Summary
Adaptation or gain control allows sensory neurons to encode diverse stimuli using a limited range of output signals. Rod vision exemplifies a general challenge facing adaptational mechanisms - balancing the benefits of averaging to create a reliable signal for adaptation with the need to adapt rapidly and locally. The synapse between rod bipolar and AII amacrine cells dominates adaptation at low light levels. We find that adaptation occurs independently at each synapse and completes in < 500 ms. This limited spatial and temporal integration suggests that the absorption of a single photon modulates gain. Indeed, responses to pairs of brief dim flashes showed directly that synaptic gain was depressed for 100–200 ms following transmission of a single-photon response. Presynaptic mechanisms mediated this synaptic depression. Thus the division of light into discrete photons controls adaptation at this synapse, and gain varies with the irreducible statistical fluctuations in photon arrival.
Introduction
Perception of sights, sounds, and smells requires that sensory systems deal with enormous changes in input signals. For example, we can see over a range of light levels a billion times greater than the range of output signals retinal neurons can produce. Even the range of intensities within a single visual scene often exceeds the number of distinct neural signals. Early sensory processing relies on adaptational mechanisms that match the range of input signals encountered to the range of distinct neural output signals (reviewed by Matthews and Reisert, 2003; Dunn and Rieke, 2006; Vollrath et al., 2007).
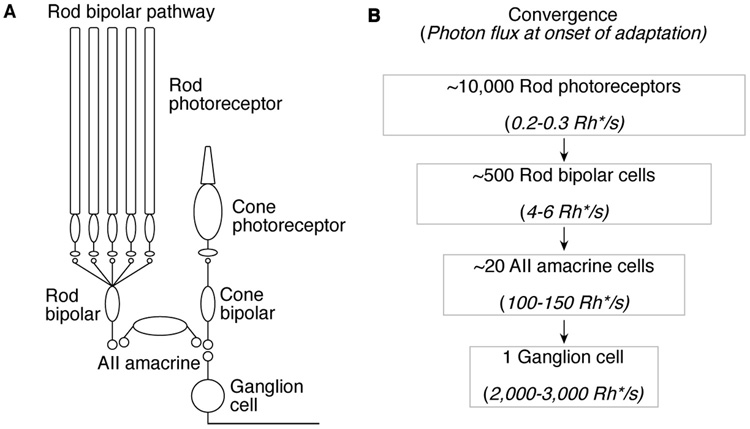
The ability of adaptation to promote efficient sensory coding depends on the extent to which it integrates over space and/or time. Too much integration raises a risk that spatially local or temporally rapid changes in input will saturate neural responses. Too little integration fails to smooth out neural noise, causing gain to fluctuate wildly. These general considerations are particularly prominent for rod vision at low light levels, where gain is controlled even when photons arrive rarely at individual rods. Single-photon responses traverse the mammalian retina through the rod bipolar pathway (rod photoreceptors → rod bipola cells → AII amacrine cells → cone bipolar cells → ganglion cells; (Dacheux and Raviola, 1986; Sterling et al., 1988; Deans et al., 2002;Volgyi et al., 2004; Figure 1A). Convergence is a salient feature of this pathway (Figure 1B). This convergence causes stimuli that produce minute signals in a small fraction of the individual rods to threaten to saturate responses of downstream cells. Adaptational mechanisms operating in the circuitry prevent such saturation; the synapse between rod bipolar and AII amacrine cells is a key site of such circuit adaptation (Dunn et al., 2006).
Figure 1. Convergence in the rod bipolar pathway.
(A) Schematic of the rod bipolar pathway. (B) Estimates of convergence and the light levels in photon absorptions per second (Rh*/s) within the receptive field of each cell at the onset of adaptation. With spatial integration, the number of photons available to control adaptation increases at each stage of processing. Electrical coupling between AII amacrine cells is not depicted but is expected to increase rod input to AII amacrines four fold (Dunn et. al., 2006).
Multiple mechanisms at the rod bipolar-to-AII amacrine synapse could contribute to adaptation, including: (1) a reduction in gain of presynaptic transmitter release; (2) a reduction in synaptic gain due to feedback inhibition from amacrine cells; (3) a reduction in the gain of postsynaptic integration in the AII amacrine cell. The broad goal of the work described here is to provide a correspondence between the functional properties that determine the effectiveness of adaptation and the synaptic mechanisms responsible.
Simultaneous recordings from pairs of synaptically-connected rod bipolar and AII amacrine cells indicated that the gain control mechanism integrates over ~20 rods. Gain control integrated temporally for < 500 ms, much faster than the seconds or minutes time scale of other adaptational mechanisms (reviewed in Barlow, 1972). From the low background levels that reduce gain, we infer that the gain control operates with single photon inputs. We tested this idea directly using pairs of dim flashes to induce gain changes; the properties of paired-flash depression were consistent with gain changes induced by steady background light. Thus single-photon responses traversing the rod bipolar-to-AII amacrine synapse evoke transient synaptic depression, and this reduction in synaptic gain extends the operational range of rod vision. The control of gain by single photons makes gain subject to the irreducible statistical variations in photon arrival, but also protects highly amplified synaptic responses from saturation.
Results
The results below are divided into three main sections: (1) characterization of the properties of gain changes induced by background light; (2) characterization of the interaction between pairs of dim flashes delivered in darkness; (3) exploration of the connection between background adaptation and paired-flash depression.
Paired recordings demonstrate gain control at the synapse between rod bipolar and AII amacrine cells
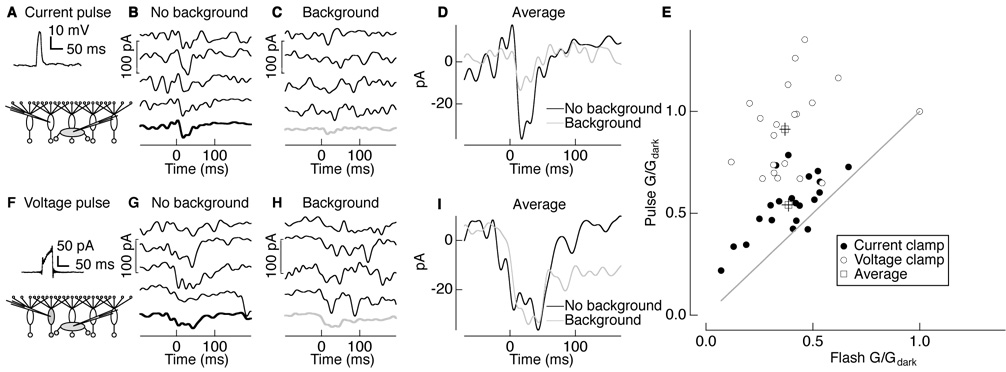
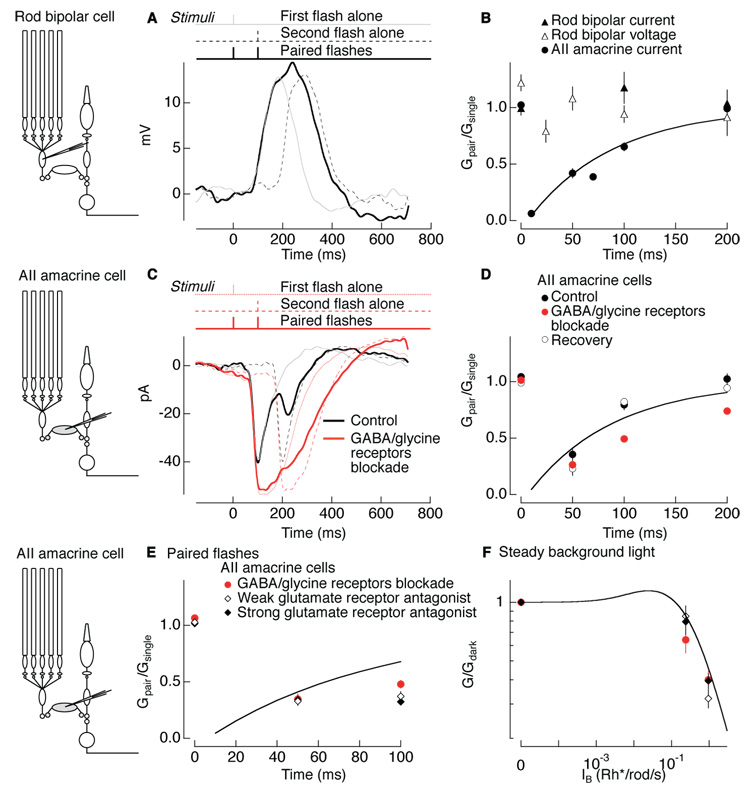
We tested directly for adaptation at the rod bipolar-to-AII amacrine synapse by measuring how synaptic gain depended on background light. We delivered brief depolarizing current pulses to a current-clamped rod bipolar cell while recording the resulting postsynaptic responses in a voltage-clamped AII amacrine cell (Figure 2A).Figure 2B and C show four individual synaptically-evoked responses in an AII amacrine cell in darkness and in the presence of a background light that approximately halved the response to a light flash (background intensity of 1 photon absorption per rod per second (Rh*/rod/s)). Thick traces show average synaptic responses; these are also overlaid in Figure 2D. Background light reduced the amplitude of the synaptically-evoked response, providing direct evidence for gain control at the rod bipolar-to-AII amacrine synapse.
