Abstract
Cellulose is the major component of plant biomass, and microbial cellulose utilization is a key step in the decomposition of plant detritus. Despite this, little is known about the diversity of cellulolytic microbial communities in soil. Fungi are well known for their cellulolytic activity and mediate key functions during the decomposition of plant detritus in terrestrial ecosystems. We developed new oligonucleotide primers for fungal exocellulase genes (cellobiohydrolase, cbhI) and used these to isolate distinct cbhI homologues from four species of litter-decomposing basidiomycete fungi (Clitocybe nuda, Clitocybe gibba, Clitopilus prunulus, and Chlorophyllum molybdites) and two species of ascomycete fungi (Xylaria polymorpha and Sarcoscypha occidentalis). Evidence for cbhI gene families was found in three of the four basidiomycete species. Additionally, we isolated and cloned cbhI genes from the forest floor and mineral soil of two upland forests in northern lower Michigan, one dominated by oak (Quercus velutina, Q. alba) and the other dominated by sugar maple (Acer saccharum) and American basswood (Tilia americana). Phylogenetic analysis demonstrated that cellobiohydrolase genes recovered from the floor of both forests tended to cluster with Xylaria or in one of two unidentified groups, whereas cellobiohydrolase genes recovered from soil tended to cluster with Trichoderma, Alternaria, Eurotiales, and basidiomycete sequences. The ability to amplify a key fungal gene involved in plant litter decomposition has the potential to unlock the identity and dynamics of the cellulolytic fungal community in situ.
The microbial decomposition of plant litter is a key ecosystem process that serves to release inorganic nutrients from plant detritus, as well as transform this material into soil organic matter (26, 37). Cellulose and lignin compose 60 to 75% of fresh plant litter (34, 37), and their degradation by heterotrophic soil microorganisms largely controls the rate of litter decay. Cellulose is a linear glucose polymer in which the subunits are linked through 1,4-β-glycosidic bonds, and it represents an important potential source of energy for saprotrophic microorganisms. Cellulose in plant cell walls is bundled into crystalline fibrils, which are linked by hemicellulose to form larger, lignin-encrusted microfibrils (21, 34). Lignin, which is composed of polymerized phenol propane monomers (10), is highly resistant to microbial attack and hence restricts microbial access to the cellulose that it protects (8). Because a wide diversity of fungi have cellulolytic capability and because the complete breakdown of lignin can only be achieved by basidiomycete fungi and some xylariaceous ascomycete fungi (5, 8), saprotrophic fungi are considered the primary agents of plant litter decomposition in terrestrial ecosystems (41).
Although fungi are key mediators of plant litter decay in terrestrial ecosystems, we have an incomplete knowledge of how the composition and function of fungal communities change during the process of litter decay. For example, litter is thought to be initially colonized by “sugar fungi” (sensu Garrett, 1963) which have neither cellulolytic or ligninolytic ability. These species are replaced over time by cellulolytic fungi, and finally the litter is colonized by ligninolytic fungi (12); however, the general validity of this scheme has been questioned (11, 20, 29, 43). Moreover, testing this hypothesis has been difficult, because determining the function of fungal species typically requires isolating organisms and examining their effects on defined substrates in pure culture (7, 15, 30-32), which has well-documented limitations (2). The recent development of degenerate primer sets for both basidiomycete (22) and ascomycete laccase genes (25) provides cultivation-independent tools for assessing the genetic diversity and activity of a “lignin-degrading guild” within fungal communities. To our knowledge, a complementary tool to examine the “cellulolytic guild” is still lacking, despite an increasing knowledge of cellulase gene structure (4, 6, 14, 33).
Because cellulose is a large, insoluble molecule with both microcrystalline and amorphous regions, it must be broken down into smaller oligosaccharides before microbial uptake, and this is achieved through the action of extracellular cellulase enzymes (24). There are two types of cellulase, endoglucanases (EC 3.2.1.4), which randomly cleave cellulose molecules, and cellobiohydrolases (EC 3.2.1.91 and EC 3.2.1.-), which remove cellobiose or glucose from the reducing or nonreducing ends of the cellulose polymer (24). These enzymes act in a coordinated and synergistic manner to hydrolyze cellulose into small oligosaccharides and ultimately glucose (24). Cellobiohydrolases are classified into three glycoside hydrolase families (GH6, GH7, and GH48) according to amino acid sequence similarity (16). Of these families, only GH7 is thought to be exclusively fungal, and this family contains CBHI cellobiohydrolases and EG1 endoglucanases from both ascomycete and basidiomycete fungi. Our goal was to develop a general primer set that would permit the recovery of GH7 cellobiohydrolase (cbhI) genes and to use this tool to examine the diversity and distribution of these genes in the forest floor and surface soil of two contrasting forest ecosystems.
MATERIALS AND METHODS
Development of primers.
Forty amino acid sequences (Table 1) representing cellobiohydrolase proteins from family GH7 were obtained from GenBank. These sequences were aligned by using the ClustalX module within Geneious Pro 3.0.5 (Biomatters Ltd.) and examined for conserved regions that might serve as primer sites. Two regions that define a 166- to 173-amino-acid-long fragment of the catalytic domain were selected (Fig. 1), and after examining the corresponding DNA sequences for evidence of codon bias, we designed degenerate primers fungcbhIF (5′-ACC AA[C,T] TGC TA[C,T] ACI [A,G]G[C,T] AA-3′; melting temperature, 53.7°C) and fungcbhIR (GC[C,T] TCC CAI AT[A,G] TCC ATC-3′; melting temperature, 52.0°C). The primers were synthesized by Integrated DNA Technologies (Coralville, IA).
TABLE 1.
