Abstract
The mechanisms by which environmental carbon sources regulate biofilm formation are poorly understood. This study investigates the roles of glucose and the catabolite repression system in Serratia marcescens biofilm formation. The abilities of this opportunistic pathogen to proliferate in a wide range of environments, to cause disease, and to resist antimicrobials are linked to its ability to form biofilms. We observed that growth of S. marcescens in glucose-rich medium strongly stimulated biofilm formation, which contrasts with previous studies showing that biofilm formation is inhibited by glucose in Escherichia coli and other enteric bacteria. Glucose uptake is known to inversely mediate intracellular cyclic AMP (cAMP) synthesis through regulation of adenylate cyclase (cyaA) activity, which in turn controls fundamental processes such as motility, carbon utilization and storage, pathogenesis, and cell division in many bacteria. Here, we demonstrate that mutation of catabolite repression genes that regulate cAMP levels (crr and cyaA) or the ability to respond to cAMP (crp) confers a large increase in biofilm formation. Suppressor analysis revealed that phenotypes of a cAMP receptor protein (crp) mutant require the fimABCD operon, which is responsible for type 1 fimbria production. Consistently, fimA transcription and fimbria production were determined to be upregulated in a cyaA mutant background by using quantitative real-time reverse transcription-PCR and transmission electron microscopy analysis. The regulatory pathway by which environmental carbon sources influence cAMP concentrations to alter production of type 1 fimbrial adhesins establishes a novel mechanism by which bacteria control biofilm development.
Serratia marcescens is a ubiquitous gram-negative bacterium capable of causing disease in diverse organisms, including humans, coral, insects, and plants (2, 11, 43, 44, 52). The abilities of S. marcescens to cause nosocomial infections and survive in the environment are attributed to its ability to form biofilms, its broad metabolic capacity, and its high natural resistance to antimicrobials and cleaning agents (18, 21, 28, 47). Biofilms are surface-attached microbial communities that afford resistance to biocides and antibiotics and are commonly involved in medical-device-associated infections (14, 20, 37).
S. marcescens readily adheres to diverse substrates, such as contact lenses and epithelial cells (23). Such interactions can be mediated through type 1 fimbriae; these large surface pili are important virulence factors in many bacteria (29). Fimbriae are versatile adhesins capable of mediating attachment to eukaryotic cell surfaces, interactions with biotic and abiotic substrates, and bacterium-bacterium interactions (4, 11, 27, 29, 41, 46, 58, 65). Previous studies of S. marcescens have indicated that biofilms are regulated by quorum sensing, require type 1 fimbriae, and differ in morphology with respect to carbon and nitrogen sources (31, 58). However, little is known about the regulation of S. marcescens biofilm formation. Thus far, type 1 fimbriae are the only surface adhesins known to mediate S. marcescens biofilm formation, where they are required for the primary step in attachment to biotic surfaces (31) and abiotic surfaces (58). Regulation of type 1 fimbriae in S. marcescens has been shown to be largely independent of quorum sensing and dependent upon the oxyR transcription factor and two genes required for normal biofilm structure in strain MG1: bsmA and bsmB (31, 58). The fimABCD operon codes for these mannose-sensitive type 1 fimbriae in S. marcescens (31, 41, 58).
First studied with Escherichia coli but well conserved in prokaryotes, uptake of preferred carbon sources and repression of genes required for utilization of less preferred carbon sources are regulated by a pathway called the catabolite repression system (CRS) (9). Catabolite regulation is generally, though not always, regulated by the second messenger cyclic AMP (cAMP) (56). The cAMP-dependent regulatory pathway has global effects on fundamental processes, including flagellum- and pilus-based motility, cell division, carbon storage, and pathogenesis (9, 10, 12, 36). It has been shown that cells grown in glucose are inhibited for cAMP production, while cells grown in less favorable carbon sources produced elevated levels of cAMP (45). Glucose uptake into bacteria by the phosphoenolpyruvate sugar phosphotransferase system (PTS) is a key regulator of cAMP production. One of the components of the PTS, coded for by the crr gene, is enzyme IIAGlc. The major regulatory signal of the PTS is mediated through the phosphorylation state of enzyme IIAGlc. When glucose is limiting, phosphorylated enzyme IIA activates adenylate cyclase (CyaA) activity, which generates cAMP. In Escherichia coli and Salmonella spp., mutations in adenylate cyclase or PTS components impart severe reductions in the amount of intracellular cAMP (17, 32, 50). Generated cAMP mediates changes through binding with cAMP receptor protein (CRP), a global regulator of transcription (9, 10). CRP can act as both a positive and a negative regulator of transcription and is required for virulence by several organisms (3, 36, 60, 66).
The CRS of S. marcescens is largely uncharacterized. An S. marcescens strain deficient in adenylate cyclase activities has previously been identified, though the mutation was not mapped, nor was the gene cloned (67). This mutant strain produced only 40% of wild-type levels of intracellular cAMP and required exogenous cAMP for use of various carbon sources (67). Data from ectopic expression of S. marcescens genes in E. coli suggest that the production of a secreted phospholipase is controlled by the CRS (19). A mutation in the PTS component gene ptsI of Serratia marcescens has previously been identified in a screen for genes required for extracellular chitinase activity (64). The defect in chitinase activity was complemented with ptsH and ptsI on a plasmid, but a role for crr in this process was not determined (64).
The effects of environmental carbon sources and carbon utilization regulatory proteins on biofilm formation have been documented for diverse bacterial species, including S. marcescens (1, 7, 25, 26, 34, 42, 51, 55). Exogenous cAMP stimulates biofilm formation in E. coli and related enteric bacteria; consistently, mutation of cyaA or crp leads to a decrease in biofilm production (25).
In this study, we demonstrate that biofilm formation by S. marcescens is regulated by components of the CRS. Conditions and mutations that reduce cAMP production or inhibit the ability of the bacterium to respond to cAMP greatly stimulate biofilm formation. The mechanism for increased biofilm formation was determined to be altered levels of type 1 fimbriae, which were upregulated when cAMP was reduced. We also show that catabolite repression genes are conserved between S. marcescens and other enterics but regulate biofilm formation in the opposite manner relative to what has been previously reported for E. coli.
MATERIALS AND METHODS
Bacterial strains, media, growth conditions, and experimental design.
The microorganisms used in this study are listed in Table 1. All bacteria were grown in LB (0.5% yeast extract, 1% tryptone, 0.5% NaCl). M63 medium supplemented with casein amino acids (0.06%, wt/vol) and either glucose, citrate, sucrose, or glycerol (0.2%, vol/vol) was used for nutritional studies. LB and M63 broth were supplemented with adenosine 3′,5′-cyclic monophosphate (A9501; Sigma-Aldrich, Inc.) where noted. The antibiotics used were ampicillin (100 μg/ml), gentamicin (10 μg/ml), kanamycin (100 μg/ml), and tetracycline (10 μg/ml). Experiments were performed at 30°C unless otherwise noted. All experiments were performed using triplicate independent cultures, and all experiments were repeated at least twice on different days.
TABLE 1.
