Abstract
Bacteriophage asccφ28 infects dairy fermentation strains of Lactococcus lactis. This report describes characterization of asccφ28 and its full genome sequence. Phage asccφ28 has a prolate head, whiskers, and a short tail (C2 morphotype). This morphology and DNA hybridization to L. lactis phage P369 DNA showed that asccφ28 belongs to the P034 phage species, a group rarely encountered in the dairy industry. The burst size of asccφ28 was found to be 121 ± 18 PFU per infected bacterial cell after a latent period of 44 min. The linear genome (18,762 bp) contains 28 possible open reading frames (ORFs) comprising 90% of the total genome. The ORFs are arranged bidirectionally in recognizable functional modules. The genome contains 577 bp inverted terminal repeats (ITRs) and putatively eight promoters and four terminators. The presence of ITRs, a phage-encoded DNA polymerase, and a terminal protein that binds to the DNA, along with BLAST and morphology data, show that asccφ28 more closely resembles streptococcal phage Cp-1 and the φ29-like phages that infect Bacillus subtilis than it resembles common lactococcal phages. The sequence of this phage is the first published sequence of a P034 species phage genome.
Bacteriophage infection of Lactococcus lactis starter cultures used in cheese making and in other dairy fermentations affects product quality and can result in fermentation failure. Because of these worldwide industrial and financial consequences, phages infecting L. lactis are among the best-studied and most commonly isolated phages infecting any bacterial species (1, 6, 9, 20). As occurs with several well-studied bacterial species (such as Escherichia coli and Bacillus subtilis), a diverse range of phage types has been found to infect L. lactis.
Taxonomic classification of lactococcal phages continues to be an area of debate. All L. lactis phages documented so far are double-stranded DNA phages, and the vast majority have a Siphoviridae morphotype (long noncontractile tail, Bradley morphotypes B1 and B2). Using mainly morphology and DNA-DNA hybridization data, Jarvis et al. (20) divided these phages into 11 or 12 species. The status of the T187 group of phages was doubtful because of DNA homology between it and phage BK5-T (34). Later, Proux et al. (33) reported recognizable modular similarities among phages infecting a range of gram-positive bacteria and proposed five genera of lactococcal phages in a phage classification scheme (not confined to lactococcal phages) partially based on the phylogeny of phage genome modules deduced from DNA sequence comparisons. However, this scheme included only phage types for which whole-genome sequence data were available. No sequence information is available for some phage types, so they cannot yet be classified in this way. Moreover, some reported types (e.g., RZh and P107) are possibly extinct (9, 20), at least in the formal taxonomic sense that reference examples are no longer available.
In the latest review of the biodiversity and classification of lactococcal phages, Deveau et al. (9) revised the species scheme proposed by Jarvis et al. (20) so that it included eight species, and they added two novel species belonging to the family Siphoviridae (1706 and Q54). The 936 (small isometric-headed) and c2 (prolate-headed) phage species each comprise only lytic phages. There is a high degree of genetic conservation within each of these two different species. By contrast, the P335 species, which contains both temperate and lytic phages, has greater genome diversity (9, 11, 28, 33). Based on an apparent high degree of genetic exchange between the formerly recognized 1483, T187, BK5-T, and P335 species, Deveau et al. (9) proposed merging these species into a revised P335 species. Recent DNA sequencing has confirmed that phages Q54 (11) and 1706 (14) are new phage species.
Most L. lactis phages isolated from dairy fermentations belong to the 936 (most common), c2, or P335 species (9, 19, 20, 23, 31). It is no coincidence, therefore, that most publicly available L. lactis phage genome sequences belong to one of these species (9). The reported ratio of phage species isolated in the dairy industry varies, perhaps depending on the geographical region, the types of starter strains used (L. lactis subsp. lactis or L. lactis subsp. cremoris), and the criteria employed for strain selection.
Distinct from these common phage types, one of the least-studied species is P034 (family Podoviridae, Bradley C2 morphotype), which is characterized by a prolate head and short tail with prominent whiskers emanating from the head-tail interface region. Historically, this species has comprised on average less than 1% of the phages isolated from dairy fermentations (1, 6). A “dot blot” DNA hybridization survey of Australian phage isolates using probes derived from reference phages detected only 3 P034 species phages, compared with 100 936 species phages, 34 P335 species phages (including the BK5-T and 1483 phage groups), and 13 c2 species phages. A further 49 phages were not classified unambiguously in that study (23).
In this study, we describe phage asccφ28 and its complete genome sequence and present evidence showing that this phage has genetic and functional similarities to the φ29-like phages of B. subtilis (30) and phage Cp-1 of Streptococcus pneumoniae (29, 35). This is the first such analysis of an L. lactis phage belonging to the P034 species. In structure, genome sequence, and mode of replication this phage species is distinctly different from other phage species infecting L. lactis.
MATERIALS AND METHODS
Phages, lactococcal strains, and growth media.
Phage asccφ28 was isolated from an Australian cheese factory whey sample, one of numerous whey samples that were surveyed during the period from 1993 to 1996 (23). L. lactis subsp. lactis ASCC99-319, a cheddar cheese starter culture component and host strain for phage asccφ28, was obtained from the Australian Starter Culture Research Centre (now the Cultures Division of Dairy Innovation Australia) collection. Phage P369 (20) was kindly provided by H.-W. Ackermann (Félix d'Hérelle Reference Center for Bacterial Viruses, Québec, Canada) as a representative of the P034 species. Phage P034 itself has apparently been lost. L. lactis strains were grown at 30°C in M17 broth (Oxoid, Basingstoke, Hampshire, United Kingdom) or on M17 agar supplemented with both 1% glucose and 10 mM CaCl2 (M17gc) for growth of phages. Phage PFU enumeration was performed in M17gc double-layer agar plates at 30°C. The phage asccφ28 burst size and latent period were determined as described by Powell and Davidson (32).
Electron microscopy.
Phage particles were purified by CsCl density gradient ultracentrifugation, stained with 0.5% uranyl acetate on a Formvar-coated copper grid (Proscitech, Brisbane, Queensland, Australia), and examined using a Hitachi H-300 electron microscope (Hitachi, Tokyo, Japan) calibrated using catalase crystals (Proscitech).
