Abstract
The success of Mycobacterium tuberculosis as one of the dreaded human pathogens lies in its ability to utilize different defense mechanisms in response to the varied environmental challenges during the course of its intracellular infection, latency, and reactivation cycle. Truncated hemoglobins trHbN and trHbO are thought to play pivotal roles in the cellular metabolism of this organism during stress and hypoxia. To delineate the genetic regulation of the M. tuberculosis hemoglobins, transcriptional fusions of the promoters of the glbN and glbO genes with green fluorescent protein were constructed, and their responses were monitored in Mycobacterium smegmatis and M. tuberculosis H37Ra exposed to environmental stresses in vitro and in M. tuberculosis H37Ra after in vivo growth inside macrophages. The glbN promoter activity increased substantially during stationary phase and was nearly 3- to 3.5-fold higher than the activity of the glbO promoter, which remained more or less constant during different growth phases in M. smegmatis, as well as in M. tuberculosis H37Ra. In both mycobacterial hosts, the glbN promoter activity was induced 1.5- to 2-fold by the general nitrosative stress inducer, nitrite, as well as the NO releaser, sodium nitroprusside (SNP). The glbO promoter was more responsive to nitrite than to SNP, although the overall increase in its activity was much less than that of the glbN promoter. Additionally, the glbN promoter remained insensitive to the oxidative stress generated by H2O2, but the glbO promoter activity increased nearly 1.5-fold under similar conditions, suggesting that the trHb gene promoters are regulated differently under nitrosative and oxidative stress conditions. In contrast, transition metal-induced hypoxia enhanced the activity of both the glbN and glbO promoters at all growth phases; the glbO promoter was induced ∼2.3-fold, which was found to be the highest value for this promoter under all the conditions evaluated. Addition of iron along with nickel reversed the induction in both cases. Interestingly, a concentration-dependent decrease in the activity of both trHb gene promoters was observed when the levels of iron in the growth media were depleted by addition of an iron chelator. These results suggested that an iron/heme-containing oxygen sensor is involved in the modulation of the trHb gene promoter activities directly or indirectly in conjunction with other cellular factors. The modes of promoter regulation under different physiological conditions were found to be similar for the trHbs in both M. smegmatis and M. tuberculosis H37Ra, indicating that the promoters might be regulated by components that are common to the two systems. Confocal microscopy of THP-1 macrophages infected with M. tuberculosis carrying the trHb gene promoter fusions showed that there was a significant level of promoter activity during intracellular growth in macrophages. Time course evaluation of the promoter activity after various times up to 48 h by fluorescence-activated cell sorting analysis of the intracellular M. tuberculosis cells indicated that the glbN promoter was active at all time points assessed, whereas the activity of the glbO promoter remained at a steady-state level up to 24 h postinfection and increased ∼2-fold after 48 h of infection. Thus, the overall regulation pattern of the M. tuberculosis trHb gene promoters correlates not only with the stresses that the tubercle bacillus is likely to encounter once it is in the macrophage environment but also with our current knowledge of their functions. The in vivo studies that demonstrated for the first time expression of trHbs during macrophage infection of M. tuberculosis strongly indicate that the hemoglobins are required, and thus important, during the intracellular phase of the bacterial cycle. The present study of transcriptional regulation of M. tuberculosis hemoglobins in vitro under various stress conditions and in vivo after macrophage infection supports the hypothesis that biosynthesis of both trHbs (trHbN and trHbO) in the native host is regulated via the environmental signals that the tubercle bacillus receives during macrophage infection and growth in its human host.
Mycobacterium tuberculosis is a phenomenally successful pathogen, having evolved mechanisms that allow it to survive inside macrophages in granulomas that are characterized by severe hypoxia, a low pH, toxic nitrogen and oxygen species, and high CO2 levels. M. tuberculosis not only appears to have the remarkable ability to adapt its metabolism to the hazardous environment of its intracellular niche but has also developed efficient ways to persist during long periods of latency (12). Understanding the molecular mechanisms by which M. tuberculosis is able to adapt its metabolism to environmental changes and enhance its persistence within the host cells would, therefore, contribute significantly to the development of new strategies to combat tuberculosis. The genome of M. tuberculosis contains the glbN and glbO genes encoding the truncated hemoglobins (trHbs) trHbN and trHbO, respectively, which belong to a recently discovered family of hemoproteins that are widely distributed in eubacteria, cyanobacteria, protozoans, and plants (36, 39, 45, 55). The existence of two trHbs in M. tuberculosis that have low sequence similarity and distinct ligand binding properties and are expressed at different stages of growth in the native host (7, 38, 43) suggests that these oxygen-binding proteins may have different physiological functions. Recent studies in our laboratory (27, 42) and the laboratory of Ouellet et al. (40, 41) have indicated that trHbN of M. tuberculosis has a potent ability to detoxify nitric oxide (NO) and relieves the toxicity of NO and nitrosative stress (42) in Escherichia coli and Mycobacterium smegmatis. The high rate of NO oxidation catalyzed by oxygenated trHbN suggests that this may be one of the vital defense systems in M. tuberculosis (41, 42) for coping with the toxic effects of NO and nitrosative stress that may be important for allowing the intracellular survival of the bacterium in macrophages. This hypothesis is supported by the observation that expression of trHbN in an NO-sensitive mutant of Salmonella enhances the survival of the mutant both in vitro under nitrosative stress conditions and in vivo during macrophage infection (44).
The physiological role of trHbO in M. tuberculosis is not obvious at present; however, the presence of trHbO during all stages of growth in the native host (43) suggests that it may be important for cellular metabolism. This suggestion is supported by the presence of trHbO in Mycobacterium leprae, which has undergone drastic genome reduction and has retained only a minimum set of genes required for intracellular survival. Since they are aerobic, the mycobacterial cells would have to have a mechanism to collect the available O2 and deliver it to the respiratory components of the cell to survive in granulomas. It has been suggested that trHbO is a membrane-associated protein, and it has been shown to interact with the CyoB subunit of the E. coli cytochrome o complex. This may be crucial for facilitating O2 availability to the component(s) of the electron transfer chain (31, 43) in order to maintain the basal metabolism during hypoxia and latency. The oxygenated trHbOs of M. tuberculosis and M. leprae also display moderate NO-scavenging activity (2), suggesting that trHbO may be tailored so that it has functional diversity and is involved in both NO detoxification and aerobic respiration.
Unlike vertebrate hemoglobins, which have only oxygen transport and storage functions, the functions of trHbs are very diverse and thus cannot be generalized; e.g., the trHbN of Chlamydomonas eugametos is induced in response to active photosynthesis (8), the trHbN of Nostoc commune is expressed only under anaerobic conditions (20), etc. The distinct functions of the proteins having similar folds and the finding that structurally similar molecules can become further differentiated functionally by being expressed at different times in the development of an organism emphasize the role of regulation by the environment. The functions of trHbN and trHbO are now emerging as important features of the survival strategy of M. tuberculosis. Adaptation to the changing environments that M. tuberculosis encounters in the course of a successful infection must require complex regulation of gene expression, and therefore, a detailed study of the regulation of the genes encoding trHbN and trHbO in response to different environmental stimuli may provide insight into the relevance of these oxygen-binding proteins in the cellular metabolism, survival, and pathogenesis of M. tuberculosis in its macrophage host. Until now, there has been no study of the transcriptional regulation of either of the M. tuberculosis trHbs, and the transcription machinery of this highly evolved pathogen still has not been thoroughly explored. In light of the function of trHbs, it seems logical to begin a study of the regulatory mechanisms governing the expression of trHb genes with an inquiry into the way that the genes respond to different environmental stresses and the macrophage environment. A study of the transcriptional responses of the genes to various stresses during growth would help us not only understand the regulation of gene expression but also establish the function of the encoded proteins.