Figure 2. Spatially local gain control at the rod bipolar-to-AII amacrine synapse.
(A–D) Paired recordings between a current-clamped presynaptic rod bipolar cell and voltage-clamped postsynaptic AII amacrine cell. (A) Rod bipolar voltage response to a current pulse of 30 pA for 50 ms. (B and C) Postsynaptic responses of an AII amacrine cell to a presynaptic current pulse in darkness (B) and on a background producing 1 Rh*/rod/s (C). Thick traces are the average of 5–10 responses. (D) Averages in darkness (black) and on background light (gray) overlaid. (E) Gain (G) in the presence of background light normalized to the gain in darkness (Gdark) for synaptic pulse and flash responses in AII amacrine cells. Closed (open) circles are individual postsynaptic responses in AII amacrine cells for presynaptic current-clamped (voltage-clamped) rod bipolar cells and squares are mean ± s.e.m. (current clamp: G/Gdark of 0.54 ± 0.03 for pulse; 0.39 ± 0.03 for flash; mean ± s.e.m.; n = 18; voltage clamp: G/Gdark of 0.91 ± 0.03 for pulse; 0.36 ± 0.03 for flash; mean ± s.e.m.; n = 17). Postsynaptic responses to voltage-clamp pulses were significantly different from those to current-clamp pulses (paired t-test; p < 0.0001) and from flash responses (p < 0.0001). (F–I) Paired recordings between a voltage-clamped presynaptic rod bipolar cell and voltage-clamped postsynaptic AII amacrine cell. (F) Rod bipolar current response to a voltage pulse of 30 mV for 50 ms. (G and H) Postsynaptic AII amacrine responses to the presynaptic voltage pulse in darkness (G) and on a background producing 1 Rh*/rod/s (H). Thick traces are the average of 5–10 responses. (I) Averages in darkness (black) and on background light (gray) overlaid. Averages begin before time 0 due to digital filtering.
To compare synaptic gain changes with those of light responses, we interleaved dim flashes with electrical pulses. We defined flash or pulse gain (Flash or Pulse G/Gdark) as the amplitude of the flash or pulse response normalized to that in darkness. Background light reduced the gain of both the AII amacrine flash (Flash G/Gdark = 0.39) and synaptic responses (Pulse G/Gdark = 0.54; black closed circles in Figure 2E; see Figure 2 legend for statistics). The smaller reduction in synaptic gain may be due to rundown of the light response in the rod bipolar cell used to probe synaptic gain compared to the other, unperturbed, rod bipolars used to probe flash response gain.
Gain control is spatially localized
Gain control at the rod bipolar-to-AII amacrine synapse could be mediated locally at each synapse or globally across synapses, e.g., through feedback from a wide-field amacrine cell. The experiment of Figure 2A–D does not distinguish between local and global mechanisms because the voltage of all rod bipolar cells, including the recorded rod bipolar, changed in concert. To distinguish between local and global gain controls, we prevented the recorded rod bipolar cell from changing its voltage in response to the background light while leaving all the other presynaptic rod bipolar cells responsive to light. If the mechanism operates locally at the synapses between a single rod bipolar and AII amacrine cell, then synaptic gain changes should be eliminated because the presynaptic rod bipolar cell we electrically stimulate is “blind” to background light. Alternatively, if signals from multiple rod bipolars are combined and used to control the gain of individual synapses, then background light should continue to reduce gain when the presynaptic rod bipolar voltage is held fixed.
Figure 2F–I show the effect of background light on the gain of signal transfer between a voltage-clamped rod bipolar cell and an AII amacrine cell. Figure 2G and H show four individual responses of the AII amacrine cell to depolarization of the rod bipolar cell in darkness and in the presence of background light (intensity 1 Rh*/rod/s). Figure 2I overlays average AII amacrine cell responses with and without the background. Voltage-clamping the presynaptic rod bipolar cell eliminated the gain changes induced by background light.
Similar to the previous experiment, flashes were interleaved with electrical pulses. Figure 2E summarizes the effect of background light on the gain of flash-evoked and synaptically-evoked responses (open circles; see Figure 2 legend for statistics). Gain changes were similar in flash-evoked responses whether the presynaptic rod bipolar was current clamped (Figure 2A–D; Flash G/Gdark = 0.39 in Figure 2E) or voltage clamped (Figure 2F–I; Flash G/Gdark = 0.36 in Figure 2E). This is expected because the voltage clamp affects only one of ~25 rod bipolar inputs to an AII amacrine cell. However, changes in the gain of synaptically-evoked responses were nearly eliminated when the presynaptic rod bipolar was voltage clamped (Pulse G/Gdark = 0.91 in Figure 2E). These results indicate that the gain of each rod bipolar-to-AII amacrine cell synapse is primarily controlled locally, without substantial influence from nearby rod bipolar cells.
Gain control is a feedforward mechanism
Local control of the gain of the rod bipolar-to-AII synapse could be implemented by a feedforward property of the synapse or by an amacrine cell that receives input from and provides a confined feedback signal to the same rod bipolar cell. The GABAergic A17 amacrine cell, in particular, has been implicated in regulating vesicle release at the rod bipolar synaptic terminal (Li et al., 2002). Further Chavez et al. (2006) suggest that reciprocal feedback between the A17 amacrine cell and rod bipolar cell may be compartmentalized to maintain synapse specificity, thus satisfying the requirement for a local mechanism.
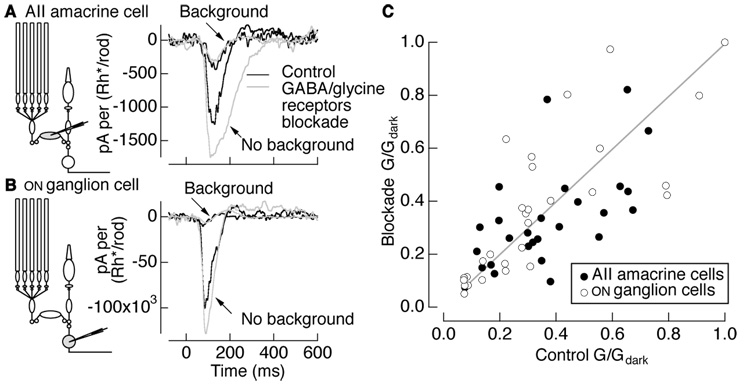
To investigate the involvement of inhibitory amacrine cells, we compared background-induced gain changes before and after blocking GABA and glycine receptors. The drugs used were effective in blocking feedback to rod bipolar cells as judged by the elimination of inhibitory post-synaptic currents in rod bipolar cells held at 0 mV (not shown). We examined the effect of blocking inhibition on the responses of both AII amacrine cells and ON ganglion cells, which exhibit similar background-dependent gain changes (Dunn et al., 2006). Figure 3A and B show flash responses of an AII amacrine cell and an ON ganglion cell in darkness and in the presence of a background of 1 Rh*/rod/s under control conditions (black) and with GABA/glycine receptors blocked (gray). Background-induced gain changes persisted with GABA/gycine receptors blocked; indeed in some cases, such as Figure 3A and B, gain changes increased, an observation we return to below in describing paired-flash depression.