Strains and plasmids used in this studya
| Designation | Description | Source or reference |
|---|---|---|
| Strain name or genotype | ||
| Serratia marcescens | ||
| RSS2 | WT pigmented strain | Presque Isle Cultures |
| cyaA1 | WT with cyaA::Tn mariner mutation | This study |
| cyaA2 | WT with cyaA::Tn mariner mutation | This study |
| cyaA3 | WT with cyaA::Tn mariner mutation | This study |
| cyaA4 | WT with cyaA::pMQ118 mutation | This study |
| fimC4 | fimC::pRMQS167 | This study |
| cyaA2 fimC4 | cyaA2 with fimC::pRMQS167 mutation | This study |
| crr-1 | WT with crr::pEJK1 mutation | This study |
| crr-1 fimC2 | fimC2 with crr::pEJK1 | This study |
| crp-1 | WT with crp::pMQ118 mutation | This study |
| crp-1 fimC2 | crp-1 with fimC::Tn mariner mutation | This study |
| fimC2 | WT with fimC::Tn mariner mutation 50E2 | 58 |
| WT with Plac-fimABCD | WT with chromosomal Plac-fimABCD | 58 |
| cyaA2 with Plac-fimABCD | cyaA2 with chromosomal Plac-fimABCD | This study |
| E. coli | ||
| S17-1 l-pir | thi pro hsdR hsdM+ ΔrecA RP4-2 Tc::Mu-Km::Tn7 pir | 40 |
| PirPlus | DH10B pir-116 | Gentaur |
| BW26386 | ΔcyaA1404::FRT | 13 |
| Plasmids | ||
| pRMQS106 | CEN6 ARSH4 URA3 aacC1 gfp-3HA; ColE1 | 59 |
| pMQ118 | Yeast oriR6K rpsL suicide vector; Kanr | * |
| pMQ131 | Yeast pBBR1 shuttle vector; aphA3 | * |
| pMQ132 | Yeast pBBR1 shuttle vector; accC1 | * |
| pRMQS157 | pMQ131 with cyaA | This study |
| pRMQS169 | pMQ118 with Plac-fimA′ | 58 |
| pRMQS167 | pMQ118 with internal fimC fragment | This study |
| pEJK1 | pMQ118 with internal crr fragment | This study |
| pRMQS173 | pMQ118 with internal crp fragment | This study |
| pRMQS174 | pMQ118 with internal cyaA fragment | This study |
WT, wild type; *, R. M. Shanks and G. A. O'Toole, unpublished.
Mutagenesis, plasmid construction, and quantitative reverse transcription-PCR (qRT-PCR).
Mariner transposon mutations were generated as previously described using pBT20 (58). The fimC4 mutagenic construct was introduced into S. marcescens by using allelic replacement vector pMQ118 as previously described (58). Briefly, pMQ118 with an internal fragment of fimC was designed to recombine with the chromosomal fimC gene, yielding a disruption of the fimC gene; the same strategy was used to mutate crp, crr, and cyaA (Table 1). Insertion mutations were verified using PCR. Revertant strains were acquired as previously described (58). Strains with pMQ118 integrations were grown to saturation three to five times without selection to maintain pMQ118 in the chromosome. Single colonies from the resulting cultures were patched onto media with and without kanamycin. Colonies sensitive to kanamycin were tested phenotypically and by PCR to verify the loss of pMQ118 and the restoration of the wild-type gene and were referred to as “revertants.”
The cyaA open reading frame of S. marcescens was cloned into pMQ131 by using Saccharomyces cerevisiae in vivo recombination and placed under the transcriptional control of Plac, generating pRMQS157 (59). pMQ131 has pBBR1 replication elements for stable replication in S. marcescens (R. Shanks and G. A. O'Toole, unpublished). Chromosomal DNA was isolated with a commercial kit (Gentra D5500A). DNA was amplified using a high-fidelity polymerase (Phusion, New England Biolabs) and cloned using yeast in vivo cloning (59).
RNA preparation and qRT-PCR were performed as described previously, with cells grown to an A600 of 1.0, and rplU transcript levels were used as an internal normalization control as previously reported (58). Three independent cultures were grown on different days for each genotype. The primers used for transcriptional analysis were F-fimA-RT (ACTACACCCTGCGTTTCGAC) and R-fimA-RT (GCGTTAGAGTTTGCCTGACC) for fimA.
Biofilm assays.
Biofilms on glass were generated using 20- by 150-mm borosilicate glass test tubes (14-961-33; Fisher Scientific) with 5 ml of LB incubated overnight at 30°C on a TC-7 tissue culture roller at full speed, 56 rpm (New Brunswick Instruments). These biofilms were stained while rotating, using 6 ml of 0.1% crystal violet, and solubilized using 6 ml of 30% glacial acetic acid, and absorbance for 150-μl aliquots was determined at 590 nm with a Synergy 2 plate reader (Biotek). Triplicate independent cultures were grown for each strain or condition, and each experiment was repeated at least two times on different days per condition. Flow cell experiments were performed using the Kadouri system 2 method as previously described (39), using LB diluted 10-fold in water and a flow rate of 16.5 ml per hour. Flow cells were inoculated with 108 bacteria and incubated at room temperature for 24 h. Confocal images were obtained as previously described (58). Flow cell experiments were performed twice with consistent results.
Detection of cAMP.
Intracellular cAMP levels were determined for stationary-phase bacteria as previously reported (53). Cells were adjusted to an optical density of 2.0 in a volume of 1 ml, washed twice in sterile distilled H2O, resuspended in 1 ml of phosphate-buffered saline (PBS), and lysed using 50 mg of CelLytic Express (Sigma, St. Louis, MO). The lysate was spun briefly in a microcentrifuge, and the supernatant was tested for cAMP levels by using a competitive enzyme immunoassay (CA-201; Sigma, St. Louis, MO). These experiments were performed three times in triplicate independent cultures on different days.
Yeast agglutination assay.
The kinetic yeast aggregation assay was performed with 1.5 ml PBS mixed with 500 μl of yeast solution (Sigma YSC2; 2%, wt/vol, in PBS) and 400 μl of bacteria in PBS (A600 = 1.0). Cultures were agitated vigorously and transferred to a cuvette (1 cm2; Sarstedt), and the A600 was measured spectrophotometrically to assess the agglutination-dependent precipitation of cultures over time (Beckman DU-70).
Nucleotide sequence accession numbers.
The GenBank accession numbers for the sequences determined in this study are EU153350 and EU183232.
RESULTS
Glucose stimulates formation of hyperbiofilms that are sensitive to exogenous cAMP.