DNA methods.
Phage DNA was prepared by solvent extraction and ethanol precipitation of a CsCl-purified phage preparation. Where indicated below, DNA was treated with proteinase K essentially as described by García et al. (12). The DNA was digested with restriction enzymes (Roche) used according to the manufacturer's instructions, electrophoresed on agarose gels, stained with ethidium bromide, and visualized using UV (38). Transfer of DNA fragments in agarose gels to nylon membranes (Hybond-N+; Amersham, Little Chalfont, Buckinghamshire, United Kingdom) for Southern blot (40) analysis was performed as described by Moineau et al. (31). Phage P369 DNA used to probe the blots was 32P labeled using random priming.
DNA sequencing and computer analyses.
Fragments (650 to 950 bp) resulting from partial AluI digestion of asccφ28 DNA were purified by agarose gel electrophoresis and cloned using the pGEM-T plasmid cloning system with E. coli SURE cells (Promega, Madison, WI). The asccφ28 genome sequence was determined by sequencing these cloned fragments, followed by designing specific primers for directed sequencing from asccφ28 genomic DNA. A Taq DyeDeoxy terminator cycle sequencing kit (Applied Biosystems, Scoresby, Victoria, Australia) and an Applied Biosystems 373A automated DNA sequencer were used. Sequences were assembled and analyzed using Sequencher 3.0 (Gene Codes, Ann Arbor, MI). The average sequence data redundancy was 4.5. DNA sequence data and deduced protein sequences were analyzed using the University of Wisconsin Genetics Computer Group suite of programs (now Accelrys GCG) and Clone software (version 9; Scientific and Educational Software, Cary, NC). Phage asccφ28 sequences were compared with sequences publicly available in databases using BLAST software (3) at the NCBI website (http://www.ncbi.nlm.nih.gov).
Protein analysis.
CsCl-purified phage samples were boiled for 5 min in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer, and then 1 to 5 μg total phage protein was electrophoresed through a polyacrylamide gel (24) and stained with Coomassie blue or Blu/T (B/T Scientific Technologies, Carlsbad, CA) used according to the manufacturer's instructions. For DNA terminal protein (TP) analysis, phage asccφ28 DNA (not treated with proteinase K) was digested with DNase I prior to boiling and electrophoresis (12). For N-terminal sequencing, proteins in SDS-PAGE gels (unstained) were electroblotted using a Bio-Rad transblot system (Bio-Rad, Hercules, CA) onto a polyvinylidene difluoride membrane (Bio-Rad) as described by Towbin et al. (42) and stained with Ponceau S (37). Bands of interest were excised from blots, destained in 200 μM NaOH, and rinsed with water. N-terminal sequence analysis was carried out with an ABI Procise 494 protein sequencer using a standard blot program (18) at the Australian Proteome Analysis Facility (Sydney, Australia).
Nucleotide sequence accession number.
The genome sequence of phage asccφ28 has been deposited in the GenBank database under accession number EU438902.
RESULTS
Species classification and physical characterization of asccφ28.
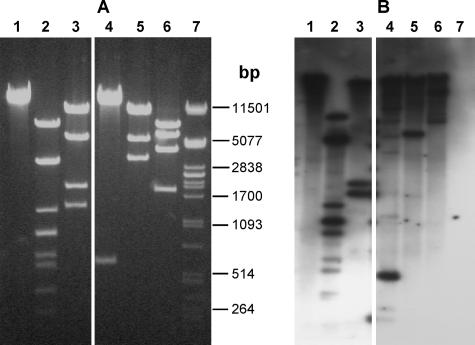
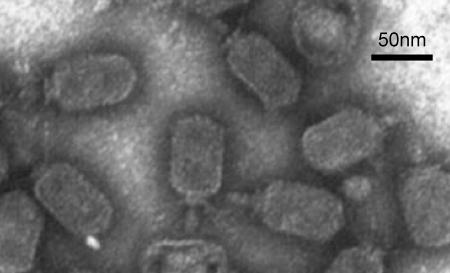
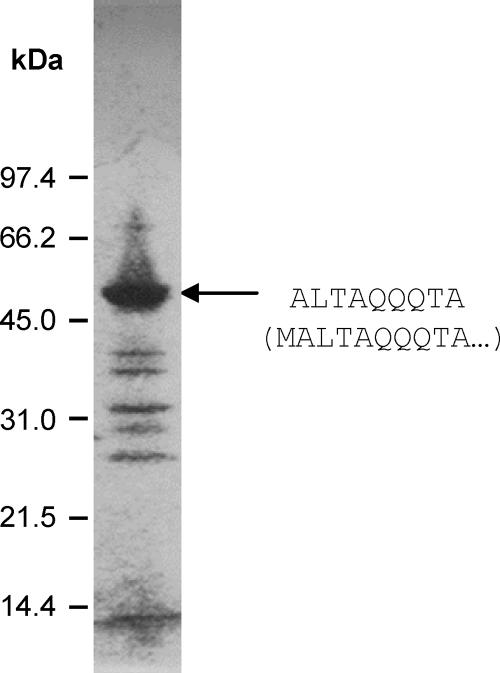
Southern hybridization showed that DNA from phage P369 (P034 species) hybridized strongly to DNA from phage asccφ28 (Fig. 1). Hybridization was not uniform to all bands, presumably reflecting differences between the two phages. The level of genetic heterogeneity within the P034 phage species is not known. Electron microscopy (Fig. 2) showed that phage asccφ28 has a prolate capsid (head) (59 by 42 nm) and a short noncontractile tail (21 by 9 nm). A collar with fibrils is located near the head-tail interface, consistent with the conclusion that it is a member of the P034 species (20). SDS-PAGE of phage asccφ28 proteins (Fig. 3) resulted in a band pattern similar to that previously reported for phage P034 (6).
FIG. 1.
