Abstract
The peroxisome proliferator-activated receptors (PPAR) α, β/δ, and γ are ligand-activated nuclear receptors involved in a number of physiological processes, including lipid and glucose homeostasis, inflammation, cell growth, differentiation, and death. PPAR agonists are used in the treatment of human diseases, like type 2 diabetes and dyslipidemia, and PPARs appear as promising therapeutic targets in other conditions, including cancer. A better understanding of the functions and regulation of PPARs in normal and pathological processes is of primary importance to devise appropriate therapeutic strategies. The ubiquitin-proteasome system (UPS) plays an important role in controlling level and activity of many nuclear receptors and transcription factors. PPARs are subjected to UPS-dependent regulation. Interestingly, the three PPAR isotypes are differentially regulated by the UPS in response to ligand-dependent activation, a phenomenon that may be intrinsically connected to their distinct cellular functions and behaviors. In addition to their effects ongene expression, PPARs appear to affect protein levels and downstream pathways also by modulating the activity of the UPS in target-specific manners. Here we review the current knowledge of the interactions between the UPS and PPARs in light of the potential implications for their effects on cell fate and tumorigenesis.
1. INTRODUCTION
Despite the everyday progress in understanding the genetic and molecular bases of cancer, this disease still strikes millions of people worldwide. The quest for new targets and more effective therapeutics is currently a major driving force in cancer research. Multiple mutations that affect critical cellular pathways lead to uncontrolled proliferation, increased survival, and block of differentiation in cancer cells [1]. Several cellular pathways (e.g., cell surface receptors, signal transduction pathways, apoptosis, angiogenesis, transcription, chromatin regulation, and proteasome-mediated degradation) have provided relevant targets and opportunities for development of clinically useful therapeutics [1]. Unfortunately, targeting each of these major pathways individually may not be sufficient. Extensive cross-talks occur between regulatory pathways and it is not unlikely that the same proteins play multiple roles in different processes. The peroxisome proliferator-activated receptor (PPAR) subfamily of nuclear receptors may represent a prime example of proteins interacting with multiple cellular pathways and exerting diverse and sometime apparently contrasting functions. Here, we review how PPARs interact with the ubiquitin-proteasome system (UPS), which is the major cellular system responsible for protein turnover, and how these two systems might reciprocally affect each other activity and functions.
2. THE UBIQUITIN-PROTEASOME SYSTEM
Ubiquitin is a 76-amino acid polypeptide that is post-transcriptionally linked to proteins via a covalent linkage to one or multiple lysine residues [2]. Several proteins including cell surface receptors, cell cycle regulators, and transcription factors are ubiquitinated and protein ubiquitination affects many cellular processes including proliferation, cell cycle progression, DNA damage repair, and cell death [2]. Ubiquitination is a regulatory signal that affects the fate and function of proteins. Ubiquitination regulates mainly protein turnover directing ubiquitinated proteins to proteasome-mediated proteolysis. Other nonproteolytic functions, like control of protein-protein interactions, cellular localization, and catalytic activity, are emerging [2]. The proteasome is a multicatalytic complex that comprises a 20S core with proteolytic activity and a 19S subunit that recognizes poly-ubiquitinated proteins, unfolds them, and passes into the 20S catalytic core for degradation. Ubiquitination is catalyzed by three types of enzymes, called E1, E2, and E3 [2, 3]. Ubiquitin is first activated by an E1 ubiquitin-activating enzyme in an ATP-dependent reaction. The activated ubiquitin is then transferred to an E2 ubiquitin-conjugating protein (UBC). Finally, E3 ubiquitin-ligases, which are the most critical enzymes in the process, catalyze the transfer and covalent attachments of the activated ubiquitin to the target protein. In human cells, a single E1 and about 60 E2 enzymes have been identified, while there are approximately a thousand E3 enzymes, which ensure a high degree of substrate specificity to the system [2, 3]. E3 enzymes are split in two major subfamilies: the Ring-H2 and the HECT domain proteins. The human genome contains also more than 70 deubiquitinating enzymes (DUBs) that remove ubiquitin chains from ubiquitinated proteins and can rescue them from proteasomal degradation [4].
Protein ubiquitination is a highly dynamic process and ubiquitination-deubiquitination cycles can serve to rapidly modulate protein level and function [4]. Ubiquitin and proteasomal components play an important role in transcription [5, 6]. Ubiquitin ligases and proteasomal subunits are present as integral components of transcription regulatory complexes [5, 6]. Histones, the main component of chromatin, are ubiquitinated and the process affects chromatin remodeling and transcription [6, 7]. RNA polymerase II is also directly regulated by ubiquitination [6, 8]. Moreover, the UPS regulates the abundance, activity, and subcellular localization of many transcription factors [5, 6]. Transcription factors are ubiquitinated and degraded by the proteasome and, paradoxically, the process is often essential for their transactivating ability [6]. In fact, transcription activation and degradation domains of transcription factors often overlap [6]. In addition, mono-ubiquitination (i.e., addition of single ubiquitin tag to a protein) can act as a post-translational modification that modulates activity of transcription factors and regulates transcription efficiency by nonproteolytic mechanisms [6]. Degradation of inhibitors of transcription factors is also often required to release active transcription factors. For example, activation of the transcription factor NF-κB is controlled by a signaling cascade based on multiple ubiquitination and proteasome-dependent events [6].
Alterations of the UPS are frequent in cancer. They are mainly due to loss or gain of function of specific components of the UPS and alterations of UPS substrates, like oncogene and tumor suppressor gene products, which become less or more susceptible to proteasomal-dependent degradation [9]. Tumor suppressor proteins are often the targets of UPS alterations. The human papillomavirus (HPV), a cause of cervical cancer, encodes two oncogenic proteins, E6 and E7. These viral proteins promote degradation of the tumor suppressor p53 via ubiquitination by the E6-associated protein (E6-AP) E3 ubiquitin ligase [10]. HDM2 is another E3 ubiquitin ligase that targets p53 to proteosomal degradation [11]. Aberrant expression of HDM2 is found in many human cancers [12]. Single nucleotide polymorphism in the HDM2 promoter leading to HDM2 overexpression has been recently associated to the development of sporadic and hereditary cancers [13]. The E3 ubiquitin ligase Skp2 is responsible for ubiquitination of the cell cycle inhibitor and tumor suppressor p27 [14]. Skp2 overexpression is observed in cancer cells leading to degradation and inactivation of this tumor suppressor protein [15]. Oncogenic proteins are also affected by alterations of UPS components. The E3 ubiquitin ligase encoded by the von Hippel-Lindau gene (pVHL) mediates the ubiquitination and degradation of the hypoxia-inducible transcription factor HIF-1α [16, 17]. Mutations in pVHL gene predispose patients to renal cell carcinoma and other cancers. In these tumors, the level of HIF-1α is increased resulting in a potent oncogenic and angiogenic stimulus.
Due to the unique mechanism of cleavage at the proteolytic active sites, selective proteasome inhibitors have been synthesized and some, like bortezomib (Velcalde, PS341), have undergone clinical evaluation as anticancer agents [18]. Bortezomib is a peptide boronate proteasome inhibitor that blocks the chymotryptic activity of the 26S proteasome [18]. The anticancer effect of bortezomib is likely to be achieved through its inhibitory effects on protein degradation and modulation of important cellular pathways, including inhibition of the NF-κB pathway [18]. Bortezomib is currently approved for clinical use for treatment of multiple myeloma. Clinical trials with bortezomib and second generation proteasome inhibitors as single agents or in combination with other chemotherapeutic agents are ongoing in various tumor types [18].