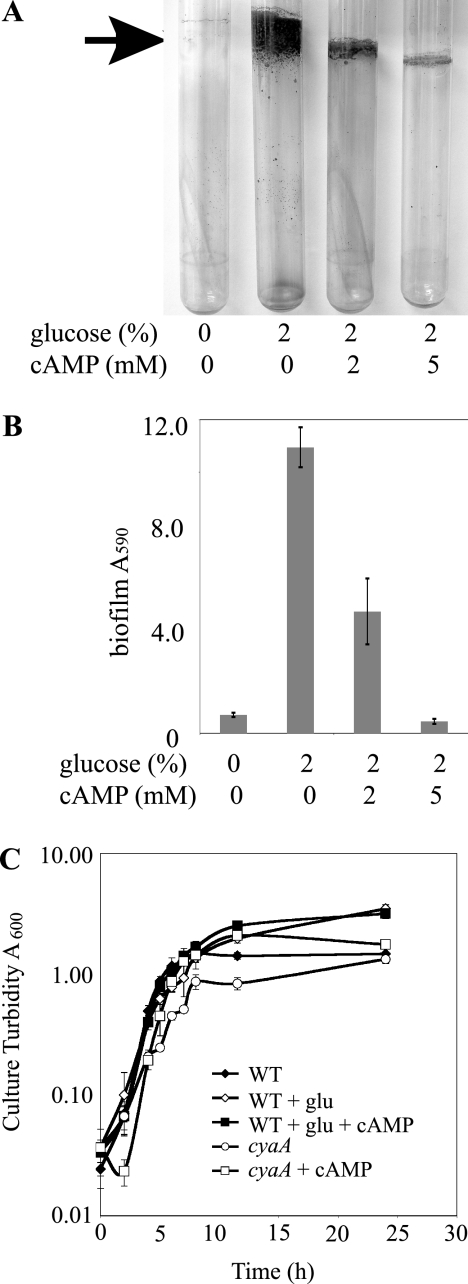
We observed that the addition of supplemental glucose to LB medium dramatically increased attachment of S. marcescens to glass test tubes under high-sheer conditions (Fig. 1A and B). No obvious biofilm was formed by the wild type grown in LB, but a robust biofilm was clearly visible with the addition of glucose (Fig. 1A). As this result differs from what has been previously reported for several enteric bacterial species (25), we decided to study the effect of glucose on S. marcescens biofilm formation.
FIG. 1.
Glucose stimulates S. marcescens biofilm formation. (A) Photographs of crystal violet-stained biofilms (black arrow) formed on glass test tubes. Biofilms were formed in at 30°C for 15 h under high-sheer conditions in LB supplemented with glucose or cAMP as indicated. (B) Biofilm levels from crystal violet-stained test tubes (results from three independent cultures per strain are shown, and error bars indicate 1 standard deviation). Growth in glucose-rich medium significantly increased crystal violet staining (P > 0.01). (C) Growth curve analysis shows culture turbidity as a function of time. Bacteria were grown in LB medium at 30°C and supplemented with 2% glucose (glu) or 10 mM cAMP as indicated. WT, wild type. This experiment was done with triplicate independent cultures. Error bars depict 1 standard deviation.
The presence of glucose in growth medium has been shown to inhibit the production of cAMP by some bacteria (9, 45). We hypothesized that S. marcescens grown in glucose-rich medium formed enhanced biofilms because of physiological changes brought about either by a reduction in cAMP levels or through an increase in growth. To help differentiate between these two models, we assessed bacterial growth (Fig. 1C). At 15 h, when biofilm formation was analyzed, there was a slight though significant increase (P > 0.01 at 11.5 and 24 h) in the amount of culture turbidity when the growth medium was supplemented with glucose compared to that for cultures grown in LB alone (Fig. 1C). To test the model in which robust biofilm formation was a result of reduced cAMP levels, increasing concentrations of exogenous cAMP were added to the growth medium. Figure 1A and B show that glucose-induced biofilm formation can be reversed by the addition of exogenous cAMP in a dose-dependent manner. To ensure that this effect is not due to inhibition of bacterial growth by cAMP, we observed that wild-type cultures grown in LB supplemented with both glucose and 10 mM cAMP grew just as well as the culture grown with LB with additional glucose (Fig. 1C). Together, these data suggest that glucose-stimulated biofilm formation is mediated by the cAMP-regulated CRS rather than caused by minor changes in growth. Mutation of predicted catabolite repression genes was used to further test this model.
Catabolite repression proteins regulate S. marcescens biofilm formation.
We had previously isolated transposon mutations in a predicted class I adenylate cyclase homolog (cyaA) in a screen for genes that modulate biofilm formation in S. marcescens, though these mutants were not described (58). The predicted CyaA polypeptide from the sequenced Db11 strain shared high amino acid identity with CyaA proteins from several enteric pathogens, including Yersinia pestis (87%), Shigella flexneri (85%), and Salmonella enterica (85%). An internal region of the cyaA gene was cloned into the pMQ118 suicide/allelic expression vector and sequenced (GenBank accession number EU153350). This sequence was ∼98% identical to the corresponding DNA of sequenced S. marcescens strain Db11. Three independent transposon mutations in the adenylate cyclase (cyaA) open reading frame were found, and allele numbers were assigned (cyaA1, -2, and -3). cyaA2 had the most 5′ integration site and was used throughout this study.
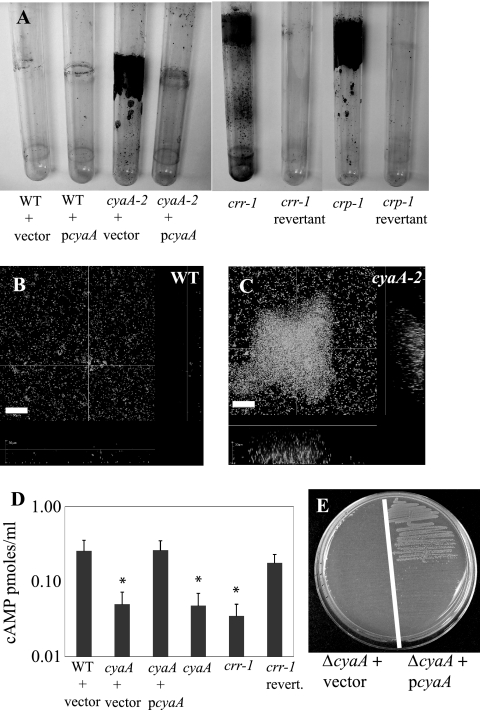
When cultures of cyaA mutants were grown in glass test tubes rotated at high speed, bacteria adhered dramatically to the sides of the tube at the air-liquid interface in a manner akin to that of the wild-type strain grown with excess glucose (Fig. 2A and Table 2). Biofilms formed under these high-sheer conditions were stained with crystal violet and quantified spectrophotometrically; a >10-fold increase was observed in the cyaA2 mutant (Fig. 2A and Table 2). In addition, the culture was full of bacterial aggregates that precipitated when the culture was stationary, suggesting increased bacterium-to-bacterium interactions. The increased interactions of cyaA mutant cells were confirmed using an assay where pelleted bacteria were vortexed and their resuspension kinetics were determined (data not shown). When the wild-type cyaA gene was added in trans to the cyaA2 mutant strain on a multicopy plasmid, biofilms returned to wild-type levels (Fig. 2A and Table 2). Growth curve analysis indicated that the hyperbiofilm phenotype was not a result of an increased growth rate caused by the cyaA mutant (Fig. 1C).
FIG. 2.
S. marcescens catabolite repression genes regulate biofilm formation and cAMP production. (A) Biofilms formed on test tubes that were grown at 30°C on a rotor for 14 to 15 h. Results are shown for mutation and complementation of cyaA, crr, and crp mutant strains. These tubes are representative images of reproducible biofilm phenotypes. WT, wild type. (B, C) Confocal microscopy of Syto-9-stained biofilms formed on a glass coverslip under constant-flow conditions at room temperature for 24 h. Panel B is a micrograph of the wild type, and panel C shows the cyaA2 mutant strain. Bar = 30 μm. This experiment was repeated with consistent results with two replicates per genotype per experiment. (D) Intracellular levels of cAMP, as determined by ELISA. Results are shown for a representative experiment done with triplicate independent samples per genotype. Error bars represent 1 standard deviation. *, P < 0.05. (E) Complementation of E. coli ΔcyaA mutant phenotypes for growth on M63 medium with glycerol as a sole carbon source by the S. marcescens cyaA gene on a multicopy plasmid.