(A) Restriction-digested asccφ28 DNA electrophoresed on a 1% agarose gel and stained with ethidium bromide. (B) Southern hybridization of the same fragments probed with DNA from phage P369. The lanes contained the following: lane 1, uncut DNA; lane 2, HindIII restriction enzyme digest; lane 3, AccI restriction enzyme digest; lane 4, ScaI restriction enzyme digest; lane 5, SpeI restriction enzyme digest; lane 6, AatII restriction enzyme digest; lane 7, marker coliphage λ DNA cut with PstI. DNA bands not obvious in the ethidium bromide-stained gel are visible in the Southern hybridization and presumably represent incomplete digestion products. Selected sizes of the marker are indicated between the panels.
FIG. 2.
Electron micrograph of uranyl acetate-stained preparation of CsCl-purified asccφ28. Bar = 50 nm.
FIG. 3.
SDS-PAGE of phage asccφ28 proteins electrophoresed on a 10 to 20% (top to bottom) polyacrylamide gradient gel. The N-terminal sequence of the dominant protein (arrow) was determined and is shown on the right. Underneath this sequence, the first 10 amino acids of the protein sequence predicted from ORF24 (encoding the MCP) are shown in parentheses.
Virulence of asccφ28.
The virulence of phage asccφ28 was assessed by performing a single-step growth curve analysis in M17gc broth using the host strain L. lactis subsp. lactis ASCC99-319 to determine the burst size and latent period. The burst size was found to be 121 ± 18 PFU per infected bacterial cell after a latent period of 44 min. Host range analysis using a plaque assay showed that asccφ28 infected 2 of 64 dairy lactococcal strains tested, both of which are strains of L. lactis subsp. lactis (data not shown).
Key features of the asccφ28 genome.
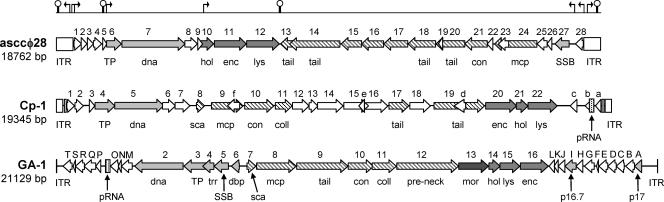
Sequence analysis showed a double-stranded DNA genome consisting of 18,762 bp. A similar genome size (approximately 18.1 kbp) has been estimated for phage P034 (20), suggesting that this small genome is typical of the P034 species. The G+C content of the asccφ28 genome is 33.7%. The DNA of phage asccφ28 does not have cohesive ends or terminal redundancy (features observed in other lactococcal phages), but inverted terminal repeat (ITR) sequences are present. A total of 28 open reading frames (ORFs) longer than 40 codons and with putative ribosomal binding sites were predicted (Table 1). Most of the stop codons are TAA. This is the smallest genome and lowest number of ORFs of the L. lactis phage genomes sequenced to date. The 28 ORFs comprise 90% of the total genome or just over 96% of the genome excluding the ITRs. Such genome compactness is common in L. lactis phages, which generally utilize 93 to 95% of their DNA as coding sequences (2). Predicted ORFs and transcriptional control signals inferred from the sequence are shown in Fig. 4. The ORFs occur on both strands, converging on bp 7709/7710. Many features of the asccφ28 genome and its ORFs (described below) show strong similarities to features of the genomes and ORFs of S. pneumoniae phage Cp-1 (29, 35) and the φ29 family of phages that infect B. subtilis, including phage φ29 itself and GA-1 (30).
TABLE 1.
Characteristics, closest GenBank homologs, and predicted functions of ORFs in the asccφ28 genome
| ORF | Ribosomal binding site and start codon sequencea | Genome positionb | Stop codon | Predicted protein
|
|||
|---|---|---|---|---|---|---|---|
| No. of amino acids | Mol wt (103) | Closest homolog (GenBank accession no.; amino acid identity; total length)c | Predicted functiond | ||||
| ORF1 | GAAAGTGAGAGAAAATG | 614-871 | TAA | 85 | 10.3 | NS | |
| ORF2 | AAAAAGGAGAATAAAGATG | 861-1061 | TAG | 66 | 8.1 | NS | |
| ORF3 | ATAGGAGATAAATG | 1069-1281 | TAA | 70 | 8.1 | NS | |
| ORF4 | GGAGAATAATATG | 1283-1564 | TAG | 93 | 10.8 | NS | |
| ORF5 | GAGGATATTATG | 1596-1742 | TAA | 48 | 6.0 | NS | DNA binding?e |
| ORF6 | AAAAAGGATTTATAACAAATG | 1736-2239 | TAA | 167 | 19.7 | NS | Terminal protein |
| ORF7 | GGAACGTTCAGAAATG | 2264-4432 | TGA | 722 | 85.8 | Bacillus phage GA-1 DNA polymerase (NP_073685; 135/511 [26%]; 578 amino acids) | DNA polymerase |
| ORF8 | GAGGTCAAACTATG | 4429-4869 | TAA | 146 | 17.3 | NS | |
| ORF9 | AAGGAGTTTTAAAATG | 4871-5041 | TAA | 56 | 6.8 | NS | |
| ORF10 | AGGAGAAAAAAACATTTATG | 5081-5467 | TAA | 128 | 14.3 | NS | Holin |
| ORF11 | GGGGGTGCTGATG | 5451-6551 | TAA | 366 | 43.5 | Streptococcus phage Cp-1 encapsidation protein (NP_044835; 75/262 [28%]; 360 amino acids) | Encapsidation protein |
| ORF12 | AAAGGAGAAAATTATG | 6563-7690 | TAA | 375 | 41.8 | Staphylococcus phage PH15 hydrolase (YP_950686; 32/97 [32%]; 633 amino acids)f | Lysin |
| ORF13 | AGATAGAGATAGTTACTCTATG | 8203-7730 | TAA | 157 | 18.4 | Streptococcus phage Cp-1 tail protein (NP_044833; 33/133 [24%]; 586 amino acids) | Tail |
| ORF14 | GAAAGGAAATTATG | 9777-8089 | TAA | 562 | 62.6 | Bacillus phage φ29 tail protein (NP_040727; 79/282 [28%]; 599 amino acids) | Tail |
| ORF15 | ATAGGAGGCTATGAGTCATG | 10527-9820 | TAA | 235 | 26.6 | Streptococcus phage Cp-1 collar protein (NP_044824; 33/154 [21%]; 194 amino acids) | Structural |
| ORF16 | AGAAAGGAAATTTAAGATG | 11282-10524 | TGA | 252 | 29.1 | L. lactis phage bIL170 putative accessory fiber (AAR26444; 22/41 [53%]; 605 amino acids)g | Structural |