Cellobiohydrolase protein sequences used to develop fungcbhI primer set
| Phylum and species | Order | Family | Gene product(s) | GenBank accession no. |
|---|---|---|---|---|
| Basidiomycota | ||||
| Agaricus bisporus | Agaricales | Agaricaceae | 1,4-β-Cellobiohydrolase | CAA90422 |
| Lentinula edodes | Agaricales | Tricholomataceae | Cel7A | AAK95563 |
| Schizophyllum commune | Agaricales | Schizophyllaceae | ScCel1P | AAX55505 |
| Volvariella volvacea | Agaricales | Pluteaceae | CBHI-I, CBHI-II | AAT64006, AAT64007 |
| Athelia rolfsii | Atheliales | Atheliaceae | Cellobiohydrolase | BAC81967 |
| Phanerochaete chrysosporium | Corticiales | Corticiaceae | CBHI, CBHI-II | AAB46373 |
| Polyporus arcularius | Polyporales | Polyporaceae | CBHI | BAF80326 |
| Irpex lacteus | Russulales | Steccherinaceae | Cel2, Cel3, Cex1 | BAA76364, BAA76365, BAD16575 |
| Ascomycota | ||||
| Aspergillus aculeatus | Eurotiales | Tricochomaceae | CBHI | BAA25183 |
| Aspergillus fumigatus | Eurotiales | Tricochomaceae | CelD, 1,4-β-cellobiohydrolase | XP750600, XP751044 |
| Aspergillus nidulans | Eurotiales | Tricochomaceae | 1,4-β-Cellobiohydrolase | AAM54069, AAM54070 |
| Aspergillus niger | Eurotiales | Tricochomaceae | 1,4-β-Cellobiohydrolase | AAF04491, AAF04492 |
| Aspergillus oryzae | Eurotiales | Tricochomaceae | Cellobiohydrolases C and D | BAC07255, BAC07256 |
| Penicillium chrysogenum | Eurotiales | Tricochomaceae | CBHI | AAV65115 |
| Penicillium funiculosum | Eurotiales | Tricochomaceae | Xylanase/cellobiohydrolase | CAC85737 |
| Penicillium janthinellum | Eurotiales | Tricochomaceae | CBHI | CAA41780 |
| Talaromyces emersonii | Eurotiales | Tricochomaceae | CBHI | AAL33603 |
| Thermoascus aurantiacus | Eurotiales | Tricochomaceae | Cellobiohydrolase | AAW27920 |
| Claviceps purpurea | Hypocreales | Clavicipitaceae | 1,4-β-Cellobiohydrolase | CAA68840 |
| Fusarium poae | Hypocreales | Mitosporic | Exoglucanase C | AAX60003 |
| Fusarium venenatum | Hypocreales | Mitosporic | Exoglucanase C | AAX60001 |
| Gibberella zeae | Hypocreales | Nectriaceae | Exoglucanase C | AAX60002 |
| Trichoderma harzianum | Hypocreales | Hypocreaceae | CBHI | AAF36391 |
| Trichoderma viride | Hypocreales | Hypocreaceae | 1,4-β-Cellobiohydrolase, CBHI | CAA37878, BAA36215 |
| Humicola grisea | Mitosporic Ascomycota | Cellulase, 1,4-β-Cellobiosidase, β-glucan cellobiohydrolase | BAA09785, AAD31545, CAA35159 | |
| Alternaria alternata | Pleosporales | Pleosporalaceae | Exoglucanase | AAF05699 |
| Chaetomium thermophilum | Sordariales | Chaetomiaceae | Cbh3, cellobiohydrolase | AAY89412, AAW64926 |
| Neurospora crassa | Sordariales | Sordariaceae | Exocellobiohydrolase | CAA54815 |
FIG. 1.
Consensus sequence of 40 fungal (CBHI) partial cellobiohydrolase amino acid sequences. The mean pairwise similarity of the alignment was 60.3%, and 83 sites were identical in all sequences. The amino acids shown are those conserved in at least 75% of the sequences. The amino acid motifs in bold type within boxes were used to design degenerate primers fungcbhIF and fungcbhIR. The mean pairwise similarity of the region defined by these two conserved motifs is 67.9%.
Fungal and soil collection.
Sporocarps of the basidiomycetes Clitocybe c.f. nuda (WASH07/03), Clitocybe c.f. gibba (Clitocybe gibba MICH, WASH07/02), Clitopilus prunulus (WASH07/01), and Chlorophylum molybdites (WASH06/10) and of the ascomycetes Xylaria polymorpha (WEX07/01) and Sarcoscypha occidentalis (WASH07/04) were collected from forests in Washtenaw and Wexford Counties, MI, during the summers and falls of 2006 and 2007. A specimen of Clitocybe gibba collected near Anchorage, AL, in 1986 (Clitocybe gibba ALASKA, voucher 5155) was obtained from the University of Michigan Herbarium. These species were chosen because they are common and widely distributed in this region and because they are known saprotrophs of wood and leaf litter; we expected them to possess cellulase genes.
In addition, forest floor and surface mineral soil were collected from a white oak-black oak (Quercus alba-Q. velutina) forest stand (MST58A; 44°18.60′N, 85°53.83′W) and a sugar maple-basswood (Acer saccharum-Tilia americana) forest stand (MST24; 44°13.40′N, 85°45.31′W) in Wexford County, MI. The soil in the oak forest is an Entic Haplorthod with a pH of 3.9 and 4.4% soil organic C, whereas the maple soil is a Typic Haplorthod with a pH of 5.5 and a carbon content of 5.5% (45). The forest floor of the oak forest has a lignin content of 34% and a cellulose content of 44%. In the maple forest, the forest floor has lower lignin (26%) and slightly higher cellulose (50%) (1). In each stand, six forest floor and six soil samples were collected at approximately 5-m intervals along each of two parallel transects, providing 12 samples of each horizon. Forest floor was collected as a grab sample, and soil was collected to a depth of 5 cm with a 2.5-cm-diameter corer. Forest floor and soil samples were separately combined to create one composite forest floor and one composite soil sample for each stand; these samples were stored on ice prior to DNA extraction.
DNA extraction.
DNA was extracted from sporocarp tissue by a modified cetyltrimethylammonium bromide method after the tissue was ground in Carlson lysis buffer (3). Soil samples were homogenized by hand and passed through a 2-mm sieve to remove roots and coarse material. Ten grams of sieved, homogenized soil was shaken with 100 ml of 0.1 M sodium pyrophosphate for 1 h, and the resulting slurry was then washed through sieves of 0.25-mm and 0.053-mm mesh with ∼4 liters of deionized water in order to remove spores and maximize the hyphal yield (23). DNA was extracted from the 0.053- to 0.25-mm fraction with PowerMax Soil DNA Isolation kits (MO BIO Laboratories, Inc., Carlsbad, CA). Forest floor samples were chopped (Hamilton Beach R 10-speed blender) to facilitate homogenization, and DNA was extracted from 2.5 g of this material as described above; DNA was stored at −80°C until use.