3. PEROXISOME PROLIFERATOR-ACTIVATED RECEPTORS
PPARs emerged in the nineties as nuclear receptors regulating transcription of genes involved in metabolic processes like lipid and glucose homeostasis [19, 20]. Later, PPARs have found to be implicated in many physiological and pathological processes [20]. PPARs belong to the nuclear hormone receptor super-family, which is one of the largest families of transcriptional regulators in the human genome with more than 40 distinct nuclear receptors [21]. Nuclear receptors bind small lipophilic molecules, such as steroid hormones, vitamins, and fatty acid derivatives, and function as ligand-activated transcription factors, interacting with specific DNA sequences (i.e., hormone response elements, HRE) in target genes and stimulating their transcription [21]. Thus, nuclear receptors provide a direct link between small lipophilic signaling molecules present in the cells or their environment and the cellular transcriptional machinery, turning on specific subsets of genes containing the appropriate HRE and inducing complex cellular responses [21, 22]. The nuclear receptor super-family includes the steroid hormone receptors (i.e., estrogen, progesterone, androgen, and glucocorticoid receptors) and receptors for nonsteroidal hormones [21–23]. The latter include the PPARs, vitamin D (VDR), and retinoic acid (RAR) receptors [21, 23]. The ligands of most nonsteroidal receptors are dietary fatty acids or generated locally by lipid metabolism within the target cell or tissue, while steroid and thyroid hormones are produced by distant endocrine organs and released in the blood [21, 22].
Nuclear receptors exhibit a characteristic modular structure comprising an N-terminal domain with the ligand-independent activation function domain (AF-1), a DNA binding domain (DBD), and a C-terminal domain containing the ligand binding (LBD), and the ligand-dependent transactivation (AF-2) domain [23]. The DBD contains two zinc finger modules and determines the DNA binding specificity of the receptors. The LBD is involved in homo- and heterodimerization and interaction with cofactors [23, 24]. The structure of the LBD is highly conserved among nuclear receptors, comprising a large hydrophobic cavity that accommodates the ligand. Variations of the size and shape of the ligand binding pocket ensure ligand specificity among receptors [23, 24]. Ligand-binding induces a conformational remodeling of the LBD that exposes surfaces required for interaction with coactivators and affects the affinity of the receptors for corepressors [23, 24]. The nonsteroidal receptors are found primarily in the cell nucleus and are bound to HRE as heterodimers with the retinoic X receptor (RXR) [19, 23]. These receptors can affect both positively and negatively transcription of target genes with the LBD mediating alternatively transcriptional activation or repression, although the mechanisms of transrepression by PPARs are still poorly understood [25]. Transcriptional repression is due to recruitment of corepressors, like NCoR/SMART, by the unliganded and DNA-bound receptor and formation of multiprotein complexes containing histone deacetylases and other chromatin remodeling enzymes [23, 25]. In the presence of ligands, corepressor complexes are released and replaced by coactivators, like SRC1 and CBP-p300, thus switching on transcription [23, 25]. Transcriptional activation is associated with histone modifications, chromatin remodeling, and assembly of the transcription initiation complex. Thus, transcriptional activation and repression by nuclear receptors are very dynamic processes involving the formation of protein complexes in which multiple coactivators and corepressors need to be rapidly exchanged [25–27]. The UPS is perhaps the major system controlling the assembly and turnover of these regulatory complexes ensuring their timely interaction with the transcriptional machinery [26]. Ubiquitin and proteasome components are associated with corepressor and coactivator complexes recruited by nuclear receptors [25, 26]. Most nuclear receptors, including thyroid hormone, estrogen, glucocorticoids receptor, RAR, and RXR receptors, as well as coactivators, corepressors, and general components of the transcription machinery are ubiquitinated and degraded by the proteasome [26, 28].
PPARs have the typical modular structure of the nuclear hormone receptors with a poorly characterized N-terminal domain with putative ligand-independent AF-1 function, a central DNA-binding domain (DBD), and a C-terminal ligand binding (LBD) and ligand-dependent AF-2 domain (Figure 1) [19, 23]. However, despite the high sequence and structural homology, the three PPAR isotypes have distinct ligand specificity, functions, and behaviors [19, 20]. PPARα is a key regulator of energy homeostasis and plays a major role in lipid metabolism and glucogenesis. PPARα is expressed in tissues with significant fatty acid and cholesterol catabolism, like brown adipose tissue, liver, kidney, intestine, heart, and skeletal muscle [29]. PPARγ exists in two isoforms (γ1 and γ2) that differ only at the N-terminus. PPARγ2 is present at high levels in adipose tissue, whereas PPARγ1 expression is broader and is present in gut, brain, vascular cells, immune cells, and retina [30]. PPARγ plays a role in adipocyte differentiation, glucose metabolism, and lipid homeostasis, and participates in monocyte/macrophage differentiation [30]. Moreover, PPARγ influences fatty acid storage in the adipose tissue and is implicated in insulin resistance and atherosclerosis [30]. PPARδ is ubiquitously expressed with high levels in colon, skin, and brain [20]. PPARδ also functions in processes linked to lipid metabolism, like fatty acid catabolism, cholesterol efflux, lipid uptake in macrophages, and preadipocyte differentiation [31]. This nuclear receptor plays also a role in placental and gut development, embryo implantation, tissue injury, and wound healing [20, 32].
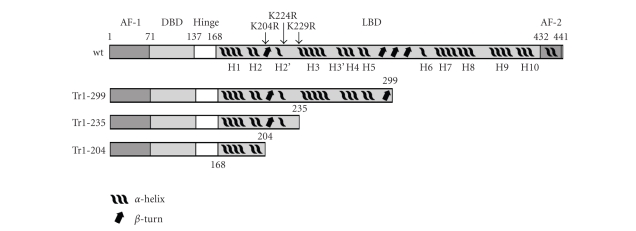
Figure 1.
Domain structure of PPARδ and truncated forms of the receptor. AF-1, N-terminal ligand-independent activation function 1 (aa 1–70). DBD, DNA binding domain (aa 71–136). Hinge, flexible hinge region (aa 137–167). LBD, ligand-binding domain (aa 168–431). AF-2, C-terminal ligand-dependent activation function-2 (aa 432–441). The position of the mutations (K204R, K224R, and K229R) introduced in the region 204–235 is shown.
PPARs possess a broad ligand-binding cavity that allows binding of a wide range of synthetic and natural lipophilic compounds [19]. Medium- and long-chain unsaturated fatty acids (e.g., linoleic acid), conjugated and oxidized fatty acids (e.g., phytanic acid), and eicosanoids bind to PPARα [19]. Fibrates, like bezafibrate, fenofibrate, and clofibrate, which are used for the treatment of dislipidemias and cardiovascular diseases, are selective PPARα agonists [29]. PPARγ binds to long-chain fatty acids, prostaglandin J2 (PG J2), and other eicosanoids [19]. Synthetic PPARγ agonists, such as pioglitazone and rosiglitazone, are insulin sensitizers used to treat type 2 diabetes [30]. PPARδ has high affinity for prostaglandin I2 (PGI2), fatty acids, and synthetic compounds [19, 31].
Beside their metabolic functions, PPARs have an important role in inflammation. PPARα and PPARγ agonists can ameliorate chronic inflammatory conditions, such as atherosclerosis, arthritis, and inflammatory bowel disease [20, 29, 30]. PPARs repress genes of the inflammatory response pathway, such as cytokines (TNFα, IL-1β, IL-6), cell adhesion molecules (MMPs), and other proinflammatory molecules (iNOS) [25]. These effects are mediated in large part by the ability of PPARs to antagonize other transcription factors, like AP-1, STAT1, and NF-κB, which have proinflammatory functions [25]. Different mechanisms have been proposed to explain the phenomenon of transrepression by PPARs, including sequestration of limiting cofactors, direct physical interaction, and antagonism between PPARs and other transcription factors, and promoter-specific block of corepressor/coactivator exchange by PPARs in selected target genes [24, 25]. The latter involves a block of the ubiquitin and proteasome-dependent processing of corepressor complexes as in the case of PPARγ-mediated repression of proinflammatory NF-κB target genes [25]. PPARγ and PPARα can also interact physically with NF-κB and c-Jun blocking transcriptional activation [33, 34]. Reciprocally, NF-κB and c-Jun can repress PPARγ and PPARα-induced transcription, respectively, by inhibiting the binding to PPRE in target genes [33–35]. Also PPARδ has a role in inflammation controlling expression of proinflammatory genes in macrophages in a ligand-dependent manner [31]. Unliganded PPARδ binds to corepressor molecules including Bcl-6, which is a repressor of inflammatory gene expression [31, 36]. Ligand binding releases the corepressor complexes resulting in transcription of PPARδ target genes. At the same time, PPARδ-bound Bcl-6 is also released and is free to repress its own target genes suppressing the inflammatory response [31, 36]. Paradoxically, PPARδ knockout has the same effects of the agonists on the expression of Bcl-6 target genes since it also leads to release the transcriptional repressor [36].
