Abstract
Four bacterial strains were isolated from a cyanophycin granule polypeptide (CGP)-degrading anaerobic consortium, identified by 16S rRNA gene sequencing, and assigned to species of the genera Pseudomonas, Enterococcus, Clostridium, and Paenibacillus. The consortium member responsible for CGP degradation was assigned as Pseudomonas alcaligenes strain DIP1. The growth of and CGP degradation by strain DIP1 under anaerobic conditions were enhanced but not dependent on the presence of nitrate as an electron acceptor. CGP was hydrolyzed to its constituting β-Asp-Arg dipeptides, which were then completely utilized within 25 and 4 days under anaerobic and aerobic conditions, respectively. The end products of CGP degradation by strain DIP1 were alanine, succinate, and ornithine as determined by high-performance liquid chromatography analysis. The facultative anaerobic Enterococcus casseliflavus strain ELS3 and the strictly anaerobic Clostridium sulfidogenes strain SGB2 were coisolates and utilized the β-linked isodipeptides from the common pool available to the mixed consortium, while the fourth isolate, Paenibacillus odorifer strain PNF4, did not play a direct role in the biodegradation of CGP. Several syntrophic interactions affecting CGP degradation, such as substrate utilization, the reduction of electron acceptors, and aeration, were elucidated. This study demonstrates the first investigation of CGP degradation under both anaerobic and aerobic conditions by one bacterial strain, with regard to the physiological role of other bacteria in a mixed consortium.
Cyanophycin (multi-l-arginyl-poly-[l-aspartic acid]), also known as cyanophycin granule polypeptide (CGP), was discovered in 1887 in cyanobacteria (7). The polymer occurs in the cytoplasm as insoluble intracellular membraneless granules (3, 22). Most genera of cyanobacteria harbor a cyanophycin synthetase gene (cphA) and synthesize CGP (26, 43, 51). Genes coding for functional, active CphA also were recently identified in heterotrophic bacteria like Acinetobacter baylyi and Desulfitobacterium hafniense (16, 23, 52). The branched polymer (Fig. 1) consists of equimolar amounts of arginine and aspartic acid arranged as a poly(aspartic acid) backbone, with arginine moieties linked to the β-carboxyl group of each aspartic acid by its α-amino group (34, 44). CGP can be converted to poly(aspartic acid). The latter is produced by the industry as a substitute for nonbiodegradable polyacrylic acid, which is used for many technical and medical applications. Thus, composition and structure make this biopolymer interesting for industry (28, 41).
FIG. 1.
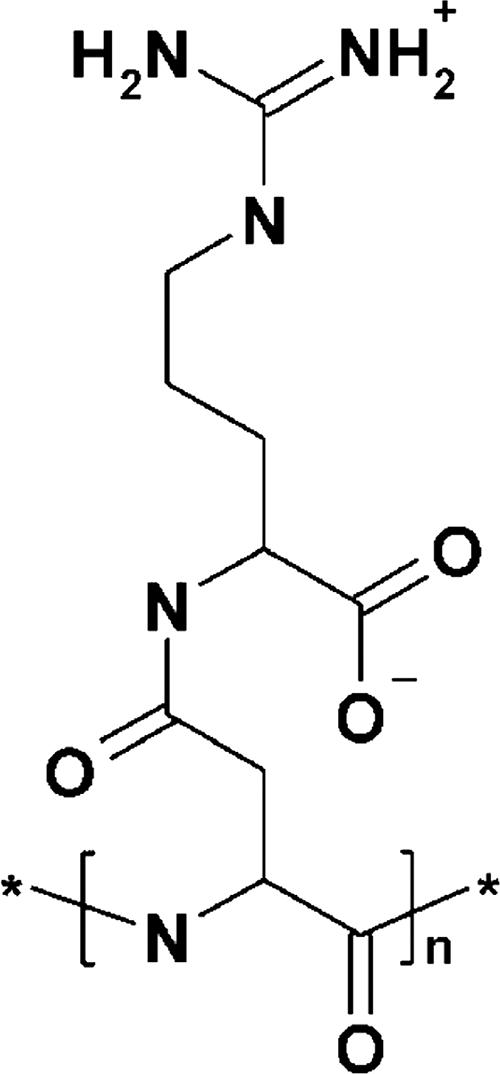
Chemical structure of the aspartic acid-arginine building block of CGP (34).
CGP accumulation is promoted by several conditions, including phosphorus limitation (46), sulfur limitation (5), low temperature, low light intensity, or a combination of these factors (32), and in the presence of translational or transcriptional inhibitors (3, 13). CGP functions as a temporary nitrogen, energy, and possibly carbon reserve (12, 26). Additionally, CGP has been suggested to play a role in nitrogen fixation in heterocysts of Anabaena species as a dynamic buffer for newly fixed nitrogen (8). It accumulates during the transition from the exponential to the stationary growth phases and degrades when balanced growth resumes (19, 26, 42). The intracellular degradation of CGP is catalyzed by cyanophycinases (CGPases) (CphB) occurring in the cytoplasm and results in the formation of β-Asp-Arg dipeptides (39).
CGP occurs in different natural habitats and represents a valuable exogenous substrate for bacteria. Like intracellular CGPases, the extracellular CGPase (CphEPa) of the gram-negative bacterium Pseudomonas anguilliseptica strain BI is a serine-type hydrolase and exhibits an α-cleavage mechanism for CGP degradation (33). Extracellular CGPases were also identified in gram-positive bacteria like Bacillus megaterium strain BAC19 (CphEBm). This enzyme is also a serine-type hydrolase and yielded β-Asp-Arg dipeptides as cleavage products; however, (Asp-Arg)2 tetrapeptides also occurred (30).
CGP degradation must also occur in anaerobic habitats, where it might be produced by anaerobic bacteria like Clostridium botulinum and D. hafniense, which harbor CGP biosynthesis genes (23, 52), or where CGP was released from a cyanobacterial biomass upon cell lysis. Recently, the anaerobic endospore-forming Sedimentibacter hongkongensis strain KI was isolated from an anaerobic CGP-degrading bacterial consortium (31). It was the first anaerobic bacterium for which the degradation of CGP was demonstrated. It hydrolyzed CGP to the dipeptides.
The aim of this study was to investigate the extracellular degradation of CGP under anaerobic conditions by bacteria able to use inorganic electron acceptors. The isolation of the most predominant four strains in an interesting anaerobic CGP-degrading consortium and their roles in CGP degradation are reported. The isolate responsible for CGP degradation is a strain of Pseudomonas alcaligenes, which is one of several Pseudomonas species known to be able to grow anaerobically in the presence of nitrate (29, 45). This is the first study of CGP degradation under both anaerobic and aerobic conditions by one bacterial strain.
MATERIALS AND METHODS
Sources of samples and enrichment of bacteria.
Sediment water samples were collected from a small pond (Schloβgraben, Münster, Germany) by completely filling 500-ml sterile glass bottles, which were kept at 4°C until use. For the enrichment of bacteria capable of CGP degradation under anaerobic conditions with special emphasis on bacteria able to reduce inorganic electron acceptors such as sulfate, Desulfovibrio medium (DSMZ 272; www.DSMZ.de) was initially used. To restrict the growth of undesired non-CGP-degrading bacteria, the medium was modified to achieve the following basal medium (BM): 1.0 g NH4Cl, 3.0 g KH2PO4, 3.0 g K2HPO4, 0.1 g KCl, 0.5 g MgCl2·6H2O, 0.1 g CaCl2·2H2O, 0.5 g NaCl, 0.5 g cysteine-HCl, 2.0 g yeast extract (unless otherwise indicated in the text), 10 ml of SL10 trace elements solution (50), and 1 mg of resazurin per liter. The pH was adjusted to 7.0 with KOH. The medium was boiled, and after Na2S·9H2O was added to a concentration of 0.04% (wt/vol), it was immediately transferred to an anaerobic chamber (Type A manual air lock; Coy, Inc., Grass Lake, MI) with a Formier gas atmosphere (N2:H2, 95%:5%, vol/vol). After the medium was cooled to room temperature, aliquots (5 or 10 ml) were dispensed into Hungate tubes (VWR International GmbH, Darmstadt, Germany), sealed, removed from the chamber and subsequently sterilized by autoclaving at 121°C for 20 min. For shaking during incubation, tubes were placed horizontally in a tube rotator (3 rpm).