PCR amplification and cloning.
Approximately 20 ng of sporocarp DNA, or 50 ng of DNA extracted from the two soil horizons, was used as the starting template for PCR amplification. In addition to the template, the reaction cocktail contained 1.5 mM MgCl2, PCR buffer, each deoxynucleoside triphosphate at 0.2 μM, each primer at 0.5 μM, 50 μg of bovine serum albumin, and 1 U of Expand High Fidelity Taq polymerase (Roche); negative controls with no DNA template were included in each batch. PCR was performed by the following protocol: initial denaturation at 94°C for 3 min, followed by 30 (sporocarps) or 35 (soil and forest floor) cycles of 94°C for 30 s, 48°C for 45 s, and 72°C for 90 s, with a final extension at 72°C for 15 min. Success of PCR was assessed by ethidium bromide visualization after agarose gel electrophoresis. We considered any lane showing a band or bands of at least 500 bp to contain a potential cbhI gene fragment.
PCR products were cleaned (PCR Cleanup; MO BIO Laboratories, Inc.) and cloned into pCR2.1-TOPO with the TOPO TA Cloning kit (Invitrogen). For the sporocarp libraries, 12 clones per species were picked and grown overnight in Luria broth before plasmid extraction (Wizard Plus Miniprep kit; Promega, Madison, WI) and sequencing at the University of Michigan Core Sequencing Facility with primers M13F and M13R. For the forest floor and soil samples, 96 clones per ecosystem (48 soil and 48 litter) were grown overnight in Luria broth supplemented with 10% glycerol and sent to the University of Georgia for bidirectional sequencing with primers M13F and M13R.
Sequence analysis.
Sequences were edited with BioEdit, and contigs were constructed in Geneious 3.0.5. For the sequences derived from each sporocarp, a multiple-sequence alignment was used to determine sequence similarity, and for sequences with similarities of >98%, a consensus sequence was derived. The coding region of each DNA sequence was deduced after alignment against known cbhI mRNA sequences (Agaricus bisporus [Z50094], Athelia rolfsii [AB103461], Lentinula edodes [AF411250], Volvariella volvacea [AY559102, AY559103], Aspergillus aculeatus [AB002821], Aspergillus fumigatus [XM745951], and Thermoascus aurantiacus [AY840982]), and partial protein sequences were predicted by translation after removal of introns. The similarity of the putative CBHI protein fragments to known CBHI proteins was assessed with BLAST P. Deduced protein sequences were aligned along with the protein sequences of the reference species (Table 1), as well as the best BLAST P matches, with the ClustalX module in Geneious Pro 3.0.5. The alignment was checked by eye, exported to PAUP*4.0 (38), and analyzed through maximum parsimony. For the maximum-parsimony analysis, all 178 characters in the alignment were included at equal weight, gaps were scored as missing data, and a heuristic search was performed with starting trees obtained by random stepwise addition and TBR branch swapping. Bootstrapping was performed with 100 replications. Trees were rooted with an outgroup composed of the GH7 endoglucanase sequences of Aspergillus oryzae BAE66197 and Aspergillus nidulans AM54071. The diversity of cellulolytic protein in each sample was estimated with p-distance (27). The p-distance values and bootstrapped standard errors were calculated with MEGA 4 (39).
Nucleotide sequence accession numbers.
Newly determined sequences were deposited in GenBank under accession numbers EU345437 to EU345472 and EU359569 to EU359616.
RESULTS
PCR amplification.
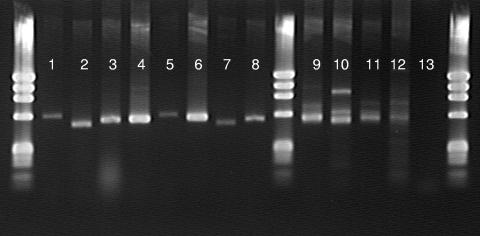
PCR with the fungcbhIF-fungcbhIR primer set successfully recovered single bands (520 to 620 bp) from the basidiomycete and ascomycete sporocarp DNA (Fig. 2). The four environmental samples produced heterogeneous product mixtures with bands ranging from 520 to 950 bp (Fig. 2).
FIG. 2.
fungcbhIF-fungcbhIR PCR amplicons recovered from sporocarp and environmental genomic DNA. The markers in the extreme left and right lanes are ΦX174 (Promega). Lanes: 1 and 5, Sarcoscypha occidentalis; 2, Xylaria polymorpha; 3, Clitopilus prunulus; 4, Clitocybe gibba (ALASKA); 6, Clitocybe gibba (MICH); 7, Clitocybe nuda; 8, Chlorophyllum molybdites; 9, oak soil horizon; 10, oak forest floor; 11, sugar maple soil horizon; 12, sugar maple forest floor; 13, no DNA.
Sporocarp clone libraries.
Sequences of 570, 571, and 580 bp were recovered from the Clitocybe nuda library. These sequences had a mean pairwise similarity of 71.9% and were therefore considered to represent three distinct genes, which we termed Clitocybe nuda cbhI570, cbhI571, and cbhI580. The presence of an intron with an identical insertion point 418 bases downstream of the 5′ primer was deduced in all three sequences, although the introns in each sequence differed in 5′ splice site, as well as in length and nucleotide composition (Table 2). Translation recovered three putative amino acid sequences of 170 residues similar to exocellobiohydrolases of Agaricus bisporus and V. volvacea (Table 2).
TABLE 2.