TABLE 2.
Biofilm formation is regulated by cAMP and cAMP-associated proteins
| Genotype or conditiona | Biofilm levelb | Growth levelc |
|---|---|---|
| No bacteria | 0.12 ± 0.02 | ND |
| WT | 0.79 ± 0.08 | ++/++ |
| WT with pMQ131 | 0.70 ± 0.34 | ++/++ |
| WT with pRMQS157 | 0.62 ± 0.32 | ND |
| cyaA | 10.12 ± 1.06 | −/+ |
| cyaA with pMQ131 | 13.88 ± 2.02 | ND |
| cyaA with pRMQS157 | 0.82 ± 0.26 | ++/++ |
| crr | 4.96 ± 0.54 | +/+ |
| crr revertant | 0.59 ± 0.15 | ++/++ |
| crp | 19.04 ± 0.95 | −/− |
| crp revertant | 0.46 ± 0.15 | ++/++ |
| fimC | 0.25 ± 0.05 | ++/++ |
| fimC with 2% glucose | 0.63 ± 0.09 | ND |
| cyaA fimC | 0.58 ± 0.17 | ND |
| crr fimC | 0.60 ± 0.06 | ND |
| crp fimC | 0.58 ± 0.17 | ND |
| WT with Plac-fimABCD | 0.44 ± 0.11 | ND |
| WT with Plac-fimABCD plus glucose | 0.35 ± 0.02 | ND |
| cyaA with Plac-fimABCD | 0.46 ± 0.05 | ND |
| cyaA with 2% mannose | 2.67 ± 1.10 | ND |
| cyaA with 2% methyl α-d-mannopyranoside | 0.42 ± 0.06 | ND |
WT, wild type. pMQ131 is an empty vector. pRMQS157 is pMQ131 with cyaA from S. marcescens.
Values are mean A590 levels ± standard deviations for crystal violet-stained biofilms from triplicate independent cultures. Experiments were repeated at least three times, and results from a representative experiment are shown.
The symbol(s) before the slash represents growth in M63-0.2% citrate-0.006% casein amino acids for 48 h at 30°C. The symbol(s) after the slash indicates growth in the same medium supplemented with 1 mM cAMP. ND, not determined; ++, ≥50% of wild-type growth (A600); +, 5 to 49% of wild-type growth; −, no obvious growth (<5% of wild-type growth).
To verify the effect of the cyaA mutation on surface attachment, biofilms were generated under continuous flow conditions at room temperature and observed using confocal laser scanning microscopy. Whereas both the wild type (Fig. 2B) and the cyaA2 mutant (Fig. 2C) produce biofilms on glass coverslips, the cyaA2 mutant exhibited exaggerated biofilm formation. An example of the difference 24 h after induction of biofilm formation is shown (Fig. 2B versus C). A site-directed cyaA mutant, cyaA4, exhibited phenotypes similar to those noted above, indicating that the transposon itself is not required for the hyperattachment phenotypes (data not shown). Together, these results suggest that cyaA mutants have elevated levels of both cell surface and bacterium-to-bacterium interactions. Although this shows that mutation of a predicted catabolite repression gene confers phenotypes similar to those caused by growth of the wild-type strain with glucose, we wished to confirm that this cyaA homolog codes for a protein that regulates cAMP levels.
The cyaA gene codes for an adenylate cyclase.
Our model for increased levels of biofilm formation by cyaA mutants predicts that increased biofilm formation is a result of reduced cAMP levels. Enzyme-linked immunosorbent assays (ELISAs) were used to determine whether the cyaA gene identified is required for regulation of cAMP production. Levels of cAMP were reproducibly fivefold lower in the cyaA2 mutant strain than in the wild-type strain (P < 0.05) (Fig. 2D). This defect was restored by the addition of pcyaA (pRMQS157) (Fig. 2D).
To further assess whether the S. marcescens cyaA gene codes for a functional adenylate cyclase, we determined whether it could complement mutant defects of a cyaA mutation of E. coli. The E. coli cyaA gene codes for a bona fide adenylate cyclase (9). CyaA activity is known to be essential for E. coli growth on many carbon sources, including glycerol (9). We found that whereas a ΔcyaA mutant of E. coli with an empty vector (pMQ131) was unable to grow on M63 minimal medium with glycerol as a sole carbon source, the same strain with the S. marcescens cyaA gene on a plasmid (pRMQS157) was able to grow on this medium (Fig. 2E). Mutations in S. marcescens cyaA also confer carbon source utilization defects in S. marcescens that can be rescued by pRMQS157 or exogenous cAMP, as expected from an adenylate cyclase mutant (Table 2). These findings support the model in which the cyaA gene of S. marcescens codes for a functional adenylate cyclase.
Mutations of catabolite repression genes crr and crp increase S. marcescens biofilm formation.
To confirm that the cAMP-dependent CRS is important for biofilm formation in S. marcescens, mutations were made in other genes involved in the catabolite repression signaling machinery. Predicted homologs of the PTS gene (crr) and the CRP gene (crp) were disrupted by targeted mutagenesis, generating the crr-1 and crp-1 mutant strains (Table 1). The crp gene from our strain of S. marcescens was cloned (GenBank accession number EU183232), and sequence analysis suggests that it codes for a protein with predicted 99% amino acid identity with CRP from E. coli, 98% with Yersinia pestis, and 95% with CRP from Vibrio cholerae. The crr homolog from S. marcescens has already been sequenced by another group (64). For a complementation control, “revertant” strains in which the suicide plasmids that had disrupted crr and crp were lost and the wild-type genes were restored were made.
The crr gene codes for a PTS component, EIIAGlc, that regulates adenylate cyclase activity in E. coli and S. enterica serovar Typhimurium. To determine whether the crr-1 mutant had reduced cAMP levels, ELISAs were performed. Analysis of triplicate independent cultures revealed that the crr-1 mutant had reduced levels of intracellular cAMP that were similar to cyaA2 levels; both exhibited ∼5-fold reductions compared to the wild type and the crr revertant (Fig. 2D). Moreover, mutation of crr confers a cyaA mutant-like biofilm phenotype (Fig. 2A and Table 2). Normal biofilm formation was observed in the crr revertant, demonstrating that the increased biofilm phenotype was dependent on the crr-1 mutation rather than a secondary mutation elsewhere in the chromosome (Fig. 2A and Table 2).
CRP is a transcription factor required for the CRS, and its activity is regulated by cellular pools of cAMP through the formation of a cAMP-CRP complex. Mutation of crp leads to altered nutritional requirements for S. marcescens growth in minimal medium that could not be rescued by exogenous cAMP (Table 2). We observed that the crp-1 mutant exhibited a significant increase in biofilm formation (P < 0.001) (Fig. 2A and Table 2). As with the crr revertant mentioned above, a restoration of normal biofilm formation in the crp revertant strain indicates that the increased biofilm phenotype was dependent on the crp-1 mutation rather than on a second-site mutation (Fig. 2A and Table 2).
Type 1 fimbriae are necessary for the enhanced biofilm phenotype exhibited by cyaA, crr, and crp mutants.