Figure 3. Inhibition is not required for background-induced gain changes.
Example current responses of (A) an AII amacrine cell and (B) ON ganglion cell to flashes in darkness and with a background producing 1 Rh*/rod/s under control conditions (black) and blockade of GABAA, GABAC, and glycine receptors (10 µM gabazine, 50 µM TPMPA, 5 µM strychnine). Current responses (pA) were normalized by the flash strength in Rh*/rod. (C) AII amacrine (n = 25) and ON ganglion cell (n = 25) gain normalized to the gain in darkness for control conditions and GABA/glycine receptor blockade conditions are similar, indicating that inhibitory synaptic inputs do not mediate background-induced gain changes. The ratio of G/Gdark under blockade of GABA/glycine receptors to control conditions was 1.02 ± 0.08 (mean ± s.e.m.) for AII amacrine cells and 1.12 ± 0.07 for ganglion cells.
Figure 3C collects results from multiple AII amacrine cells and ganglion cells; average gain changes did not differ significantly with and without GABA/glycine receptors blocked (see Figure 3 legend for statistics). Individual cells show considerable spread about the mean behavior, some of which is attributed to uncertainty in measuring gain. The persistence of gain control in the absence of GABA/glycinergic-mediated inhibition suggests that it is largely or entirely a feedforward property of the rod bipolar-to-AII amacrine synapse.
Temporal properties of gain control
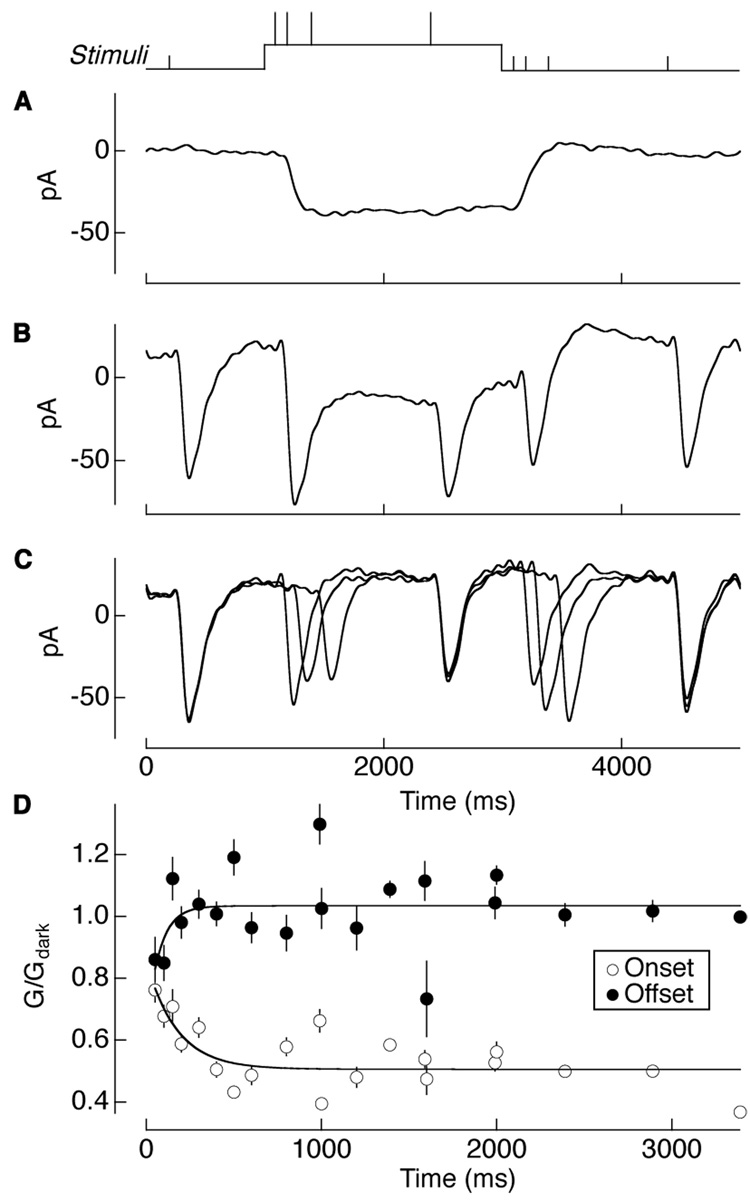
We examined the kinetics of gain control by delivering brief flashes of light at different times relative to a background step (see Stimulus trace in Figure 4). Gain control operated within a few hundred milliseconds. Figure 4A shows the response of an AII amacrine cell to the background step alone. Figure 4B shows responses to flashes superimposed on the background, and Figure 4C shows the flash responses after subtracting the average background step response. The gradual decrease (increase) in the response amplitude following the onset (offset) of the background indicates the time course of gain control.
Figure 4. Temporal properties of gain control.
(A) Current responses of an AII amacrine cell to a step of 1 Rh*/rod/s, (B) with 10 ms flashes superimposed on the step response, and (C) with the step response subtracted from the superimposed flashes. Stimulus timing is shown above. (D) Gain of the subtracted flash responses normalized to the gain of the first flash in darkness to show the kinetics of gain changes following the onset and offset of the 0.3 Rh*/rod/s background light. Error bars are s.e.m. (n = 11). Data points for onset and offset were fit with a single exponential (solid line), Equation 1 (Experimental Procedures), with G0 = 0.5 for onset and 1.0 for offset, A = 0.3 for onset and −0.4 for offset, and τ = 170 ± 90 ms for onset and 80 ± 50 ms for offset (mean ± s.d.).
To quantify the kinetics of adaptation onset and offset, the amplitude of each isolated flash response (Figure 4C) was normalized by the amplitude of the flash response in darkness. These normalized amplitudes were plotted against time and fit with a single exponential (Figure 4D; time constants of 170 ms for onset and 80 ms for offset; see Experimental Procedures and Figure 4 legend for statistics). Backgrounds of 0.3 and 1 Rh*/rod/s produced similar onset and offset time constants (not shown).
Gain modulated by single photons: paired-flash depression
We found previously that gain control becomes prominent for backgrounds producing 0.2–0.3 Rh*/rod/s (Dunn et al., 2006). The paired rod bipolar-AII amacrine cell recordings (Figure 2) indicate that the gain control mechanism integrates signals from the ~20 rods that provide input to a single rod bipolar cell. The rapid gain changes following background onset and offset (Figure 4) indicate that the integration time is 100–200 ms. Together, these results imply the gain control operates with ~1 Rh* per integration area per integration time (Rh*/Σspace/Σtime). The elimination of a substantial fraction of the rod’s single-photon responses by a nonlinearity at the rod-to-rod bipolar synapse (Field and Rieke, 2002; Berntson et al., 2004) will lower this estimate.
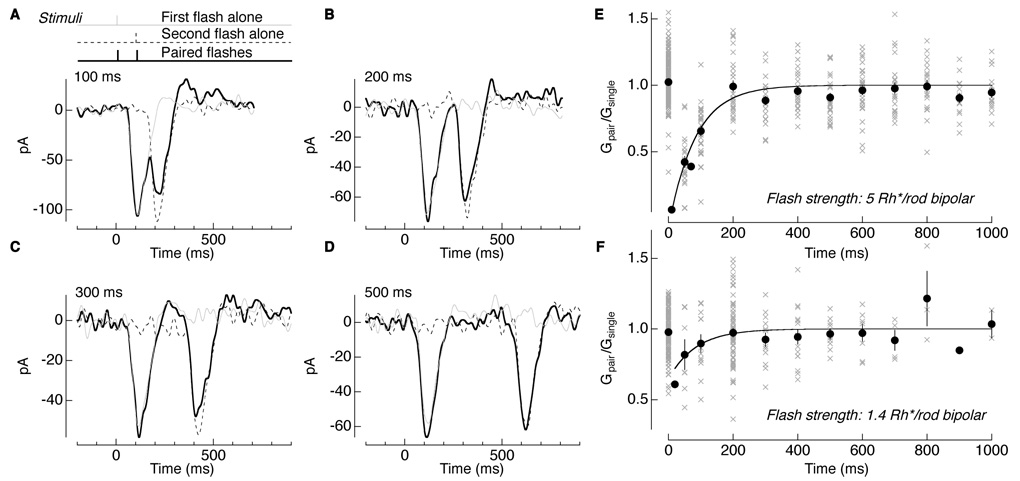
The calculation above predicts that each single-photon response traversing the rod bipolar-to-AII amacrine cell synapse reduces synaptic gain for a few hundred milliseconds. We tested this hypothesis directly by delivering pairs of brief flashes and determining whether the first flash changed the gain of the response to the second flash. Figure 5A shows the response of an AII amacrine cell to two flashes separated by 100 ms. The gray and dashed lines show interleaved single flashes delivered at early and late times. Pairs of flashes were delivered for a range of time separations (Figure 5A–D). For short separations, the response to the second pulse was diminished relative to the first, an indication of paired-flash depression. This effect diminished for separations exceeding a few hundred milliseconds.