In the present work, we conducted a systematic study of regulation of the trHb genes of M. tuberculosis by constructing transcriptional fusions with green fluorescent protein (GFP) and monitoring the promoter activity both in vitro under various stress conditions and in vivo after infection of THP-1 macrophages.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
Unless otherwise noted, M. smegmatis mc2155 and M. tuberculosis H37Ra were grown in Middlebrook 7H9 medium or on Middlebrook 7H10 agar plates (Difco) supplemented with 0.05% Tween, 0.2% glycerol, and the OADC (10% bovine serum albumin fraction V, dextrose, catalase, oleic acid) enrichment for M. tuberculosis. All transformations in E. coli were performed with strain JM109. E. coli was grown in Luria broth (Difco) at 37°C in shaking cultures. For E. coli and mycobacteria, 100 and 50 μg/ml of hygromycin, respectively, were added to the medium when required.
Construction of transcriptional fusions.
The transcriptional fusions were constructed by PCR amplification of a sequence encompassing positions −240 to 26 and positions −410 to 70 relative to the start codons of glbN and glbO, respectively, from M. tuberculosis H37Rv genomic DNA using primers that incorporated a 5′ XbaI site and a 3′ EcoRV site. The XbaI- and EcoRV-digested PCR products were ligated to XbaI- and EcoRV-cut pSC301 (9) from which the SOD promoter had been excised. pSC301b, the GFP read-through control vector, was constructed by digestion of pSC301 with XbaI and EcoRV to remove the SOD promoter, end filling with Vent polymerase, and religation. The constructs were electroporated into M. smegmatis mc2155 and M. tuberculosis H37Ra as previously described (54).
Determination of activities of transcriptional fusions.
The M. smegmatis clones, transformed with the transcriptional fusions, were grown for 3 to 4 days and were then inoculated into fresh medium at an optical density at 600 nm (OD600) of 0.05. The cultures were allowed to grow for another 8 to 10 h, resulting in an OD600 of around 0.5. The cells were then exposed to different physiological stresses, including 10 and 30 mM nitrite and 0.5 and 1.0 mM sodium nitroprusside (SNP) for nitrosative stress; 1 and 5 mM H2O2 for oxidative stress; 0.2, 0.3, and 0.4 mM 2,2′dipyridyl as an iron chelator; and medium whose pH was adjusted to 7 and 4. One hundred microliters of each culture was removed at an OD600 of 0.6 (early exponential phase), 1.2 (late exponential phase), and 2.0 (early stationary phase). The samples were diluted into 1 to 2 ml of phosphate-buffered saline (PBS), and the numbers of relative fluorescence units (RFU) were determined using a 490-nm excitation filter and a 520-nm emission filter with a spectrofluorometer (Perkin Elmer). The OD600 of each culture was determined with a UV-1601 Shimadzu spectrophotometer. The background fluorescence due to read-through transcription of the transcriptional fusion vector was determined by determining the number of RFU for strains that harbored the pSC301b plasmid and were subjected to the same conditions. The experiments with M. tuberculosis were done similarly. The RFU/OD600 values were plotted versus time. All measurements were obtained for triplicate cultures (means ± standard deviations) and were corrected for background fluorescence by subtracting the number of RFU for the control strain carrying pSC301b incubated under identical conditions.
Confocal microscopy.
THP-1 cells were grown in RPMI 1640 containing 5% fetal bovine serum. In a 24-well tissue culture plate, 105 cells were seeded onto glass coverslips in RPMI 1640 containing 5% fetal bovine serum and then activated using 25 ng/ml phorbol myristate acetate (PMA) (Sigma) for 24 h. Infection was performed as previously described (14). After 4 h of infection, the cells were washed extensively with PBS to remove the extracellular bacteria. The cells were then fixed with 4% formaldehyde and visualized by confocal fluorescence microscopy.
Infection of THP-1 macrophages with M. tuberculosis and preparation of bacteria for FACS analysis.
For fluorescence-activated cell sorting (FACS) analysis, THP-1 cell monolayers were established in 100-mm tissue culture petri dishes with 107 cells. After overnight incubation, the cells were activated using 25 ng/ml PMA for 24 h. The M. tuberculosis cells were prepared for infection by washing them three times with PBS under sterile conditions. The bacteria were then resuspended in 1 ml of PBS, and macrophages were infected using a multiplicity of infection of 1:20 in two duplicate sets. The cells were incubated at 37°C in 95% air-5% CO2 for 4 h, after which they were extensively washed with PBS to remove the noningested bacteria. The infected macrophage monolayers were then incubated for 12, 24, and 48 h.
Each macrophage monolayer was washed five times with PBS. In vivo-grown mycobacteria were prepared using a previously described protocol (13). Briefly, the macrophage cells were harvested by using a scraper, collected by centrifugation (1,200 rpm for 10 min at 4°C), and then broken by 20 passages through a 23-gauge needle. Intact cells and nuclei were removed by pelleting them by centrifugation at 3,000 rpm for 5 min, and the supernatant containing bacteria was then centrifuged at 13,000 rpm for 5 min. The pellet obtained was resuspended in PBS for FACS analysis. The in vitro-grown mycobacteria were washed three times with PBS and resuspended in 1 ml of PBS by passage through a 23-gauge needle. For autofluorescence control, M. tuberculosis cells transformed with control vector pSC301b were prepared similarly.
Flow cytometry.
Flow cytometric analysis was carried out using a Becton Dickinson FACScan and the Cell Quest software (Becton Dickinson). Data were collected for 10,000 individual particles per sample, with gating for size to eliminate interference due to potential clumping using the previously described procedure (13).
RESULTS
Genetic organization and promoter structure of M. tuberculosis trHbs.
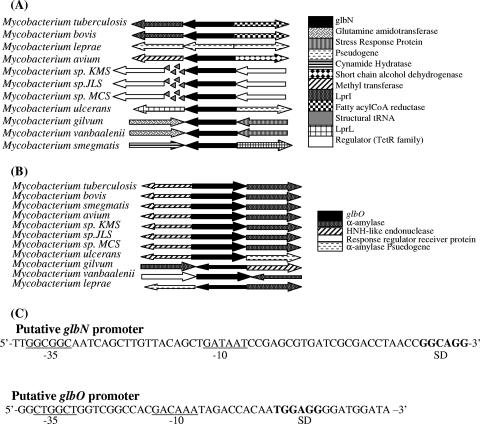
Conservation of gene order in prokaryotes has been considered one of the important predictors of gene function that helps in speculating about the probable function of a gene based on its positional counterparts or gene organization. The genomic organization around glbN (Fig. 1A) appeared to be similar only in the pathogenic members of the M. tuberculosis complex, and the nonpathogenic members had an altogether different genetic arrangement (Fig. 1A). Interestingly, the genomic organization of the glbO gene in various mycobacterial species was fairly well conserved (Fig. 1B), and 9 of the 11 species had identical loci flanking the glbO gene. Upstream intergenic regions of the glbN (228 bp) and glbO (143 bp) genes did not contain any obvious recognition sequences for mycobacterial stress response or oxygen-dependent transcriptional regulators. Analysis of the upstream region using the promoter prediction program available at http://www.fruitfly.org/seq_tools/promoter.html predicted the presence of putative promoter elements characterized by scores of 0.87 and 0.66, respectively (Fig. 1C). The transcription start sites were predicted to be 11 and 50 bp upstream of the translational start sites of glbO and glbN, respectively. The putative promoters showed that a distinct Shine-Dalgarno sequence and probable −10 and −35 sites were present.