We hypothesized that the hyperbiofilm phenotype caused by growth in glucose or by mutation of catabolite repression genes was due to altered gene expression of a biofilm-promoting factor(s). The increased expression of this factor would enable S. marcescens to better adhere to abiotic substrates. To determine the mechanism by which the CRS regulates biofilm formation, we took a genetic approach. Mutations of crp, cya, or crr led to matte colonies that were very dense and slid across the surface of an agar plate when prodded, whereas wild-type colonies were smooth, shiny, and pliable. We predicted that the mechanisms for increased biofilm formation and altered colony morphology were dependent upon the same factor(s). This colony morphology phenotype was chosen for suppressor analysis because it provides higher throughput than a screen for suppressors of the biofilm phenotype on glass test tubes. Random mutations were made in a crp-1 background by using a mariner-based transposon. Approximately 30 suppressors of this dense-colony phenotype were identified from 35,000 candidates. These crp(Su) (suppressor of crp-1) mutants generate smooth and pliable colonies similar to those of the wild type. Thus far, multiple crp(Su) mutations have been mapped to genes in the fimABCD operon, which is required for generation of type 1 fimbriae in S. marcescens. One crp(Su) mutation has been mapped to fimB, four independent crp(Su) mutations were found in fimC, and two independent crp(Su) mutations were mapped to fimD.
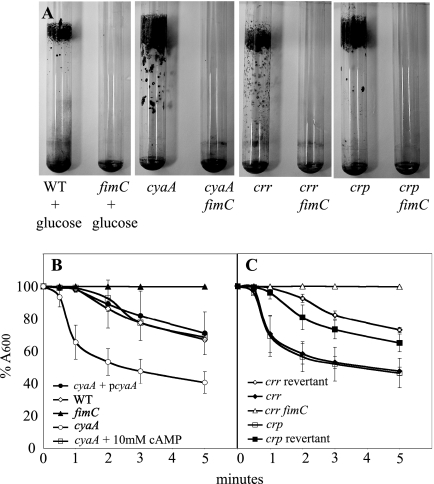
Previous studies have indicated that type 1 fimbrial surface adhesins coded by the fimABCD operon are important for S. marcescens biofilm formation (31, 58). Additionally, several studies indicate that cAMP levels regulate fimbria production in other species of bacteria (15, 57). Directed mutations in the fimC usher protein tested the contribution of fimbriae to biofilm formation in cells grown in glucose and the cya, crr, and crp mutants (Fig. 3A and Tables 1 and 2). Fimbriae were found to be necessary for the hyperbiofilm phenotype on glass test tubes in all cases (Fig. 3A and Table 2). Consistently, downregulation of fimABCD expression by placement of the chromosomal fimABCD operon under the control of Plac from E. coli also eliminated the hyperbiofilm effect caused by cyaA mutation and growth in glucose (Table 2). We have previously shown that integration of the pRMQS169 plasmid into the S. marcescens chromosome places fimABCD under the control of Plac and supports fimbria production at levels indistinguishable from those for the wild-type in LB medium (58). We note that Plac is positively regulated by cAMP-CRP in E. coli and consistently see lower levels of fimbriae in the cyaA mutant with Plac driving fimABCD expression than in the wild type (data not shown).
FIG. 3.
Fimbriae are necessary for catabolite repression mutant biofilm phenotypes. (A) Mutation of fimC suppressed the hyperbiofilm phenotype caused by high levels of glucose or mutation of catabolite repression genes. For panels B and C, a kinetic assessment of yeast agglutination was performed. Positive agglutination results in lower absorbance readings. Here, absorbance is shown relative to time zero. WT, wild type. (B) The ability of S. marcescens to agglutinate yeast was dependent on fimbriae (fimC). Mutation of cyaA led to an increase in yeast agglutination that can be complemented by wild-type cyaA on a plasmid (pRMQS157) or by the addition of exogenous cAMP. (C) Kinetic agglutination of yeast by crr-1, mutants, and control strains. A crr fimC double mutant did not aggregate yeast (identical results were found with cya fimC and crp fimC double mutants [not shown]). All experiments were done with triplicate independent cultures on at least three different days, and the representative data from 1 day's experiment are shown. Error bars represent 1 standard deviation.
S. marcescens type 1 fimbriae are inhibited by mannose (41, 54). It follows that if type 1 fimbriae are important for the biofilm phenotype of cyaA mutants then exogenous mannose should inhibit biofilm formation. This assumes that the mannose-binding portion of the fimbriae is also responsible for attachment to glass. Two percent mannose and 2% nonhydrolyzable mannose (methyl α-d-mannopyranoside) both severely inhibited cyaA2 mutant biofilm-forming capacity (Table 2).
Since the wild-type strain does not form robust biofilms on glass test tubes and because fimbriae are required for the hyperbiofilm phenotype, we predicted that the reduced ability to produce cAMP (high glucose and cya and crr mutations) or respond to cAMP (crp mutation) would lead to increased fimbria production, leading to increased biofilm formation. To test this prediction, we used yeast agglutination as a functional assay of fimbria production. Agglutination of Saccharomyces cerevisiae by S. marcescens has been shown to require type 1 fimbriae (Fig. 3B) (58). The cyaA2, crr-1, and crp-1 strains all produced significant increases in the amount of yeast agglutination at ≥1 min (P < 0.03) (Fig. 3B and C). The cyaA fimC, crp fimC, crr fimC, and fimC mutants completely lose their abilities to agglutinate yeast cells over the course of the experiment, confirming that hyperagglutination of yeast cells is fimABCD dependent (Fig. 3B and C and data not shown). As a complementation control, we found that the amount of cyaA mutant agglutination could be restored to wild-type levels by the wild-type S. marcescens cyaA gene on a plasmid (Fig. 3B). Similarly, the crr revertant and crp revertant strains established wild-type levels of agglutination (Fig. 3C).
TEM indicates that cyaA and crp mutants have elevated levels of type 1 fimbriae.
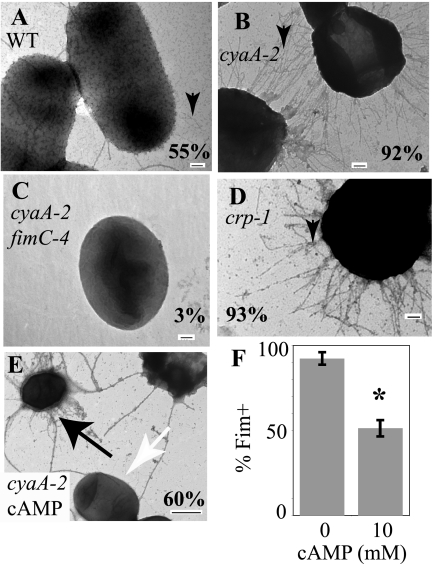
Transmission electron microscopy (TEM) was used to directly visualize the extents of fimbriation of cells with cyaA and crp mutations. Negatively stained cyaA2 mutant cells exhibited a dramatic, hyperpiliated phenotype compared to wild-type cells (Fig. 4A versus B). The majority of the cyaA2 culture population had numerous fimbriae (92.1% ± 3.2% of cells; n = 279) (Fig. 4B). Wild-type cells were more sparsely decorated with fimbriae, with only 55% (n = 140) exhibiting surface pili (Fig. 4A), yet when grown with 2% glucose, the wild type exhibited an intermediate phenotype, with 80% (n = 147) appearing to be hyperfimbriate like the cyaA mutant. FimC is required to produce type 1 fimbriae in our strain background (58). Directed mutation of fimC conferred an absence of obvious fimbriae (<1% Fim+; n = 140). A cyaA2 fimC4 double mutant was generated and was largely devoid of surface pili (3.3%; n = 272), confirming that CyaA negatively regulates the production of type 1 fimbriae rather than some other surface pili (Fig. 4C). Cells deficient in Crp were also tested for fimbria production by TEM, and a cya-like phenotype was observed (Fig. 4D). The percentage of the crp-1 mutant culture exhibiting fimbriae was found to be 93.2% ± 0.5% (n = 148 cells), while a crp-1 fimC2 mutant exhibited no fimbriae (n = 28).