For the preparation of the solid BM agar plates, the sterile-filtered reducing agent (Na2S·9H2O) was added directly to autoclaved BM containing 1.2% (wt/vol) agar. The medium was cooled before it was poured into petri dishes inside the anaerobic chamber. After inoculation, the plates were transferred to 3.5-liter anaerobic jars (Oxoid, Wesel, Germany) and incubated at the desired temperature.
In addition, Luria-Bertani (LB) medium (40) agar plates were used for anaerobic experiments (with added reducing agents) and aerobic experiments. Liquid or solid nitrogen-free RCV medium (2) was used to examine the ability of Paenibacillus odorifer PNF4 to fix dinitrogen.
Source and isolation of CGP.
“Recombinant” CGP was isolated and purified from lyophilized cells of recombinant Escherichia coli strain DH1 harboring plasmid pMa/c5-914::cphAPCC6803 according to the acid extraction method (15). “Cyanobacterial” CGP was isolated from the cells of Synechococcus sp. strain MA19 as described previously (44).
Sterilization of CGP.
CGP was sterilized by diethyl ether (33). Alternatively, CGP was first dissolved in 0.1 N HCl, passed through a filter (pore size, 0.2 μm; Millipore GmbH, Eschborn, Germany), and finally reprecipitated at pH 7.3 by adding 1 volume of sterile 0.1 N NaOH. Sterile CGP suspensions were injected directly into sterile Hungate tubes containing the anaerobically prepared BM to final concentrations up to 1 g liter−1. For the CGP overlays, 1.2% (wt/vol) Bacto agar was added to a CGP suspension; this mixture was sterilized by autoclaving and poured in a thin layer onto Desulfovibrio medium or BM agar plates.
Experiments to determine the optimum conditions for CGP degradation were conducted at 30°C in anaerobically prepared Hungate tubes containing 10 ml BM and 1 g liter−1 CGP in addition to different concentrations of yeast extract in the presence or absence of sodium nitrate. The lowest optical density value at 578 nm (OD578 nm) was considered a sign of completed CGP degradation and was reached usually 1 to 2 days after the visual disappearance of CGP in the tubes.
Strain enrichment and purification.
Small aliquots of the sediment sample were spread on Desulfovibrio medium (DSMZ 272) agar plates with CGP overlays and incubated anaerobically at 30°C to enrich sulfate-reducing bacteria able to degrade CGP. The plates were checked in the anaerobic chamber for the appearance of halos caused by CGP-degrading bacteria.
The direct sequencing of 16S rRNA genes was applied to the total DNA isolated from the consortium. Individual purification strategies were then developed, depending on the main characteristics revealed for each member. A facultative anaerobic non-spore-forming Enterococcus sp. was enriched and purified on LB medium agar plates under aerobic conditions until an axenic culture was microscopically confirmed. A facultative anaerobic spore-forming species of the genus Paenibacillus was obtained as an axenic culture after pasteurization at 80°C, followed by several purification steps on LB agar plates under an aerobic atmosphere. A strictly anaerobic spore-forming species of the genus Clostridium was purified using serial dilutions in liquid BM and 30-min pasteurization; dilutions were then spread on LB agar and incubated aerobically at 30°C for at least 48 h to allow any present facultative anaerobic bacteria to grow. From the plate with the lowest total colony count, a swab was taken from a colony-free area of the agar surface using a sterile cotton swab to collect nongerminated Clostridium spores. The swab was used to inoculate fresh anaerobically prepared LB agar plates or a Hungate tube with LB medium, which was incubated anaerobically at 30°C for 48 h to obtain an axenic culture of Clostridium sp. The CGP-degrading Pseudomonas sp. was obtained as an axenic culture after several purification steps on BM agar plates (0.5 g liter−1 yeast extract) with CGP overlays under anaerobic conditions.
Utilization of electron acceptors and substrates.
Clostridium thiosulfatireducens DSM 13105T and Clostridium peptidivorans DSM 12505T were used as reference strains to investigate the ability to use inorganic electron acceptors for growth. These experiments were independent from those which examined CGP degradation and were conducted with BM in which 0.5% (wt/vol) Casamino Acids were substituted for yeast extract, as this change enabled better growth of all strains than when yeast extract was present. Cultivations were performed at 30°C for at least 15 days in Hungate tubes containing 10 ml of this modified BM and one of the following electron acceptors: 20 mM sodium sulfate, 20 mM sodium thiosulfate, 2 mM sodium sulfite, 2% (wt/vol) elemental sulfur, 10 mM sodium nitrate, or 10 mM sodium nitrite.
Substrate utilization was investigated at 30°C in cysteine-free BM containing 0.5 g liter−1 yeast extract. Substrates were injected into Hungate tubes to a final concentration of 10 mM (amino acids), 5 mM (dipeptides), or 20 mM (organic acids and sugars) after sterilization by filtration (pore size, 0.2 μm; Millipore, Eschborn, Germany). Experiments were performed in duplicate, and cultures were inoculated with a preculture grown under the same conditions.
Analytical techniques.
Bacterial growth was monitored by measuring the increase in turbidity at 578 nm after the insertion of the Hungate tubes into an Eppendorf 1101 M spectrophotometer (Eppendorf, Hamburg, Germany). An increase in the OD578 nm in the tubes containing the tested compound over the control tubes lacking the compound indicated its utilization. The levels of H2S and nitrate were determined spectrophotometrically as colloidal CuS (9) and by using a Nanocolor Nitrate 50 kit (Macherey-Nagel, Düren, Germany), respectively.
Free amino acids and dipeptides were detected by the high-pressure liquid chromatography (HPLC) system described previously (1). The system was equipped with a B801 column (Prep Nova-Pak HR [3.9 by 300 mm]; Knauer GmbH, Berlin, Germany). The CGP samples were subjected in advance to acid hydrolysis (6 N HCl, 95°C, overnight).
Organic acids, alcohols, and sugars were measured by HPLC (LaChrom Elite HPLC system; VWR-Hitachi International, Darmstadt, Germany), using a 300- by 6.5-mm Metacarb 67 H advanced C column (Varian, Palo Alto, CA), a Hitachi type 22350 oven (VWR), and a Hitachi type 2490 refractive index detector (VWR). Compounds were eluted with 0.005 N particle-free H2SO4 buffer at a flow rate of 0.8 ml/min; peaks were analyzed by EZ Chrome Elite software (VWR International, Darmstadt, Germany).
DNA extraction and analysis of 16S rRNA genes.
The isolation of total genomic DNA was performed as described previously (38). The 16S rRNA genes were amplified by PCR from total DNA using standard oligonucleotide primers (37). The PCR products were purified using a Nucleo-trap CR kit (Macherey-Nagel, Düren, Germany) and were then directly sequenced or cloned into pGEM-T Easy vectors (Promega, Manheim, Germany), transformed into CaCl2-competent cells (18) of E. coli TOP10 (Invitrogen, San Diego, CA), and then sequenced (only the axenic cultures). E. coli clones harboring hybrid plasmids were identified on LB agar plates containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; 0.004%, wt/vol) and ampicillin (75 μg/ml). Plasmids were isolated by the alkaline lysis method (6).