FIG. 1.
Schematic representation of the genomic organization of (A) the glbN gene and (B) the glbO gene. (C) In silico-predicted putative promoter sequences of the glbN and glbO genes. The −10 and −35 sequences are underlined, and the Shine-Dalgarno (SD) sequence is indicated by bold type.
Transcriptional fusion of M. tuberculosis trHb gene promoters with the gfp gene.
To study the transcriptional activity of the putative promoters of the glbN and glbO genes of M. tuberculosis, we constructed transcriptional fusions with the promoterless gfp gene in the E. coli-Mycobacterium shuttle vector pSC301 (9). The glbN and glbO transcriptional fusions were designated pSCNP and pSCOP, respectively. The glbN- and glbO-GFP promoter fusions exhibited low but distinct transcriptional activity in E. coli; however, their transcriptional responses were not studied in E. coli due to the great differences in the transcriptional machinery and regulation of the two organisms (1). Therefore, pSCNP and pSCOP were transformed into M. smegmatis, which has been widely used as a suitable heterologous host for studying transcriptional control of M. tuberculosis promoters (15, 23). The assay of the promoter activities in M. smegmatis using spectrofluorometry showed that there was significant up-regulation of the glbN promoter during the late exponential and stationary phases of growth, whereas the glbO promoter was constitutively active at a constant level throughout the growth cycle. The transcriptional activities of the trHb gene promoters were recorded following exposure to a variety of stress conditions and compared with the untreated control activities at early exponential phase (OD600, 0.6), late exponential phase (OD600, 1.2), and stationary phase (OD600, 2.0) of growth.
Activities of M. tuberculosis trHb gene promoters in M. smegmatis under different environmental conditions.
To study the implications of trHbs for intracellular growth and survival of M. tuberculosis, expression of the glbN and glbO promoters was first studied in vitro under conditions that simulated the major environmental challenges that the tubercle bacillus faces during its pathogenic stage, including various levels of oxidative and nitrosative stresses, pH challenge, and oxygen and nutrient limitation, in order to carry out aerobic metabolism.
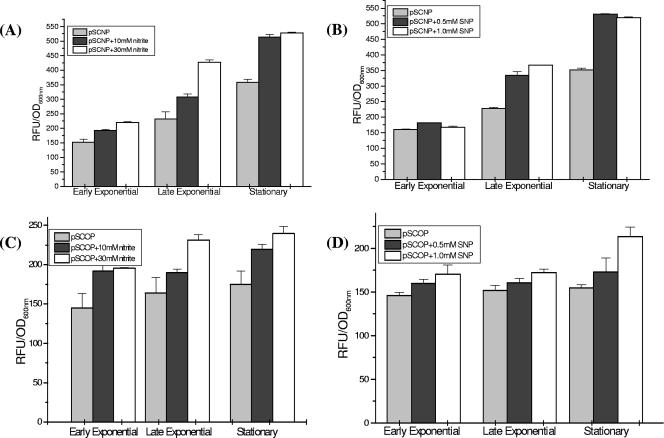
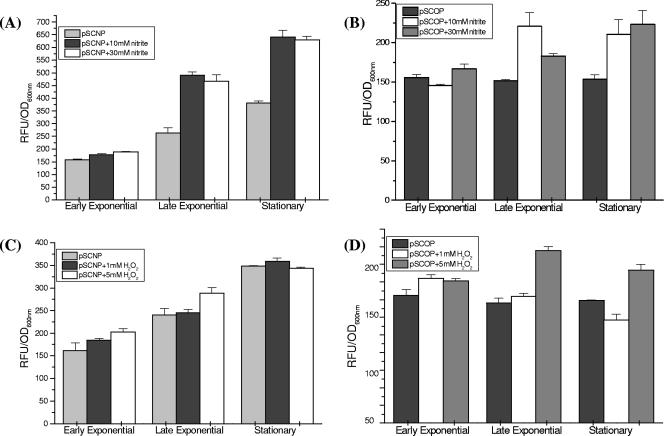
The first stress faced by M. tuberculosis during infection is exposure to oxidizing agents, principally represented by the reactive oxygen intermediates (ROIs) and the reactive nitrogen intermediates (RNIs), produced by the activated macrophages. For induction of nitrosative stress, we used nitrite, which protonates to form HNO2, which quickly dismutates to produce several species of nitrogen oxides, including NO (nitric oxide). There was a dose-dependent increase in glbN promoter activity in the presence of nitrite, with the levels of induction ranging from 1.3- to 1.5-fold with 10 mM nitrite and from 1.5- to 1.9-fold with 30 mM nitrite, at different growth phases (Fig. 2A). To evaluate whether the induction resulting from addition of nitrite was due to the combined effect of reactive nitrogen species generated during protonation of nitrite or due solely to release of NO during nitrite reduction, SNP, which specifically releases NO, was employed. The glbN promoter activity was induced 1.4- to 1.7-fold at all three growth phases after addition of both 0.5 and 1.0 mM SNP (Fig. 2B), suggesting that NO may be one of the key factors for modulating the glbN promoter activity.
FIG. 2.
Transcriptional activities of the trHb gene promoters in M. smegmatis under nitrosative stress conditions. M. smegmatis mc2155 cells transformed with pSCNP or pSCOP were grown to an OD600 of around 0.5, and then 10 or 30 mM sodium nitrite or 0.5 or 1.0 mM SNP was added. GFP fluorescence was measured at OD600 of 0.6 (early exponential phase), 1.2 (late exponential phase), and 2.0 (early stationary phase). (A and B) glbN promoter activities at different growth phases of M. smegmatis mc2155 (A) in the presence of nitrite and (B) in the presence of SNP. (C and D) glbO promoter activities at different growth phases of M. smegmatis mc2155 (C) in the presence of nitrite and (D) in the presence of SNP. Statistical significance was established using a P value of <0.05.
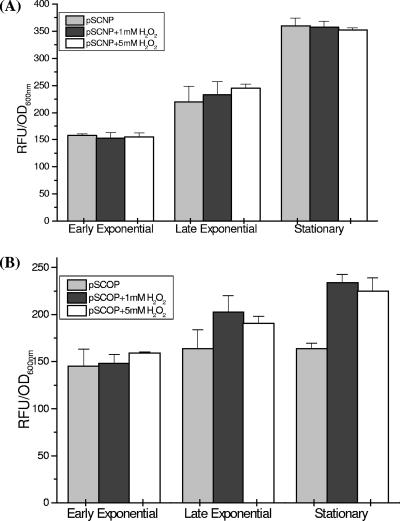
The activity of the glbO promoter was enhanced in the presence of nitrite at all the growth phases in a more or less concentration-dependent manner (Fig. 2C). The level of induction (1.4-fold) was lower than that observed for the glbN promoter (1.5- to 1.9-fold). With SNP, a noticeable difference in the glbO promoter activity was observed only at the stationary phase, with only ∼1.3-fold up-regulation (Fig. 2D). Addition of H2O2 for induction of oxidative stress did not result in any significant difference in the promoter activities of the two trHbs during the exponential phase of growth. However, the glbO promoter consistently showed moderate (∼1.3-fold) up-regulation in the presence of H2O2 during the stationary phase (Fig. 3A and B).