FIG. 4.
TEM analysis of fimbria production. TEM micrographs of negatively stained bacteria grown under shaking conditions for 15 h in LB medium at 30°C. The percentage value represents the percentage of cells that exhibit fimbriae (n ≥ 140 cells per strain) from multiple independent cultures. Black arrowheads indicate fimbriae. (A) Two wild-type (WT) cells with moderate levels of type 1 fimbriae are shown. (B) Most cells in the cyaA2 mutant culture exhibit high levels of fimbriae. (C) cyaA2 fimC4 double mutant cell devoid of fimbriae. (D) Bacteria from crp-1 mutant cultures are highly fimbriate. (E) TEM micrograph of the cyaA2 mutant grown in the presence of 10 mM cAMP. Note typical cyaA mutant morphology (black arrow) on one cell and lack of obvious fimbriae (white arrow) on another cell. The bar represents 100 nm, except in panel E, where the bar represents 500 nm. (F) Quantitation of TEM images from triplicate independent cya-2 cultures showing the percentage of cells with fimbriae grown in medium supplemented with (10 mM) or without cAMP. The asterisk represents a significant reduction in the percentage of cells with surface fimbriae (P < 0.02).
To test the possibility that the mannose-associated reduction in biofilm formation (Table 2) was due to a reduction in fimbria production rather than an inhibition of fimbria binding activity, we assessed the cyaA mutant incubated with 2% mannose by TEM and found that >90% of the cells were hyperfimbriate (n > 100) and were indistinguishable from the cyaA mutant grown without mannose (data not shown).
Exogenous cAMP inhibits fimbria production.
Since mutation of cyaA results in an increase in production of fimbriae and reduced levels of cAMP, it follows that cAMP has a negative impact on fimbria production. To test this prediction, the yeast agglutination assay was used. Levels of ≥10 mM but not ≤2 mM significantly reduced the ability of the cyaA mutant to agglutinate yeast at 30 seconds or more (P < 0.05) (Fig. 3B and data not shown). To confirm data from the indirect yeast agglutination assay (Fig. 3B), exogenous cAMP (up to 20 mM) was added to the cyaA2 mutant cultures, and the effect upon fimbria production was assessed by TEM (Fig. 4E and F). The presence of cAMP (10 to 20 mM) reduced the percentage of fimbria-positive cells in a dose-dependent manner (98.2% at 0 mM, 59.5% at 10 mM, and 35.2% at 20 mM; n > 100 per condition) (Fig. 4F). The addition of 10 to 20 mM of cAMP did not lead to a uniform decrease in the number of fimbriae on all cells; many cyaA mutant cells were coated with fimbriae (Fig. 4E), whereas others were devoid of obvious fimbriae (Fig. 4E).
CyaA regulates transcription of fimbria genes.
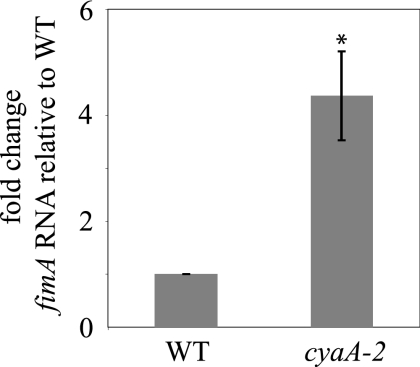
Our data suggest that cAMP has a negative impact on fimbria production. To test this model, real time qRT-PCR was utilized to determine fimA transcript levels. A cyaA2 mutant was used as a strain with reduced cAMP for comparison to the wild-type strain. We found that the absence of Cya leads to a significant (4.4-fold) increase in fimA transcription (P < 0.03) (Fig. 5).
FIG. 5.
Transcription of fimA is negatively regulated by adenylate cyclase. Real-time qPCR was used to assess transcription of type 1 fimbria subunit coding gene fimA. RNA levels relative to those for the wild type (WT) are shown. The difference is significant (P < 0.03). The experiment shows an average of three independent cultures made on different days. Error bars indicate standard errors.
DISCUSSION
In this report, we demonstrate that biofilm formation by S. marcescens is regulated by CRS control of type 1 fimbrial adhesins. Both conditions and mutations that reduced cAMP production (cyaA and crr) or cAMP-mediated responses (crp) stimulated biofilm and attachment phenotypes. Contrary to what has been reported for E. coli, we observed that glucose stimulates S. marcescens biofilm formation and that this phenotype is sensitive to cAMP (Fig. 1A and B and Table 2), suggesting that biofilm formation is antagonized by cAMP and catabolite repression genes. We concluded that this effect was not a result of increased growth rates due to elevated glucose. Hyperbiofilms were not observed in wild-type cultures grown with both exogenous cAMP and glucose, though they grew at rates indistinguishable from those for cultures supplemented with only glucose (Fig. 1C).
We provide genetic and biochemical evidence that the S. marcescens cyaA homolog codes for a functional adenylate cyclase. The cyaA mutant was not only defective in production of cAMP but also unable to grow with citrate, glycerol, or sucrose as a sole carbon source (Fig. 2C and D, Table 2, and data not shown). These growth phenotypes were rescued by exogenous cAMP (≥400 μM) or wild-type cyaA on a plasmid. Moreover, the S. marcescens cyaA gene, which is highly similar to cyaA from E. coli, was sufficient to complement cAMP-dependent mutant defects in a ΔcyaA mutant of E. coli.
The catabolite repression genes crr and crp were mutated for the first time in S. marcescens. We noted that mutation of the CRS component crr homolog led to a deficiency in intracellular cAMP levels similar to the cyaA mutant. This suggests that the crr gene product has a conserved function with the corresponding E. coli protein in positive regulation of adenylate cyclase activity. Interestingly, mutation of crr reduced, but did not eliminate, the ability of bacteria to grow in minimal media supplemented with citrate, whereas cyaA and crp mutants were completely unable to grow in citrate medium (Table 2). These data suggest that adenylate cyclase has some activity in a crr mutant background sufficient for partial growth in citrate medium and a less extreme hyperbiofilm phenotype; however, no difference between cyaA and crr mutants was evident when cAMP levels in stationary-phase cells were measured. As predicted, the crp mutant of S. marcescens was unable to grow on citrate (or sucrose or glycerol) as a sole carbon source, and this effect could not be rescued by exogenous cAMP. Together, mutational analysis of cyaA, crr, and crp and the effects of growth in exogenous glucose and cAMP indicate that the CRS of S. marcescens regulates biofilm formation. This suggests that extracellular carbon sources have a profound role in regulation of S. marcescens biofilm formation in vivo. Consistently, Rice and colleagues have shown a major role for carbon source type and availability in S. marcescens biofilm development (51).