Cellobiohydrolase (cbhI) gene fragments recovered from sporocarp DNAs of six fungal species and best BLAST P matches of putative CBHI protein fragments recovered after removal of introns and translationa
| Species (putative gene) | Length of amplicon (bp) | Intron
|
BLAST P match (% identity) | |||
|---|---|---|---|---|---|---|
| Insertion position(s) | Length(s) (bp) | Splice site(s)
|
||||
| 5′ | 3′ | |||||
| Clitocybe nuda cbhI570 | 570 | 418 | 58 | GTAAGC | CAG | Agaricus bisporus cellobiohydrolase, CAA90422 (82.4) |
| Clitocybe nuda cbhI580 | 580 | 418 | 68 | GTGAGT | TAG | Volvariella volvacea cbhI-II, AAT64007 (81.2) |
| Clitocybe nuda cbhI571 | 571 | 418 | 59 | GTAAGT | CAG | Volvariella volvacea cbhI-II, AAT64007 (76.5) |
| Clitocybe gibba cbhI562 | 562 | 418 | 49 | GTGAGA | CAG | Volvariella volvacea cbhI-II, AAT64007 (81.2) |
| Clitocybe gibba cbhI564 | 564 | 418 | 51 | GTCAGC | TAG | Volvariella volvacea cbhI-II, AAT64007 (81.2) |
| Clitocybe gibba cbhI566 | 566 | 418 | 53 | GTCAGT | TAG | Volvariella volvacea cbhI-I, AAT64006 (82) |
| Clitocybe c.f. gibba cbhI562 | 562 | 418 | 49 | GTGAGA | CAG | Volvariella volvacea cbhI-II, AAT64007 (81.2) |
| Clitocybe c.f. gibba cbhI564 | 564 | 418 | 51 | GTCAGC | TAG | Volvariella volvacea cbhI-II, AAT64007 (82.3) |
| Clitocybe c.f. gibba cbhI566 | 566 | 418 | 53 | GTCAGT | TAG | Volvariella volvacea cbhI-I, AAT64006 (81.2) |
| Clitopilus prunulus cbhI | 564 | 418 | 51 | GTAAGC | CAG | Volvariella volvacea cbhI-II, AAT64007 (76.5) |
| Chlorophylum molybdites cbhI568 | 568 | 418 | 55 | GTGAGT | TAG | Agaricus bisporus exocellobiohydrolase, CAA90422 (82.9) |
| Chlorophylum molybdites cbhI567 | 567 | 418 | 55 | GTAAGC | TAG | Volvariella volvacea cbhI-II, AAT64007 (80.0) |
| Sarcoscypha occidentalis cbhI | 615 | 146, 442 | 42, 46 | GTATGA, GTTCGA | AAG, CAG | Pleurotus ostreatus sp. “Florida” 1,4-β-cellobiosidase (76.7) |
| Xylaria polymorpha cbhI | 518 | Sclerotinia sclerotiorum hypothetical GH7 protein (75.6) | ||||
Intron positions were inferred by alignment against known cbhI mRNA sequences. Gene fragments were amplified by PCR with the fungcbh primer set.
Nucleotide sequences 562, 564, and 566 bp in length were recovered in both the Clitocybe gibba (ALASKA) and Clitocybe gibba (MICH) libraries and were subsequently termed C. gibba cbhI562, cbhI564, and cbhI566. The cbhI562, -564, and -566 sequences had a mean pairwise similarity of 80.6% in the C. gibba (ALASKA) library and 81.3% similarity in the C. gibba MI library. Comparing across the two C. gibba libraries, the cbhI562 sequences were 97.1% similar, the cbhI564 sequences were 97.9% similar, and the cbhI566 sequences were nearly identical (99.1% similar). All sequences contained an intron which, as for C. nuda, was inserted downstream at 418 bp (Table 2). Clitocybe gibba intron length, composition, and splice sites also differed between sequence size classes (Table 2). Translation recovered putative amino acid sequences 170 residues in length that were similar to V. volvacea cellobiohydrolases CBHI-I and CBHI-II (Table 2).
Two sequences 568 and 569 bp in length and 84.1% identical were recovered from the Chlorophyllum molybdites library and termed cbhI568 and cbhI569 (Table 2). Both sequence types had 55-bp introns that differed in splice site (Table 2) and in sequence. Translation recovered 170-amino-acid-long sequences with similarity to Agaricus bisporus exocellobiohydrolase and V. volvacea CBHI-II (Table 2).
For the remaining basidiomycete, Clitopilus prunulus, a 564-bp sequence was recovered (Table 1). The presence of an intron was inferred, and translation recovered a 170-residue protein fragment with similarity to V. volvacea CBHI-II (Table 2).
A 615-bp sequence was recovered in the library of the ascomycete Sarcoscypha occidentalis, and the presence of two short introns was inferred (Table 2). Removal of these introns and subsequent translation produced a 173-amino-acid protein fragment with similarity to the 1,4-β-cellobiosidase of the basidiomycete Pleurotus ostreatus sp. “Florida” (Table 2).
Finally, for the ascomycete Xylaria polymorpha, a 519-bp sequence was recovered. There was no evidence of an intron, and translation recovered a 172-residue putative protein sequence with similarity to the GH7 protein of Sclerotinia sclerotiorum (Table 2).
Environmental clone library.
A total of 160 clones containing inserts of 521 to 692 bp obtained from the four sublibraries (black oak-white oak litter, black oak-white oak soil, sugar maple-basswood litter, and sugar maple-basswood soil) were sequenced, and 50 unique DNA sequences were recovered. Alignments against mRNA revealed that the majority of the sequences contained a single intron; however, introns were absent in seven sequences and five contained two introns. Translation of the 50 sequences uncovered 45 putative amino acid sequences (21 from soil, 23 from litter, and 1 from both soil and litter) ranging in length from 165 to 174 residues, and all 45 of these putative amino acid sequences gave BLAST P matches (65.5 to 90.6% identity) to known GH7 cellobiohydrolase sequences (Table 3). Pairwise similarity of the deduced protein fragments ranged from 54.2 to 99.4%. The p-distance values were 0.257 ± 0.023, 0.294 ± 0.022, 0.322 ± 0.023, and 0.323 ± 0.023 for the oak litter, maple litter, oak soil, and maple soil, respectively.
TABLE 3.