| ORF17 | GAAAGATATG | 12118-11297 | TAG | 273 | 30.2 | Streptococcus phage Cp-1 hypothetical protein p18 (NP_044830; 57/222 [25%]; 253 amino acids)h | Structural |
| ORF18 | AAGGAGATTTTAAATG | 13081-12128 | TAG | 317 | 35.2 | Burkholderia cenocepacia phage tail protein (EAY61823; 31/88 [35%]; 1,301 amino acids) | Tail |
| ORF19 | AAGGAAATAAAAATG | 13322-13176 | TAG | 48 | 5.5 | NS | |
| ORF20 | GAGGAAGATTAAATG | 14085-13093 | TAA | 330 | 36.1 | L. lactis phage KSY1 protein gp73 (ABG21616; 99/153 [64%]; 347 amino acids)i | Tail |
| ORF21 | AAGAAATGATG | 14856-14086 | TAA | 256 | 29.0 | Streptococcus phage Cp-1 connector protein (NP_044823; 60/217 [27%]; 337 amino acids) | Upper collar or connector |
| ORF22 | AAAGGGTTTGTATG | 15056-14856 | TGA | 66 | 8.0 | NS | |
| ORF23 | GAAAGTAGATG | 15594-15244 | TAA | 116 | 14.1 | NS | |
| ORF24 | AAGGAGAATAAAAATG | 16570-15110 | TAG | 486 | 53.1 | L. lactis subsp. cremoris MG1363 cell wall surface anchor family protein (YP_001032441; 38/85 [44%]; 1,349 amino acids)j | Major capsid protein |
| ORF25 | AGGAGTAAATAATG | 16914-16573 | TAA | 113 | 12.8 | NS | |
| ORF26 | AAAGGAATTAAAATTATG | 17082-16918 | TAA | 54 | 6.3 | NS | |
| ORF27 | AAAGGAAAACACAAAAATG | 17682-17191 | TAA | 163 | 18.6 | Bacillus phage GA-1 SSB (NP_073688; 53/163 [32%]; 170 amino acids) | SSB |
| ORF28 | ATAAAGACAGCAAATG | 18168-17896 | TGA | 90 | 10.8 | NS | |
The sequence complementary to the 3′ end of the L. lactis 16S rRNA (43) is underlined, and the start codon is indicated by bold type.
ORF13 to ORF28 (inclusive) are on the complementary strand.
The amino acid identity for the region shown and the number of amino acids (total length) for each homolog are indicated. NS, no significant homology found.
Based on a BLAST search of databases or other experimental data described in the text.
The ORF5 predicted protein contains a zinc finger motif which suggests a DNA binding function (27).
The highest sequence similarity occurs in the N-terminal part of the predicted protein, the region likely to be responsible for lytic activity (10, 13, 39).
There was also homology to structural or tail antireceptor (host-binding) proteins in other L. lactis phages, including bIL170 (GenBank accession no. AAR26444), bIL41 (AAB41477), r1t (NP_695075), TP901-1 (NP_112714), and 4268 (NP_839936), as well as to Streptococcus thermophilus phage antireceptor proteins. This homology occurred in the same region consisting of up to 41 amino acids centrally located in the ORF16 predicted protein.
The gene encoding hypothetical protein p18 in phage Cp-1 is located between two other phage tail genes.
There was also homology to the lysins (amidases) of Lactobacillus casei phages A2 (GenBank accession no. NP_680500) and PL-1 (BAA96749) (41% identity for 299 of 350 amino acids for both proteins).
There was also homology to the major capsid or other major structural proteins of various L. lactis phages, including F4-1 (GenBank accession no. P26596), p2 (AAF85633), sk1 (NP_044957), and bIL170 (NP_047126) (37 to 44% identity for 81 to 83 amino acids located at the C-terminal end of the predicted protein encoded by ORF24), and to the major capsid protein of Streptococcus phage Cp-1 (NP_044821) (20% identity for 267 of 360 amino acids).
FIG. 4.
Deduced genetic map of phage asccφ28 compared with maps for phages Cp-1 (29) and GA-1, a φ29-like phage (30). The maps for Cp-1 and GA-1 are derived from data in the GenBank database (accession numbers Z47794 and X96987, respectively). Gene functions are abbreviated as follows: TP, terminal protein; dna, DNA polymerase; hol, holin; enc, encapsidation protein; lys, lysin; tail, tail protein; coll, collar protein; mcp, major capsid (head) protein; con, collar or connector protein; SSB, single-stranded DNA-binding protein; sca, scaffolding protein; trr, transcriptional regulator; dbp, DNA-binding protein; pre-neck, preneck appendage protein; mor, morphogenesis protein. The genes encoding DNA replication proteins p16.7 and p17 in GA-1 are indicated. Light gray shading indicates genes involved in DNA replication or transcriptional regulation; medium gray shading indicates genes involved in cell lysis; diagonal lines indicate genes encoding structural proteins; and dark gray shading in the GA-1 map indicates the gene encoding the morphogenesis protein. Proteins with no known function are indicated by open arrows. Packaging RNAs and inverted terminal repeat regions are indicated by pRNA and ITR, respectively. Above the asccφ28 map, putative promoters and rho-independent transcriptional terminators (see the GenBank accession number EU438902 sequence for details) are indicated by arrows and hairpin structures, respectively.
Terminal repeats and DNA replication.
A distinctive feature of the asccφ28 genome is the presence at both ends of 577-bp ITRs. A long ITR (236 bp) is present in phage Cp-1 (12, 29), whereas a short ITR (6 to 8 bp) is present in the φ29-like phages (30). These phages require ITRs and a terminal replication protein (TP) that binds to their ITRs for DNA replication. Neither of these features has been observed in any other sequenced L. lactis phage, including the recently sequenced phage KSY1 belonging to the Podoviridae (8).