While crr has a role in the CRS of E. coli and there is a link between the CRS and biofilm formation in several organisms, this is the first report to directly link a crr homolog (enzyme IIAGlc) to regulation of biofilm formation. Recent work with other organisms shows that other PTS components play a role in biofilm formation. PTS enzyme I is important in regulation of Vibrio cholerae biofilm formation, but mutation of enzyme IIAGlc had no effect on biofilm growth (22). A multicomponent, fructose-specific PTS component, FruI, has been found to positively regulate Streptococcus gordonii biofilm formation (35). Mutations in PTS component IIB (glucose specific) and enzyme IIC (cellobiose specific), but not enzyme IIA, of Klebsiella pneumoniae were isolated in a signature-tagged mutagenesis screen for genes required for attachment to extracellular matrix components (7).
In gram-negative bacteria, the effects of glucose and mutations in genes that code for catabolite repression functions have a range of effects on biofilm formation. The first evidence that a catabolite repression gene could contribute to biofilm formation was found in Pseudomonas aeruginosa, where mutation of the catabolite repression control gene (crc) led to a severe reduction in biofilm formation due to a reduction in type IV pilus production (42). Interestingly, Crc controls catabolite repression in a cAMP-independent manner (56, 68). P. aeruginosa and S. marcescens both control surface attachment through CRSs; however, Crc appears to be a positive regulator of surface adhesins, whereas CRS genes of S. marcescens are shown here to negatively regulate type 1 fimbria production. Likewise, Salmonella enterica serovar Enteritidis biofilm formation and virulence potential are stimulated by growth in glucose-rich medium, suggesting that lower levels of cAMP could influence these phenotypes (8). In V. cholerae, mutations of some CRS genes, including the CRP homolog, have negative impacts on biofilm formation (22, 33); however, these changes are thought to be mediated through alteration of exopolysaccharide production (22, 33). Lastly, a CRP homolog of Shewanella oneidensis has been found to regulate biofilm detachment in response to environmental stimuli (63).
Cyclic nucleotides have previously been reported to regulate pilus formation in other organisms (6, 30, 62). In Salmonella enterica serovar Typhimurium and E. coli, cAMP production was shown to be a positive regulator of type 1 fimbria production (15, 57), K99 pilus production (24), and pap pilus production (3). In P. aeruginosa, the CRP homolog Vfr is required for positive regulation of type IV pili (5), and cyclic di-GMP levels positively regulate CupA fimbria production (38).
We report here that the addition of exogenous cAMP was correlated with a reduction in the percentage of fimbriate bacterial cells in the cyaA mutant background, further supporting the conclusion that cAMP inhibits S. marcescens fimbria production (Fig. 4E and F). However, in this case, the extents of fimbriation varied greatly in those cells with fimbriae, with most cells displaying reduced levels similar to those in the wild type and a subset of cells remaining indistinguishable from the parental cyaA mutant (Fig. 4E). This pleiotropic effect suggests that either there is variable penetration of cAMP into Serratia cells or cAMP can modulate phase variation of fimbria production. Fimbria production in several species of bacteria is controlled in a phase-variable manner (6), though this phenomenon has never been shown to occur in Serratia spp.
Evidence presented here indicates that catabolite repression proteins direct fimbria production, but it is unknown whether there are intermediate regulators between CRP and fimABCD transcription. We found no obvious CRP consensus binding site in the region upstream of the fimABCD operon; however, we were able to find consensus sequences upstream of predicted sugar transporters. This suggests that the CRP consensus binding site is conserved in S. marcescens, as would be predicted by the >99% identity between the CRP proteins from E. coli and S. marcescens. We are currently screening for factors that may be intermediates between CRP and the fimABCD operon.
The significance of this report is that a central environmental carbon regulatory circuit, the CRS, plays a profound role in the surface behaviors of the opportunistic pathogen S. marcescens through regulation of a large surface adhesin. Carbon source and availability have a large impact on the way S. marcescens interacts with its environment and are critical elements in the surface behaviors of this organism. Carbon levels have previously been shown to affect S. marcescens biofilm formation (51). The interaction of S. marcescens with other organisms can also be regulated by carbon sources. One study showed that S. marcescens inhibitory effects on fungi proliferation can be suppressed by added glucose (54). In the human environment, glucose levels are elevated at several sites associated with infections, including the bloodstream, vaginal fluid, synovial fluid, nasopharyngeal tissue, and aqueous humor (16, 61). The presence of sugars in these environments can have a major impact on bacterial adherence (48). Under environmental conditions, S. marcescens-derived, polymer-degrading enzymes, such as cellulase and chitinases, could influence its surface attachment behaviors in niches with high levels of cellulose or chitin through alteration of sugar levels in its microenvironment (49). Future studies will include determining the role of the CRS in the control of biofilm formation and fimbria production in response to environmental cues.
Acknowledgments
We thank the Campbell and Kinchington laboratories and Dayna Helvick for helpful discussion, Kira L. Lathrop for expert assistance with microscopy, Joseph Horzempa and Paul Carlson for technical advice, and Shannon Hinsa and the CGSC for kindly providing strains. We acknowledge the Mednansky Institute, iHOP, and the Sanger Center for helpful online information.
This work was supported by the Campbell Laboratory of Ophthalmic Microbiology, the Eye and Ear Foundation of Pittsburgh, Research to Prevent Blindness, and an NEI Core Grant for Vision Research, EY08098.
Footnotes
Published ahead of print on 18 April 2008.