Cellobiohydrolase genotypes recovered with fungcbh primer set from soil and litter samples of two northern hardwood forests in Michigan
| Library and clone | Size (bp) | Intron 5′ splice site(s) (size [bp]) | Protein size (amino acids) | BLAST P (% identity)a |
|---|---|---|---|---|
| Black oak-white oak | ||||
| M02 | 512 | None | 170 | Penicillium janthinellum CBHI, CAA41780 (90) |
| N08 | 515 | None | 171 | Nectria hematococca ExoC, AAS82856 (77) |
| P12 | 521 | None | 173 | Botryotinia fuckeliana, EDN25878 (83) |
| O03 | 521 | None | 173 | Botryotinia fuckeliana, EDN25878 (83) |
| M08 | 556 | 409 (57) | 166 | Thermoascus aurantiacus 1,4-β-cellobiosidase, AAW27920 (73) |
| P03 | 557 | 420 (48) | 169 | Sclerotinia sclerotiorum, EDN99480 (73) |
| O11 | 560 | 409 (63) | 165 | Phanerochaete chrysosporium Cel7D, 1GPI_A (75) |
| P10 | 560 | 409 (64) | 165 | Phanerochaete chrysosporium Cel7D, 1GPI_A (74) |
| P02 | 562 | 409 (69) | 164 | Irpex lacteus cellulase, BAA76364 (76) |
| M04 | 563 | 261 (60) | 167 | Trichoderma reesei Cel7A, 1Q2E (85) |
| N12 | 565 | 424 (51) | 171 | Sclerotinia sclerotiorum, EDN99480 (73) |
| M11 | 569 | 424 (51) | 172 | Alternaria alternata exoglucanase, AAF05699 (85) |
| A01 | 571 | 418 (58) | 171 | Volvariella volvacea CBHI-I, AAT64006 (83) |
| P01 | 576 | 423 (59) | 172 | Botryotinia fuckeliana, EDN25878 (83) |
| M06 | 623 | 421 (108) | 171 | Sclerotinia sclerotiorum, EDN99480 (79) |
| O05 | 637 | 364 (122) | 171 | Sclerotinia sclerotiorum, EDN99480 (82) |
| N06 | 645 | 98 (63), 523 (61) | 173 | Lentinula edodes cellulase Cel7A, AAK95563 (78) |
| N01 | 690 | 421 (76), 571 (103) | 170 | Acremonium thermophilum 1,4-β-cellobiosidase, CAM98446 (76) |
| Sugar maple-basswood | ||||
| G12 | 519 | None | 173 | Sclerotinia sclerotiorum, EDN99480 (75) |
| F08 | 522 | None | 174 | Penicillium chrysogenum, AAV65115 (67) |
| F12 | 522 | None | 174 | Penicillium chrysogenum, AAV65115 (65) |
| K06 | 545 | 404 (52) | 164 | Phanerochaete chrysosporium CBHI, AAB46373 (75) |
| G09 | 561 | 409 (58) | 167 | Irpex lacteus cellulase, BAA76363 (79) |
| G08 | 562 | 409 (58) | 167 | Irpex lacteus cellulase, BAA76363 (80) |
| G03 | 563 | 368 (50) | 170 | Phaeosphaeria nodorum SN15, EAT83824 (82) |
| I02 | 563 | 261 (60) | 167 | Trichoderma reesei Cel7A, 1Q2E (85) |
| I11 | 563 | 418 (51) | 170 | Fusarium venenatum exoglucanase C, AAX60001 (80) |
| L05 | 564 | 418 (52) | 170 | Thermoascus aurantiacus cellobiosidase, CAM98447 (73) |
| L06 | 564 | 475 (50) | 172 | Alternaria alternata exoglucanase, AAF05699 (78) |
| J08 | 568 | 403 (71) | 165 | Botryotinia fuckeliana, EDN25878 (83) |
| H05 | 569 | 421 (53) | 171 | Aspergillus clavatus cellobiohydrolase D, EAW11196 (76) |
| E04 | 569 | 424 (50) | 173 | Alternaria alternata exoglucanase, AAF05699 (86) |
| E10 | 569 | 418 (56) | 171 | Volvariella volvacea CBHI-II, AAT64007 (80) |
| F04 | 569 | 418 (56) | 171 | Agaricus bisporus 1,4-β-cellobiohydrolase, CAA90422 (81) |
| F03 | 569 | 418 (56) | 171 | Agaricus bisporus 1,4-β-cellobiohydrolase, CAA90422 (81) |
| L09 | 572 | 424 (54) | 172 | Botryotinia fuckeliana, EDN25878 (78) |
| L03 | 572 | 424 (55) | 172 | Botryotinia fuckeliana, EDN25878 (80) |
| K11 | 572 | 424 (54) | 172 | Sclerotinia sclerotiorum, EDN99480 (77) |
| L02 | 574 | 424 (56) | 172 | Sclerotinia sclerotiorum, EDN99480 (78) |
| K09 | 591 | 427 (70) | 173 | Sclerotinia sclerotiorum, EDN99480 (82) |
| H04 | 616 | 261 (53), 462 (59) | 167 | Neosartorya fischeri cellobiohydrolase D, EAW15926 (75) |
| I04 | 616 | 25 (61), 462 (52) | 167 | Talaromyces emersonii CBHI, AAL33603 (74) |
| K05 | 627 | 421 (112) | 171 | Sclerotinia sclerotiorum, EDN99480 (77) |
| I06 | 640 | 98 (57), 481(62) | 173 | Polyporus arcularius CBHI, BAF80326 (87) |
Identity calculated over the full length of the translated protein fragment.
Phylogenetic analysis.
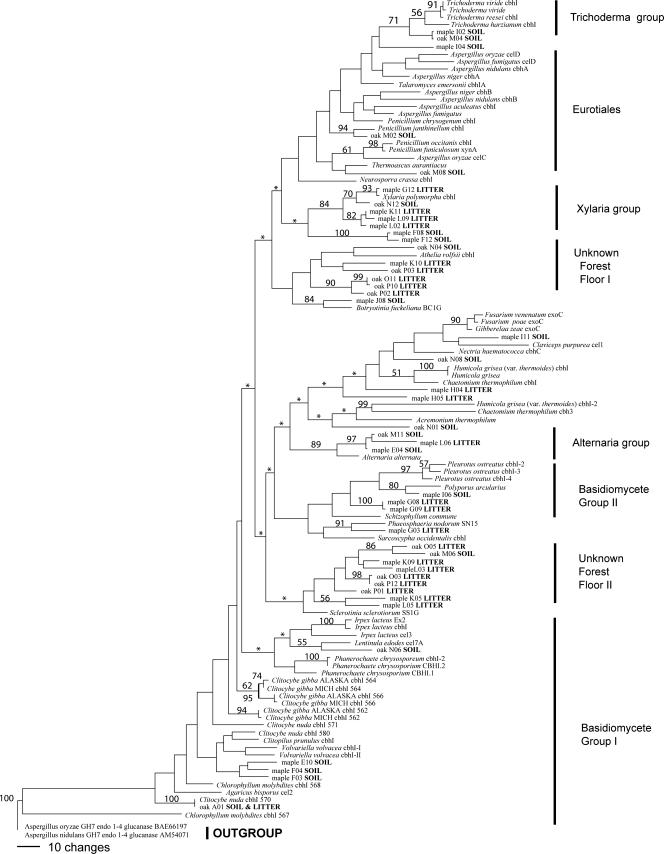
Of the 178 characters in the protein sequence alignment, 31 were constant, 14 were variable but not parsimony informative, and 133 were parsimony informative. A total of 411 most parsimonious trees of 2,161 steps were found (Fig. 3), with the next most parsimonious trees having 2,166 steps. Generally, bootstrap support for many of the terminal nodes was good, but most internal nodes were either poorly supported or collapsed to polytomy under strict consensus (Fig. 3).