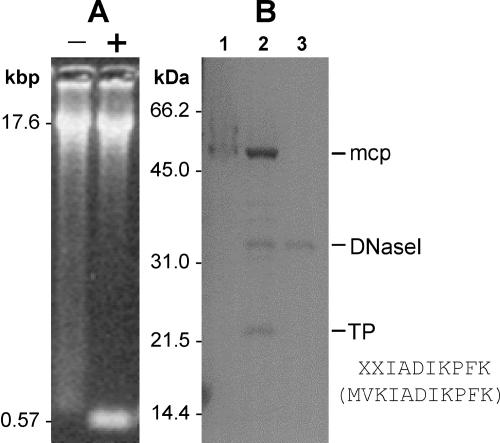
Phage asccφ28 was examined for a TP using methods similar to those described by García et al. (12). The terminal ScaI DNA fragments of phage asccφ28 were seen only after proteinase K treatment of a sample prior to loading on an agarose gel (Fig. 5A). A smear of retarded DNA was seen for the control (untreated) sample. This result is consistent with removal of a DNA-bound protein by proteinase treatment, enabling the terminal ScaI fragments to migrate normally and be seen as a clear band in the gel. Similarly, SDS-PAGE of asccφ28 DNA showed a protein band (estimated molecular mass, 22 kDa) that appeared only after DNase I treatment of the sample (Fig. 5B), consistent with release of a DNA-bound protein by DNase I treatment. Matching the N-terminal sequence of the protein isolated from the gel (Fig. 5B) showed that it is encoded by ORF6.
FIG. 5.
TP detection. (A) Phage asccφ28 DNA was restriction digested with ScaI (each ITR has a ScaI site close to its inner end), resulting in two 573-bp terminal DNA fragments. Digested DNA was treated (lane +) or not treated (lane −) with proteinase K and then electrophoresed on a 1.2% agarose gel. (B) Phage asccφ28 DNA (not treated with proteinase K) was electrophoresed on a 12% polyacrylamide gel. Lane 1, asccφ28 DNA carrying the bound TP; lane 2, asccφ28 DNA digested with 1 U of DNase I, which released the TP; lane 3, 1 U of DNase I (control). Lanes 1 and 2 also show that there was residual MCP in the DNA preparation. The N-terminal sequence of the TP was determined and is shown along with the first 10 amino acids of the protein predicted from ORF6 (in parentheses).
By analogy with the Bacillus φ29 phages and streptococcal phage Cp-1, these data suggest that phage asccφ28 replicates via a covalently linked replication protein attached to the termini of its genome and that the ITR of phage asccφ28 contains the origin of phage replication.
Functional assignment of ORFs.
Where possible based on homologies detected by BLAST or other sequence analyses or on experimental data, functions were inferred for proteins predicted to be expressed from ORFs (Table 1). Genes with related functions appear to be grouped in discrete modules.
The predicted proteins encoded by ORF1 to ORF5, ORF8, and ORF9 (Fig. 4) do not show any significant BLAST homology with previously sequenced or predicted proteins in public databases. ORF6 encodes the TP involved in the replication of phage asccφ28 (Fig. 5). The phage DNA polymerase is encoded by ORF7 and shares significant amino acid homology with the protein-primed, DNA-dependent type B DNA polymerases of phage Cp-1 and the φ29-like phages. The tandem arrangements of the DNA polymerase and TP genes in asccφ28, Cp-1, and the φ29-like phages are identical (Fig. 4).
Kyte-Doolittle analysis of the predicted amino acid sequence encoded by ORF10 (not shown) indicated the presence of two transmembrane domains and a highly charged C-terminal region. These features are common in phage holins (26, 46). Along with the position of ORF10 upstream from the putative lysin gene (ORF12) (Table 1), this suggests that ORF10 encodes the holin protein of asccφ28. Holins of different L. lactis phages do not share any sequence similarity (26), and no sequence similarity was observed for the putative holin of asccφ28 and any previously sequenced phage holin. ORF11 encodes a predicted protein homologous to the encapsidation (DNA packaging) protein of phage Cp-1 and the φ29-like phages. This protein functions to encapsidate phage DNA into the mature phage capsid by an ATP-driven process at the end of the phage replicative cycle (16, 30).
ORF13 to ORF28 are on the complementary DNA strand (Fig. 4) and are discussed below in the presumed order of gene transcription. ORF28 encodes a predicted protein of unknown function. The ORF27 predicted protein has homology to a single-stranded DNA-binding protein (SSB) of Bacillus phage GA-1 (15). ORF26 encodes a small protein containing seven direct repeats of the tetrapeptide sequence VDEK or VNEK in its C-terminal region (amino acids 22 to 54), plus an imperfect eighth repeat at the C terminus. The significance of this is unknown.
Most of the remaining ORFs in asccφ28 appear to be structural genes. The predicted protein encoded by ORF25 is responsible for serological cross-reactivity between phages c2 and asccφ28 (22). However, it showed no BLAST homology to proteins from phage c2 or to any other proteins. ORF24 putatively encodes the major capsid protein (MCP), and its product corresponds to the major protein band shown in Fig. 3. The predicted asccφ28 MCP (53.1 kDa) is almost exactly the same size as that of B. subtilis phage GA-1 (53.0 kDa), but it is larger than that of phage Cp-1 (42 kDa). N-terminal sequence data (Fig. 3) indicate that there is no posttranslational cleavage, in contrast to the MCP of Cp-1 (29). The MCP gene in phage Cp-1 is followed downstream by a gene encoding an upper collar (or connector) protein (29). Genes encoding similar proteins occur downstream of the MCP gene in the φ29-like phages (30). In phage asccφ28 this gene order is conserved, since the predicted protein encoded by ORF21 shows homology to the upper collar proteins from both phage Cp-1 and the φ29-like phages. With the exception of ORF19 (no BLAST homology was found for the predicted protein), ORF20 to ORF13 also appear to encode structural proteins (Table 1). ORF20 encodes a predicted protein with significant homology to predicted protein gp73 in L. lactis phage KSY1 (8) and to Lactobacillus phage amidases (Table 1).