The involvement of PPARs in carcinogenesis has been widely discussed, although it is still controversial whether the different isotypes either favor or inhibit tumorigenesis [37, 38]. This may still represent a major concern for developing PPAR-targeted therapeutics for clinical applications because of the potential risk of promoting tumorigenesis as indicated by studies in rodents [39]. PPARs are expressed in several human cancers and PPAR ligands have been shown to modulate tumor growth [37, 38]. Inactivating mutations, deletions and chromosomal translocations of PPARγ have been found in various cancers pointing to a tumor suppressor role of this nuclear receptor [40–42]. PPARγ ligands promote differentiation, growth arrest, and death of cancer cells in vitro [38]. PPARγ ligands reduce growth of human tumor xenografts and spontaneous and carcinogen-induced tumors in rodents [38]. PPARα is also expressed in various tumors and cancer cell lines [43, 44]. Activation of PPARα in cancer cells inhibits proliferation and suppresses metastatic potential [45–47]. PPARα ligands have shown antitumor activity also in murine models [46, 48, 49]. PPARδ participates in a number of important pathways controlling adhesion, proliferation, differentiation, and survival [37]. Unlike the other isotypes, PPARδ has been shown to prevent apoptosis and induce cell growth in normal cell types, like primary mouse keratinocytes, preadipocytes, vascular smooth muscle cells, hepatic stellate cells [37]. Consistent with an antiapoptotic role, PPARδ increases the expression of antiapoptotic genes and activates prosurvival signaling pathways in keratinocytes [50]. PPARδ agonists stimulate proliferation and survival of cancer cells in vitro and promote tumor growth in mice [51–57]. PPARδ is a downstream target of β-catenin/T cell factor-4, which is central in colon cancer pathogenesis and regulates other cancer-promoting genes like c-myc and cyclin D1 [58]. Cyclooxygenase-2 (Cox-2) modulates PPARδ activity and nonsteroidal anti-inflammatory drugs that have chemopreventive effects in colon cancer inhibit PPARδ activity and expression [58–60]. Cox-2 is frequently upregulated in cancer and preneoplastic lesions, and Cox-2 products like PGI2 act as selective agonists of PPARδ [58–60]. To further support a protumorigenic role of PPARδ, PPARδ expression is elevated in cancers, like colorectal, endometrial, and head and neck cancers [58, 59, 61]. Additional evidence pointing to a tumor promoting function of PPARδ comes from experiments in mice where disruption of PPARδ decreased tumorigenicity of cancer cells in nude mice and PPARδ activation increased tumor growth [55, 57, 62].
Despite this large body of evidence, some controversial results in animal experiments cast doubts both on the anti- and protumorigenic activities of PPARs [37, 38]. Experiments in rodents have shown increased frequency and enhanced tumor growth by PPARγ agonists [38, 63, 64]. Similar contradictory data have been reported for PPARα, whereby prolonged administration of PPARα agonists caused hepatocarcinogenesis in rats and mice [65]. The frequency of intestinal tumors also increased in PPARδ knockout mice [66, 67] or decreased upon treatment of the animals with PPARδ ligands [68]. These contradictory results between cellular and animal models and different animal models suggest that the function of these nuclear receptors is more complex than that has been assumed so far and may depend heavily on cell and tissue context, cross-talks with multiple signaling pathways and noncell autonomous mechanisms. A hint to this complexity is given by recent studies of the role of PPARs in tumor angiogenesis. In addition to cancer cell-autonomous effects, PPARs affect strongly tumor angiogenesis and inflammation, two processes that have a critical role in tumor pathogenesis and progression. PPARγ and PPARα agonists have anti-inflammatory properties, which may contribute greatly to their in vivo antitumor activity under certain circumstances. PPARγ ligands are also potent angiogenic inhibitors [69, 70] and PPARα agonists suppress VEGF production, endothelial cell proliferation, and tumor growth in mice [48, 49]. PPARδ activation stimulates VEGF production in mice, which at least in part had an autocrine prosurvival effect on cancer cells [71]. PPARδ has been recently identified as a critical node in a tumor angiogenic network linking angiogenesis to inflammation and carcinogenesis [72]. Knockout of PPARδ in host tissues but not in tumor cells reduced tumor growth by impairing angiogenesis [72]. Interestingly, the in vivo antitumor activity of PPARα agonists also depended heavily on the effects of host endothelial and stromal cells rather than cancer cells blocking angiogenesis and inflammation [48, 49]. Paradoxically, PPARα knockout impaired tumor growth in mice, because it resulted in a strong inflammatory response and production of anti-angiogenic factors, like TSP-1 and endostatin [73]. This paradoxical response is similar to the effects of PPARδ on inflammatory gene expression in macrophages, where both receptor activation and knockout suppressed expression of a subset of target genes [31, 36]. This dual mode of regulation of gene expression, whereby ligand- dependent and independent mechanisms lead to transrepression, derepression, or trans-activation of distinct subsets of genes, seems a common theme for these nuclear receptors and needs to be taken into account when examining their functions in physiological and pathological processes.
4. THE UBIQUITIN-PROTEASOME SYSTEM AND PEROXISOME PROLIFERATOR-ACTIVATED RECEPTORS
4.1. UPS and control of PPAR turnover
Important factors to consider when studying the multiple and complex functions of PPARs are their connections with other cellular systems and how these interactions reciprocally impact on each system activity. Recent reports suggest that the activity of PPARs is linked in many ways to the UPS [28]. All three PPARs are short-lived proteins that undergo ubiquitination and proteosomal degradation and the UPS is mainly responsible for the turnover of these nuclear receptors [28]. However, the three PPAR isotypes have different behaviors with respect to ligand-dependent receptor turnover. PPARγ undergoes negative autoregulation upon agonist binding. PPARγ is ubiquitinated and degraded by the proteasome in a negative feedback loop that probably serves to attenuate receptor-mediated gene transactivation [74]. PPARα turnover is controlled by ligands in a slightly different manner. Instead of enhancing ubiquitination and degradation, PPARα ligands prevent ubiquitination and lead to increased stability of the receptor [75]. The protective effect of the ligand, however, is maximal during the first 3 hours of exposure to the ligand and the receptor is then rapidly degraded [75].
We have recently examined the ligand-dependent turnover of PPARδ and the role of the UPS in this process [76]. Our study revealed interesting differences between PPARδ and other PPAR isotypes with respect to ligand-dependent receptor turnover and interaction with the UPS. We found that PPARδ, like other nuclear receptors, is ubiquitinated and rapidly degraded by the proteasome [76]. Brief incubation of cells expressing both endogenous and recombinant PPARδ with proteasome inhibitors led to rapid accumulation of the receptor in cell nuclei. Interestingly, in the presence of proteasome inhibitors, PPARδ was transcriptionally competent as shown by luciferase reporter assays and assessment of endogenous target genes by RT-PCR [76]. Thus, PPARδ was different from other nuclear receptors, including the estrogen, androgen, thyroid hormone, and retinoic acid receptors, whose transcriptional activity is reduced by proteasome inhibitors [26]. Furthermore, while in the absence of ligands PPARδ had a very short half life (~30 minutes), the addition of ligand increased considerably the receptor half life [76]. The effects of the synthetic and natural ligands were rapid with an increase of PPARδ protein level within 4 hours upon addition to the cell culture medium. The receptor level remained high as long as the ligands were present [76]. Removal of the ligands was followed by rapid reversal with return to the baseline level within few hours. Once again, PPARδ behavior was unique among nuclear receptors, whose turnover is generally accelerated by their own ligands [26, 77]. The progesterone receptor, thyroid receptor, estrogen receptor, RAR, and RXR all show ligand-dependent increase of degradation associated with transcriptional activation [26, 77]. The direct consequence of these events is a rapid decrease of the receptor half life and switching-off the transcriptional response. Only vitamin D3 receptor is known to be stabilized by the ligand with a similar kinetics [78]. As mentioned above, PPARγ is also rapidly degraded upon exposure to ligands [74] and PPARα is stabilized only transiently by ligands [75]. Further work demonstrated that ligand-induced stabilization of PPARδ was due to a selective block of receptor ubiquitination [76]. This ubiquitination block depended on the continuous presence of the ligand, was rapidly reversed after removal of the ligand, and was due to the direct interaction of the ligand with the receptor [76]. Disruption of the LBD in PPARδ/Tr1-299 abolished the effect of the ligand on ubiquitination and proteolysis, although the truncated form of the receptor was still ubiquitinated and degraded by the proteasome [76]. Thus, binding of the ligand to the LBD induced a conformational change that, in addition to allowing receptor trans-activation, blocked the interaction of PPARδ with an ubiquitin ligase or, alternatively, promoted binding of a deubiquitinating enzyme.