Figure 5. Paired-flash depression induced by dim flashes.
(A–D) Current responses of an AII amacrine cell to a single 10 ms flash delivered at time 0 (gray), at 100 ms (dashed line), and paired flashes in sequence (solid). In each trial the interval between the two flashes was varied. The interval between trials was at least 800 ms, allowing light response amplitudes to recover before the delivery of subsequent flashes. (E and F) Summary of gain of the paired response (Gpair) normalized to the gain of the single response (Gsingle) across interstimulus intervals. Points at time 0 represent the normalized gain values of the first flash. All other points represent gain for the second flash. Points fit with a single exponential, Equation 1 (Experimental Procedures), with G0 fixed at 1, A = −1.1, and time constant τ = 83 ± 11 ms (mean ± s.d.) for a flash strength of 5 Rh*/rod bipolar (n = 26) (E) and G0 fixed at 1, A = −0.4 and τ = 84 ± 40 ms for a flash strength of 1.4 Rh*/rod bipolar (n = 15) (F).
We calculated the effect of the first flash on the response to the second flash by subtracting the response to the first flash alone from the response to the pair of flashes and comparing the amplitude of the result to that of the response to the second flash alone (see Experimental Procedures). Figure 5E summarizes the normalized gain as a function of flash separation (gray crosses for individual trials and black closed circles for averages). As a control, we calculated the normalized gain for the first flash in the pair, which, on average, should be 1 (points at 0 s in Figure 5E and F). Gain changes at flash strengths of 5 Rh*/rod bipolar (Figure 5E) and 1.4 Rh*/rod bipolar (Figure 5F) were fit with a single exponential with a time constant of ~85 ms (see Figure 5 legend). The kinetics of paired-flash depression provide another estimate of the kinetics of the offset of gain control, comparable to that obtained from light steps (Figure 4D).
The nonlinearity at the rod-to-rod bipolar synapse causes the number of effective photon absorptions to be less than that based on simple convergence (see Experimental Procedures); thus the flashes in Figure 5F produced 0.3–0.7 Rh*effective/rod bipolar. The persistance of paired-flash depression for these dim flashes indicates that each effective photon absorption in the rod bipolar receptive field reduces the gain of the AII amacrine cell response for ~100 ms.
Properties of paired-flash depression
Next we compared the site and properties of paired-flash depression and background adaptation. If the same mechanism mediates paired-flash depression and the background adaptation characterized in Figure 1–Figure 4, paired-flash depression should occur at the rod bipolar-to-AII amacrine cell synapse. To test this prediction, we compared paired-flash depression in rod bipolar cell responses with that in AII amacrine cell responses. Figure 6A shows voltage responses of a rod bipolar cell to flashes (5 Rh*/rod bipolar) separated by 100 ms. Figure 6B compares the normalized gain as a function of flash separation for the rod bipolar cell currents (closed triangles) and voltages (open triangles) and for the AII amacrine cell currents (closed circles and fit from Figure 5E). Neither the rod bipolar currents nor voltages showed significant paired-flash depression. The absence of paired-flash depression in the rod bipolar input currents and output voltages and presence in the AII amacrine cell input currents locate paired-flash depression to the rod bipolar-to-AII amacrine cell synapse.
Figure 6. Paired-flash depression involves changes in glutamate concentration at the rod bipolar-to-AII amacrine synapse.
(A) Voltage responses of a rod bipolar cell to a single 10 ms flash delivered at time 0 (gray), at 100 ms (dotted line), and paired flashes in sequence (solid). (B) Summary of gain of the paired response (Gpair) normalized to the gain of single response (Gsingle) for rod bipolars currents (closed triangles; n = 22), voltages (open triangles; n = 27), and AII amacrine currents (closed circles; n = 26) from Fig 5E (see Experimental Procedures). At 100 ms interval, the rod bipolar cell currents and voltages are not significantly different (paired t-test; p > 0.13). Rod bipolar cell currents are significantly different than AII amacrine cell currents (paired t-test; p < 3e-7 at 50 ms; p < 0.008 at 100 ms). Flash strength was 5 Rh*/rod bipolar across all conditions. (C) Current responses of an AII amacrine cell under control conditions (black) and under blockade of GABA/glycine receptors (red) to a single flash delivered at time 0 (gray or dotted red line), at 100 ms (dashed line), and paired flashes in sequence (solid). (D) Summary of gain of the paired response normalized to the gain of the single response for AII amacrine cell currents (n = 26) under control conditions (black closed circles), under blockade of GABA/glycine receptors (red closed circles), and after recovery (black open circles). (E) Summary of gain of paired response normalized to the gain of the single response for AII amacrine cell currents (n = 20) under constant blockade of GABA/glycine receptors (red circles), and in the presence of the weak glutamate receptor kynurenic acid (700 µM; open diamonds), and in the presence of the strong glutamate receptor antagoinist NBQX (300 nM; closed diamonds). Line in B, D, E is the fit to the AII amacrine currents taken from Fig 5E. (F) AII amacrine cell (n = 18) gain (G) normalized to the gain in darkness (Gdark) induced by steady background light under the same three drug conditions used in E. Line shown previously in (Dunn et al., 2006). Error bars are s.e.m.
To determine if paired-flash depression required inhibitory synaptic input, we delivered pairs of flashes with GABA and glycine receptors blocked (Figure 6C–D). Suppressing GABA/glycine receptors affected response kinetics and enhanced overall response amplitude (Figure 6C), but failed to eliminate depression induced by a pair of flashes (compare red and black circles in Figure 6D). Paired-flash depression was instead exacerbated without GABA and glycinergic inhibition, similar to the gain changes induced by steady background light in some cells (points below equality line in Figure 3C). These results indicate that paired-flash depression is a feedforward property of the synapse, like the gain control induced by steady background light (Figure 3).
Paired-flash depression could reflect a decrease in glutamate concentration in the synaptic cleft or a decreased sensitivity of postsynaptic currents to released transmitter due to desensitization or saturation of postsynaptic glutamate receptors. To distinguish between these possibilities, we compared the effect of weak and strong glutamate receptor antagonists on paired-flash depression. Rapidly dissociating weak antagonists minimize the effects of receptor desensitization and saturation, unlike slowly dissociating strong antagonists (see Experimental Procedures). Thus if synaptic depression occurs by a decrease in glutamate concentration, it should persist in the presence of both weak and strong receptor antagonists. If depression occurs by receptor desensitization or saturation, it should be decreased by the weak (but not strong) antagonist.
Figure 6E shows that both weak and strong glutamate receptor antagonists (kynurenic acid and NBQX) had little effect on paired-flash depression. We blocked GABA and glycine receptors in these experiments to eliminate the confounding effect of altering inhibition by suppressing glutamatergic transmission to amacrine cells. Both weak and strong glutamate antagonists also had little effect on gain changes produced by steady background light (Figure 6F). Thus neither desensitization nor saturation of postsynaptic receptors appears to contribute substantially to gain control; consistent with this result, recovery of AII amacrine AMPA receptors from desensitization (Veruki et al., 2003) is ~5 times faster than recovery from paired-flash depression. Instead, the lack of effect of glutamate receptor antagonists indicates that changes in glutamate concentration, likely due to changes in transmitter release, cause paired-flash depression and adaptation to background light.