Transcription.
Putative transcript initiation and termination signals in the asccφ28 genome (as detailed in the GenBank accession no. EU438902 sequence) are shown in Fig. 4. No transcript mapping or analysis of temporal control of gene expression was done in this study, so any transcript model remains speculative.
DISCUSSION
In this study we present the first complete genome sequence of an L. lactis bacteriophage belonging to the P034 species of phages (family Podoviridae). Phages belonging to this group have a C2 morphotype encountered only rarely in the dairy industry. The reason for this rarity is not clear. Phage asccφ28 has a narrow host range, but narrow host ranges have been observed for many lactococcal phages (data not shown). Its virulence (measured by latent period and burst size) is comparable to that of other lactococcal phages (21).
Morphologically and in gene arrangement, mode of replication, and protein sequence homology, phage asccφ28 more closely resembles phage Cp-1 that infects S. pneumoniae (29) and the φ29 family of phages that infect B. subtilis (30) than it resembles other sequenced L. lactis phages. Phage Cp-1 and the φ29-like phage GA-1 are the phages most similar to asccφ28, as determined by homology at the protein level.
Phage asccφ28 presumably evolved from a common ancestor of the Streptococcus Cp-1 and Bacillus φ29-like phages, but it is not clear at what point in its evolution it acquired the ability to infect L. lactis. In the G+C content of its DNA, phage asccφ28 more closely resembles L. lactis than either S. pneumoniae or B. subtilis. However, only three ORFs (ORF16, ORF20, and ORF24) in asccφ28 encode proteins with recognizable homology to known proteins from L. lactis and its phages (Table 1), presumably due to recombination that occurred at some stage during evolution. Some of the homologies may be associated with host specificity. Two of these ORFs (ORF16 and ORF20) encode tail proteins that could be directly involved in phage-host surface interaction.
Further evidence for the relatedness of asccφ28 to phage Cp-1 and the φ29-like phages is in the mode of DNA replication. All these phages have a phage-encoded DNA polymerase, TP, and ITRs, indicating that they replicate in similar ways (not previously documented for an L. lactis phage). The known mechanism of DNA replication for phage Cp-1 and the φ29-like phages requires formation of a stable complex between the TP, the phage-encoded DNA polymerase, and the phage DNA ITR regions, with the TP itself initially priming the DNA polymerase. During replication no multiple-length DNA concatemers are formed (they are formed in other lactococcal phages), and single-stranded DNA is generated (15, 17, 29, 30, 36). Consistent with this, the asccφ28 genome appears to encode an SSB protein (Table 1). The predicted DNA polymerase of asccφ28 is large (722 amino acids) compared with the corresponding proteins of phages Cp-1 (568 amino acids), GA-1 (578 amino acids), and φ29 (575 amino acids). A ClustalW alignment (not shown) of these DNA polymerases revealed significant differences for the asccφ28 DNA polymerase in the nine semiconserved domains described by Meier et al. (30), although the three aspartic acid residues needed for catalysis at the active site remain conserved.
Critical components of the active process of packaging DNA into mature phage heads in the Cp-1 and φ29-like phages include a phage-encoded packaging (or prohead) RNA (pRNA) that is transcribed from a specific phage promoter, as well as an encapsidation protein (4, 30). The pRNAs have been described for Cp-1 and φ29-like phages (4, 17, 29, 30) and are 160 to 180 nucleotides long. Although there is little sequence homology between these pRNAs, their secondary structures are remarkably similar (4, 29). Noncoding regions of the asccφ28 genome were examined for potential transcripts exhibiting secondary structure consistent with the properties of these pRNAs, but none was found. The location of the presumed pRNA gene of asccφ28 remains unknown. However, BLAST homologies indicate the presence of an encapsidation protein in asccφ28, encoded by ORF11 (Table 1).
A comparison of complete gene maps for phages asccφ28, Cp-1, and GA-1 (Fig. 4) shows that genes with similar functions are arranged in modules (operon-like structures) and that the order of these modules in the genome varies, consistent with the modular evolution of phages (5, 33). Despite rearrangements that change the gene order, the essential nature of each functional module is preserved. Considering likely transcripts and ORF functions (Table 1 and Fig. 4), four discrete types of modules can be hypothesized for the asccφ28 genome: (i) ITR regions, (ii) DNA replication modules, (iii) a structural protein module, and (iv) a cell lysis module. It is suggested that, as in phage Cp-1 (29) and the φ29-like phages (30), the outer regions of the asccφ28 genome encode proteins produced early in the phage life cycle, while the center region of the asccφ28 genome encodes proteins needed later in the life cycle (Fig. 4). The deduced promoter and terminator positions are consistent with this modular arrangement and imply that there is modular control of gene expression.
The hypothesized “early” modules located near the ends of the asccφ28 genome contain ORFs encoding proteins involved DNA replication (TP, DNA polymerase, SSB). However, several predicted proteins encoded in these regions show no recognizable BLAST homologies (Table 1), making functional assignments on this basis impossible. Presumably, some early genes are involved in interactions with host elements or in the regulation of transcription or translation. Curiously, the functions of many early genes of other L. lactis phages also cannot be ascertained by sequence homology (7, 26).
The structural protein genes in asccφ28, comprising mostly the MCP, collar, and tail protein genes, are contiguous in the genome (Fig. 4). Unlike the MCP of Cp-1, but similar to that of φ29, the MCP in asccφ28 is not subject to N-terminal posttranslational cleavage during the packaging process. Coding regions for two of the putative tail proteins (ORF13 and ORF14) overlap extensively, suggesting that translation of these two ORFs may be regulated or coupled in some way. This has been reported for assembly of tail genes in a number of other phages (25, 41).
The cell lysis module comprises the holin (ORF10), encapsidation protein (ORF11), and lysin (ORF12) genes. The gene order within this module is different in phages Cp-1 and the φ29-like phages (Fig. 4). Holins mediate the semispecific transport across the plasma membrane of the lysin and, when expressed, lead to rapid death of the producing cell (44, 45). ORF10 was not represented in our plasmid library, consistent with lethality of the ORF10 protein. Production of holins and lysins must be tightly controlled, since premature production is deleterious to the efficiency of phage replication. In most phages a holin-lysin cassette is found near the end of a late RNA transcript, since these genes are not expressed until they are needed late in the phage infection cycle (45).