FIG. 3.
Transcriptional activities of the trHb gene promoters in M. smegmatis under oxidative stress conditions. (A) glbN and (B) glbO promoter activities at different growth phases of M. smegmatis mc2155 in the presence of 1 and 5 mM H2O2. Statistical significance was established using a P value of <0.05.
Even if M. tuberculosis is able to block phagosome acidification, the block is not complete as there is slight decrease in the pH of the mycobacterial phagosomes (13). Therefore, the effect of culture pH on the transcriptional activity of the trHb gene promoters was investigated by using media in which the initial pH value was adjusted to 7 and 4. Both promoters were found to be independent of regulation by pH (data not shown).
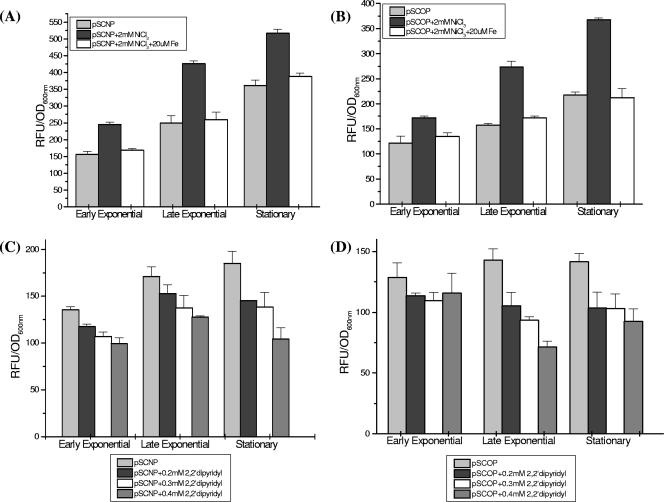
It is generally accepted that the environment within the tuberculous granuloma is hypoxic (32), and extensive redirection of gene expression was noted in M. tuberculosis cultures in response to a brief period of oxygen limitation (11). Recent reports suggested that addition of transition metals could induce hypoxic genes under normoxia (5, 21, 26, 53). When we added 2 mM nickel chloride to the growth medium to induce hypoxia, there was ∼1.7-fold induction of glbN promoter activity at the early and late exponential growth phases (Fig. 4A). In the case of the glbO promoter, the level of induction was higher and reached ∼2.3-fold at the stationary phase (Fig. 4B). The induction of the trHb gene promoters was reversed when iron (20 μM FeCl3) was added along with 2 mM nickel chloride to the growth medium, indicating that an iron/heme-containing factor(s) is involved in regulation of the transcriptional activity of the glbN and glbO promoters (Fig. 4A and B).
FIG. 4.
Transcriptional activities of the trHb gene promoters under transition metal-induced hypoxia and iron depletion conditions. (A) glbN and (B) glbO promoter activities at different growth phases of M. smegmatis mc2155 in the presence of 2 mM NiCl3 and/or 20 μM FeCl3 and (C) glbN and (D) glbO promoter activities at different growth phases of M. smegmatis mc2155 in the presence of different concentrations of the iron chelator 2,2′dipyridyl. Statistical significance was established using a P value of <0.05.
Inside the phagosomes and granulomas, the availability of nutrients and essential elements, especially iron (17) and magnesium (4), is greatly reduced. We examined the effects of diminished iron levels on the promoters of the trHbs by using the iron chelator 2,2′-dipyridyl at three different concentrations. A concentration-dependent decrease in the promoter activity was observed for both the trHb gene promoters. This effect of iron depletion was most pronounced during the stationary phase, when maximum down-regulation (twofold) was observed for the glbN promoter (Fig. 4C). A greater concentration-dependent decrease was observed for the activity of the glbO promoter compared to the activity of the glbN promoter, with values ranging from 1.7- to 2.3-fold (Fig. 4D).
Activities of trHb gene promoters in M. tuberculosis H37Ra.
M. smegmatis has safely been used as a surrogate host for expression of genes from slow-growing pathogenic mycobacteria, but there are several promoters which do not follow the same pattern in M. tuberculosis and M. smegmatis (30). In addition to this, there are some differences in the patterns of the sigma factors of the two organisms (33). Since the most homologous system available for M. tuberculosis H37Rv is M. tuberculosis H37Ra, we also evaluated trHb promoter activity in this host. The promoter-GFP fusions were transformed in M. tuberculosis H37Ra, where the activities exhibited the same pattern that they exhibited in M. smegmatis, with the glbN gene promoter up-regulated at stationary phase and the glbO gene promoter constitutively active (data not shown). The trHb gene promoter activities were assayed under all stress conditions employed for M. smegmatis. The results obtained showed that the patterns were very similar and that there were similar changes in the trHb gene promoter activities. Representative results for trHb gene promoter activities in the presence of nitrite and H2O2 are presented here. The results showed that the glbN promoter behaves more or less similarly in the two mycobacterial species (Fig. 5A and C). The glbO promoter, however, showed slightly higher levels of induction under both nitrosative and oxidative stress conditions in M. tuberculosis than in M. smegmatis (Fig. 5B, 5D).
FIG. 5.
Transcriptional activity of the trHb gene promoters in M. tuberculosis H37Ra under nitrosative and oxidative stress: (A) glbN and (B) glbO promoter activities at different growth phases of M. tuberculosis H37Ra in the presence of 10 and 30 mM sodium nitrite and (C) glbN and (D) glbO promoter activities at different growth phases of M. tuberculosis H37Ra in the presence of 1 and 5 mM H2O2. Statistical significance was established using a P value of <0.05.
Activities of M. tuberculosis trHb promoters during intracellular infection.
In vitro studies of transcriptional regulation of the trHb gene promoters clearly indicated that the responses of the promoters of the glbN and glbO genes were distinct under different stress conditions, suggesting that the promoters are relevant under in vivo growth conditions. Additionally, for the function of trHbN and trHbO to be relevant to the intracellular growth of M. tuberculosis, it is important to determine if these compounds are synthesized during the intracellular phase. The observation that GFP is efficiently expressed in mycobacteria and the observation that fluorescent bacteria can be directly observed in infected macrophages (13) suggested that such a strategy may be applicable to studies of gene induction by mycobacteria in host cells. To study the intracellular expression of the hemoglobin genes, PMA-activated THP-1 cells were used, which have been reported to closely model the primary human alveolar macrophages (46) that are the critical growth niche for intracellular M. tuberculosis in the lungs (29). The PMA-activated THP-1 cells were infected with M. tuberculosis H37Ra carrying the transcriptional fusion of the glbN and glbO genes. Confocal microscopy of the infected THP-1 cells showed that there was distinct fluorescence in the phagocytosed M. tuberculosis H37Ra cells carrying glbN and glbO transcriptional fusions (data not shown).