Paired-flash depression predicts background-induced gain changes
Can paired-flash depression explain the gain changes induced by steady background light? To answer this question, we predicted the effect of steady background light on gain using the measured amplitude and kinetics of paired-flash depression.
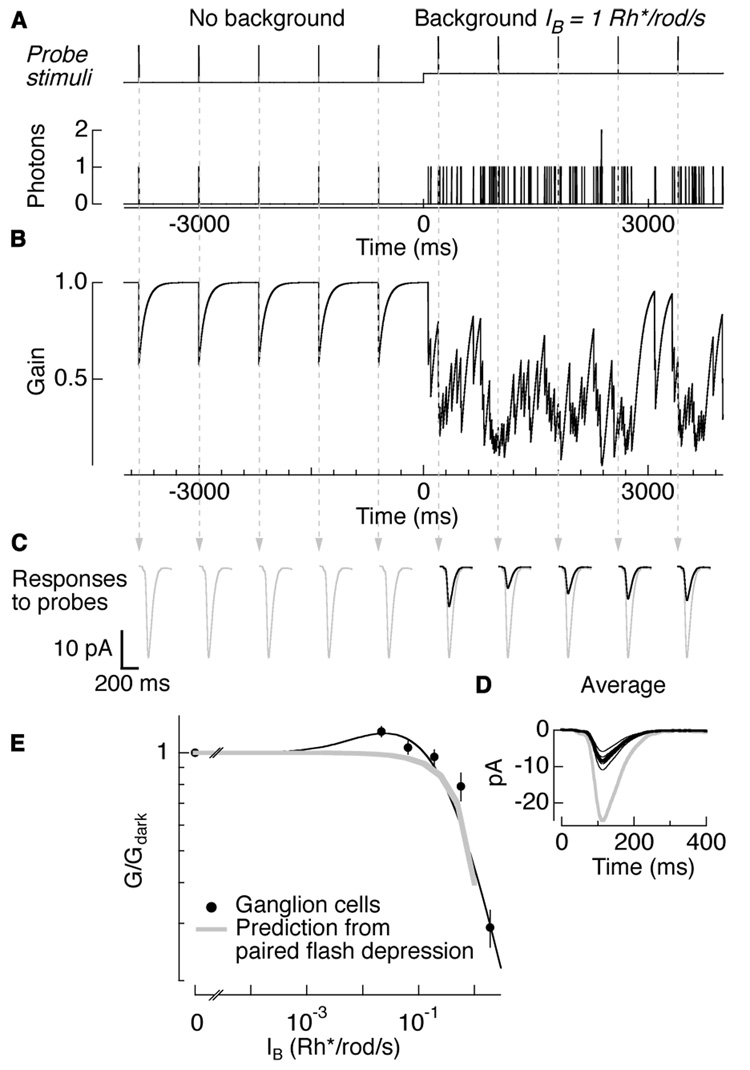
The predicted gain changes made two assumptions: (1) gain changes occur independently in each rod bipolar cell (demonstrated in Figure 2), and (2) the absorption of 1 Rh*/rod bipolar is enough to induce depression in the response to a subsequent flash delivered within a certain time window (demonstrated in Figure 5F). Figure 7 illustrates the gain model. Background light produced a train of randomly timed photon events; probe stimuli delivered periodically monitored gain (Figure 7A). Figure 7B plots the predicted gain over time assuming that gain following each photon absorption recovered exponentially with a 85 ms time constant. The gain of the measured flash responses will depend on the relative timing of probe stimuli (arrows) and background photon events (Figure 7C). On average, backgrounds producing 1 Rh*/rod/s halved the gain of flash responses (G/Gdark = 0.47; Figure 7D). For comparison, responses in darkness are shown in gray.
Figure 7. Amplitude and kinetics of paired-flash depression can predict background-dependent gain changes.
(A) Schematic of probe stimuli delivered every 800 ms in darkness and on a background of 1 Rh*/rod/s (top panel). Poisson train of single photon events for probe and background photons (bottom panel). (B) Predicted gain values for each photon absorption, obtained from paired-flash depression of a single photon (1 Rh*/rod bipolar). (C) Responses to probe flashes (arrows) in an AII amacrine cell in darkness (first 5 responses) and on the background (last 5 responses). Average responses in darkness are shown in gray for comparison. (D) Overlaid individual trials (thin lines) and average (thick lines) responses in darkness (gray) and on the background (black). Gain at simulated background normalized to gain in darkness is 0.47 ± 0.08 (mean ± s.e.m; n = 5). (E) Background-dependent gain changes for ON ganglion cells (black circles). Results and fit shown previously in (Dunn et al., 2006). Overlaid in gray is the gain change prediction made by paired-flash depression induced by a single photon. Prediction was for backgrounds dimmer than at which rod photoreceptor adaptation began.
Figure 7E compares the background-dependence of the predicted gain changes from paired-flash depression (gray) with the measured gain changes in ganglion cells (black circles, similar to those in AII amacrine cells; data from Dunn et al., 2006). The predictions fail to capture the increase in gain for weakest backgrounds, but are otherwise in good agreement with experimental results. This correspondence holds for backgrounds up to 1 Rh*/rod/s; at brighter backgrounds rod photoreceptors adapt. Because the interaction between receptor and network gain control mechanisms is unknown we did not predict gain at brighter backgrounds. The ability of paired-flash depression to account for adaptation produced by steady background lights provides another line of evidence that background adaptation is mediated by depression of the rod bipolar-to-AII amacrine synapse following transmission of a single-photon response.
Discussion
We examined the functional properties of gain control at the rod bipolar-to-AII amacrine synapse by measuring how background light changed the AII amacrine flash response and the synaptic gain. We found that gain is controlled independently at each rod bipolar-to-AII amacrine cell synapse and that each single-photon response traversing the synapse reduces gain for 100–200 ms. Thus, at these light levels, adaptation is controlled by the division of light into discrete photons and synaptic gain is modulated by the irreducible quantal fluctuations in photon absorption. Below we discuss what properties of the rod bipolar pathway are specialized for transmission of single-photon responses and why the rod bipolar-to-AII amacrine synapse is a necessary location for gain control. We consider the consequences of minimal spatial and temporal integration in gain control as well as how these properties may be relevant for encoding and viewing statistics. Finally, we consider possible mechanisms underlying gain control.
Strategies for transmitting single-photon responses through the rod bipolar pathway: amplification and convergence
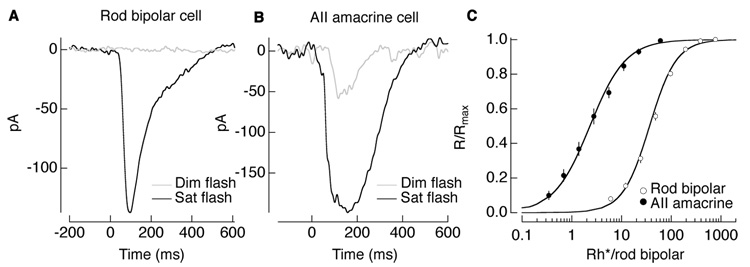
Rod photoreceptors generate highly amplified responses to single photon absorptions. Two issues make reliable transmission of these signals a challenge. First, at low light levels, convergence threatens to obscure signals from the few rods absorbing photons with noise from the remaining rods. A thresholding nonlinearity at the rod-to-rod bipolar synapse serves to retain selectively signals from those rods absorbing photons, thus substantially improving the signal-to-noise ratio of the rod bipolar responses (Field and Rieke, 2002). Second, downstream of the rods, noise introduced by elements of the rod bipolar circuitry threatens to swamp the small responses to single absorbed photons. Amplification at the rod bipolar-to-AII amacrine synapse (Pang et al., 2004) helps mitigate the impact of these downstream noise sources. Thus a flash producing 1 Rh*/rod bipolar (0.25–0.5 Rh*effective/rod bipolar) produces a barely discernible response in a rod bipolar (~3% of maximal response) but produces a nearly half-maximal response in an AII amacrine cell (Figure 8A–C).
Figure 8. Light responses of AII amacrine cells are more amplified than those of rod bipolar cells.