In contrast to ORF12 (which putatively encodes the phage asccφ28 endolysin), the similarity of the ORF20 predicted protein to lactococcal phage KSY1 protein gp73 and to Lactobacillus phage amidases (Table 1) occurs toward the C-terminal end. For most lysins/autolysins this region controls substrate binding specificity (10, 13, 39). A possible explanation is that asccφ28 has a protein in its tail structure to facilitate binding to the cell wall, followed by DNA injection, like in some other phages. Phage KSY1 (8) also has two putative endolysins, the gp53a and gp73 predicted proteins (the latter shows similarity to the ORF20 predicted protein of asccφ28). However, for both of these lysins in phage KSY1, putative holin genes are immediately upstream, suggesting that they function from within the host cell.
In conclusion, the complete 18,762-bp genome sequence of phage asccφ28 belonging to a rare L. lactis phage species (P034) has been determined. Phage asccφ28 resembles the φ29-like phages of B. subtilis and phage Cp-1 of S. pneumoniae more closely than it resembles other L. lactis phage species. Sequence analysis revealed 28 ORFs, 13 of which encode predicted proteins with observable BLAST similarity to other proteins or predicted proteins in the GenBank database. The relative rarity and distinctive characteristics of this phage type raise the question of whether future adaptations might increase the infectivity, potential host range, or prevalence of such phages in dairy industry fermentations.
Acknowledgments
This work was supported by the Australian Research Council through an Australian Postgraduate Award (Industry) to S.E.K., by the Dairy Research and Development Corporation (now Dairy Australia), and by member companies of the Australian Starter Culture Research Centre (now the Cultures Division of Dairy Innovation Australia).
We are grateful for the assistance of Michelle Taylor and Andrea Merrall with whey sample testing that led to the isolation of phage asccφ28, with phage stock maintenance, and with host range analysis.
Footnotes
Published ahead of print on 4 April 2008.
REFERENCES
- 1.Ackermann, H. W. 2001. Frequency of morphological phage descriptions in the year 2000. Arch. Virol. 146:843-857. [DOI] [PubMed] [Google Scholar]
- 2.Allison, G. E., and T. R. Klaenhammer. 1998. Phage resistance mechanisms in lactic acid bacteria. Int. Dairy J. 8:207-226. [Google Scholar]
- 3.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, D. L., and B. E. Reilly. 1993. Morphogenesis of bacteriophage φ29, p. 859-867. In A. L. Sonenshien, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, DC.
- 5.Botstein, D. 1980. A theory of modular evolution for bacteriophages. Ann. N. Y. Acad. Sci. 354:484-491. [DOI] [PubMed] [Google Scholar]
- 6.Braun, V., S. Hertwig, H. Neve, A. Geis, and M. Teuber. 1989. Taxonomic differentiation of bacteriophages of Lactococcus lactis by electron microscopy, DNA-DNA hybridization, and protein profiles. J. Gen. Microbiol. 135:2551-2560. [Google Scholar]
- 7.Chandry, P. S., S. C. Moore, J. D. Boyce, B. E. Davidson, and A. J. Hillier. 1997. Analysis of the DNA sequence, gene expression, origin of replication and modular structure of the Lactococcus lactis lytic bacteriophage sk1. Mol. Microbiol. 26:49-64. [DOI] [PubMed] [Google Scholar]
- 8.Chopin, A., H. Deveau, S. D. Ehrlich, S. Moineau, and M.-C. Chopin. 2007. KSY1, a lactococcal phage with a T7-like transcription. Virology 365:1-9. [DOI] [PubMed] [Google Scholar]
- 9.Deveau, H., S. J. Labrie, M.-C. Chopin, and S. Moineau. 2006. Biodiversity and classification of lactococcal phages. Appl. Environ. Microbiol. 72:4338-4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischetti, V. A. 2004. The use of phage lytic emzymes to control bacterial infections, p. 321-334. In E. Kutter and A. Sulakvelidze (ed.), Bacteriophages—biology and applications. CRC Press, Boca Raton, FL.
- 11.Fortier, L.-C., A. Bransi, and S. Moineau. 2006. Genome sequencing and global gene expression of Q54, a new phage species linking the 936 and c2 phage species of Lactococcus lactis. J. Bacteriol. 188:6101-6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.García, E., A. Gómez, C. Ronda, C. Escarmis, and R. López. 1983. Pneumococcal bacteriophage Cp-1 contains a protein bound to the 5′ termini of its DNA. Virology 128:92-104. [DOI] [PubMed] [Google Scholar]
- 13.García, P., J. L. García, R. López, and E. García. 2005. Pneumococcal phages, p. 335-361. In M. K. Waldor, D. I. Friedman, and S. L. Adhya (ed.), Phages: their role in bacterial pathogenesis and biotechnology. ASM Press, Washington, DC.
- 14.Garneau, J. E., D. M. Tremblay, and S. Moineau. 2008. Characterization of 1706, a virulent phage from Lactococcus lactis with similarities to prophages from other Firmicutes. Virology 373:298-309. [DOI] [PubMed] [Google Scholar]
- 15.Gascón, I., J. M. Lázaro, and M. Salas. 2000. Differential functional behavior of viral φ29, Nf and GA-1 SSB proteins. Nucleic Acids Res. 28:2034-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimes, S., and D. Anderson. 1997. The bacteriophage φ29 packaging proteins supercoil the DNA ends. J. Mol. Biol. 266:901-914. [DOI] [PubMed] [Google Scholar]
- 17.Guo, P., S. Erickson, and D. Anderson. 1987. A small viral RNA is required for in vitro packaging of bacteriophage φ29 DNA. Science 236:690-694. [DOI] [PubMed] [Google Scholar]
- 18.Hewick, R. M., M. W. Hunkapiller, L. E. Hood, and W. J. Dreyer. 1981. A gas-liquid solid phase peptide and protein sequenator. J. Biol. Chem. 256:7990-7997. [PubMed] [Google Scholar]
- 19.Jarvis, A. W. 1984. Differentiation of lactic streptococcal phages into phage species by DNA-DNA homology. Appl. Environ. Microbiol. 47:343-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarvis, A. W., G. F. Fitzgerald, M. Mata, A. Mercenier, H. Neve, I. B. Powell, C. Ronda, M. Saxelin, and M. Teuber. 1991. Species and type phages of lactococcal bacteriophages. Intervirology 32:2-9. [DOI] [PubMed] [Google Scholar]
- 21.Klaenhammer, T. R., and G. F. Fitzgerald. 1994. Bacteriophages and bacteriophage resistance, p. 106-168. In M. J. Gasson and W. M. de Vos (ed.), Genetics and biotechnology of lactic acid bacteria. Blackie Academic and Professional, London, United Kingdom.