M. tuberculosis cells harboring glbN or glbO promoter fusions were used to infect PMA-activated THP-1 cells, and at 4, 12, 24, and 48 h postinfection, THP-1 cells were lysed and the intracellular bacteria were harvested for analysis by flow cytometry. Two peaks at which the low-fluorescence fraction was composed mainly of cell debris were observed, as has also been observed by Dhandayuthapani et al. (13) for Mycobacterium bovis. The peak showing a higher level of fluorescence consisted of intact bacterial cells isolated from macrophages. The overall maximum for this peak was much lower than that obtained with the bacteria used to infect the macrophages, which probably reflected differences in the total numbers of particles in the samples.
The FACS analysis of bacteria collected immediately after infection (for 4 h) revealed the presence of highly fluorescent cells, suggesting that both the trHb gene promoters were active. The distribution of fluorescence for the bacteria grown in macrophages was found to be similar to the distribution of fluorescence for the in vitro-grown bacteria. Time course FACS analysis of in vivo-grown M. tuberculosis after 12, 24, and 48 h of intracellular growth indicated that functionally active trHb gene promoters were present during these periods. The mean fluorescence intensities of mycobacteria collected at various time points during infection relative to the mean fluorescence intensities of mycobacteria grown in vitro showed that there was a slight but consistent increase in the activity of the glbN promoter from 4 to 48 h (Table 1). The glbO promoter showed a much more drastic change; the mean fluorescence intensities of the in vivo- and in vitro-grown mycobacteria were more or less similar until 24 h after infection, but the level of fluorescence of bacteria isolated from infected macrophages was found to be almost twice that of the in vitro-grown bacteria after 48 h of infection (Table 2). Overall, the in vivo studies of transcriptional regulation of the M. tuberculosis trHb gene promoters clearly indicated the presence of active glbN and glbO promoters whose activity increased after continued growth in the intramacrophage environment. The results, therefore, indicate that there is a steady expression of both trHbs and that both trHbs may be present during macrophage infection.
TABLE 1.
Flow cytometric analysis of M. tuberculosis carrying the glbN transcriptional fusion
| Time postinfection (h) | Fluorescence intensity (mean ± SE) of M. tuberculosis with pSCNPa
|
|
|---|---|---|
| Grown in vitro | Grown in vivo | |
| 4 | 49.73 ± 0.35 | 45.57 ± 0.32 |
| 12 | 48.30 ± 0.31 | 48.46 ± 0.25 |
| 24 | 48.95 ± 0.28 | 49.82 ± 0.23 |
| 48 | 50.00 ± 0.41 | 61.44 ± 0.52 |
The M. tuberculosis cells carried the glbN transcriptional fusion (pSCNP) and were harvested from shake flasks (grown in vitro) or infected THP-1 macrophages (grown in vivo). Three independent experiments were carried out, each in duplicate. The data are from one representative experiment.
TABLE 2.
Flow cytometric analysis of M. tuberculosis carrying the glbO transcriptional fusion
| Time postinfection (h) | Fluorescence intensity (mean ± SE) of M. tuberculosis with pSCOPa
|
|
|---|---|---|
| Grown in vitro | Grown in vivo | |
| 4 | 23.41 ± 0.20 | 25.00 ± 0.32 |
| 12 | 29.82 ± 0.13 | 29.79 ± 0.16 |
| 24 | 28.90 ± 0.22 | 29.84 ± 0.03 |
| 48 | 23.41 ± 0.27 | 42.41 ± 0.44 |
The M. tuberculosis cells carried the glbO transcriptional fusion (pSCOP) and were harvested from shake flasks (grown in vitro) or infected THP-1 macrophages (grown in vivo).Three independent experiments were carried out, each in duplicate. The data are from one representative experiment.
DISCUSSION
In M. tuberculosis, during its intracellular phase, there is coordinated gene expression in response to signals associated with various stages of the infection cycle. Although a number of virulence genes and their response regulators have recently been identified (19, 56), the mechanisms governing the intracellular survival of this organism and its persistence within the human host are poorly understood. Global analysis of gene expression in several pathogenic bacteria, including M. tuberculosis, has revealed that large-scale changes occur upon in vitro exposure to the environmental conditions that simulate the intracellular milieu (28, 35, 49). trHbN and trHbO are produced by M. tuberculosis at different stages of growth (7, 38, 43), and it has been proposed that they confer an in vivo survival advantage by evading toxic effects of reactive oxygen and nitrogen species in the hypoxic environment of macrophages due to their distinct ability to scavenge reactive oxygen and nitrogen species (44) and trap oxygen with high efficiency, which may contribute significantly to overcoming the intracellular host defense mechanisms.
Mycobacterial genome sequence analysis has indicated that trHbs may be widely distributed among pathogenic and nonpathogenic species. Interestingly, the trHbO-encoding gene (glbO) has been identified in all mycobacterial species, including M. leprae, and its genetic organization is quite conserved across various species compared to glbN, suggesting that trHbO might be involved in a more basic and/or crucial function needed for mycobacterial cellular metabolism. In contrast, the genomic organization of glbN is not very well conserved across mycobacterial species. It is quite possible that trHbN may have distinct roles in different mycobacterial species, as has recently been shown for the function of trHbN of M. smegmatis and M. tuberculosis (27); thus, the conservation of the glbN gene organization among different species of mycobacteria may not be very relevant.
The tubercle bacillus encounters the potentially hypoxic and hostile environment of the granuloma, which is enriched with various reactive oxygen and nitrogen species, by using specific and general stress responses involving regulation of the components of its defense mechanisms. The transcriptional activities of the glbN and glbO promoters during aerobic growth of M. smegmatis and M. tuberculosis were consistent with the protein expression pattern observed for trHbN and trHbO in M. bovis (38, 43). The glbN promoter was up-regulated both by the general nitrosative stress inducer nitrite and by a specific NO releaser (SNP), suggesting that there is induction of trHbN biosynthesis during exposure to NO and other reactive nitrogen species. We have previously demonstrated that trHbN of M. tuberculosis has a potent NO dioxygensase activity that converts toxic NO into harmless nitrate in the presence of oxygen and relieves toxicity due to NO and nitrosative stress (42). It has been proposed that because of its high oxygen affinity, trHbN retains bound oxygen and ensures availability of a critical level of oxygen to carry out NO detoxification in the hypoxic and NO-enriched environment of macrophages, ensuring intracellular survival of M. tuberculosis. The observed regulation behavior can be correlated to the previously observed function of the encoded trHbN, which detoxifies NO and, therefore, should be available to the mycobacteria whenever toxic levels of NO are present. No change in glbN promoter activity was observed in M. smegmatis or M. tuberculosis under oxidative stress conditions or after a change in pH, which ruled out the possibility that there was regulation of the trHbN gene by these conditions.