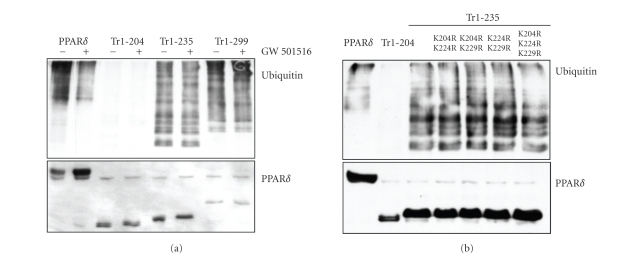
Using site-directed mutagenesis, we investigated further the role of distinct PPARδ domains in the ligand-dependent regulation of receptor turnover [76]. This analysis revealed additional differences between PPARδ and other PPAR isotypes. Mutations in the DBD of PPARδ reduced the effect of ligands on receptor ubiquitination [76]. This suggested that the ligand acted preferentially on the DNA-bound receptor preventing its ubiquitination. Interestingly, mutations in DBD of PPARγ did not affect ligand-dependent turnover, indicating that DNA binding was not a prerequisite for ligand-induced degradation of this receptor [74]. On the other hand, we showed that the AF-2 domain of PPARδ was not required for ligand-induced block of ubiquitination, indicating that the effect was independent of coactivator binding [76]. For most nuclear receptors, the transactivating function is linked to proteolytic degradation and mutations in the transactivating domain affect also receptor ubiquitination and proteolysis [77]. The AF-2 domain of PPARγ has a similar role and mediates ligand-induced degradation of the receptor [74]. For PPARγ and other nuclear receptors, conformational changes induced by the ligands may favor the concomitant interaction with coactivators and components of the UPS. Overexpression of transcriptional coactivators led also to a decrease of PPARα level in the presence of ligand, showing that the interaction with coactivators via the AF-2 domain promoted proteolysis of the α isotype [79]. Thus, for PPARα the initial stabilization is probably followed by the recruitment of coactivators along with other factors that trigger proteolysis of the receptor. In contrast, in the case of PPARδ we showed that transactivation and receptor ubiquitination are functionally separated [76]. The absence of a link between these two processes allows independent control of receptor transactivation and ubiquitination upon ligand binding and may be a prerequisite to avoid rapid degradation and sustain its transcriptional activity once it is engaged in transcriptional activation complexes. Further analysis of PPARδ mutants indicates that the region between amino acid 204 and 235 may play a role in controlling ubiquitination and proteolytic degradation of the receptor (Figure 1). This region has a poor secondary structure, forms a loop exposed to the surface, and may be in an environment prone to ubiquitination [80, 81]. In addition, the region is quite diverse between the PPAR isotypes, possibly explaining the divergent responses in terms of ligand-dependent turnover. Pull-down experiments showed that the truncated PPARδ/Tr1-235 was ubiquitinated, while the shorter PPARδ/Tr1-204 was not (Figure 2(a)). Different scenarios can explain these results and are under consideration. The region between amino acid 204 and 235 may contain lysine residues that are the major sites of ubiquitination of PPARδ. However, mutations of the three lysines present in this region (K204R, K224R and K229R) did not affect ubiquitination of the PPARδ/Tr1-235 (Figure 2(b)). Thus, alternatively the region 204–235 may be needed for the binding of an ubiquitin ligase or cofactors that mediate the interaction of the receptor with the UPS.
Figure 2.
Ubiquitination of truncated and mutated forms of PPARδ. (a) U2OS cells were transfected with HA-ubiquitin expressing vector along with wild type His-PPARδ or truncated forms of the receptor (PPARδ/Tr1-204, Tr1-235 and Tr1-299). After 24 hours, cells were incubated overnight with vehicle or the PPARδ ligand GW501516 (5 μM) and subsequently all samples were incubated with 10 μM the proteasome inhibitor PS341 for 4 hours. His-tagged wild type and truncated PPARδ were pulled-down with nickel affinity gel under denaturing conditions. PPARδ was detected in pull-down fractions using an anti-His antibody and ubiquitinated proteins with an anti-HA antibody. (b) U2OS cells were transfected with HA-ubiquitin vector along with the indicated PPARδ expressing vectors. PPARδ/Tr1-235 had wild type sequence or the indicated double or triple mutations (K204R, K224R and K229R). Cells were treated and analyzed as above.
Thus, even if the PPAR isotypes are structurally very similar, binding to specific ligands induces divergent responses as far as receptor turnover. PPARγ upon ligand binding becomes ubiquitinated and prone to degradation, whereas ligands prevent or delay ubiquitination and degradation of PPARδ and PPARα. Most nuclear receptors exhibit negative autoregulation upon interaction with the respective ligands [26, 77]. Ligand-induced stabilization is a less common and has been observed only for very few nuclear receptors. The system in place for PPARδ may be geared to prevent both accumulation of high levels of the receptor and its prolonged activation [76]. Overactivity of PPARδ may be detrimental to cells, perhaps due to its antiapoptotic and potentially tumorigenic activity [32, 37]. The level of PPARδ is low and constantly controlled via UPS-dependent proteolysis, which may affect greatly the ligand-independent functions of the receptor like transrepression of other transcription factor target genes. Under physiological conditions, the low abundance and short half life of natural PPARδ ligands, like PGI2, would contribute to keep the receptor in the unbound state [32]. In the presence of high concentrations of ligands, the DNA-bound and liganded PPARδ is protected from proteasomal degradation by the inhibition of its ubiquitination [76]. The stabilized DNA-bound receptor would be able to transactivate target genes as long as enough ligand is present. This would be consistent with the fact that in processes, such as wound healing, inflammation, and cancer, PPARδ levels seem to increase concomitantly with upregulation of cyclooxygenase-2 and other enzymes for the production of lipid metabolites capable of stabilizing and activating PPARδ [32, 37, 55, 58, 59]. In the absence of this coordinated increase of ligand and receptor levels, PPARδ might not be able to act as antiapoptotic and growth-promoting factor. How ligand-induced stabilization of PPARδ affects ligand-dependent interactions with other transcription factors leading to transrepression or derepression of gene expression is still unknown.