DNA sequencing was performed with a SequiTherm Excel TM II long-read cycle sequencing kit (Epicentre Technologies, Madison, WI) and the following primers: sequencing primers 27f, 343r, 357f, 519r, 536f, 803f, 907r, 1114f, 1385r, and 1525r; universal primer 5′-GTAAAACGACGGCCAGT-3′; and reverse primer 5′-CAGGAAACAGCTATGAC-3′ (MWG-Biotech AG, Ebersberg, Germany). Sequence reactions were generated by a GeneReadIR 4200 DNA analyzer (LI-COR) and run on a LI-COR 4000L automatic sequencer (MWG-Biotech, Ebersberg, Germany). Nucleic acid sequence data were analyzed with the Contig Assembly Program (CAP) online software (20). The 16S rRNA gene sequences were aligned with previously published sequences of representative strains using the BLASTn function from the National Center for Biotechnology Information (NCBI) database. Reference sequences were aligned using ClustalX 1.8 software (48). Positions of sequence and alignment uncertainty were omitted from the analysis using the program BioEdit v7.0.5 (T. Hall, North Carolina State University [http://www.mbio.ncsu.edu/BioEdit/bioedit.html]). Phylogenetic trees were constructed using the programs TreeView 1.6.5 (35) and NJplot (36).
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences were deposited in the NCBI database under the following accession numbers: EF199996 for Pseudomonas alcaligenes strain DIP1, EF199997 for Enterococcus casseliflavus strain ELS3, EF199998 for Clostridium sulfidogenes strain SGB2, and EF199999 for Paenibacillus odorifer strain PNF4.
RESULTS
Isolation of an anaerobic CGP-degrading mixed consortium.
Four new bacterial isolates were purified from a CGP-degrading consortium. The latter was enriched from pond sediments under anaerobic conditions favoring bacteria capable of reducing sulfate. When the presence of three bacterial strains in the consortium was microscopically confirmed, direct 16S rRNA gene sequencing of the total DNA isolated from this consortium clearly revealed that species of the genera Enterococcus, Clostridium, and Paenibacillus constitute this consortium. To identify the CGP-degrading strain and to investigate the possible physiological roles of the other members, axenic cultures of three different bacteria were obtained.
Identification of the consortium member responsible for CGP degradation.
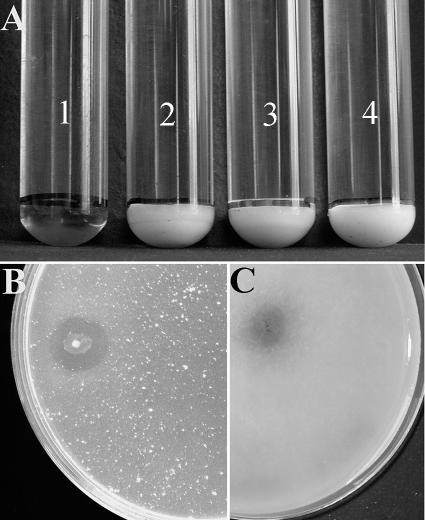
Axenic cultures of the three consortium members were tested for their ability to degrade CGP separately and also in cocultivations under both anaerobic and aerobic conditions. Surprisingly, CGP degradation did not occur in any of the tested cultures (Fig. 2A, tubes 2, 3, and 4). This suggested the presence of an additional, not-yet-detected bacterium in the CGP-degrading consortium. By applying further purification steps to the last CGP-degrading mixed consortium using CGP overlay agar plates, an axenic culture capable of anaerobic CGP degradation was obtained. 16S rRNA gene sequencing identified this isolate as a member of the genus Pseudomonas. Confirmatory experiments on CGP showed the stable ability of this isolate to degrade CGP under anaerobic conditions as well as under aerobic conditions.
FIG. 2.
Degradation of CGP in liquid and solid media. (A) Four anaerobic Hungate tubes, which contained initial concentrations of 1 g liter−1 CGP plus 0.5 g liter−1 yeast extract in 10 ml BM, after incubation at 30°C for 15 days. Tube 1 contains P. alcaligenes strain DIP1 and shows CGP degradation after the first 48 h of incubation. Tubes 2, 3, and 4 contain E. casseliflavus strain ELS3, C. sulfidogenes strain SGB2, and P. odorifer strain PNF4, respectively. None of the three strains could degrade CGP. (B) Degradation halo by P. alcaligenes strain DIP1 appearing after 24 h of incubation on an aerobic CGP overlay agar plate. (C) Degradation halo by P. alcaligenes strain DIP1 appearing after 3 to 4 days of incubation on an anaerobic CGP overlay agar plate.
Cell morphology of the mixed consortium members.
Colonies of the CGP-degrading Pseudomonas sp. appeared after a 24-h incubation on LB agar plates at 30°C under anaerobic as well as aerobic conditions. Microscopy revealed straight rods 1 to 3 μm in length and approximately 0.5 μm in diameter. Cells were single, non-spore-forming, and highly motile and showed a negative Gram reaction.
Colonies of the facultative anaerobic Enterococcus isolate appeared after a 24-h incubation on LB agar plates when grown aerobically at 37°C. Microscopy revealed the characteristic coccal cell form of this genus. Cells were 0.4 μm in diameter, single or in pairs, and showed a positive Gram reaction.
The strictly anaerobic Clostridium member grew on LB agar after a 24-h incubation at 37°C. Microscopy showed straight or slightly curved rods 3 to 6 μm in length and 0.5 μm in diameter. Cells appeared singly or in pairs, motile until sporulation, and showed a positive Gram reaction. Spores were oval, subterminal, or terminal in aged cultures.
The fourth member of the consortium, belonging to the genus Paenibacillus, exhibited a special colony form under anaerobic conditions. After the third incubation day on LB agar plates at 30°C, the round colonies with irregular margins sank into the agar surface, which is characteristic of agar-utilizing Paenibacillus species (49). Microscopy showed straight rods 2 to 7 μm in length and 0.4 to 0.6 μm in diameter. Cells appeared single, motile until sporulation, and gram positive when grown aerobically on LB agar plates for 48 h at 30°C. Spores were rectangular to oval and subterminal and then central just prior to release from the sporangia.
Taxonomic affiliation of the consortium members.
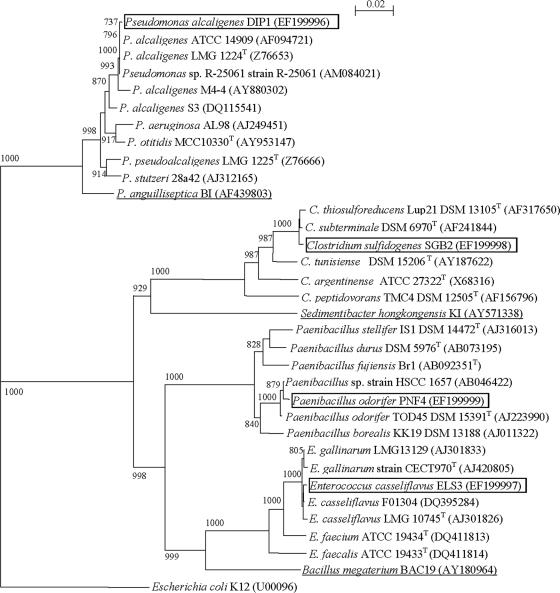
The CGP-degrading isolate (with a complete 16S rRNA gene sequence of 1,503 bp) was taxonomically assigned as a member of the genus Pseudomonas with sequence similarity of 99% to the denitrifying bacterium Pseudomonas sp. strain R-25061 (1,513 bp), strain R-25209 (1,513 bp), and strain R-25208 (1,500 bp). The isolate also showed 99% sequence similarity to P. alcaligenes LMG 1224T (1,492 bp). Therefore, it was classified as a new strain (DIP1) of the species P. alcaligenes.