The response of the glbO promoter in the mycobacterial hosts M. smegmatis and M. tuberculosis appeared to be very different from that of the glbN promoter under nitrosative and oxidative stress conditions. The transcriptional activity of the glbO promoter increased under nitrosative stress conditions, but only to a level lower than that of the glbN promoter. During stationary phase, the M. tuberculosis glbO promoter exhibited nearly 1.3-fold induction in the presence of SNP, which is very similar to the level observed for the glbO promoter of M. leprae (15). The promoter activity of glbO was also enhanced under oxidative stress conditions, which suggests that the activity of the glbO promoter might be modulated by the cumulative effect of general stress. trHbO seems to be required for aerobic respiration (31, 43), and therefore, its levels are increased by the general stresses that the bacteria come across once they are inside phagosomes, which include RNIs, ROIs, and hypoxia. It has been proposed that in M. leprae trHbO has a dual function, respiration and NO detoxification (15, 50, 51). Such a role for M. tuberculosis trHbO has not been observed, but the fact that M. tuberculosis trHbO displays a low level of NO dioxygensase activity in E. coli and the fact that its promoter is induced under both oxidative and nitrosative stress conditions during stationary phase suggest that it might also have functional diversity very similar to that of trHbO of M. leprae. Additionally, a trHbN knockout strain of M. bovis retained NO uptake activity, albeit with lower efficiency, which might be attributed to the presence of trHbO (41).
In contrast to the results under oxidative and nitrosative stress conditions, the transcriptional activities of both the glbN and glbO promoters increased under transition metal-induced hypoxia. It has been observed that transition metals, like nickel or cobalt, induce a number of hypoxic genes under normoxic conditions (5, 21, 53) by serving as substrates for ferrochelatase and being incorporated into protoporphyrin IX in place of iron (48). Nickel-substituted hemoproteins, therefore, do not bind oxygen and cannot function as oxygen sensors. Addition of nickel chloride induced the glbO promoter to the highest level observed for this promoter under all the conditions evaluated. These observations suggest that there is a hemosensor in mycobacteria that may up-regulate the activity of the trHb gene promoters. This suggestion was substantiated by a decrease in the up-regulation of the glbN and glbO promoters when iron, which is known to reverse the effect of addition of nickel by competing with ferrochelatase, was included along with nickel (26).
In vertebrates, hemoproteins have been reported to act as oxygen sensors (5, 18, 22, 34, 52), and recently, heme has also been shown to be involved in regulating transcription of oxygen-responsive genes in Saccharomyces cerevisiae (25, 26). In fact, the heme-containing proteins DosS and DosT of M. tuberculosis have been shown to function as redox and hypoxia sensors, respectively (24). The concentration-dependent decrease in the activity of both trHb gene promoters after depletion of iron, further indicating involvement of an iron homeostasis regulator that may modulate the activity of the trHb gene promoters directly or indirectly by regulating general response regulators of hypoxia in mycobacteria. The overall responses of the trHb gene promoters in M. tuberculosis H37Ra and M. smegmatis were more or less similar under all the stress conditions used, which suggests that at least these two promoters behave in a similar manner in the two mycobacterial species and, therefore, might be regulated by components which are common to both systems.
The levels of induction of the trHb promoters ranged from 1.2- to 2.3-fold under the different growth conditions, which is quite a significant change at the protein level considering that GFP does not require any substrate and, therefore, does not require an amplification step for visualization of fluorescence. Most studies examining gene regulation in mycobacteria have shown a similar level of induction; for example, 11 of 12 Mycobacterium marinum promoters known to be induced exhibited 1.4- to 3.4-fold GFP induction (3). The trHb gene promoters were found to be maximally responsive to RNIs, iron depletion, and hypoxia. The multicellular structure within which the tubercle bacillus resides is also known to express NOS2 and to produce RNIs in situ in tuberculosis tissues of infected mice and humans (6, 16, 47). Apart from oxidative and nitrosative stresses inside the phagosomes and granulomas, the availability of nutrients and essential elements may be reduced (4, 17). Therefore, all these stresses are likely to be biologically relevant signals encountered by M. tuberculosis in vivo, which the tubercle bacillus then exploits as signals for regulation of the expression of specific genes that promote survival in the host.
The pattern of gene expression is modified for adaptation to an intracellular environment, as has been shown previously for Salmonella and Listeria, both of which are intracellular pathogens (37). By analogy, similar mechanisms are expected to be operative in M. tuberculosis. Therefore, identification of the genes which are expressed or up-regulated within the host cells would contribute to our understanding of the pathogenicity of this organism. We showed here for the first time using confocal microscopy that the trHb gene promoters were active during intracellular growth. FACS analysis showed that the glbN promoter exhibited much higher activity than the glbO promoter, and the glbN promoter was found to be active after intracellular growth for about 48 h, with a slow but steady increase in activity. However, we cannot rule out the possibility of further up-regulation of the glbN promoter after periods of infection longer than those studied here. The glbO promoter activity, like the in vitro expression pattern, remained at a steady-state level up to 24 h postinfection and, interestingly, increased ∼2-fold after 48 h of infection. During the course of infection, there are several changes in the macrophage environment due to the release of cytokines in a time-dependent manner (for example, NF-κB [14]), and there is activation of various protection mechanisms (for example, generation of ROIs and RNIs against the Mycobacterium). Therefore, the increase in glbO promoter activity might be due to induction of some host-specific factor in Mycobacterium itself or might be due to variation in the host environment. Most physiological studies have stressed the importance of trHbN for the survival of M. tuberculosis inside the host; however, the specific up-regulation of the trHbO promoter during growth inside macrophages places it on center stage along with trHbN. As previously observed (10), the activity of the SOD promoter, used as control, decreased significantly after just 4 h of intracellular growth of M. tuberculosis cells carrying pSC301 (data not shown). This suggests that the stresses that the Mycobacterium cells face during growth inside macrophages keep the trHb gene promoters active and that there might be a requirement for constant expression of both of the trHb proteins during intracellular growth of the Mycobacterium.
Together, the in vitro and in vivo studies in this work provide a comprehensive picture of the regulation of M. tuberculosis hemoglobin genes. The regulation of each promoter correlates not only with the stresses that the tubercle bacillus is likely to encounter once it is in the macrophage environment but also with our current knowledge of the functions of the promoters. M. tuberculosis is a phenomenally successful pathogen, having evolved mechanisms to precisely regulate the expression of proteins that allow it to survive in the ever-changing macrophage environment. To date, all the speculation about the functions and importance of the trHbs has been centered on the information obtained from studies conducted in vitro. The present study demonstrated for the first time expression of trHbs in Mycobacterium during growth inside macrophages. The specific up-regulation of the glbO promoter during growth inside macrophages reinforces the importance of this promoter for the survival of the mycobacterium. The in vivo studies presented here strongly indicate that the trHbs of M. tuberculosis are required and, thus, important in the natural habitat of the bacterium.
Acknowledgments
We are very grateful to Yossef Av-Gay for the generous gift of the pSC301 plasmid used in this work. The help provided by S. Kumar and R. Dhiman is acknowledged with thanks.
We are grateful to the Department of Biotechnology and the Council for Scientific and Industrial Research, Government of India, for financial support.
Footnotes
Published ahead of print on 4 April 2008.