- 22.Kotsonis, S. E. 1999. Detection and characterization of bacteriophages infecting Lactococcus lactis. Ph.D. thesis. University of Melbourne, Melbourne, Australia.
- 23.Kotsonis, S. E., I. B. Powell, M. L. Billinghurst, A. J. Hillier, G. K. Y. Limsowtin, and B. E. Davidson. 1998. Differentiation of 189 bacteriophages infecting Lactococcus lactis. Aust. J. Dairy Technol. 53:120. [Google Scholar]
- 24.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of bacteriophage T4. Nature 227:680. [DOI] [PubMed] [Google Scholar]
- 25.Levin, M. E., R. W. Hendrix, and S. R. Casjens. 1993. A programmed translational frameshift is required for the synthesis of a bacteriophage λ tail assembly protein. J. Mol. Biol. 234:124-139. [DOI] [PubMed] [Google Scholar]
- 26.Lubbers, M. W., N. R. Waterfield, T. P. J. Beresford, R. W. F. Le Page, and A. W. Jarvis. 1995. Sequencing and analysis of the prolate-headed lactococcal bacteriophage c2 genome and identification of the structural genes. Appl. Environ. Microbiol. 61:4348-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacKay, J. P., and M. Crossley. 1998. Zinc fingers are sticking together. Trends Biochem. Sci. 23:1-4. [DOI] [PubMed] [Google Scholar]
- 28.Mahony, J., H. Deveau, S. McGrath, M. Ventura, C. Canchaya, S. Moineau, G. F. Fitzgerald, and D. van Sinderen. 2006. Sequence and comparative genomic analysis of lactococcal bacteriophages jj50, 712 and P008: evolutionary insights into the 936 phage species. FEMS Microbiol. Lett. 261:253-261. [DOI] [PubMed] [Google Scholar]
- 29.Martín, A. C., R. López, and P. García. 1996. Analysis of the complete nucleotide sequence and functional organization of the genome of Streptococcus pneumoniae bacteriophage Cp-1. J. Virol. 70:3678-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meijer, W. J. J., J. A. Horcajadas, and M. Salas. 2001. φ29 family of phages. Microbiol. Mol. Biol. Rev. 65:261-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moineau, S., J. Fortier, H. W. Ackermann, and S. Pandian. 1992. Characterization of lactococcal bacteriophages from Quebec cheese plants. Can. J. Microbiol. 38:875-882. [Google Scholar]
- 32.Powell, I. B., and B. E. Davidson. 1985. Characterization of streptococcal bacteriophage c6A. J. Gen. Virol. 66:2737-2741. [DOI] [PubMed] [Google Scholar]
- 33.Proux, C., D. van Sinderen, J. Suarez, P. Garcia, V. Ladero, G. F. Fitzgerald, F. Desiere, and H. Brüssow. 2002. The dilemma of phage taxonomy illustrated by comparative genomics of Sfi21-like Siphoviridae in lactic acid bacteria. J. Bacteriol. 184:6026-6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Relano, P., M. Mata, M. Bonneau, and P. Ritzenthaler. 1987. Molecular characterization and comparison of 38 virulent and temperate bacteriophages of Streptococcus lactis. J. Gen. Microbiol. 133:3053-3063. [DOI] [PubMed] [Google Scholar]
- 35.Ronda, C., R. López, and E. García. 1981. Isolation and characterization of a new bacteriophage, Cp-1, infecting Streptococcus pneumoniae. J. Virol. 40:551-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salas, M., and F. Rojo. 1993. Replication and transcription of bacteriophage φ29 DNA, p. 843-857. In A. L. Sonenshien, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology and molecular genetics. American Society for Microbiology, Washington, DC.
- 37.Salinovich, O., and R. C. Montelaro. 1986. Reversible staining and peptide mapping of proteins transferred to nitrocellulose after separation by SDS-PAGE. Anal. Biochem. 156:341-347. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 39.Sheehan, M. M., J. L. García, R. López, and P. García. 1997. The lytic enzyme of the pneumococcal phage Dp-1: a chimeric lysin of intergeneric origin. Mol. Microbiol. 25:717-725. [DOI] [PubMed] [Google Scholar]
- 40.Southern, E. M. 1975. Detection of specific binding sequence among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 98:503-517. [DOI] [PubMed] [Google Scholar]
- 41.Torgov, M. Y., D. M. Janzen, and M. K. Reddy. 1998. Efficiency and frequency of translational coupling between the bacteriophage T4 clamp loader genes. J. Bacteriol. 180:4339-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van de Guchte, M., J. Kok, and G. Venema. 1992. Gene expression in Lactococcus lactis. FEMS Microbiol. Rev. 88:73-92. [DOI] [PubMed] [Google Scholar]
- 44.Wilson, D. B. 1982. Effect of the lambda S gene product on properties of the Escherichia coli inner membrane. J. Bacteriol. 151:1403-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young, R. 1992. Bacteriophage lysis: mechanism and regulation. Microbiol. Rev. 56:430-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young, R., and U. Bläsi. 1995. Holins: form and function in bacteriophage lysis. FEMS Microbiol. Rev. 17:191-205. [DOI] [PubMed] [Google Scholar]