4.2. UPS, PPARs, and interactions with other signaling pathways
In addition to ligand-dependent receptor turnover, the UPS is an important way to control PPAR activity in response to upstream signal transduction pathways (Figure 3). Receptor phosphorylation by cellular kinases can regulate both basal and ligand-induced activity of PPARs as well as modulate their protein level by indirectly controlling proteasome-dependent degradation [82]. In colorectal cancer cells, the polypeptide hormone gastrin promotes cell proliferation and the effect is associated with decreased PPARγ level. This was mediated by phosphorylation of PPARγ involving the epidermal growth factor receptor and ERK1/2 kinase leading to increased PPARγ proteasome-mediated degradation [83]. In fat cells IFN-γ treatment induces a rapid reduction of PPARγ protein level, which is blocked by proteasome inhibitors [84]. On the other hand, there are instances in which PPARs enhance stabilization or degradation of proteins by affecting their susceptibility to UPS-mediated degradation. Perhaps the best example of a signaling pathway in which both PPARs and the UPS are implicated is the Wnt pathway. Suppression of the canonical Wnt signaling is required for differentiation of preadipocytes into adipocytes. The process is in part mediated by PPARγ-induced degradation of β-catenin, which is a central element in the Wnt pathway. Activation of PPARγ promotes degradation of β-catenin in glycogen synthase kinase 3β (GSK3B)-dependent or independent manner [85, 86]. β-catenin mutations that inhibit degradation block expression of a subset of adipogenic and PPARγ target genes [85]. PPARγ-dependent degradation of β-catenin requires an active APC-containing destruction-complex. Mutations of the T cell factor/lymphocyte enhancer factor (TCF/LEF) binding domain of β-catenin or of a catenin-binding domain (CBD) within PPARγ block proteasomal degradation of β-catenin [87]. The interaction between β-catenin and PPARγ affect their respective oncogenic and tumor suppressor function [87]. A functional APC was found to be required also for PPARγ-mediated suppression of colon carcinogenesis [88]. Activation of PPARγ induces degradation of cyclin D1, which has a critical role in cell cycle regulation, along with β-catenin in hepatocytes [89]. Reduced cyclin D1 protein level was observed also in breast cancer cells upon PPARγ activation by selective ligands and cyclin D1 downregulation was blocked by inhibition of the proteasome [90]. However, the ability of thiazolidinedione ligands to reduce β-catenin and cyclin D1 levels might be in part PPARγ-independent and determined by direct effects of these compounds on protein degradation [91, 92]. Beside the induction of proteosomal degradation, activation of PPARγ has been shown to increase the level of proteins by blocking their proteolysis. Activation of PPARγ in human hepatocarcinoma cells inhibits proteosomal degradation of p27, a cyclin-dependent kinase inhibitor, with consequent inhibition of cell proliferation [93]. Similarly, PPARγ inhibits claudin 4 degradation resulting in urothelial cell differentiation [94]. In both cases, the increased protein level is probably due to reduced ubiquitination. Interestingly, transcriptome analysis of ovarian cancer cells exposed to a PPARγ agonist revealed that PPARγ activation resulted in upregulation of several genes involved in protein modification and ubiquitination, including many ubiquitin ligases and ubiquitin-conjugating enzymes [95]. This finding may provide a plausible explanation for the broad effects that PPAR-γ agonists have on protein ubiquitination and turnover and clearly deserves further investigation [95].
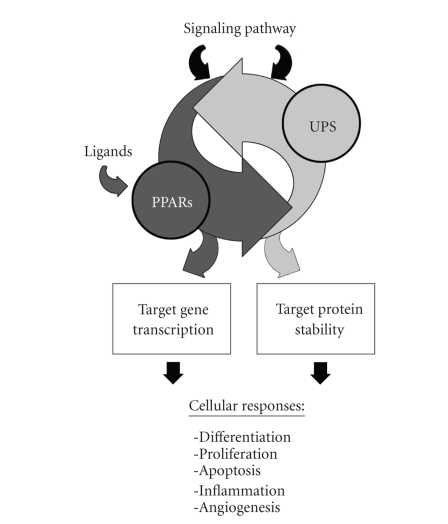
Figure 3.
Interactions between PPARs and the ubiquitin-proteasome system (UPS) affect multiple cellular pathways. The UPS regulates activity of PPARs by controlling receptor turnover in ligand dependent and independent manners and affecting the ability of PPARs to regulate target gene transcription. Signaling pathways can modulate PPAR activity by affecting UPS-mediated turnover (e.g., increased PPARγ degradation in response to growth factors or hormones). PPAR can also affect biological pathways and cellular responses by increasing or decreasing susceptibility of proteins to proteasomal degradation (e.g., enhanced degradation of β-catenin and suppression of the Wnt pathway by PPARγ).
PPARα agonists also enhance protein degradation. In LPS-treated macrophages PPARα agonists enhance degradation of inducible nitric oxide synthase (iNOS), reducing nitric oxide (NO) production, which is an important mediator in inflammatory processes. PPARα agonists did not affect iNOS expression and proteasome inhibitors reversed the effect on iNOS protein levels, indicating that PPARα agonists enhanced degradation of this protein by the proteasome [96]. PPARδ has been found to regulate ubiquitin C expression and this has been linked to the modulation of protein kinase Cα (PKCα) and attenuation of cell proliferation in the skin. The level of PKCα was lower in the skin of PPARδ wild-type mice treated with TPA compared to the skin of PPARδ-null mice [97]. On the other hand, the amount of ubiquitinated PKCα was lower in skin of TPA-treated PPARδ-null mice compared to wild-type mice and inhibition of the proteasome prevented TPA-induced downregulation of PKCα. Thus, the effects of PPARδ on cell proliferation in the skin could be due to ubiquitin-dependent turnover of PKCα that in turn modulated the activity of the PKCα-dependent pathways [97].
Finally, the UPS is involved in the reciprocal regulation of PPARs and other transcription factors. Activation of NF-κB is achieved when the inhibitor IκB, which normally holds NF-κB in the cytoplasm, is phosphorylated and recognized by the E3-β-transducin repeat containing protein (β-TRCP). Ubiquitinated IκB is degraded by the proteasome, allowing NF-κB to translocate to the nucleus and induce gene transcription [98]. NF-κB has a critical role in inflammation. In experimental rat models of autoimmune myocarditis stabilization and translocation of NF-κB were inhibited by PPARγ-dependent expression of IκB [99]. Likewise, PPARα activation induced IκB in aortic smooth muscle cells and in human hepatocytes [100]. The transcription factor AP-1, which is another key player in inflammation, interacts with the PPARs and may be regulated in a similar combinatorial manner by PPARs and the UPS [33, 34].
5. CONCLUSIONS
Here, we have presented the current evidence linking PPARs and the UPS. Ubiquitination and proteasomal degradation control the level and modulate the activity of PPARs in many ways. Ligand binding and proteolytic degradation affect turnover and transcriptional activity of the PPAR isotypes in distinct ways. PPARδ ubiquitination is selectively blocked by agonist ligands ensuring the accumulation of DNA-bound receptor engaged in transcriptional activation complexes. The opposite is true for the other PPAR isotypes. Distinct cellular pathways can exploit the UPS to modulate PPAR turnover and activity affecting their multiple functions. Furthermore, PPARs can control the level of specific proteins by modulating the activity of the UPS. This could be mediated by their ability to control the expression of components of the UPS, like ubiquitin ligases, or via protein-protein interactions. Controlling turnover of the receptors, the UPS can affect also the ligand-independent functions of PPARs. In this context, the control operated by the UPS on nuclear receptor levels might affect their ability to modulate activity of other transcriptional regulators. Increased proteolysis might reduce PPAR levels and produce apparently paradoxical responses with derepression or transrepression of distinct subsets of genes as seen in certain PPAR knockout experiments. The contribution of the multiple interactions between PPARs and the UPS need to be taken in consideration when examining the effects of PPAR overexpression, knock down or ligand-dependent activation on complex biological processes, like inflammation, angiogenesis, and tumorigenesis.