REFERENCES
- 1.Agarwal, N., and A. K. Tyagi. 2006. Mycobacterial transcriptional signals: requirements for recognition by RNA polymerase and optimal transcriptional activity. Nucleic Acids Res. 34:4245-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ascenzi, P., M. Milani, and P. Visca. 2006. Peroxynitrite scavenging by ferrous truncated hemoglobin GlbO from Mycobacterium leprae. Biochem. Biophys. Res. Commun. 351:528-533. [DOI] [PubMed] [Google Scholar]
- 3.Barker, L. P., D. M. Brooks, and P. L. Small. 1998. The identification of Mycobacterium marinum genes differentially expressed in macrophage phagosomes using promoter fusions to green fluorescent protein. Mol. Microbiol. 29:1167-1177. [DOI] [PubMed] [Google Scholar]
- 4.Buchmeier, N., A. Blanc-Potard, S. Ehrt, D. Piddington, L. Riley, and E. A. Groisman. 2000. A parallel intraphagosomal survival strategy shared by Mycobacterium tuberculosis and Salmonella enterica. Mol. Microbiol. 35:1375-1382. [DOI] [PubMed] [Google Scholar]
- 5.Bunn, H. F., and R. O. Poyton. 1996. Oxygen sensing and molecular adaptation to hypoxia. Physiol. Rev. 76:839-885. [DOI] [PubMed] [Google Scholar]
- 6.Choi, H. S., P. R. Rai, H. W. Chu, C. Cool, and E. D. Chan. 2002. Analysis of nitric oxide synthase and nitrotyrosine expression in human pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 166:178-186. [DOI] [PubMed] [Google Scholar]
- 7.Couture, M., S. R. Yeh, B. A. Wittenberg, J. B. Wittenberg, Y. Ouellet, D. L. Rousseau, and M. A. Guertin. 1999. Cooperative oxygen-binding hemoglobin from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 96:11223-11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couture, M., T. K. Das, H. C. Lee, J. Peisach, D. L. Rousseau, B. A. Wittenberg, J. B. Wittenberg, and M. Guertin. 1999. Chlamydomonas chloroplast ferrous hemoglobin. Heme pocket structure and reactions with ligands. J. Biol. Chem. 274:6898-6910. [DOI] [PubMed] [Google Scholar]
- 9.Cowley, S. C., and Y. Av-Gay. 2001. Monitoring promoter activity and protein localization in Mycobacterium spp. using green fluorescent protein. Gene 264:225-231. [DOI] [PubMed] [Google Scholar]
- 10.Cowley, S. C., R. Babakaiff, and Y. Av-Gay. 2002. Expression and localization of the Mycobacterium tuberculosis protein tyrosine phosphatase PtpA. Res. Microbiol. 153:233-241. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham, A. F., and C. L. Spreadbury. 1998. Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and localization of the 16-kilodalton alpha-crystallin homolog. J. Bacteriol. 180:801-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deretic, V., and R. A. Fratti. 1999. M. tuberculosis phagosome. Mol. Microbiol. 31:1603-1609. [DOI] [PubMed] [Google Scholar]
- 13.Dhandayuthapani, S., L. E. Via, C. A. Thomas, P. M. Horowitz, D. Deretic, and V. Deretic. 1995. Green fluorescent protein as a marker for gene expression and cell biology of mycobacterial interactions with macrophages. Mol. Microbiol. 17:901-912. [DOI] [PubMed] [Google Scholar]
- 14.Dhiman, R., M. Raje, and S. Majumdar. 2007. Differential expression of NF-κB in mycobacteria infected THP-1 affects apoptosis. Biochim. Biophys. Acta 1770:649-658. [DOI] [PubMed] [Google Scholar]
- 15.Fabozzi, G., P. Ascenzi, S. D. Renzi, and P. Visca. 2006. Truncated hemoglobin GlbO from Mycobacterium leprae alleviates nitric oxide toxicity. Microb. Pathog. 40:211-220. [DOI] [PubMed] [Google Scholar]
- 16.Flynn, J. L., C. L. Scanga, K. E. Tanaka, and J. Chan. 1998. Effects of aminoguanidine on latent murine tuberculosis. J. Immunol. 160:1796-1803. [PubMed] [Google Scholar]
- 17.Gold, B., G. M. Rodriguez, S. A. Marras, M. Pentecost, and I. Smith. 2001. The Mycobacterium tuberculosis IdeR is a dual functional regulator that controls transcription of genes involved in iron acquisition, iron storage and survival in macrophages. Mol. Microbiol. 42:851-865. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg, M. A., S. P. Dunning, and H. F. Bunn. 1988. Regulation of the erythropoietin gene: evidence that the oxygen sensor is a heme protein. Science 242:1412-1415. [DOI] [PubMed] [Google Scholar]
- 19.Gupta, S., and A. K. Tyagi. 1993. Sequence of newly identified Mycobacterium tuberculosis gene encoding a protein with sequence homology to virulence regulating proteins. Gene 126:157-158. [DOI] [PubMed] [Google Scholar]
- 20.Hill, D. R., T. J. Belbin, M. V. Thorsteinsson, D. Bassam, S. Brass, A. Ernst, P. Boger, H. Paerl, M. E. Mulligan, and M. Potts. 1996. GlbN (cyanoglobin) is a peripheral membrane protein that is restricted to certain Nostoc spp. J. Bacteriol. 178:6587-6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho, V. T., and H. F. Bunn. 1996. Effects of transition metals on the expression of the erythropoietin gene: further evidence that the oxygen sensor is a heme protein. Biochem. Biophys. Res. Commun. 223:175-180. [DOI] [PubMed] [Google Scholar]
- 22.Hochachka, P. W., L. T. Buck, C. J. Doll, and S. C. Land. 1996. Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc. Natl. Acad. Sci. USA 93:9493-9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain, V., S. Sujatha, A. K. Ojha, and D. Chatterji. 2005. Identification and characterization of rel promoter element of M. tuberculosis. Gene 351:149-157. [DOI] [PubMed] [Google Scholar]
- 24.Kumar, A., J. C. Toledo, R. P. Patel, J. R. Lancaster, Jr., and A. J. Steyn. 2007. Mycobacterium tuberculosis DosS is a redox sensor and DosT is a hypoxia sensor. Proc. Natl. Acad. Sci. USA 104:11568-11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwast, K. E., P. V. Burke, and R. O. Poyton. 1998. Oxygen sensing and the transcriptional regulation of oxygen-responsive genes in yeast. J. Exp. Biol. 201:1177-1195. [DOI] [PubMed] [Google Scholar]
- 26.Kwast, K. E., P. V. Burke, B. T. Staahl, and R. O. Poyton. 1999. Oxygen sensing in yeast: evidence for the involvement of the respiratory chain in regulating the transcription of a subset of hypoxic genes. Proc. Natl. Acad. Sci. USA 96:5446-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lama, A., S. Pawaria, and K. L. Dikshit. 2006. Oxygen binding and NO scavenging properties of truncated hemoglobin, HbN, of Mycobacterium smegmatis. FEBS Lett. 580:4031-4041. [DOI] [PubMed] [Google Scholar]
- 28.Lee, B. Y., and M. A. Horwitz. 1995. Identification of macrophage and stress-induced proteins of Mycobacterium tuberculosis. J. Clin. Investig. 96:245-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leemans, J. C., N. P. Juffermans, S. Florquin, N. van Rooijen, M. J. Vervoordeldonk, A. Verbon, S. J. van Deventer, and T. van der Poll. 2001. Depletion of alveolar macrophages exerts protective effects in pulmonary tuberculosis in mice. J. Immunol. 166:4604-4611. [DOI] [PubMed] [Google Scholar]