Light responses in the AII amacrine cell more amplified than in the rod bipolar cell. (A) Current responses of a rod bipolar cell to a 10 ms dim flash (gray; 0.06 Rh*/rod) and saturating flash (black; 37.8 Rh*/rod). (B) Current responses of an AII amacrine cell to a 10 msec dim flash (gray; 0.07 Rh*/rod) and saturating flash (black; 4 Rh*/rod). Signals are amplified in the AII amacrine cell. (C) Intensity-response relations for rod bipolars and AII amacrine cells taken from (Dunn et al., 2006) and shown on a scale for photon absorptions per rod bipolar. Flash strength does not account for the nonlinearity at the rod-to-rod bipolar synapse to show effective photon absorptions; this would shift the data points leftward by a factor of 0.3–0.5. AII amacrines cell responses reach saturation at light levels 100 times less than responses of rod bipolar cells.
Consistent with its high amplification, the rod bipolar-to-AII amacrine synapse is the element of the rod bipolar pathway most threatened by saturation at low light levels (Dunn et al., 2006). Indeed as light levels increase, the threat of saturation arises as soon as more than 1 photon is absorbed in the collection of rods providing input to a rod bipolar cell within the ~200 ms integration time for rod signals. Thus the gain of the synaptic inputs to the AII amacrine cell input must be controlled to prevent saturation of the AII amacrine cell and downstream retinal neurons.
Consequences of controlling gain with single photons
While gain control at the rod bipolar-to-AII amacrine cell synapse is required to avoid saturation, nothing about the amplification of signals at the synapse indicates how many photons are required for the adapting signal. For example, the gain control mechanism could, in principal, rely on a wide-field amacrine cell that integrates photon absorptions over a spatial region larger than the rod bipolar cell. Greater integration would increase the reliability of signal controlling adaptation. To prevent saturation of the rod bipolarto- AII amacrine synapse, such a mechanism would likely need to operate on transmitter release or on postsynaptic receptors rather than on the integration of synaptic inputs by the AII amacrine cell. Consistent with this view, electrical coupling between AII amacrine cells, which influences synaptic integration, contributes minimally to gain control at the rod bipolar-to-AII amacrine synapse (Figure 8 in Dunn et al., 2006).
We find that gain control at the rod bipolar-to-AII amacrine cell synapse is a spatially localized, rapid mechanism that operates with single photons. The reduction of gain for single photon absorptions implies gain will be inherently noisy. In particular, the random arrival of individual photons at a nominally constant background intensity will necessarily produce fluctuations in gain (Figure 7C, compare last 5 flash responses). This presents a different view of gain control than the more common examples of a volume control on a radio or exposure setting on a camera. In particular, rather than acting deterministically, synaptic gain fluctuates rapidly. Gain fluctuations add to the other sources of noise and compound the challenges facing the visual system at low light levels.
Noisy gain control seems a poor strategy for reliably representing the world around us. Noise is an obvious drawback of limited spatial and temporal integration, but this limited integration is not without benefits. For example, gain control presents an opportunity to weight responses according to their reliability (Rudd and Brown, 1996; Brown and Rudd, 1998). The properties of gain control at the rod bipolar-to-AII amacrine synapse cause the noise in the AII amacrine responses to be relatively independent of background (Dunn et al., 2006), instead of increasing with increasing background as expected from quantal fluctuations in photon absorption. Thus signals from nearby rod bipolar cells stimulated with different photon fluxes will be subject to independent gain controls that approximately equate their noise. This effective normalization of rod bipolar inputs implies that noise in the integrated signal produced in the AII amacrine cell will be efficiently reduced by averaging across rod bipolar inputs rather than being dominated by a few inputs with high noise (e.g. due to high photon fluxes).
The short temporal integration of gain control makes sense considering how we view visual scenes. Saccades occur every 100–400 ms during free viewing of natural scenes and in darkness (results reported in cat (Maldonado and Babul, 2007)), thereby setting a limit on the relevant temporal integration of local light stimuli. Additional integration, while producing a more reliable signal, makes adaptation inappropriate once the eyes have moved to a new location.
Possible mechanisms underlying paired-flash depression and background-induced gain changes
Manipulation of glutamate receptors on AII amacrine cells suggested that paired-flash depression was due to changes in presynaptic vesicle release. Previous work at the rod bipolar-to-AII amacrine synapse, using presynaptic electrical stimulation rather than flashes of light, also suggests that a presynaptic mechanism underlies paired-pulse depression (von Gersdorff and Matthews, 1997; Singer et al., 2004; Singer and Diamond, 2006). This mechanism could involve vesicle depletion (von Gersdorff and Matthews, 1997; Singer and Diamond, 2006), changes in vesicle release through a neurotransmitter such as dopamine affecting intracellular calcium levels (Heidelberger and Matthews 1994), or direct modulation of presynaptic calcium channels (Palmer et al., 2003).
To test further the possibility that presynaptic mechanisms underlie depression, one could modulate the rod bipolar release probability by reducing extracellular calcium locally (so as not to affect transmitter release from the rod photoreceptors). For a presynaptic mechanism, paired-flash depression is expected to decrease, similarly to results reported for paired-pulse depression (Singer et al., 2004).
Electrically-evoked paired-pulse depression cannot be compared directly with our light-evoked paired-flash depression because the synaptic signals are very different; nonetheless there are similarities. Paired-pulse depression reduces the second postsynaptic response by ~50% for intervals of less than 100 ms (Singer and Diamond, 2006), consistent with our measurements in Figure 5. In addition, one component of paired-pulse depression recovered with a time constant of ~200 ms, roughly similar to the time constant characteristic of paired-flash depression. Thus the presynaptic mechanisms underlying paired-pulse depression measured with electrical pulses may underlie paired-flash depression.
What is the connection between paired-flash depression and gain changes with steady background light? We have located the mechanism for paired-flash depression to the rod bipolar-to-AII amacrine cell synapse (Figure 6A–B), a key site for adaptation produced by background light (Dunn et al., 2006). Furthermore neither paired-flash depression nor background-induced gain changes require GABA or glycine receptors (Figure 6C–D and Figure 3), suggesting the mechanism(s) is a feedforward property of the rod bipolar-to-AII amacrine cell synapse. Inhibition contributes to this signaling pathway, as evidenced by the slower and larger responses of the AII amacrine cell under blockade of inhibitory receptors (compare red to black traces in Figure 6C). The exacerbation of paired-flash depression (Figure 6C–D) may result if, for example, inhibition plays a role in preventing vesicle depletion by truncating the light-induced depolarization of the rod bipolar synaptic terminal (Dong and Hare, 2003). The similarities in location, feedforward properties, presynaptic mechanisms, and the ability of paired-flash depression to predict gain changes induced by steady background light suggest that the regulation of transmitter release serves light adaptation at low light levels. In other parts of the nervous system, synaptic depression also underlies adaptation, for example in the mammalian cortex and avian auditory brainstem (Abbott et al., 1997; Tsodyks and Markram, 1997; Cook et al., 2003).
The spatial, temporal, and mechanistic properties of gain control at the rod bipolar-to-AII amacrine synapse indicate that a single photon modulates gain. In a limited sense of efficient coding, this adaptive mechanism, subject to fluctuations in the stimulus, fails to produce reliable signals. However in considering natural vision, this adaptive mechanism seems appropriate. The spatial inhomogeneity of a natural scene requires minimal spatial integration so as not to degrade the resolution necessary to pick out the locations of photons in a dark environment. Likewise, natural eye movements impose constraints on temporal integration. These spatial and temporal constraints on natural vision perhaps dictate that an efficient, perceptually meaningful control of gain acts locally and rapidly on single photons.
Experimental Procedures
Tissue
We recorded from mouse (C57BL/6) retinas. Mice were dark-adapted for 12 hrs, and the retinas were isolated under infrared light (≥ 950 nm) following procedures approved by the Administrative Panel on Laboratory Animal Care at the University of Washington.