ACKNOWLEDGMENT
The authors wish to acknowledge the support received from the Tessin Foundation for Cancer Research.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Ciechanover A, Orian A, Schwartz AL. Ubiquitin-mediated proteolysis: biological regulation via destruction. BioEssays. 2000;22(5):442–451. doi: 10.1002/(SICI)1521-1878(200005)22:5<442::AID-BIES6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 3.Hershko A, Ciechanover A. The ubiquitin system. Annual Review of Biochemistry. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 4.Baek K-H. Conjugation and deconjugation of ubiquitin regulating the destiny of proteins. Experimental and Molecular Medicine. 2003;35(1):1–7. doi: 10.1038/emm.2003.1. [DOI] [PubMed] [Google Scholar]
- 5.Conaway RC, Brower CS, Conaway JW. Emerging roles of ubiquitin in transcription regulation. Science. 2002;296(5571):1254–1258. doi: 10.1126/science.1067466. [DOI] [PubMed] [Google Scholar]
- 6.Muratani M, Tansey WP. How the ubiquitin-proteasome system controls transcription. Nature Reviews Molecular Cell Biology. 2003;4(3):192–201. doi: 10.1038/nrm1049. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y. Transcriptional regulation by histone ubiquitination and deubiquitination. Genes and Development. 2003;17(22):2733–2740. doi: 10.1101/gad.1156403. [DOI] [PubMed] [Google Scholar]
- 8.Lee K-B, Wang D, Lippard SJ, Sharp PA. Transcription-coupled and DNA damage-dependent ubiquitination of RNA polymerase II in vitro. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(7):4239–4244. doi: 10.1073/pnas.072068399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reinstein E, Ciechanover A. Narrative review: protein degradation and human diseases: the ubiquitin connection. Annals of Internal Medicine. 2006;145(9):676–684. doi: 10.7326/0003-4819-145-9-200611070-00010. [DOI] [PubMed] [Google Scholar]
- 10.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 11.Freedman DA, Levine AJ. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Molecular and Cellular Biology. 1998;18(12):7288–7293. doi: 10.1128/mcb.18.12.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giglio S, Mancini F, Gentiletti F, et al. Identification of an aberrantly spliced form of HDMX in human tumors: a new mechanism for HDM2 stabilization. Cancer Research. 2005;65(21):9687–9694. doi: 10.1158/0008-5472.CAN-05-0450. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Carbayo M, Socci ND, Kirchoff T, et al. A polymorphismin HDM2 (SNP309) associates with early onset in superficial tumors, TP53 mutations, and poor outcome in invasive bladder cancer. Clinical Cancer Research. 2007;13(11):3215–3220. doi: 10.1158/1078-0432.CCR-07-0013. [DOI] [PubMed] [Google Scholar]
- 14.Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nature Cell Biology. 1999;1(4):193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 15.Bloom J, Pagano M. Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Seminars in Cancer Biology. 2003;13(1):41–47. doi: 10.1016/s1044-579x(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 16.Maynard MA, Ohh M. Molecular targets from VHL studies into the oxygen-sensing pathway. Current Cancer Drug Targets. 2005;5(5):345–356. doi: 10.2174/1568009054629672. [DOI] [PubMed] [Google Scholar]
- 17.Metzen E, Ratcliffe PJ. HIF hydroxylation and cellular oxygen sensing. Biological Chemistry. 2004;385(3-4):223–230. doi: 10.1515/BC.2004.016. [DOI] [PubMed] [Google Scholar]
- 18.Adams J. The proteasome: a suitable antineoplastic target. Nature Reviews Cancer. 2004;4(5):349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- 19.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocrine Reviews. 1999;20(5):649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 20.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405(6785):421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 21.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294(5548):1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 22.Sonoda J, Pei L, Evans RM. Nuclear receptors: decoding metabolic disease. FEBS Letters. 2008;582(1):2–9. doi: 10.1016/j.febslet.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiological Reviews. 2001;81(3):1269–1304. doi: 10.1152/physrev.2001.81.3.1269. [DOI] [PubMed] [Google Scholar]
- 24.Gronemeyer H, Gustafsson J-Å, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nature Reviews Drug Discovery. 2004;3(11):950–964. doi: 10.1038/nrd1551. [DOI] [PubMed] [Google Scholar]
- 25.Glass CK, Ogawa S. Combinatorial roles of nuclear receptors in inflammation and immunity. Nature Reviews Immunology. 2006;6(1):44–55. doi: 10.1038/nri1748. [DOI] [PubMed] [Google Scholar]
- 26.Rochette-Egly C. Dynamic combinatorial networks in nuclear receptor-mediated transcription. Journal of Biological Chemistry. 2005;280(38):32565–32568. doi: 10.1074/jbc.R500008200. [DOI] [PubMed] [Google Scholar]
- 27.Perissi V, Aggarwal A, Glass CK, Rose DW, Rosenfeld MG. A corepressor/coactivator exchange complex required for transcriptional activation by nuclear receptors and other regulated transcription factors. Cell. 2004;116(4):511–526. doi: 10.1016/s0092-8674(04)00133-3. [DOI] [PubMed] [Google Scholar]
- 28.Genini D, Catapano CV. Control of peroxisome proliferator-activated receptor fate by the ubiquitin-proteasome system. Journal of Receptors and Signal Transduction. 2006;26(5-6):679–692. doi: 10.1080/10799890600928202. [DOI] [PubMed] [Google Scholar]
- 29.Lefebvre P, Chinetti G, Fruchart J-C, Staels B. Sorting out the roles of PPARα in energy metabolism and vascular homeostasis. The Journal of Clinical Investigation. 2006;116(3):571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semple RK, Chatterjee VKK, O’Rahilly S. PPARγ and human metabolic disease. The Journal of Clinical Investigation. 2006;116(3):581–589. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barish GD, Narkar VA, Evans RM. PPARδ: a dagger in the heart of the metabolic syndrome. The Journal of Clinical Investigation. 2006;116(3):590–597. doi: 10.1172/JCI27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michalik L, Wahli W. Involvement of PPAR nuclear receptors in tissue injury and wound repair. The Journal of Clinical Investigation. 2006;116(3):598–606. doi: 10.1172/JCI27958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delerive P, De Bosscher K, Besnard S, et al. Peroxisome proliferator-activated receptor α negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-κB and AP-1. Journal of Biological Chemistry. 1999;274(45):32048–32054. doi: 10.1074/jbc.274.45.32048. [DOI] [PubMed] [Google Scholar]
- 34.Grau R, Punzón C, Fresno M, Iñiguez MA. Peroxisome-proliferator-activated receptor α agonists inhibit cyclo-oxygenase 2 and vascular endothelial growth factor transcriptional activation in human colorectal carcinoma cells via inhibition of activator protein-1. Biochemical Journal. 2006;395(1):81–88. doi: 10.1042/BJ20050964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Planavila A, Laguna JC, Vázquez-Carrera M. Nuclear factor-κB activation leads to down-regulation of fatty acid oxidation during cardiac hypertrophy. Journal of Biological Chemistry. 2005;280(17):17464–17471. doi: 10.1074/jbc.M414220200. [DOI] [PubMed] [Google Scholar]
- 36.Lee C-H, Chawla A, Urbiztondo N, Liao D, Boisvert WA, Evans RM. Transcriptional repression of atherogenic inflammation: modulation by PPARδ . Science. 2003;302(5644):453–457. doi: 10.1126/science.1087344. [DOI] [PubMed] [Google Scholar]
- 37.Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nature Reviews Cancer. 2004;4(1):61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- 38.Koeffler HP. Peroxisome proliferator-activated receptor γ and cancers. Clinical Cancer Research. 2003;9(1):1–9. [PubMed] [Google Scholar]
- 39.Kopelovich L, Fay JR, Glazer RI, Crowell JA. Peroxisome proliferator-activated receptor modulators as potential chemopreventive agents. Molecular Cancer Therapeutics. 2002;1(5):357–363. [PubMed] [Google Scholar]