FIG. 3.
Phylogenetic relationships among the reference cbhI-encoded protein sequences, environmental clones, and best BLAST P matches. The data set included 178 characters. Shown is 1 of 411 most-parsimonious trees. Bootstrap frequencies of >50% are shown at supported branches. Asterisks indicate branches that collapse in strict consensus. The tree is rooted with an outgroup of GH7 endocellulases.
DISCUSSION
Family-specific degenerate primers for fungal cellulases have been proposed before (35); however, these earlier primers were developed with less sequence information, amplified small regions of the catalytic domain, were highly degenerate, or failed to discriminate between cellobiohydrolase and endoglucanase genes (18, 35). In contrast to these, the degenerate primers developed here effectively recovered cbhI genes from fungal tissue and environmental samples and therefore provide a new tool to examine the diversity and distribution of novel genes in the environment.
Multiple forms of cbhI genes were recovered from nearly all of the fungal species that we examined. The same is true of fungal laccase genes or basidiomycete actin genes recovered from individual species, and this observation has been attributed to a combination of allelic variation and the presence in the fungal genome of distinct gene families derived from different ancestral genes (13, 40). In three of the four basidiomycete species that we examined, we found multiple forms of the cbhI gene that differ in length and DNA sequence, as well as intron size and sequence (Table 1), suggesting that they are distinct cellobiohydrolase genes. These distinct forms encode different CBHI proteins (Fig. 3), further suggesting that they may represent distinct gene families. This result extends previous studies which have documented the presence of cbhI gene families in several fungal species (6, 19, 28), as well as from recently sequenced fungal genomes which have revealed two distinct GH7 genes in Aspergillus terreus, A. fumigatus, and A. flavus, three in Sclerotinia sclerotiorum, six in Coprinopsis cinerea, and eight in Puccinia graminis. Ecologically, having multiple different forms of the cbhI gene may be advantageous, if the proteins encoded by these genes have different environmental optima (42). For example, in the basidiomycete Phanerochaete chrysosporium, which has six forms of the cbhI gene, different patterns of gene expression are observed, depending on whether the fungus is metabolizing powdered cellulose or wood chips (36, 42). In all of the fungal species that we examined, many of the cbhI genes were represented by sequences exhibiting minor coding variation; for example, the Clitocybe nuda cbhI570 gene was represented by three sequence types with a mean pairwise similarity of 98.6%. Although we used a proofreading polymerase, we cannot entirely exclude the possibility of sequencing error. Nevertheless, most of the observed sequence variation was concentrated in third codon positions and putative protein sequences rarely varied by more than a single amino acid. In combination, these observations suggest that allelic forms of the cbhI genes may exist in these species. Further studies with single spore isolates are required to more conclusively determine the allelic status of these cbhI genes.
Cellobiohydrolase genes were recovered from the soil and forest floor of both ecosystems that we sampled. The majority of these environmental cbhI genotypes could not be linked to a known species, which was not unexpected given that fewer than 100 known or presumed cbhI genes reside in public databases and the mean BLAST P score obtained was 79.8% (Table 3). By means of comparison, the similarity of the cbhI genes of Neurospora crassa (Ascomycota, Sordariales) and Phanerochaete chrysosporium (Basidiomycota, Corticiales) across the region defined by the fungcbhI primer set is 73.5%. The best matches we obtained were actually to cbhI genes isolated as part of this study from Clitocybe nuda (100% match to oak clone A01) and Xylaria polymorpha (98.8% to maple clone G12), showing that increased sampling from culture and herbarium collections is required to improve cbhI identification in the future.
Phylogenetic analysis (Fig. 3) provided some insight into the taxonomic affinity of the environmental clones; however, the small number of known sequences relative to the taxonomic breadth of cellulolytic fungi made identification of deep relationships difficult or even impossible. Within the two ecosystems that we studied, more than 70% of the CBHI protein fragments recovered from the forest floor libraries clustered in just three groups (Fig. 3). One group, with good bootstrap support, clustered with Xylaria polymorpha (Xylaria group, Fig. 3). The other two groups, Unknown Forest Floor I and II (Fig. 3), are poorly supported and show the highest similarities (65 to 75% similarity at best) to the CBHI proteins of the basidiomycete Athelia rolfsii and the ascomycete Sclerotinia sclerotiorum. Given the example above of Neurospora crassa and Phanerochaete chrysosporium, the true taxonomic affiliations of these environmental sequences remain unknown. Of the remaining forest floor protein fragments, only one (oak A01, Fig. 3) can be unequivocally identified as a basidiomycete, and only one clustered in a well-supported clade (Alternaria group, Fig. 3). Interestingly, despite the differences in litter biochemistry between oak and maple forests, the cellulolytic communities seem to show a high degree of overlap. Soil-derived CBHI protein fragments from both the maple and oak ecosystems were also placed in the Alternaria group, and this soil horizon of both ecosystems was also represented in a well-supported Trichoderma clade (Trichoderma group, Fig. 3). CBHI protein fragments potentially derived from other microfungal ascomycete species within the Eurotiales, Helotiales, and Sordariales, as well as from Basidiomycetes, were also recovered from the soils of both ecosystems (Fig. 3).