Recording procedures
Rod bipolar cells and AII amacrine cells were recorded in retinal slices as previously described (Field and Rieke, 2002; Armstrong-Gold and Rieke, 2003; Dunn et al., 2006). ON alpha-like ganglion cells were recorded in flat mount preparations as previously described (Dunn et al., 2006). Rod bipolar cells were recorded under whole-cell current or voltage clamp (held at −60mV) with an internal solution containing (in mM) 110 K-Aspartate, 10 HEPES, 5 NMG-HEDTA, 1 MgCl2, 0.5 CaCl2, 4 Mg-ATP, 0.5 Tris-GTP, 10 L-glutamic acid, 10 sodium phosphocreatine; pH was adjusted to 7.2 with K-OH and osmolarity was ~280 mOsm. Currents of AII amacrine cells and ganglion cells were measured under voltage clamp (held at −60mV) with an internal solution containing (in mM) 105 CsCH3SO3, 10 TEA-Cl, 20 HEPES, 10 Cs2-EGTA, 2 QX314-Br, 5 Mg-ATP and 0.5 Tris-GTP; pH was adjusted to 7.2 with CsOH and osmolarity was ~280mOsm. Junction potentials (~ −10 mV) have not been corrected.
Current-clamped rod bipolar cells had resting membrane potentials of −59 ± 1 mV (mean ± s.e.m; n = 18). For rod bipolar cells, typical access resistances were 20–50 MΩ and input resistances were 1000–1500 MΩ. For AII amacrine cells, typical access resistances were 40–60 MΩ and input resistances were 300–500 MΩ. For ON alpha-like ganglion cells, typical access resistances were 15–25 MΩ and input resistances were 100–150 MΩ. Access resistances were not compensated. Light responses in rod bipolar cells persisted for 140 ± 40 s (mean ± s.d.; n = 22) after whole-cell recording began; data was collected during the initial 88 ± 45 s. Light responses in AII amacrine and ganglion cells persisted for the duration of the recording.
Electrical stimulation
For paired recordings between synaptically-coupled rod bipolar cells and AII amacrine cells, 50 ms electrical pulses were delivered to the rod bipolar cell to elicit a robust, but not saturating, postsynaptic response in the AII amacrine cell. Injected pulses ranged from 10–40 pA under current-clamp and 20–40 mV under voltage-clamp.
Light stimuli
Light stimuli were delivered from light emitting diodes (LEDs) with peak output wavelengths of 470 nm (10 msec flashes) and 513 nm (background light). Calibrated photon fluxes were converted to rates of rhodopsin isomerizations (Rh*/s) using the measured LED spectra and photon flux at the preparation (Dunn et al., 2006).
Pharmacology
To block inhibitory synaptic transmission, we added the GABAA receptor antagonist gabazine (10 µM), the GABAC receptor antagonist TPMPA (50 µM), and the glycine receptor antagonist strychnine (5 µM) (Figure 3 and 6C–F) to the superfusion solution.
To determine whether changes in glutamate concentration and/or desensitization or saturation of postsynaptic receptors mediate gain control, we compared the effects of strong and weak glutamate receptor antagonists. The key distinction between the strong and weak antagonists is the difference in their dissociation rate from the receptors. Rapidly dissociating weak antagonists minimize the effects of receptor desensitization and saturation, unlike slowly dissociating strong antagonists. The basis for this difference is described below.
We blocked about half of the AII amacrine cell AMPA receptors using subsaturating concentrations of the weak antagonist kynurenic acid (700 µM) and the strong antagonist NBQX (300 nM) (Figure 6E–F). We estimated the dissociation rate of the antagonist from koff = kon K, where K is the affinity and kon is the binding rate constant (Sampath and Rieke, 2004). The binding rate constant for glutamate receptor antagonists to the AMPA receptor, kon, ranges from 1 to 50 µM−1s−1 (Clements et al., 1998), and the affinity, K, is 172 µM for kynurenic acid (Weber et al., 2001) and 24 nM for NBQX (Kovacs et al., 2004). Using these previously reported values, we calculated that kynurenic acid has a koff of 170–8600 s−1 and that NBQX has a koff of 0.024–1.2 s−1. Thus kynurenic acid dissociates from the AMPA receptor much more rapidly than the ~100 ms duration of the flash response and the 50–100 ms separation between flashes in the paired-flash experiments (Figure 6E), whereas NBQX dissociates much more slowly.
The rapid dissociation of kynurenic acid means that: (1) some receptors bound to antagonist and protected from desensitization during the response to the first flash become available during the response to the second flash; and (2) competition between kynurenic acid and glutamate decreases the effective glutamate affinity of the receptors. Thus kynurenic acid protects some receptors from desensitization and saturation. The slow dissociation of NBQX means that the same complement of receptors (those not bound to the antagonist) is largely responsible for responses to both flashes; these receptors are subject to desensitization and saturation. Thus for a mechanism relying on AMPA receptor desensitization or saturation, the degree of paired-flash depression measured under kynurenic acid should be less than that measured under NBQX. For a mechanism involving changes in glutamate concentration, the degree of paired-flash depression measured in the presence of kynurenic acid should be at least equivalent to that measured in the presence of NBQX.
Measuring gain
Gain was determined from the correlation between individual responses and a template formed from the average of all responses measured under the same conditions (see Methods of (Dunn et al., 2006)). Gain (G) was normalized to 1 for a single-photon response in the dark (Gdark). Gain values below 1 indicate the action of a gain control.
Kinetics time constants
The dependence of normalized gain, Gnorm on time, t, was fit with a single exponential:
| (1) |
where Gnorm is either G/Gdark in Figure 4D or Gpair/Gsingle in Figure 5E and F. G0 is the asymptotic value of the gain, A is a scale factor and τ is the time constant for Gnorm to reach 37% of its final value.
Quantification of the degree of paired-flash depression
To quantify paired-flash depression, we subtracted the response to the first flash alone (gray in Figure 5A) from the response to the flash pair (solid line in Figure 5A) and divided the result (Gpair) by the response to the second flash (Gsingle; dashed line in Figure 5A). As a control, we calculated the gain of the response to the first flash. As expected, the resulting ratio, Gpair/Gsingle, was near 1 (Figure 5E, F and Figure 6B, D, E).
Paired-flash depression can explain background-dependent changes in gain
To determine whether the amplitude and kinetic properties of paired-flash depression could predict the background-dependent gain changes measured previously (Dunn et al., 2006; Figure 7E), we first extrapolated the paired-flash depression properties for the absorption of 1 photon in the rod bipolar cell receptive field. Paired-flash depression was probed at three different flash strengths (1.4, 5, 10 Rh*/rod bipolar). Assuming linearity, we extrapolated the paired-flash depression expected for 1 Rh*effective/rod bipolar (Figure 5). The effective photon absorption in the rod bipolar, Rh*effective, accounted for the nonlinearity at the rod-to-rod bipolar synapse that eliminates 0.5–0.75 of the single-photon responses (Field and Rieke, 2002; Field et al., 2005).
After describing the properties of paired-flash depression for 1 Rh*effective/rod bipolar as Gnorm (from equation 1), we transformed this into a time-dependent gain change, Δ g(t) = 1- Gnorm. The predicted instantaneous gain change, Δ g, was taken as the probability that a photon was absorbed at time t, ΣP(t), multiplied by the time-dependent gain change caused by a single photon absorption, Δ g(t).
| (2) |
The first term describes the mean background light, which can be written as the rate of photon absorptions, R, multiplied by the discrete time step, Δτ. The second term becomes the integral of the time-dependent gain change caused by a single photon absorption, ΣΔg(t).
| (3) |
The predicted gain changes from paired-flash depression could only be calculated up to 1 Rh*/rod/s, at which rod adaptation began. The current calculations ignore spontaneous rod isomerizations as their rate is ~30 times less than the backgrounds where adaptation begins (Burns et al., 2002; Baylor et al., 1984).
Model of gain modulation of single photons
We modeled the effect of single photons on the gain of probe stimuli in Figure 7A–D. First we generated a Poisson train of photon events in darkness and on a mean background light. Then we added probe stimuli delivered at regular intervals (Figure 7A). Gain modulations for each photon event were calculated by multiplying the train of photon events by the function Δ g(t) obtained from paired-flash depression induced by a single photon (Figure 7B). At each time point that a probe stimulus was delivered, we multiplied the gain value over a 100 ms template following the time of the flash by the average dim flash response from an AII amacrine cell (Figure 7C and D).