The Enterococcus isolate of the consortium, with a complete 16S rRNA gene sequence of 1,513 bp, showed similarity of 99% to E. casseliflavus F01304 (1,482 bp) and the type strain E. casseliflavus LMG 10745 (1,559 bp). Consequently, it was taxonomically assigned as E. casseliflavus strain ELS3.
The strictly anaerobic Clostridium member of the consortium had a complete 16S rRNA gene sequence of 1,504 bp and a 99% sequence similarity to both C. subterminale DSM 6970T (1,504 bp) and C. thiosulfatireducens DSM 13105T (1,447 bp). This isolate was assigned as C. sulfidogenes SGB2 (A. Sallam and A. Steinbüchel, submitted for publication).
Similarly, the complete 16S rRNA gene sequence (1,530 bp) of the Paenibacillus isolate showed 99% sequence similarity to Paenibacillus sp. strain HSCC 1657 (1,504 bp) and P. odorifer TOD45T (1,438 bp). Thus, the Paenibacillus isolate was assigned as P. odorifer strain PNF4. The phylogenetic tree shown in Fig. 3 demonstrates the relationship between the four consortium members and their related strains.
FIG. 3.
Neighbor-joining tree based on 16S rRNA gene sequences showing the estimated phylogenetic relationships of the four consortium members to their closely related species and other bacteria. The underlined strains were previously investigated for CGP degradation (30, 31, 33). The names of species investigated in this study are boxed in the diagram. E. coli K-12 was used as an out-group. Accession numbers are given in parentheses. Bootstrap values are shown as percentages of 1,000 replicates. Scale bar = 2% sequence divergence.
Degradation of CGP by P. alcaligenes strain DIP1 under anaerobic conditions.
No CGP degradation was observed after 60 days of static or shaken incubation in liquid BM with CGP as the sole carbon source when cells of strain DIP1 (washed twice with sterile water) were used for inoculation. When tubes were inoculated directly from an anaerobic liquid preculture grown on LB medium, and only under shaking conditions, CGP was degraded slowly and disappeared after 22 days, indicating that yeast extract and shaking are necessary for CGP degradation. CGP degradation was enhanced in cultures containing 0.5 g liter−1 yeast extract and showed a visual disappearance of CGP after 4 days. A total of 10 mM sodium nitrate in addition to 0.5 g liter−1 yeast extract reduced the time to the disappearance of CGP to only 48 h.
Optimum conditions for CGP degradation by P. alcaligenes DIP1 in the absence of oxygen.
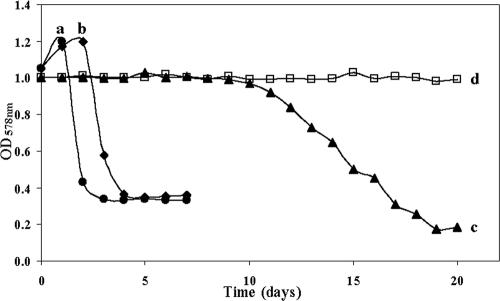
The shortest period for CGP degradation by P. alcaligenes strain DIP1 occurred in tubes containing 0.5 g liter−1 yeast extract plus 10 mM sodium nitrate, for which the minimum OD578 nm was reached after four incubation days (Fig. 4). Tubes containing sodium nitrate plus 1 or 1.5 to 2 g liter−1 yeast extract reached the lowest OD578 nm after 6 or 7 days, respectively. In tubes with liquid BM without yeast extract or sodium nitrate, CGP degradation was retarded but not impaired (Fig. 4).
FIG. 4.
Degradation of 1 g liter−1 CGP in 10 ml BM by axenic cultures of P. alcaligenes strain DIP1 in Hungate tubes cultivated anaerobically at 30°C under shaking conditions. a, tube containing 0.5 g liter−1 yeast extract and 10 mM sodium nitrate and showing the fastest CGP degradation by P. alcaligenes strain DIP1 with visual disappearance of CGP and the lowest OD578 nm after one and four incubation days, respectively; b, tube containing 0.5 g liter−1 yeast extract and no sodium nitrate and showing the visual disappearance of CGP and the lowest OD578 nm after four and five incubation days, respectively; c, tube containing 10 mM sodium nitrate and no yeast extract and showing the visual disappearance of CGP and the lowest OD578 nm after 12 and 19 incubation days, respectively; d, tube containing neither nitrate nor yeast extract (negative control).
Effect of the other consortium members on CGP degradation by P. alcaligenes DIP1.
In the absence of yeast extract and nitrate, CGP was degraded by P. alcaligenes DIP1 only if it was cocultivated with E. casseliflavus strain ELS3, C. sulfidogenes strain SGB2, or both. However, relatively long incubation periods were required; during cocultivations of all four consortium members, CGP degradation started only after an incubation period of 29 ± 2 days, while in similar tubes containing, in addition, 10 mM sodium nitrate, 10 ± 1 days was required, which is not much shorter than the period required by strain DIP1 alone under similar conditions. Also, in the presence of sodium nitrate and 0.5 g liter−1 yeast extract, the time course of CGP degradation by the defined four-member coculture did not diverge significantly from that of axenic cultures of P. alcaligenes strain DIP1. Similarly, cocultivations with strain ELS3 and/or strain SGB2 under the same conditions did not affect the degradation course of CGP. However, the consumption of CGP dipeptides was faster (consumption was parallel to degradation) due to the fast anaerobic growth of both strains. The presence of P. odorifer strain PNF4 did not induce any effect in all tested cocultivations.
Degradation of CGP by P. alcaligenes DIP1 under aerobic conditions.
When strain DIP1 was cultivated in 10 ml liquid BM with 1 g liter−1 CGP in 100-ml Klett flasks with baffles and incubated aerobically under shaking conditions, HPLC analysis indicated CGP degradation within 24 h. Unlike the CGP degradation rate under anaerobic conditions, the CGP degradation rate under aerobic conditions correlated with yeast extract concentration.
Utilization of electron acceptors under anaerobic conditions.
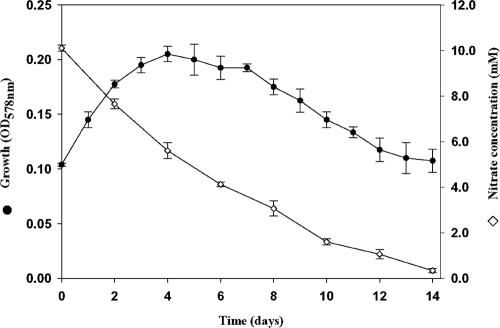
Nitrate and nitrite could be used as electron acceptors only by the CGP-degrading isolate P. alcaligenes strain DIP1. Quantitative monitoring of nitrate concentration revealed that the highest reduction rate occurred during the first 24 h of incubation with a decrease of 2.5 mM in the concentration of sodium nitrate, whereas the concentration decreased by only 1.65 mM during either of the two following days. From the fourth day onward, the nitrate reduction rate decreased progressively until a complete reduction of nitrate had occurred after 15 days of incubation (Fig. 5).