- 40.Sarraf P, Mueller E, Smith WM, et al. Loss-of-function mutations in PPARγ associated with human colon cancer. Molecular Cell. 1999;3(6):799–804. doi: 10.1016/s1097-2765(01)80012-5. [DOI] [PubMed] [Google Scholar]
- 41.Mueller E, Smith M, Sarraf P, et al. Effects of ligand activation of peroxisome proliferator-activated receptor γ in human prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(20):10990–10995. doi: 10.1073/pnas.180329197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kroll TG, Sarraf P, Pecciarini L, et al. PAX8-PPAR γ 1 fusion in oncogene human thyroid carcinoma. Science. 2000;289(5483):1357–1360. doi: 10.1126/science.289.5483.1357. [DOI] [PubMed] [Google Scholar]
- 43.Collett GP, Betts AM, Johnson MI, et al. Peroxisome proliferator-activated receptor α is an androgen-responsive gene in human prostate and is highly expressed in prostatic adenocarcinoma. Clinical Cancer Research. 2000;6(8):3241–3248. [PubMed] [Google Scholar]
- 44.Suchanek KM, May FJ, Robinson JA, et al. Peroxisome proliferator-activated receptor α in the human breast cancer cell lines MCF-7 and MDA-MB-231. Molecular Carcinogenesis. 2002;34(4):165–171. doi: 10.1002/mc.10061. [DOI] [PubMed] [Google Scholar]
- 45.Saidi SA, Holland CM, Charnock-Jones DS, Smith SK. In vitro and in vivo effects of the PPAR-alpha agonists fenofibrate and retinoic acid in endometrial cancer. Molecular Cancer. 2006;5, article 13 doi: 10.1186/1476-4598-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yokoyama Y, Xin B, Shigeto T, et al. Clofibric acid, a peroxisome proliferator-activated receptor α ligand, inhibits growth of human ovarian cancer. Molecular Cancer Therapeutics. 2007;6(4):1379–1386. doi: 10.1158/1535-7163.MCT-06-0722. [DOI] [PubMed] [Google Scholar]
- 47.Grabacka M, Plonka PM, Urbanska K, Reiss K. Peroxisome proliferator-activated receptor α activation decreases metastatic potential of melanoma cells in vitro via down-regulation of Akt. Clinical Cancer Research. 2006;12(10):3028–3036. doi: 10.1158/1078-0432.CCR-05-2556. [DOI] [PubMed] [Google Scholar]
- 48.Pozzi A, Ibanez MR, Gatica AE, et al. Peroxisomal proliferator-activated receptor-α-dependent inhibition of endothelial cell proliferation and tumorigenesis. Journal of Biological Chemistry. 2007;282(24):17685–17695. doi: 10.1074/jbc.M701429200. [DOI] [PubMed] [Google Scholar]
- 49.Panigrahy D, Kaipainen A, Huang S, et al. PPARα agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(3):985–990. doi: 10.1073/pnas.0711281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di-Po N, Tan NS, Michalik L, Wahli W, Desvergne B. Antiapoptotic role of PPARβ in keratinocytes via transcriptional control of the Akt1 signaling pathway. Molecular Cell. 2002;10(4):721–733. doi: 10.1016/s1097-2765(02)00646-9. [DOI] [PubMed] [Google Scholar]
- 51.Cutler NS, Graves-Deal R, LaFleur BJ, et al. Stromal production of prostacyclin confers an antiapoptotic effect to colonic epithelial cells. Cancer Research. 2003;63(8):1748–1751. [PubMed] [Google Scholar]
- 52.Glinghammar B, Skogsberg J, Hamsten A, Ehrenborg E. PPARδ activation induces COX-2 gene expression and cell proliferation in human hepatocellular carcinoma cells. Biochemical and Biophysical Research Communications. 2003;308(2):361–368. doi: 10.1016/s0006-291x(03)01384-6. [DOI] [PubMed] [Google Scholar]
- 53.Shureiqi I, Jiang W, Zuo X, et al. The 15-lipoxygenase-1 product 13-S-hydroxyoctadecadienoic acid down-regulates PPAR-δ to induce apoptosis in colorectal cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(17):9968–9973. doi: 10.1073/pnas.1631086100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stephen RL, Gustafsson MCU, Jarvis M, et al. Activation of peroxisome proliferator-activated receptor δ stimulates the proliferation of human breast and prostate cancer cell lines. Cancer Research. 2004;64(9):3162–3170. doi: 10.1158/0008-5472.can-03-2760. [DOI] [PubMed] [Google Scholar]
- 55.Gupta RA, Wang D, Katkuri S, Wang H, Dey SK, DuBois RN. Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-δ accelerates intestinal adenoma growth. Nature Medicine. 2004;10(3):245–247. doi: 10.1038/nm993. [DOI] [PubMed] [Google Scholar]
- 56.Yin Y, Russell RG, Dettin LE, et al. Peroxisome proliferator-activated receptor δ and γ agonists differentially alter tumor differentiation and progression during mammary carcinogenesis. Cancer Research. 2005;65(9):3950–3957. doi: 10.1158/0008-5472.CAN-04-3990. [DOI] [PubMed] [Google Scholar]
- 57.Wang D, Wang H, Shi Q, et al. Prostaglandin E2 promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor δ . Cancer Cell. 2004;6(3):285–295. doi: 10.1016/j.ccr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 58.He T-C, Chan TA, Vogelstein B, Kinzler KW. PPARδ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99(3):335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta RA, Tan J, Krause WF, et al. Prostacyclin-mediated activation of peroxisome proliferator-activated receptor δ in colorectal cancer. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(24):13275–13280. doi: 10.1073/pnas.97.24.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gupta RA, DuBois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nature Reviews Cancer. 2001;1(1):11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 61.Tong BJ, Tan J, Tajeda L, et al. Heightened expression of cyclooxygenase-2 and peroxisome proliferator-activated receptor-δ human endometrial adenocarcinoma. Neoplasia. 2000;2(6):483–490. doi: 10.1038/sj.neo.7900119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park BH, Vogelstein B, Kinzler KW. Genetic disruption of PPARδ decreases the tumorigenicity of human colon cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(5):2598–2603. doi: 10.1073/pnas.051630998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saez E, Tontonoz P, Nelson MC, et al. Activators of the nuclear receptor PPARγ enhance colon polyp formation. Nature Medicine. 1998;4(9):1058–1061. doi: 10.1038/2042. [DOI] [PubMed] [Google Scholar]
- 64.Lefebvre A-M, Chen I, Desreumaux P, et al. Activation of the peroxisome proliferator-activated receptor γ promotes the development of colon tumors in C57BL/6J-APCMin/+ mice. Nature Medicine. 1998;4(9):1053–1057. doi: 10.1038/2036. [DOI] [PubMed] [Google Scholar]
- 65.Peters JM, Cheung C, Gonzalez FJ. Peroxisome proliferator-activated receptor-α and liver cancer: where do we stand? Journal of Molecular Medicine. 2005;83(10):774–785. doi: 10.1007/s00109-005-0678-9. [DOI] [PubMed] [Google Scholar]
- 66.Harman FS, Nicol CJ, Marin HE, Ward JM, Gonzalez FJ, Peters JM. Peroxisome proliferator-activated receptor-δ attenuates colon carcinogenesis. Nature Medicine. 2004;10(5):481–483. doi: 10.1038/nm1026. [DOI] [PubMed] [Google Scholar]
- 67.Reed KR, Sansom OJ, Hayes AJ, et al. PPARδ status and Apc-mediated tumourigenesis in the mouse intestine. Oncogene. 2004;23(55):8992–8996. doi: 10.1038/sj.onc.1208143. [DOI] [PubMed] [Google Scholar]
- 68.Marin HE, Peraza MA, Billin AN, et al. Ligand activation of peroxisome proliferator-activated receptor β inhibits colon carcinogenesis. Cancer Research. 2006;66(8):4394–4401. doi: 10.1158/0008-5472.CAN-05-4277. [DOI] [PubMed] [Google Scholar]
- 69.Panigrahy D, Singer S, Shen LQ, et al. PPARγ ligands inhibit primary tumor growth and metastasis by inhibiting angiogenesis. The Journal of Clinical Investigation. 2002;110(7):923–932. doi: 10.1172/JCI15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xin X, Yang S, Kowalski J, Gerritsen ME. Peroxisome proliferator-activated receptor γ ligands are potent inhibitors of angiogenesis in vitro and in vivo. Journal of Biological Chemistry. 1999;274(13):9116–9121. doi: 10.1074/jbc.274.13.9116. [DOI] [PubMed] [Google Scholar]