Acknowledgments
We thank Mike Ahlquist, CJ Bell, Paul Newman, and Maura Wixey for technical assistance; Jon Cafaro, Thuy Doan, Gabe Murphy, David Perkel, Michael Shadlen, Joshua Singer, and Barry Wark for comments on the manuscript; Adrienne Fairhall, Peter Detwiler, Alapakkam Sampath, and Joshua Tewksbury for interesting discussion. This work was supported by the National Institutes of Health (FR; EY11850), Achievement Rewards for College Scientists (FAD), and the Howard Hughes Medical Institute (FR, and FAD with a predoctoral fellowship).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- Armstrong-Gold CE, Rieke F. Bandpass filtering at the rod to second-order cell synapse in salamander (Ambystoma tigrinum) retina. J Neurosci. 2003;23:3796–3806. doi: 10.1523/JNEUROSCI.23-09-03796.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB. Dark and Light Adaptation: Psychophysics. Handbook of Sensory Physiology. 1972;8:1–24. [Google Scholar]
- Baylor DA, Nunn BJ, Schnapf JL. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson A, Smith RG, Taylor WR. Transmission of single photon signals through a binary synapse in the mammalian retina. Vis Neurosci. 2004;21:693–702. doi: 10.1017/S0952523804215048. [DOI] [PubMed] [Google Scholar]
- Brown LG, Rudd ME. Evidence for a noise gain control mechanism in human vision. Vision Res. 1998;38:1925–1933. doi: 10.1016/s0042-6989(97)00400-8. [DOI] [PubMed] [Google Scholar]
- Burns ME, Mendez A, Chen J, Baylor DA. Dynamics of cyclic GMP synthesis in retinal rods. Neuron. 2002;36:81–91. doi: 10.1016/s0896-6273(02)00911-x. [DOI] [PubMed] [Google Scholar]
- Chavez AE, Singer JH, Diamond JS. Fast neurotransmitter release triggered by Ca influx through AMPA-type glutamate receptors. Nature. 2006;443:705–708. doi: 10.1038/nature05123. [DOI] [PubMed] [Google Scholar]
- Clements JD, Feltz A, Sahara Y, Westbrook GL. Activation kinetics of AMPA receptor channels reveal the number of functional agonist binding sites. J. Neurosci. 1998;18:119–127. doi: 10.1523/JNEUROSCI.18-01-00119.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DL, Schwindt PC, Grande LA, Spain WJ. Synaptic depression in the localization of sound. Nature. 2003;421:66–70. doi: 10.1038/nature01248. [DOI] [PubMed] [Google Scholar]
- Dacheux RF, Raviola E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J Neurosci. 1986;6:331–345. doi: 10.1523/JNEUROSCI.06-02-00331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans MR, Volgyi B, Goodenough DA, Bloomfield SA, Paul DL. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 2002;36:703–712. doi: 10.1016/s0896-6273(02)01046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong CJ, Hare WA. Temporal modulation of scotopic visual signals by A17 amacrine cells in mammalian retina in vivo. J Neurophysiol. 2003;89:2159–2166. doi: 10.1152/jn.01008.2002. [DOI] [PubMed] [Google Scholar]
- Dunn FA, Doan T, Sampath AP, Rieke F. Controlling the gain of rod-mediated signals in the Mammalian retina. J Neurosci. 2006;26:3959–3970. doi: 10.1523/JNEUROSCI.5148-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FA, Rieke F. The impact of photoreceptor noise on retinal gain controls. Curr Opin Neurobiol. 2006;16:363–370. doi: 10.1016/j.conb.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Field GD, Rieke F. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron. 2002;34:773–785. doi: 10.1016/s0896-6273(02)00700-6. [DOI] [PubMed] [Google Scholar]
- Field GD, Sampath AP, Rieke F. Retinal processing near absolute threshold: from behavior to mechanism. Annu Rev Physiol. 2005;67:491–514. doi: 10.1146/annurev.physiol.67.031103.151256. [DOI] [PubMed] [Google Scholar]
- Heidelberger R, Matthews G. Dopamine enhances Ca2+ responses in synaptic terminals of retinal bipolar neurons. Neuroreport. 1994;5:729–732. doi: 10.1097/00001756-199402000-00018. [DOI] [PubMed] [Google Scholar]
- Kovacs I, Simon A, Szarics E, Barabas P, Heja L, Nyikos L, Kardos J. Cyclothiazide binding to functionally active AMPA receptor reveals genuine allosteric interaction with agonist binding sites. Neurochem Int. 2004;44:271–280. doi: 10.1016/s0197-0186(03)00137-2. [DOI] [PubMed] [Google Scholar]
- Li W, Zhang J, Massey SC. Coupling pattern of S1 and S2 amacrine cells in the rabbit retina. Vis Neurosci. 2002;19:119–131. doi: 10.1017/s0952523802191115. [DOI] [PubMed] [Google Scholar]
- Maldonado PE, Babul CM. Neuronal activity in the primary visual cortex of the cat freely viewing natural images. Neuroscience. 2007;144:1536–1543. doi: 10.1016/j.neuroscience.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Matthews HR, Reisert J. Calcium, the two-faced messenger of olfactory transduction and adaptation. Curr Opin Neurobiol. 2003;13:469–475. doi: 10.1016/s0959-4388(03)00097-7. [DOI] [PubMed] [Google Scholar]
- Palmer MJ, Hull C, Vigh J, von Gersdorff H. Synaptic cleft acidification and modulation of short-term depression by exocytosed protons in retinal bipolar cells. J Neurosci. 2003;23:11332–11341. doi: 10.1523/JNEUROSCI.23-36-11332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Light-evoked current responses in rod bipolar cells, cone depolarizing bipolar cells and AII amacrine cells in dark-adapted mouse retina. J Physiol. 2004;558:897–912. doi: 10.1113/jphysiol.2003.059543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd ME, Brown LG. Stochastic retinal mechanisms of light adaptation and gain control. Spat Vis. 1996;10:125–148. doi: 10.1163/156856896x00097. [DOI] [PubMed] [Google Scholar]
- Sampath AP, Rieke F. Selective transmission of single photon responses by saturation at the rod-to-rod bipolar synapse. Neuron. 2004;41:431–443. doi: 10.1016/s0896-6273(04)00005-4. [DOI] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Vesicle depletion and synaptic depression at a mammalian ribbon synapse. J Neurophysiol. 2006;95:3191–3198. doi: 10.1152/jn.01309.2005. [DOI] [PubMed] [Google Scholar]
- Singer JH, Lassova L, Vardi N, Diamond JS. Coordinated multivesicular release at a mammalian ribbon synapse. Nat Neurosci. 2004;7:826–833. doi: 10.1038/nn1280. [DOI] [PubMed] [Google Scholar]
- Sterling P, Freed MA, Smith RG. Architecture of rod and cone circuits to the on-beta ganglion cell. J Neurosci. 1988;8:623–642. doi: 10.1523/JNEUROSCI.08-02-00623.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsodyks MV, Markram H. The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc Natl Acad Sci U S A. 1997;94:719–723. doi: 10.1073/pnas.94.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veruki ML, Mørkve SH, Hartveit E. Functional properties of spontaneous EPSCs and non-NMDA receptors in rod amacrine (AII) cells in rat retina. J Physiol. 2003;543:759–774. doi: 10.1113/jphysiol.2003.039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgyi B, Deans MR, Paul DL, Bloomfield SA. Convergence and segregation of the multiple rod pathways in mammalian retina. J Neurosci. 2004;24:11182–11192. doi: 10.1523/JNEUROSCI.3096-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath MA, Kwan KY, Corey DP. The micromachinery of mechanotransduction in hair cells. Annu Rev Neurosci. 2007;30:339–365. doi: 10.1146/annurev.neuro.29.051605.112917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Depletion and replenishment of vesicle pools at a ribbon-type synaptic terminal. J Neurosci. 1997;17:1919–1927. doi: 10.1523/JNEUROSCI.17-06-01919.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Dietrich D, Grasel I, Reuter G, Seifert G, Steinhauser C. 6-Hydroxykynurenic acid and kynurenic acid differently antagonise AMPA and NMDA receptors in hippocampal neurons. J Neurochem. 2001;77:1108–1115. doi: 10.1046/j.1471-4159.2001.00340.x. [DOI] [PubMed] [Google Scholar]