FIG. 5.
Growth of and nitrate reduction by P. alcaligenes DIP1 when grown at 30°C under anaerobic conditions. Duplicate Hungate tubes containing 10 ml BM with 0.5% (wt/vol) Casamino Acids and 10 mM sodium nitrate were used. Growth (measured by the OD578 nm) and the concentration of nitrate in the medium during the incubation period are shown.
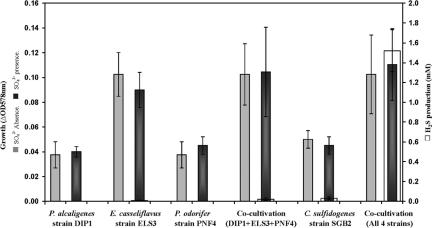
C. sulfidogenes strain SGB2 showed an unstable ability to reduce sulfate in few cultivation experiments. Other members of the consortium and Clostridium strains, which were used as a reference, did not show such activity with sulfate. However, sulfate reduction during the cocultivation of the four members of the consortium in a defined mixed culture was stable and led over several repetitions to the production of around 1.5 mM H2S from the initial 20 mM sodium sulfate. Meanwhile, no significant amounts of H2S were detected when C. sulfidogenes strain SGB2 was cocultivated with only one or two other consortium members under the same conditions (Fig. 6).
FIG. 6.
Growth and H2S production by the consortium and individually by the four isolates. Cells were incubated at 30°C in duplicate BM tubes containing 0.5% (wt/vol) Casamino Acids with or without 20 mM sodium sulfate as an electron acceptor. C. sulfidogenes strain SGB2 produced 1.5 mM H2S only when cocultivated with the other three consortium members. Axenic cultures of the four members or other combinations thereof did not produce significant amounts of H2S.
Thiosulfate was also used as a terminal electron acceptor by C. sulfidogenes strain SGB2, as well as by the two reference strains C. thiosulfatireducens and C. peptidivorans. Sulfur could be reduced only by C. sulfidogenes strain SGB2 and C. thiosulfatireducens, whereas sulfite was not used as a terminal electron acceptor by any of the tested strains.
Substrate utilization.
P. alcaligenes strain DIP1 utilized l-aspartate, l-arginine, CGP dipeptides, pyruvate, and succinate under an anaerobic atmosphere, while l-lysine, l-ornithine, lactate, and acetate were not utilized. Under aerobic conditions, P. alcaligenes strain DIP1 utilized l-aspartate, l-arginine, l-lysine, CGP dipeptides, pyruvate, lactate, acetate, and succinate as carbon sources but not l-ornithine.
Under an anaerobic atmosphere, E. casseliflavus strain ELS3 utilized l-arginine, l-lysine, l-ornithine, CGP dipeptides, pyruvate, acetate, and succinate as carbon sources but not l-aspartate or lactate. Under aerobic conditions, this strain utilized l-arginine, l-lysine, l-ornithine, CGP dipeptides, pyruvate, lactate, acetate, and succinate but not l-aspartate.
Among all investigated substrates, P. odorifer strain PNF4 utilized only pyruvate under aerobic as well as anaerobic conditions as a carbon source. Additionally, glucose, sucrose, and fructose were utilized during the dinitrogen fixation experiments by P. odorifer strain PNF4 regardless of the state of aeration.
The strict anaerobic C. sulfidogenes strain SGB2 utilized l-arginine, l-ornithine, CGP dipeptides, and pyruvate under an anaerobic atmosphere as carbon sources, whereas l-aspartate, l-lysine, lactate, acetate, and succinate were not utilized.
Fate of CGP degradation products within the consortium.
Monitoring the degradation of 1 g liter−1 CGP by axenic cultures of P. alcaligenes strain DIP1 by HPLC revealed the formation of 0.75 ± 0.05 g liter−1 of dipeptides and the absence of free aspartate and arginine when incubated under an aerobic or anaerobic atmosphere. Dipeptides disappeared 20 ± 2 days after the complete degradation of CGP under anaerobic conditions and only 4 to 5 days under aerobic conditions and was accompanied by a pH increase from 7.0 to 7.7 or 8.3, respectively.
(i) P. alcaligenes strain DIP1.
Under anaerobic conditions, immediately after the completion of CGP degradation and concomitant with the formation of 0.75 g liter−1 CGP dipeptides (corresponding to 1.2 mM aspartate and 1.6 mM arginine), small amounts of approximately 0.1 mM alanine and 0.42 mM succinate were the only other products that could be detected by HPLC. After incubation for 7 additional days, the concentrations of alanine and succinate increased to 0.92 and 0.84 mM, respectively. In addition, 0.5 mM of ornithine was detected, whereas the concentration of CGP dipeptides decreased to 1.7 mM. After the complete utilization of the CGP dipeptides (21 days after CGP degradation), the concentration of succinate increased further to 2.3 mM, alanine to 1.2 mM, and ornithine to 1 mM. Free arginine was never detected. When strain DIP1 was grown separately on aspartate or arginine as a carbon source in independent experiments, alanine and succinate occurred in aspartate cultures, while ornithine occurred only in arginine cultures. This confirmed that during CGP degradation, alanine and succinate originated from aspartate utilization, whereas ornithine originated from arginine.
(ii) E. casseliflavus strain ELS3.
After the complete utilization of 5 mM CGP dipeptides by E. casseliflavus strain ELS3 under an anaerobic atmosphere, HPLC analysis revealed the presence of 1.9 mM free aspartate in addition to 0.2 mM succinate and traces of acetate and ornithine. However, as in the case of strain DIP1, no free arginine could be detected. Lactate utilization was only possible under aerobic conditions, and acetate occurred as the main product. Lactate itself was the major product in addition to traces of acetate and ethanol when strain ELS3 was cultivated on pyruvate under anaerobic conditions. In the presence of oxygen, acetate became the main product from pyruvate, and no lactate was detected by HPLC analysis.
(iii) C. sulfidogenes strain SGB2.
After the complete utilization of 5 mM CGP dipeptides by C. sulfidogenes strain SGB2, 2.3 mM aspartate and 0.2 mM arginine were detected in the medium in addition to 0.9 mM acetate, 0.4 mM succinate, and traces of propionate and ethanol.
(iv) P. odorifer strain PNF4.
This bacterium could not utilize CGP dipeptides or any of the free amino acids tested. However, pyruvate was utilized under both anaerobic and aerobic conditions with traces of ethanol as the only detectable end product in the medium. P. odorifer strain PNF4 possessed the capability of fixing gaseous dinitrogen, which is a common trait for most species of the genus Paenibacillus (2).
DISCUSSION
During this study, the isolated bacterium capable of CGP degradation under an anaerobic atmosphere was unexpectedly affiliated with the denitrifying P. alcaligenes. However, while nitrate enhanced the overall growth of P. alcaligenes strain DIP1 and subsequently the degradation of CGP, the ability to grow anaerobically in the absence of nitrate occurs only in a few exceptional cases with members of the genus Pseudomonas: P. aeruginosa exhibits anaerobic growth in the presence of arginine plus small amounts of yeast extract, and P. chloritidismutans utilizes chlorate (ClO3−) as an alternative electron acceptor (29).
Although the course of CGP degradation in the presence of nitrate and yeast extract in cocultivations of all consortium members did not diverge sharply from that of axenic cultures of P. alcaligenes strain DIP1, in the absence of nitrate and yeast extract, CGP was degraded only by the consortium. A possible reason for this is that any trace amounts of substrates released by cells of the other three members before sporulation or upon cell lysis could be sufficient for cells of strain DIP1 to grow and secrete minimal amounts of CGPase to start the degradation of CGP and make the dipeptides available for further growth. This is in accordance with the known ability of P. alcaligenes to utilize a wide range of substrates and to need only traces thereof to produce the necessary extracellular enzymes. This feature made such species attractive for applications in extracellular degradation processes (17, 29).