- 30.Lei, J., H. Zhang, C. Wu, X. Wang, Y. Yang, X. Zhang, Y. Huang, and H. Wang. 2005. The influence of Mycobacterium tuberculosis sigma factors on the promotion efficiency of ptpAt promoter in Mycobacterium smegmatis. Curr. Microbiol. 51:141-147. [DOI] [PubMed] [Google Scholar]
- 31.Liu, C., Y. He, and Z. Chang. 2004. Truncated hemoglobin O of Mycobacterium tuberculosis: the oligomeric state change and the interaction with membrane components. Biochem. Biophys. Res. Commun. 316:1163-1172. [DOI] [PubMed] [Google Scholar]
- 32.Loebel, R. O., E. Shorr, and H. B. Richardson. 1933. The influence of adverse conditions upon the respiratory metabolism and growth of human tubercle bacilli. J. Bacteriol. 26:167-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manganelli, R., R. Proveddi, S. Rodrigue, J. Beaucher, L. Gaudreau, and I. Smith. 2004. Sigma factors and global gene regulation in Mycobacterium tuberculosis. J. Bacteriol. 186:895-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maxwell, P. H., C. W. Pugh, and P. J. Ratcliffe. 1993. Inducible operation of the erythropoietin 3′ enhancer in multiple cell lines: evidence for a widespread oxygen-sensing mechanism. Proc. Natl. Acad. Sci. USA 90:2423-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mekalanos, J. J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moens, L., J. Vanfleteren, Y. Van de Peer, K. Peeters, O. Kapp, J. Czeluzniak, M. Goodman, M. Blaxter, and S. Vinogradov. 1996. Globins in nonvertebrate species: dispersal by horizontal gene transfer and evolution of the structure-function relationships. Mol. Biol. Evol. 13:324-333. [DOI] [PubMed] [Google Scholar]
- 37.Moors, M. A., and D. A. Portnoy. 1995. Identification of bacterial genes that contribute to survival and growth in an intracellular environment. Trends Microbiol. 3:83-85. [DOI] [PubMed] [Google Scholar]
- 38.Mukai, M., P. V. Savard, H. Ouellet, M. Guertin, and S. R. Yeh. 2002. Unique ligand-protein interactions in a new truncated hemoglobin from Mycobacterium tuberculosis. Biochemistry 41:3897-3905. [DOI] [PubMed] [Google Scholar]
- 39.Nardini, M., A. Pesce, M. Milani, and M. Bolognesi. 2007. Protein fold and structure in the truncated (2/2) globin family. Gene 398:2-11. [DOI] [PubMed] [Google Scholar]
- 40.Ouellet, H., L. Juszczak, D. Dantsker, U. Samuni, Y. H. Ouellet, P. Y. Savard, J. B. Wittenberg, B. A. Wittenberg, J. B. Friedman, and M. Guertin. 2003. Reaction of Mycobacterium tuberculosis truncated HbO with ligand reveals a novel ligand-inclusive hydrogen bond network. Biochemistry 42:5764-5774. [DOI] [PubMed] [Google Scholar]
- 41.Ouellet, H., Y. Ouellet, C. Richard, M. Labarre, B. A. Wittenberg, J. B. Wittenberg, and M. Guertin. 2002. Truncated hemoglobin HbN protects Mycobacterium bovis from nitric oxide. Proc. Natl. Acad. Sci. USA 99:5902-5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pathania, R., N. K. Navani, A. M. Gardner, P. R. Gardner, and K. L. Dikshit. 2002. Nitric oxide scavenging and detoxification by the Mycobacterium tuberculosis hemoglobin, HbN in Escherichia coli. Mol. Microbiol. 45:1303-1314. [DOI] [PubMed] [Google Scholar]
- 43.Pathania, R., N. K. Navani, G. Rajmohan, and K. L. Dikshit. 2002. Mycobacterium tuberculosis hemoglobin HbO associates with membranes and stimulates cellular respiration of recombinant Escherichia coli. J. Biol. Chem. 277:5293-5302. [DOI] [PubMed] [Google Scholar]
- 44.Pawaria, S., G. Rajamohan, V. Gambhir, A. Lama, G. C. Varshney, and K. L. Dikshit. 2007. Intracellular growth and survival of Salmonella enterica serovar Typhimurium carrying truncated hemoglobins of Mycobacterium tuberculosis. Microb. Pathog. 42:119-128. [DOI] [PubMed] [Google Scholar]
- 45.Pesce, A., M. Nardini, M. Milani, and M. Bolognesi. 2007. Protein structure in the truncated (2/2) hemoglobin family. IUBMB Life 59:535-541. [DOI] [PubMed] [Google Scholar]
- 46.Riendeau, C. J., and H. Kornfeld. 2003. THP-1 cell apoptosis in response to mycobacterial infection. Infect. Immun. 71:254-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scanga, C. A., V. P. Mohan, K. Yu, H. Joseph, K. Tanaka, J. Chan, and J. L. Flynn. 2000. Depletion of CD4+ T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J. Exp. Med. 192:347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinclair, P., A. H. Gibbs, J. F. Sinclair, and F. de Matteis. 1979. Formation of cobalt protoporphyrin in the liver of rats. A mechanism for the inhibition of liver haem biosynthesis by inorganic cobalt. Biochem. J. 178:529-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sturgill-Koszycki, S., P. H. Schlesinger, P. Chakraborty, P. L. Haddix, H. L. Collins, A. K. Fok, R. D. Allen, S. L. Gluck, J. Heuser, and D. G. Russell. 1994. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263:678-681. [DOI] [PubMed] [Google Scholar]
- 50.Visca, P., G. Fabozzi, A. Petrucca, C. Ciaccio, M. Coletta, G. D. Sanctis, M. Bolognesi, M. Milani, and P. Ascenzi. 2002. The truncated hemoglobin from Mycobacterium leprae. Biochem. Biophys. Res. Commun. 294:1064-1070. [DOI] [PubMed] [Google Scholar]
- 51.Visca, P., G. Fabozzi, M. Milani, M. Bolognesi, and P. Ascenzi. 2002. Nitric oxide and Mycobacterium leprae pathogenicity. IUBMB Life 54:95-99. [DOI] [PubMed] [Google Scholar]
- 52.Wang, G. L., and G. L. Semenza. 1993. General involvement of hypoxia-inducible factor-1 in transcriptional response to hypoxia. Proc. Natl. Acad. Sci. USA 90:4304-4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, G. L., B. H. Jiang, E. A. Rue, and G. L. Semenza. 1995. Hypoxia-Inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 92:5510-5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wards, B. J., and D. M. Collins. 1996. Electroporation at elevated temperatures substantially improves transformation efficiency of slow-growing mycobacteria. FEMS Microbiol. Lett. 145:101-105. [DOI] [PubMed] [Google Scholar]
- 55.Wittenberg, J. B., M. Bolognesi, B. A. Wittenberg, and M. Guertin. 2002. Truncated hemoglobins: a new family of hemoglobins widely distributed in bacteria, unicellular eukaryotes, and plants. J. Biol. Chem. 277:871-874. [DOI] [PubMed] [Google Scholar]
- 56.Wren, B. W., S. M. Colby, R. R. Cubberley, and M. J. Pallen. 1992. Degenerate PCR primers for the amplification of fragments from genes encoding response regulators from a range of pathogenic bacteria. FEMS Microbiol. Lett. 99:287-292. [DOI] [PubMed] [Google Scholar]