scholar]
- Shen P, Campagnoni CW, Kampf K, Schlinger BA, Arnold AP, Campagnoni AT. Isolation and characterization of a zebra finch aromatase cDNA: in situ hybridization reveals high aromatase expression in brain. Mol Brain Res. 1994;24:227–237. doi: 10.1016/0169-328x(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Shinoda K, Nagano M, Osawa Y. Neuronal aromatase expression in preoptic, strial, and amygdaloid regions during late prenatal and early postnatal development in the rat. J Comp Neurol. 1994;343:113–129. doi: 10.1002/cne.903430109. [DOI] [PubMed] [Google Scholar]
- Sierra A, Azcoitia I, Garcia-Segura LM. Endogenous estrogen formation is neuroprotective in model of cerebellar ataxia. Endocrine. 2003;21:43–51. doi: 10.1385/endo:21:1:43. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B, Watzka M, Schramm J, Bidlingmaier F, Klingmuller D. Expression of CYP19 (aromatase) mRNA in different areas of the human brain. J Steroid Biochem Mol Biol. 1999;70:237–241. doi: 10.1016/s0960-0760(99)00114-4. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B. Neurosteroid metabolism in the human brain. Eur J Endocrinol. 2001;145:669–679. doi: 10.1530/eje.0.1450669. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Gerhold LM, Bottner M, Rau SW, DelaCruz C, Yang E, Zhu H, Yu J, Cashion AB, Kindy MS, Merchenthaler I, Gage FH, Wise PM. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta. J Comp Neurol. 2007;500:1064–1075. doi: 10.1002/cne.21240. [DOI] [PubMed] [Google Scholar]
- Veiga S, Garcia-Segura LM, Azcoitia I. Neuroprotection by the steroids pregnenolone and dehydroepiandrosterone is mediated by the enzyme aromatase. J Neurobiol. 2003;56:398–406. doi: 10.1002/neu.10249. [DOI] [PubMed] [Google Scholar]
- Veiga S, Melcangi RC, Doncarlos LL, Garcia-Segura LM, Azcoitia I. Sex hormones and brain aging. Exp Gerontol. 2004;39:1623–1631. doi: 10.1016/j.exger.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Wehrenberg U, Prange-Kiel J, Rune GM. Steroidogenic factor-1 expression in marmoset and rat hippocampus: colocalization with StAR and aromatase. J Neurochem. 2001;76:1879–1886. doi: 10.1046/j.1471-4159.2001.00207.x. [DOI] [PubMed] [Google Scholar]
- White EL. Synaptic connections between identified elements. In: White EL, editor. Cortical circuits Synaptic organization of the cerebral cortex. Structure, function and theory. Birkhäuser; Boston: 1989. pp. 46–105. [Google Scholar]
- Wise PM, Dubal DB, Wilson ME, Rau SW. Estradiol is a neuroprotective factor in in vivo and in vitro models of brain injury. J Neurocytol. 2000;29:401–410. doi: 10.1023/a:1007169408561. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm Behav. 1998;34:140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- Wynne RD, Saldanha CJ. Glial aromatization descreases neural injury in the zebra finch (Taeniopygia guttata): influence on apoptosis. J Neuroendocrinol. 2004;16:676–683. doi: 10.1111/j.1365-2826.2004.01217.x. [DOI] [PubMed] [Google Scholar]
- Yague JG, Munoz A, de Monasterio-Schrader P, Defelipe J, Garcia-Segura LM, Azcoitia I. Aromatase expression in the human temporal cortex. Neuroscience. 2006;138:389–401. doi: 10.1016/j.neuroscience.2005.11.054. [DOI] [PubMed] [Google Scholar]
- Yamada K, Harada N, Tamaru M, Takagi Y. Effects of changes in gonadal hormones on the amount of aromatase messenger RNA in mouse brain diencephalon. Biochem Biophys Res Commun. 1993;195:462–468. doi: 10.1006/bbrc.1993.2066. [DOI] [PubMed] [Google Scholar]
- Yamada-Mouri N, Hirata S, Hayashi M, Kato J. Analysis of the expression and the first exon of aromatase mRNA in monkey brain. J Steroid Biochem Mol Biol. 1995;55:17–23. doi: 10.1016/0960-0760(95)00157-u. [DOI] [PubMed] [Google Scholar]
- Yang SH, Liu R, Wu SS, Simpkins JW. The use of estrogens and related compounds in the treatment of damage from cerebral ischemia. Ann NY Acad Sci. 2003;1007:101–107. doi: 10.1196/annals.1286.010. [DOI] [PubMed] [Google Scholar]
- Zhou L, Lehan N, Wehrenberg U, Disteldorf E, von Lossow R, Mares U, Jarry H, Rune GM. Neuroprotection by estradiol: a role of aromatase against spine synapse loss after blockade of GABA(A) receptors. Exp Neurol. 2007;203:72–81. doi: 10.1016/j.expneurol.2006.07.020. [DOI] [PubMed] [Google Scholar]