The notable effect of shaking the Hungate tubes during CGP degradation experiments was not observed for the strict anaerobic bacterium S. hongkongensis (31). A possible reason is the tendency of P. alcaligenes strain DIP1 cells to form biofilms. This reduces its cell substrate contact with the insoluble CGP sediment in statically incubated tubes. Shaking obviously retarded cell aggregation for several days during CGP degradation. This is clearly coupled with laboratory conditions, while in nature, the formation of biofilms is of advantage for surviving and attaching other organisms as well as utilizable substrates (29) such as CGP.
The reduction of inorganic electron acceptors by C. sulfidogenes strain SGB2 was thoroughly investigated (Sallam and Steinbüchel, submitted). In contrast to the unstable reduction of sulfate by axenic cultures of strain SGB2, cocultivations with the other three consortium members led to stable and reproducible sulfate reduction (Fig. 6). Even though this dependency could not be completely elucidated under laboratory conditions, it provides another exemplar of the complex bacterial syntrophy of anaerobic habitats.
Unlike the strictly aerobic Bacillus megaterium BAC19 (30), no dipeptide oligomers of higher order like (β-Asp-Arg)2 tetrapeptides were produced from CGP by P. alcaligenes strain DIP1. This is in accordance with the effect of CphEPa of P. anguilliseptica (33) and the CGPase of S. hongkongensis (31), and it indicates a high similarity between the three enzymes. The ability of strains ELS3 and SGB2 to transport aspartate only in the form of a dipeptide into cells supports the theory that amino acids occurring in dipeptides have a higher bioavailability than free amino acids (11, 14, 47).
Besides dipeptides, the production of succinate and alanine from CGP by P. alcaligenes strain DIP1, and the detection of the latter in cultures grown on free aspartate, indicates that aspartate from CGP dipeptides was metabolized intracellularly into l-alanine by l-aspartate β-decarboxylase (l-aspartate 4-carboxy-lyase [EC 4.1.1.12]), which catalyzes the β-decarboxylation of l-aspartic acid to alanine and CO2. This turnover pathway of aspartate was also reported during previous studies of CGP production (12) and in several other microorganisms like Pseudomonas dacunhae, which was selected for the industrial production of alanine due to its strong aspartate β-decarboxylase activity (25).
Succinate was most probably produced from aspartate by aspartate ammonia-lyase (EC 4.3.1.1) and fumarate reductase (EC 1.3.99.1). Both enzymes were reported for several Pseudomonas species (4, 27). The latter one reduces fumarate to succinate in an energy-generating process of electron transport-coupled phosphorylation (24).
Arginine deaminase (ADI, arginine dihydrolase; EC 3.5.3.6) (10) provides the organism with energy, carbon, and nitrogen and protects cells from acidic conditions by ammonia production; therefore, the ADI pathway is assayed by the disappearance of arginine and the increase of pH in medium (29). Via the ADI pathway, arginine is converted first into citrulline, which is then converted to ornithine and carbamoyl-phosphate. Degradation of the latter by carbamate kinase (EC 2.7.2.2) yields ATP, allowing Pseudomonas cells to maintain their motility for an extended time period during anaerobiosis (29). This pathway provides the most plausible explanation for the presence of ornithine and not arginine after CGP degradation by strain DIP1. In addition, cultivations on free arginine revealed ornithine as the only metabolic end product. Also, the elevated medium pH and the strong ammonia odor of lyophilized samples indicated the presence of the ADI pathway in P. alcaligenes strain DIP1.
After the degradation of 1 g liter−1 CGP by P. alcaligenes strain DIP1 in anaerobic cultures, the concentrations of alanine and succinate corresponded to approximately 12.5% and 46.1%, respectively, of the aspartate content in the amount of CGP dipeptides used (0.83 mM). The remaining fraction of aspartate ended most probably as direct building blocks. The early appearance of end products from both amino acids indicated that cells of P. alcaligenes strain DIP1 acquire their energy for growth from the catabolism of both aspartate and arginine in parallel. After an additional 7 days of incubation, the concentrations of alanine and succinate corresponded to 47.6% and 43.7%, respectively, of the aspartate content in the used dipeptides (1.7 mM), while the detected ornithine concentration corresponded to 30.7% of the arginine content of the dipeptides. Finally, after the complete utilization of the CGP dipeptides, the ornithine concentration increased to 34.8% of the consumed arginine content of CGP. On the other hand, the succinate concentration increased to 60.6%, while the concentration of alanine represented only 32% of the aspartate content of the utilized 1 g liter−1 CGP.
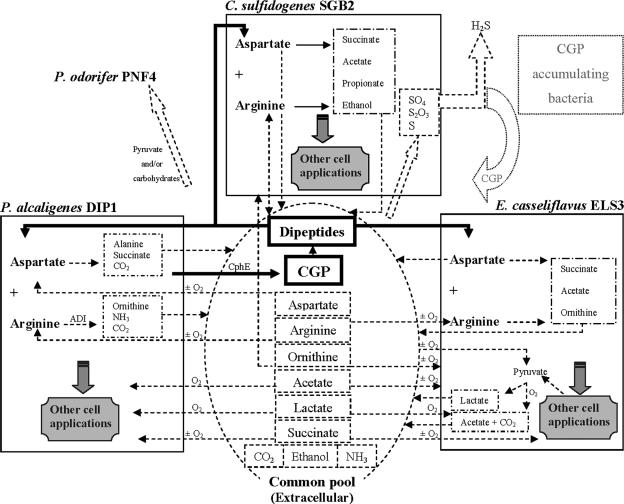
Natural biofilms, mats, and aggregates are well-organized communities, and their functions are characterized by interactions between different populations within these communities; in addition, these microbial communities are often sensitive to disturbances (29). Thus, monitoring the physiological relationships between such species helps in understanding and subsequently directing, controlling, or modifying these systems. In the case of the anaerobic consortium investigated in this study (Fig. 7), and starting with CGP in the extracellular common pool, P. alcaligenes strain DIP1 excretes an extracellular CGPase which degrades CGP to dipeptides. The latter are subsequently taken up by the same strain, strain ELS3 or strain SGB2. Strain DIP1 then releases alanine, succinate, and ornithine to the common pool. The released succinate becomes available for utilization by the same strain or by E. casseliflavus strain ELS3, regardless of the degree of aeration. P. alcaligenes strain DIP1 is the only strain of the consortium capable of taking up and utilizing the free aspartate produced by strains ELS3 or SGB2 during growth on the CGP dipeptides. Arginine is probably excreted to the common pool by C. sulfidogenes strain SGB2 and is later consumed by the same strain, strain DIP1 or strain ELS3. Ornithine, which is the end product of arginine metabolism of P. alcaligenes strain DIP1, serves E. casseliflavus strain ELS3 as a substrate. Under anaerobic conditions, C. sulfidogenes strain SGB2 may also profit from ornithine.
FIG. 7.
Ecological and physiological interactions between the four members of the consortium. Various aspects of contribution between P. alcaligenes DIP1, E. casseliflavus ELS3, C. sulfidogenes SGB2, and P. odorifer PNF4 are shown with CGP degradation in the center. See the text for a detailed description. O2, only under an aerobic atmosphere; ±O2, regardless of the state of aeration. The dotted fields represent hypothetical aspects.