- 71.Wang D, Wang H, Guo Y, et al. Crosstalk between peroxisome proliferator-activated receptor δ and VEGF stimulates cancer progression. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(50):19069–19074. doi: 10.1073/pnas.0607948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abdollahi A, Schwager C, Kleeff J, et al. Transcriptional network governing the angiogenic switch in human pancreatic cancer. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(31):12890–12895. doi: 10.1073/pnas.0705505104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaipainen A, Kieran MW, Huang S, et al. PPARα deficiency in inflammatory cells suppresses tumor growth. PLoS ONE. 2007;2(2):e260 pages. doi: 10.1371/journal.pone.0000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hauser S, Adelmant G, Sarraf P, Wright HM, Mueller E, Spiegelman BM. Degradation of the peroxisome proliferator-activated receptor γ is linked to ligand-dependent activation. Journal of Biological Chemistry. 2000;275(24):18527–18533. doi: 10.1074/jbc.M001297200. [DOI] [PubMed] [Google Scholar]
- 75.Blanquart C, Barbier O, Fruchart J-C, Staels B, Glineur C. Peroxisome proliferator-activated receptor α (PPARα) turnover by the ubiquitin-proteasome system controls the ligand-induced expression level of its target genes. Journal of Biological Chemistry. 2002;277(40):37254–37259. doi: 10.1074/jbc.M110598200. [DOI] [PubMed] [Google Scholar]
- 76.Genini D, Catapano CV. Block of nuclear receptor ubiquitination: a mechanism of ligand-dependent control of peroxisome proliferator-activated receptor δ activity. Journal of Biological Chemistry. 2007;282(16):11776–11785. doi: 10.1074/jbc.M609149200. [DOI] [PubMed] [Google Scholar]
- 77.Dennis AP, Haq RU, Nawaz Z. Importance of the regulation of nuclear receptor degradation. Front Biosci. 2001;6:D954–D959. doi: 10.2741/dennis. [DOI] [PubMed] [Google Scholar]
- 78.Li X-Y, Boudjelal M, Xiao J-H, et al. 1,25-dihydroxyvitamin D3 increases nuclear vitamin D3 receptors by blocking ubiquitin/proteasome-mediated degradation in human skin. Molecular Endocrinology. 1999;13(10):1686–1694. doi: 10.1210/mend.13.10.0362. [DOI] [PubMed] [Google Scholar]
- 79.Blanquart C, Mansouri R, Fruchart J-C, Staels B, Glineur C. Different ways to regulate the PPARα stability. Biochemical and Biophysical Research Communications. 2004;319(2):663–670. doi: 10.1016/j.bbrc.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 80.Xu HE, Lambert MH, Montana VG, et al. Structural determinants of ligand binding selectivity between the peroxisome proliferator-activated receptors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(24):13919–13924. doi: 10.1073/pnas.241410198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Catic A, Collins C, Church GM, Ploegh HL. Preferred in vivo ubiquitination sites. Bioinformatics. 2004;20(18):3302–3307. doi: 10.1093/bioinformatics/bth407. [DOI] [PubMed] [Google Scholar]
- 82.Burns KA, Vanden Heuvel JP. Modulation of PPAR activity via phosphorylation. Biochimica et Biophysica Acta. 2007;1771(8):952–960. doi: 10.1016/j.bbalip.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chang AJ, Song DH, Wolfe MM. Attenuation of peroxisome proliferator-activated receptor γ (PPARγ) mediates gastrin-stimulated colorectal cancer cell proliferation. Journal of Biological Chemistry. 2006;281(21):14700–14710. doi: 10.1074/jbc.M602623200. [DOI] [PubMed] [Google Scholar]
- 84.Waite KJ, Floyd ZE, Arbour-Reily P, Stephens JM. Interferon-γ-induced regulation of peroxisome proliferator-activated receptor γ and STATs in adipocytes. Journal of Biological Chemistry. 2001;276(10):7062–7068. doi: 10.1074/jbc.M007894200. [DOI] [PubMed] [Google Scholar]
- 85.Liu J, Farmer SR. Regulating the balance between peroxisome proliferator-activated receptor γ and β-catenin signaling during adipogenesis: a glycogen synthase kinase 3β phosphorylation-defective mutant of β-catenin inhibits expression of a subset of adipogenic genes. Journal of Biological Chemistry. 2004;279(43):45020–45027. doi: 10.1074/jbc.M407050200. [DOI] [PubMed] [Google Scholar]
- 86.Sharma C, Pradeep A, Wong L, Rana A, Rana B. Peroxisome proliferator-activated receptor γ activation can regulate β-catenin levels via a proteasome-mediated and adenomatous polyposis coli-independent pathway. Journal of Biological Chemistry. 2004;279(34):35583–35594. doi: 10.1074/jbc.M403143200. [DOI] [PubMed] [Google Scholar]
- 87.Liu J, Wang H, Zuo Y, Farmer SR. Functional interaction between peroxisome proliferator-activated receptor γ and β-catenin. Molecular and Cellular Biology. 2006;26(15):5827–5837. doi: 10.1128/MCB.00441-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Girnun GD, Smith WM, Drori S, et al. APC-dependent suppression of colon carcinogenesis by PPARγ . Proceedings of the National Academy of Sciences of the United States of America. 2002;99(21):13771–13776. doi: 10.1073/pnas.162480299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sharma C, Pradeep A, Pestell RG, Rana B. Peroxisome proliferator-activated receptor γ activation modulates cyclin D1 transcription via β-catenin-independent and cAMP-response element-binding protein-dependent pathways in mouse hepatocytes. Journal of Biological Chemistry. 2004;279(17):16927–16938. doi: 10.1074/jbc.M309045200. [DOI] [PubMed] [Google Scholar]
- 90.Qin C, Burghardt R, Smith R, Wormke M, Stewart J, Safe S. Peroxisome proliferator-activated receptor γ agonists induce proteasome-dependent degradation of cyclin D1 and estrogen receptor α in MCF-7 breast cancer cells. Cancer Research. 2003;63(5):958–964. [PubMed] [Google Scholar]
- 91.Huang J-W, Shiau C-W, Yang Y-T, et al. Peroxisome proliferator-activated receptor γ-independent ablation of cyclin D1 by thiazolidinediones and their derivatives in breast cancer cells. Molecular Pharmacology. 2005;67(4):1342–1348. doi: 10.1124/mol.104.007732. [DOI] [PubMed] [Google Scholar]
- 92.Wei S, Lin L-F, Yang C-C, et al. Thiazolidinediones modulate the expression of β-catenin and other cell-cycle regulatory proteins by targeting the F-box proteins of Skp1-Cul1-F-box protein E3 ubiquitin ligase independently of peroxisome proliferator-activated receptor γ . Molecular Pharmacology. 2007;72(3):725–733. doi: 10.1124/mol.107.035287. [DOI] [PubMed] [Google Scholar]
- 93.Motomura W, Takahashi N, Nagamine M, et al. Growth arrest by troglitazone is mediated by p27kip1 accumulation, which results from dual inhibition of proteasome activity and Skp2 expression in human hepatocellular carcinoma cells. International Journal of Cancer. 2004;108(1):41–46. doi: 10.1002/ijc.11561. [DOI] [PubMed] [Google Scholar]
- 94.Varley CL, Garthwaite MAE, Cross W, Hinley J, Trejdosiewicz LK, Southgate J. PPARγ-regulated tight junction development during human urothelial cytodifferentiation. Journal of Cellular Physiology. 2006;208(2):407–417. doi: 10.1002/jcp.20676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vignati S, Albertini V, Rinaldi A, et al. Cellular and molecular consequences of peroxisome proliferator-activated receptor-γ activation in ovarian cancer cells. Neoplasia. 2006;8(10):851–861. doi: 10.1593/neo.06433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Paukkeri E-L, Leppänen T, Sareila O, Vuolteenaho K, Kankaanranta H, Moilanen E. PPARα agonists inhibit nitric oxide production by enhancing iNOS degradation in LPS-treated macrophages. British Journal of Pharmacology. 2007;152(7):1081–1091. doi: 10.1038/sj.bjp.0707477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim DJ, Murray IA, Burns AM, Gonzalez FJ, Perdew GH, Peters JM. Peroxisome proliferator-activated receptor-β/δ inhibits epidermal cell proliferation by down-regulation of kinase activity. Journal of Biological Chemistry. 2005;280(10):9519–9527. doi: 10.1074/jbc.M413808200. [DOI] [PubMed] [Google Scholar]
- 98.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annual Review of Immunology. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 99.Yuan Z, Liu Y, Liu Y, et al. Cardioprotective effects of peroxisome proliferator activated receptor γ activators on acute myocarditis: anti-inflammatory actions associated with nuclear factor κB blockade. Heart. 2005;91(9):1203–1208. doi: 10.1136/hrt.2004.046292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Delerive P, De Bosscher K, Berghe WV, Fruchart J-C, Haegeman G, Staels B. DNA binding-independent induction of IκBα gene transcription by PPARα . Molecular Endocrinology. 2002;16(5):1029–1039. doi: 10.1210/mend.16.5.0826. [DOI] [PubMed] [Google Scholar]