We used p-distance values as a measurement of cellobiohydrolase protein diversity within our ecosystems, and on the basis of these p-distance values, the soil horizons appear to harbor a greater diversity of cellulolytic proteins than the forest floor. Because it is possible that cbhI genes from groups not represented during primer design are not being recovered, comparisons of diversity must be considered speculative. Our primers were designed with 45 of the 62 full-length or nearly full-length cellobiohydrolase sequences curated at the CAZy database (http://www.cazy.org/fam/GH7.html), including all of the sequences for which cellobiohydrolase activity has been confirmed. However, as revealed by inspection of Table 1, this selection is biased toward Ascomycetes, especially from the Sordariomycetes, Eurotiomycetes, and Dothidiomycetes, as well as toward Agaricales within the Basidiomycetes. Nevertheless, our results demonstrate that the primers can amplify cbhI genes from species, such as Sarcoscypha occidentalis (Pezizomycetes), not represented during primer design.
Fungi are key agents of plant litter decay, and we have successfully developed a culture-independent method for studying the community of cellulolytic fungi in soil. We selected the gene for cellobiohydrolase as the target gene because it encodes a key enzyme mediating cellulose decomposition; it also likely plays a critical role in the initial phases of litter colonization and exploitation, when crystalline cellulose is present in the plant cell wall (9). Moreover, by focusing on a single cellulase type, our primers also offer the potential to examine relationships between gene expression and enzyme activity. In addition to cbhI genes, many fungi also possess cbhII genes which encode the cellobiohydrolase enzymes of family GH6. However, coordinated expression of both cbhI and cbhII genes has been observed in the basidiomycete Polyporus arcularius (28), and similar results have been obtained for Trichoderma reesei (17), V. volvacea (19), and Agaricus bisporus (4, 44); thus, focusing solely on the cbhI gene should provide a reasonable indication of cellobiohydrolase potential. The application of the fungcbhI primers in gene expression studies has the potential to provide an indication of fungal cellobiohydrolase activity, as well as to allow the factors that affect cellobiohydrolase activity to be examined in complex communities.
Acknowledgments
This work was supported by grants (DE-FG02-93ER61666 and DE-FG02-95ER62125) from the Office of Science (BER), U.S. Department of Energy.
Footnotes
Published ahead of print on 11 April 2008.
REFERENCES
- 1.Blackwood, C. B., M. P. Waldrop, D. R. Zak, and R. L. Sinsabaugh. 2007. Molecular analysis of fungal communities and laccase genes in decomposing litter reveals differences among forest types but no impact of nitrogen deposition. Environ. Microbiol. 9:1306-1316. [DOI] [PubMed] [Google Scholar]
- 2.Bose, R. G. 1963. A modified cellulosic medium for the isolation of cellulolytic fungi from infected materials and soils. Nature 198:505-506. [Google Scholar]
- 3.Carlson, J. E., L. K. Tulsieram, J. C. Glaubitz, V. M. K. Luk, C. Kauffeldt, and R. Rutledge. 1991. Segregation of random amplified DNA markers in F1 progeny of conifers. Theor. Appl. Genet. 83:194-200. [DOI] [PubMed] [Google Scholar]
- 4.Chow, C.-M., E. Yagüe, S. Raguz, D. A. Wood, and C. F. Thurston. 1994. The cel3 gene of Agaricus bisporus codes for a modular cellulase and is transcriptionally regulated by the carbon source. Appl. Environ. Microbiol. 60:2779-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooke, R. C., and A. D. M. Rayner. 1984. Ecology of saprotrophic fungi. Longman, London, United Kingdom.
- 6.Covert, S. F., A. Vanden Wymelenberg, and D. Cullen. 1992. Structure, organization, and transcription of a cellobiohydrolase gene cluster from Phanerochaete chrysosporium. Appl. Environ. Microbiol. 58:2168-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox, P., S. P. Wilkinson, and J. M. Anderson. 2001. Effects of fungal inocula on the decomposition of lignin and structural polysaccharides in Pinus sylvestris litter. Biol. Fertil. Soils 33:246-251. [Google Scholar]
- 8.Cullen, D. 1997. Recent advances on the molecular genetics of ligninolytic fungi. J. Biotechnol. 53:273-289. [DOI] [PubMed] [Google Scholar]
- 9.Ding, S., W. Ge, and J. A. Buswell. 2006. Cloning of multiple cellulase cDNAs from Volvariella volvacea and their differential expression during substrate colonization and fruiting. FEMS Microbiol. Lett. 263:207-213. [DOI] [PubMed] [Google Scholar]
- 10.Fengel, D., and G. Wegener. 1989. Wood: chemistry, ultrastructure, reactions, 2nd ed. de Gruyter, Berlin, Germany.
- 11.Frankland, J. C. 1998. Fungal succession—unraveling the unpredictable. Mycol. Res. 102:1-15. [Google Scholar]
- 12.Garrett, S. D. 1963. Soil fungi and soil fertility. Pergamon Press, Oxford, United Kingdom.
- 13.Germann, U., G. Muller, P. Hunziker, and K. Lerch. 1988. Characterization of two allelic forms of Neurospora crassa laccase. J. Biol. Chem. 263:885-896. [PubMed] [Google Scholar]
- 14.Gielkens, M. M. C., E. Dekkers, J. Visser, and L. H. De Graaf. 1999. Two cellobiohydrolase-encoding genes from Aspergillus niger require d-xylose and the xylanolytic transcriptional activator XlnR for their expression. Appl. Environ. Microbiol. 65:4340-4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hao, J.-J., X.-J. Tian, F.-Q. Song, X.-B. He, Z.-J. Zhang, and P. Zhang. 2006. Involvement of lignocellulolytic enzymes in the decomposition of leaf litter in a subtropical forest. J. Eukaryot. Microbiol. 53:193-198. [DOI] [PubMed] [Google Scholar]
- 16.Henrissat, B. 1991. A classification of glycosyl hydrolases based on amino acid sequence similarity. Biochem. J. 280:309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ilmén, M., A. Saloheimo, M.-L. Onnela, and M. E. Pentillä. 1997. Regulation of cellulase gene expression in the filamentous fungus Trichoderma reesei. Appl. Environ. Microbiol. 63:1298-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobsen, J., M. Lydolph, and L. Lange. 2005. Culture independent PCR: an alternative enzyme discovery strategy. J. Microbiol. Methods 60:63-71. [DOI] [PubMed] [Google Scholar]
- 19.Jia, J., P. S. Dyer, J. A. Buswell, and J. F. Peberdy. 1999. Cloning of the cbhI and cbhI genes involved in cellulose utilization by the straw mushroom Volvariella volvacea. Mol. Gen. Genet. 261:985-993. [DOI] [PubMed] [Google Scholar]
- 20.Kjøller, A., and S. Struwe. 1982. Microfungi in ecosystems: fungal occurrence and activity in litter and soil. Oikos 39:391-422. [Google Scholar]
- 21.Koshijima, T., and T. Watanabe. 2003. Association between lignin and carbohydrates in wood and other plant tissues. Springer, Berlin, Germany.