Under anaerobic conditions, E. casseliflavus strain ELS3 converts pyruvate mainly to lactate. The latter becomes finally available for the same strain again as acetate and CO2 or for the CGP-degrading P. alcaligenes strain DIP1 when the atmosphere becomes aerobic. Under aerobic conditions, acetate and CO2 are direct end products of pyruvate metabolism by strain ELS3; both are released and further metabolized.
The presence of P. odorifer strain PNF4 in the investigated consortium is probably a case of syntrophy, but on the other hand, Paenibacillus is the only dinitrogen-fixing genus among the aerobic endospore-forming bacilli (2); therefore, strain PNF4 might play an active role in natural habitats by providing a nitrogen source. Another probable form of interaction within the consortium is the effect of nitrate concentration on the reduction of sulfur compounds by C. sulfidogenes strain SGB2. Nitrate concentrations over 20 mM were found to inhibit sulfate-reducing populations (21). This may lead to a temporary inhibition of strain SGB2 to allow P. alcaligenes strain DIP1 to flourish and to reduce the concentration of nitrate. Consequently, strain SGB2 would flourish and again contribute to the reduction of sulfur compounds, thereby stimulating CGP-producing bacteria (if present) to supply the mixed consortium with additional CGP.
Other factors like aeration and temperature affect such consortia greatly. The state of aeration, for example, may have small effects on the utilization of substrates and electron acceptors in the consortium (Fig. 7) or have a severe effect through the complete inactivation of C. sulfidogenes strain SGB2 (in the presence of oxygen). Thus, the effects of environmental alterations make mixed communities very complex and not easily explored. However, the ecological, biotechnological, and often economical significance of such consortia make it worthy to investigate their role in biodegradation processes, such as that of CGP.
Acknowledgments
We thank Tran Hai and Kay Frey for the frequent provision of cyanobacterial as well as noncyanobacterial CGP and/or CGP-containing biomasses.
Footnotes
Published ahead of print on 18 April 2008.
REFERENCES
- 1.Aboulmagd, E., F. B. Oppermann-Sanio, and A. Steinbüchel. 2000. Molecular characterization of the cyanophycin synthetase from Synechocystis sp. strain PCC6308. Arch. Microbiol. 174:297-306. [DOI] [PubMed] [Google Scholar]
- 2.Achouak, W., P. Normand, and T. Heulin. 1999. Comparative phylogeny of rrs and nifH genes in the Bacillaceae. Int. J. Syst. Bacteriol. 49:961-967. [DOI] [PubMed] [Google Scholar]
- 3.Allen, M. M., and M. A. Hawley. 1983. Protein degradation and synthesis of cyanophycin granule polypeptide in Aphanocapsa sp. J. Bacteriol. 154:1480-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appanna, V. D., R. Hamel, C. Mackenzie, P. Kumar, and S. Kalyuzhnyi. 2003. Adaptation of Pseudomonas fluorescens to Al-citrate: involvement of tricarboxylic acid and glyoxylate cycle enzymes and the influence of phosphate. Curr. Microbiol. 47:521-527. [DOI] [PubMed] [Google Scholar]
- 5.Ariño, X., J. J. Ortega-Calvo, M. Hernandez-Marine, and C. Saiz-Jimenez. 1995. Effect of sulfur starvation on the morphology and ultrastructure of the cyanobacterium Gloeothece sp. PCC 6909. Arch. Microbiol. 163:447-453. [Google Scholar]
- 6.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline procedure for screening recombinant plasmid DNA. Nucleic Acid Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borzi, A. 1887. Le comunicazioni intracellulari delle Nostochinee. Malpighia 1:28-74. [Google Scholar]
- 8.Carr, N. G. 1988. Nitrogen reserves and dynamics reservoirs in cyanobacteria, p. 13-21. In L. J. Rogers and J. R. Gallon (ed.), Biochemistry of the algae and cyanobacteria. Annual Proceedings of the Phytochemical Society of Europe. Phytochemical Society of Europe, Clarendon, Oxford.
- 9.Cord-Ruwisch, R. 1985. A quick method for the determination of dissolved and precipitated sulfides in cultures of sulfate-reducing bacteria. J. Microbiol. Methods 4:33-36. [Google Scholar]
- 10.Cunin, R., N. Glansdorff, A. Piérard, and V. Stalon. 1986. Biosynthesis and metabolism of arginine in bacteria. Microbiol. Rev. 50:314-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dock, D. B., J. E. Aguilar-Nascimento, and M. Q. Latorraca. 2004. Probiotics enhance the recovery of gut atrophy in experimental malnutrition. Biocell 28:143-150. [PubMed] [Google Scholar]
- 12.Elbahloul, Y., K. Frey, J. Sanders, and A. Steinbüchel. 2005. Protamylasse, a residual compound of industrial starch production, provides a suitable medium for large-scale cyanophycin production. Appl. Environ. Microbiol. 71:7759-7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elbahloul, Y., M. Krehenbrink, R. Reichelt, and A. Steinbüchel. 2005. Physiological conditions conducive to high cyanophycin content in biomass of Acinetobacter calcoaceticus strain ADP1. Appl. Environ. Microbiol. 71:858-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foucaud, C., S. Furlan, P. Bellengier, V. Juillard, and J. Richard. 1998. Nutritional value of the non-protein N that accumulates during growth of Lactococcus lactis in milk for dairy lactococcal and leuconostoc isolates. J. Dairy Res. 65:491-501. [Google Scholar]
- 15.Frey, K. M., F. B. Oppermann-Sanio, H. Schmidt, and A. Steinbüchel. 2002. Technical scale production of cyanophycin with recombinant strains of Escherichia coli. Appl. Environ. Microbiol. 68:3377-3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Füser, G., and A. Steinbüchel. 2007. Analysis of genome sequences for genes of cyanophycin metabolism: identifying putative cyanophycin metabolizing prokaryotes. Macromol. Biosci. 7:278-296. [DOI] [PubMed] [Google Scholar]
- 17.Gerritse, G., R. W. Hommes, and W. J. Quax. 1998. Development of a lipase fermentation process that uses a recombinant Pseudomonas alcaligenes strain. Appl. Environ. Microbiol. 64:2644-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 19.Hejazi, M., K. Piotukh, J. Mattow, R. Deutzmann, R. Volkmer-Engerts, and W. Lockau. 2002. Isoaspartyl dipeptidase activity of plant-type asparaginases. Biochem. J. 364:129-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang, X. 1992. A contig assembly program based on sensitive detection of fragment overlaps. Genomics 14:18-25. [DOI] [PubMed] [Google Scholar]
- 21.Jenneman, G. E., M. J. Mcinerney, and R. M. Knapp. 1986. Effect of nitrate on biogenic sulfide production. Appl. Environ. Microbiol. 51:1205-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koop, A., I. Voss, A. Thesing, H. Kohl, R. Reichelt, and A. Steinbüchel. 2007. Identification and localization of cyanophycin in bacteria cells via imaging of the nitrogen distribution using energy-filtering transmission electron microscopy. Biomacromolecules 8:2675-2683. [DOI] [PubMed] [Google Scholar]
- 23.Krehenbrink, M., F. B. Oppermann-Sanio, and A. Steinbüchel. 2002. Evaluation of non-cyanobacterial genome sequences for occurrence of genes encoding proteins homologous to cyanophycin synthetase and cloning of an active cyanophycin synthetase from Acinetobacter sp. strain DSM 587. Arch. Microbiol. 177:371-380. [DOI] [PubMed] [Google Scholar]
- 24.Kröger, A., V. Geisler, E. Lemma, F. Theis, and R. Lenger. 1992. Bacterial fumarate respiration. Arch. Microbiol. 158:311-314. [Google Scholar]
- 25.Kumagai, H. December 2004, posting date. Amino acid production, p. 756-765. In M. Dworkin et al. (ed.), The prokaryotes: an evolving electronic resource for the microbiological community, 3rd ed., release 3.18. Springer, New York, NY. doi: 10.1007/0-387-30741-9_20. [DOI]
- 26.Mackerras, A. H., N. M. de Chazal, and G. D. Smith. 1990. Transient accumulation of cyanophycin in Anabaena cylindrical and Synechocystis 6308. J. Gen. Microbiol. 136:2057-2065. [Google Scholar]
- 27.Miyamoto, K., and H. Katsuki. 1992. Possible physiological roles of aspartase, NAD- and NADP-requiring glutamate dehydrogenases of Pseudomonas fluorescens. J. Biochem. (Tokyo) 112:52-56. [DOI] [PubMed] [Google Scholar]
- 28.Mooibroek, H., N. Osterhuis, M. Giuseppin, M. Toonen, H. Franssen, E. Scott, J. Sanders, and A. Steinbüchel. 2007. Assessment of technological options and economical feasibility for cyanophycin biopolymer and high-value amino acid production. Appl. Microbiol. Biotechnol. 77:257-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore, E. R. B., B. J. Tindall, V. A. P. Martins Dos Santos, D. R. H. Pieper, J. Ramos, and N. J. Palleroni. May 1999, posting date. Pseudomonas: nonmedical, p. 646-703. In M. Dworkin et al. (ed.), The prokaryotes: an evolving electronic resource for the microbiological community, 3rd ed., release 3.0. Springer, New York, NY. doi: 10.1007/0-387-30746-X_21. [DOI]
- 30.Obst, M., A. Sallam, H. Luftmann, and A. Steinbüchel. 2004. Isolation and characterization of gram-positive cyanophycin-degrading bacteria-kinetic studies on cyanophycin depolymerase activity in aerobic bacteria. Biomacromolecules 5:153-161. [DOI] [PubMed] [Google Scholar]
- 31.Obst, M., A. Krug, H. Luftmann, and A. Steinbüchel. 2005. Degradation of cyanophycin by Sedimentibacter hongkongensis strain KI and Citrobacter amalonaticus strain G isolated from an anaerobic bacterium consortium. Appl. Environ. Microbiol. 71:3642-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obst, M., and A. Steinbüchel. 2004. Microbial degradation of poly(amino acid)s. Biomacromolecules 5:1166-1176. [DOI] [PubMed] [Google Scholar]
- 33.Obst, M., F. B. Oppermann-Sanio, H. Luftmann, and A. Steinbüchel. 2002. Isolation of cyanophycin-degrading bacteria, cloning and characterization of an extracellular cyanophycinase gene (cphE) from Pseudomonas anguilliseptica strain BI. The cphE gene from P. anguilliseptica BI encodes a cyanophycin-hydrolyzing enzyme. J. Biol. Chem. 277:25096-25105. [DOI] [PubMed] [Google Scholar]
- 34.Oppermann-Sanio, F. B., and A. Steinbüchel. 2003. Cyanophycin, p. 83-106. In S. R. Fahnestock and A. Steinbüchel (ed.), Biopolymers, vol. 7. Wiley-VCH, Weinheim, Germany. [Google Scholar]
- 35.Page, R. D. M. 1996. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 36.Perrière, G., and M. Gouy. 1996. WWW-query: an on-line retrieval system for biological sequence banks. Biochimie 78:364-369. [DOI] [PubMed] [Google Scholar]
- 37.Rainey, F. A., N. Ward-Rainey, R. M. Kroppenstedt, and E. Stackebrandt. 1996. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. nov. Int. J. Syst. Bacteriol. 46:1088-1092. [DOI] [PubMed] [Google Scholar]
- 38.Rao, R. N., M. A. Richardson, and S. Kuhstoss. 1987. Cosmid shuttle vectors for cloning and analysis of Streptomyces DNA. Methods Enzymol. 153:166-198. [DOI] [PubMed] [Google Scholar]
- 39.Richter, R., M. Hejazi, R. Kraft, K. Ziegler, and W. Lockau. 1999. Cyanophycinase, a peptidase degrading the cyanobacterial reserve material multi-L-arginyl-poly-L-aspartic acid (cyanophycin): molecular cloning of the gene of Synechocystis sp. PCC 6803, expression in Escherichia coli, and biochemical characterization of the purified enzyme. Eur. J. Biochem. 263:163-169. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 41.Scott, E., F. Peter, and J. Sanders. 2007. Biomass in the manufacture of industrial products—the use of proteins and amino acids. Appl. Microbiol. Biotechnol. 75:751-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sherman, D. M., D. Tucker, and L. A. Sherman. 2000. Heterocyst development and localization of cyanophycin in N2-fixing cultures of Anabaena sp. PCC7120 (cyanobacteria). J. Phycol. 36:932-941. [Google Scholar]
- 43.Simon, R. D. 1987. Inclusion bodies in the cyanobacteria: cyanophycin, polyphosphate, polyhedral bodies, p. 199-225. In P. Fay and C. van Baalen (ed.), The cyanobacteria. Elsevier, Amsterdam, The Netherlands.
- 44.Simon, R. D., and P. Weathers. 1976. Determination of the structure of the novel polypeptide containing aspartic acid and arginine which is found in cyanobacteria. Biochim. Biophys. Acta 420:165-176. [DOI] [PubMed] [Google Scholar]
- 45.Song, B. K., N. J. Palleroni, and M. M. Häggblom. 2000. Isolation and characterization of diverse halobenzoate-degrading denitrifying bacteria from soils and sediments. Appl. Environ. Microbiol. 66:3446-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stephan, D. P., H. G. Ruppel, and E. K. Pistorius. 2000. Interrelation between cyanophycin synthesis, L-arginine catabolism and photosynthesis in the cyanobacterium Synechocystis sp. strain PCC 6803. Z. Naturforsch. Sect. C 55:927-942. [DOI] [PubMed] [Google Scholar]
- 47.Sussman, A. J., and C. Gilvarg. 1971. Peptide transport and metabolism in bacteria. Annu. Rev. Biochem. 40:397-408. [DOI] [PubMed] [Google Scholar]
- 48.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uetanabaro, P., C. Wahrenburg, W. Hunger, R. Pukall, C. Spröer, E. Stackebrandt, V. P. de Canhos, D. Claus, and D. Fritze. 2003. Paenibacillus agarexedens sp. nov., nom. rev., and Paenibacillus agaridevorans sp. nov. Int. J. Syst. Evol. Microbiol. 53:1051-1057. [DOI] [PubMed] [Google Scholar]
- 50.Widdel, F., and F. Bak. 1992. Gram-negative mesophilic sulfate-reducing bacteria, p. 3352-3378. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes, 2nd ed., vol. 4. Springer, New York, NY. [Google Scholar]
- 51.Wingard, L. L., S. R. Miller, J. M. Sellker, E. Stenn, M. M. Allen, and A. M. Wood. 2002. Cyanophycin production in a phycoerythrin-containing marine Synechococcus strain of unusual phylogenetic affinity. Appl. Environ. Microbiol. 68:1772-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ziegler, K., R. Deutzmann, and W. Lockau. 2002. Cyanophycin synthetase-like enzymes of non-cyanobacterial eubacteria: characterization of the polymer produced by a recombinant synthetase of Desulfitobacterium hafniense. Z. Naturforsch. Sect. C 57:522-529. [DOI] [PubMed] [Google Scholar]