- 22.Luis, P., G. Walther, H. Kellner, F. Martin, and F. Buscot. 2004. Diversity of laccase genes from basidiomycetes in a forest soil. Soil. Biol. Biochem. 36:1025-1036. [Google Scholar]
- 23.Lynch, M. D. J., and R. G. Thorn. 2006. Diversity of basidiomycetes in Michigan forest soil. Appl. Environ. Microbiol. 72:7050-7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lynd, L. R., P. J. Weimer, W. H. van Zyl, and I. S. Pretorius. 2002. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 66:506-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyons, J. I., S. Y. Newell, A. Buchan, and M. A. Moran. 2003. Diversity of ascomycete laccase gene sequences in southeastern US salt marsh. Microb. Ecol. 45:270-281. [DOI] [PubMed] [Google Scholar]
- 26.Moorhead, D. L., and R. L. Sinsabuagh. 2006. A theoretical model of litter decay and microbial interaction. Ecol. Monogr. 76:151-174. [Google Scholar]
- 27.Nei, M., and S. Kumar. 2000. Molecular evolution and phylogenetics. Oxford University Press, New York, NY.
- 28.Ohnishi, Y., M. Nagase, T. Ichiyanagi, Y. Kitamoto, and T. Aimi. 2007. Transcriptional regulation of two cellobiohydrolase genes (cel1 and cel2) from the wood-degrading basidiomycete Polyporus arcularius. Appl. Microbiol. Biotechnol. 76:1069-1078. [DOI] [PubMed] [Google Scholar]
- 29.Osono, T. 2005. Colonization and succession of fungi during decomposition of Swida controversa leaf litter. Mycologia 97:589-597. [DOI] [PubMed] [Google Scholar]
- 30.Osono, T., and H. Takeda. 2002. Comparison of litter degrading ability among diverse fungi in a cool temperate deciduous forest in Japan. Mycologia 94:421-427. [PubMed] [Google Scholar]
- 31.Osono, T., and H. Takeda. 2006. Fungal decomposition of Abies needle and Betula leaf litter. Mycologia 98:172-179. [DOI] [PubMed] [Google Scholar]
- 32.Osono, T., Y. Fukasawa, and H. Takeda. 2003. Roles of diverse fungi in larch needle-litter decomposition. Mycologia 95:820-826. [PubMed] [Google Scholar]
- 33.Poças-Fonseca, M. J., I. Silva-Pereira, B. B. Rocha, and M. O. de Azevedo. 2000. Substrate-dependent differential expression of Humicola grisea var. thermoidea cellobiohydrolase genes. Can. J. Microbiol. 46:749-752. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt, O. 2006. Wood and tree fungi. Springer-Verlag, Berlin, Germany.
- 35.Sheppard, P. O., F. J. Grant, P. J. Oort, C. A. Sprecher, D. C. Foster, F. S. Hagen, A. Upshall, G. L. McKnight, and P. J. O'Hara. 1994. The use of conserved family-specific sequences to clone cellulose homologue cDNAs from Fusarium oxysporum. Gene 150:163-167. [DOI] [PubMed] [Google Scholar]
- 36.Sims, P. F. G., M. S. Soares-Felipe, Q. Wang, M. E. Gent, C. Tempelaars, and P. Broda. 1994. Differential expression of multiple exo-cellobiohydrolase I-like genes in the lignin-degrading fungus Phanerochaete chrysosporium. Mol. Microbiol. 12:209-216. [DOI] [PubMed] [Google Scholar]
- 37.Swift, M. J., O. W. Heal, and J. M. Anderson. 1979. Decomposition in terrestrial ecosystems. Blackwell Scientific Publications, Oxford, United Kingdom.
- 38.Swofford, D. L. 1998. PAUP*. Phylogenetic analysis using parsimony (*and other methods), version 4. Sinauer Associates, Sunderland, MA.
- 39.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software, version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 40.Tarkka, M. T., R. Vasara, M. Gorfer, and M. Raudaskoski. 2000. Molecular characterization of actin genes from homobasidiomycetes: two different actin genes from Schizophyllum commune and Suillus bovinus. Gene 251:27-35. [DOI] [PubMed] [Google Scholar]
- 41.Thorn, R. G., C. A. Reddy, D. Harris, and E. A. Paul. 1996. Isolation of saprophytic basidiomycetes from soil. Appl. Environ. Microbiol. 62:4288-4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vallim, M. A., B. J. H. Janse, J. Gaskell, A. A. Pizzirani-Kleiner, and D. Cullen. 1998. Phanerochaete chrysosporium cellobiohydrolase and cellobiose dehydrogenase transcripts in wood. Appl. Environ. Microbiol. 64:1924-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webster, J. 1970. Coprophilous fungi. Trans. Br. Mycol. Soc. 54:161-180. [Google Scholar]
- 44.Yagüe, E., M. Mehak-Zunic, L. Morgan, D. A. Wood, and C. F. Thurston. 1997. Expression of CEL2 and CEL4, two proteins from Agaricus bisporus with similarity to fungal cellobiohydrolase I and β-mannanase, respectively, is regulated by the carbon source. Microbiology 143:239-244. [DOI] [PubMed] [Google Scholar]
- 45.Zak, D. R., and K. S. Pregitzer. 1990. Spatial and temporal variability of nitrogen cycling in northern lower Michigan. For. Sci. 36:367-380. [Google Scholar]