REFERENCES
- 1.Abranches, J., M. M. Candella, Z. T. Wen, H. V. Baker, and R. A. Burne. 2006. Different roles of EIIABMan and EIIGlc in regulation of energy metabolism, biofilm development, and competence in Streptococcus mutans. J. Bacteriol. 188:3748-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamo, S. A. 2004. Estimating disease resistance in insects: phenoloxidase and lysozyme-like activity and disease resistance in the cricket Gryllus texensis. J. Insect Physiol. 50:209-216. [DOI] [PubMed] [Google Scholar]
- 3.Båga, M., M. Göransson, S. Normark, and B. E. Uhlin. 1985. Transcriptonal activation of a pap pilus virulence operon from uropathogenic Escherichia coli. EMBO J. 30:3887-3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnich, N., J. Boudeau, L. Claret, and A. Darfeuille-Michaud. 2003. Regulatory and functional co-operation of flagella and type 1 pili in adhesive and invasive abilities of AIEC strain LF82 isolated from a patient with Crohn's disease. Mol. Microbiol. 48:781-794. [DOI] [PubMed] [Google Scholar]
- 5.Beatson, S. A., C. B. Whitechurch, J. L. Sargent, R. C. Levesque, and J. S. Mattick. 2002. Differential regulation of twitching motility and elastase production by Vfr in Pseudomonas aeruginosa. J. Bacteriol. 184:3605-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blomfield, I. C. 2001. The regulation of pap and type 1 fimbriation in Escherichia coli. Adv. Microb. Physiol. 45:1-49. [DOI] [PubMed] [Google Scholar]
- 7.Boddicker, J. D., R. A. Anderson, J. Jagnow, and S. Clegg. 2006. Signature-tagged mutagenesis of Klebsiella pneumoniae to identify genes that influence biofilm formation on extracellular matrix material. Infect. Immun. 74:4590-4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonafonte, M. A., C. Solano, B. Sesma, M. Alvarez, L. Montuenga, D. Garcia-Ros, and C. Gamazo. 2000. The relationship between glycogen synthesis, biofilm formation and virulence in Salmonella enteritidis. FEMS Microbiol. Lett. 191:31-36. [DOI] [PubMed] [Google Scholar]
- 9.Botsford, J. L., and J. G. Harman. 1992. Cyclic AMP in prokaryotes. Microbiol. Rev. 56:100-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruckner, R., and F. Titgemeyer. 2002. Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol. Lett. 209:141-148. [DOI] [PubMed] [Google Scholar]
- 11.Castro, D. P., S. H. Seabra, E. S. Garcia, W. D. Souza, and P. Azambuja. 2007. Trypanosoma cruzi: ultrastructural studies of adhesion, lysis and biofilm formation by Serratia marcescens. Exp. Parasitol. 117:201-207. [DOI] [PubMed] [Google Scholar]
- 12.Crasnier, M. 1996. Cyclic AMP and catabolite repression. Res. Microbiol. 147:479-482. [DOI] [PubMed] [Google Scholar]
- 13.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.del Pozo, J. L., and R. Patel. 2007. The challenge of treating biofilm-associated bacterial infections. Clin. Pharmacol. Ther. 82:204-209. [DOI] [PubMed] [Google Scholar]
- 15.Eisenstein, B. I., E. H. Beachey, and S. S. Solomon. 1981. Divergent effects of cyclic adenosine 3′,5′-monophosphate on formation of type 1 fimbriae in different K-12 strains of Escherichia coli. J. Bacteriol. 145:620-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Exley, R. M., L. Goodwin, E. Mowe, J. Shaw, H. Smith, R. C. Read, and C. M. Tang. 2005. Neisseria meningitidis lactate permease is required for nasopharyngeal colonization. Infect. Immun. 73:5762-5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feucht, B. U., and M. H. Saier. 1980. Fine control of adenylate cyclase by the phosphoenolpyruvate:sugar phosphotransferase system in Escherichia coli and Salmonella typhimurium. J. Bacteriol. 141:603-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandhi, P. A., A. D. Sawant, L. A. Wilson, and D. G. Ahearn. 1993. Adaptation and growth of Serratia marcescens in contact lens disinfectant solutions containing chlorhexidine gluconate. Appl. Environ. Microbiol. 59:183-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Givskov, M., and S. Molin. 1992. Expression of extracellular phospholipase from Serratia liquefaciens is growth-phase dependent, catabolite repressed and regulated by anaerobisis. Mol. Microbiol. 6:1363-1374. [DOI] [PubMed] [Google Scholar]
- 20.Hall-Stoodley, L., J. W. Costerton, and P. Stoodley. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2:95-108. [DOI] [PubMed] [Google Scholar]
- 21.Hejazi, A., and F. R. Falkiner. 1997. Serratia marcescens. J. Med. Microbiol. 46:903-912. [DOI] [PubMed] [Google Scholar]
- 22.Houot, L., and P. I. Watnick. 2008. A novel role for enzyme I of the Vibrio cholerae phosphoenolpyruvate phosphotransferase system in regulation of growth in a biofilm. J. Bacteriol. 190:311-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hume, E. B., F. Stapleton, and M. D. Willcox. 2003. Evasion of cellular ocular defenses by contact lens isolates of Serratia marcescens. Eye Contact Lens 29:108-112. [DOI] [PubMed] [Google Scholar]
- 24.Isaacson, R. E. 1980. Factors affecting expression of the Escherichia coli pilus K99. Infect. Immun. 28:190-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson, D. W., J. W. Simecka, and T. Romeo. 2002. Catabolite repression of Escherichia coli biofilm formation. J. Bacteriol. 184:3406-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson, D. W., K. Suzuki, L. Oakford, J. W. Simecka, M. E. Hart, and T. Romeo. 2002. Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J. Bacteriol. 184:290-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jagnow, J., and S. Clegg. 2003. Klebsiella pneumoniae MrkD-mediated biofilm formation on extracellular matrix- and collagen-coated surfaces. Microbiology 149:2397-2405. [DOI] [PubMed] [Google Scholar]
- 28.Jones, G. L., C. T. Muller, M. O'Reilly, and D. J. Stickler. 2006. Effect of triclosan on the development of bacterial biofilms by urinary tract pathogens on urinary catheters. J. Antimicrob. Chemother. 57:266-272. [DOI] [PubMed] [Google Scholar]
- 29.Jonson, A.-B., S. Normark, and M. Rhen. 2005. Fimbriae, pili, flagella and bacterial virulence. Contrib. Microbiol. 12:67-89. [DOI] [PubMed] [Google Scholar]
- 30.Kurn, N., and L. Shapiro. 1976. Effect of 3′:5′-cyclic GMP derivatives on the formation of Caulobacter crescentus surface structures. Proc. Natl. Acad. Sci. USA 73:3303-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labbate, M., H. Zhu, L. Thung, R. Bandara, M. R. Larsen, M. D. P. Willcox, M. Givskov, S. A. Rice, and S. Kjelleberg. 2007. Quorum-sensing regulation of adhesion in Serratia marcescens MG1 is surface dependent. J. Bacteriol. 189:2702-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy, S., G. Q. Zeng, and A. Danchin. 1990. Cyclic AMP synthesis in Escherichia coli strains bearing known deletions in the pts phosphotransferase operon. Gene 86:27-33. [DOI] [PubMed] [Google Scholar]
- 33.Liang, W., A. Pascual-Montano, A. J. Silva, and J. A. Benitez. 2007. The cyclic AMP receptor protein modulates quorum sensing, motility and multiple genes that affect intestinal colonization in Vibrio cholerae. Microbiology 153:2964-2975. [DOI] [PubMed] [Google Scholar]
- 34.Lim, Y., M. Jana, T. T. Luong, and C. Y. Lee. 2004. Control of glucose- and NaCl-induced biofilm formation by rbf in Staphylococcus aureus. J. Bacteriol. 186:722-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loo, C. Y., K. Mitrakul, I. B. Voss, C. V. Hughes, and N. Ganeshkumar. 2003. Involvement of an inducible fructose phosphotransferase operon in Streptococcus gordonii biofilm formation. J. Bacteriol. 185:6241-6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lory, S., M. Wolfgang, V. Lee, and R. Smith. 2004. The multi-talented bacterial adenylate cyclases. Int J. Med. Microbiol. 293:479-482. [DOI] [PubMed] [Google Scholar]
- 37.Mah, T. F., and G. A. O'Toole. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34-39. [DOI] [PubMed] [Google Scholar]
- 38.Meissner, A., V. Wild, R. Simm, M. Rohde, C. Erck, F. Bredenbruch, M. Morr, U. Romling, and S. Haussler. 2007. Pseudomonas aeruginosa cupA-encoded fimbriae expression is regulated by a GGDEF and EAL domain-dependent modulation of the intracellular level of cyclic diguanylate. Environ. Microbiol. 9:2475-2485. [DOI] [PubMed] [Google Scholar]
- 39.Merritt, J. H., D. E. Kadouri, and G. A. O'Toole. 2005. Growing and analyzing static biofilms, p. 1B.1.1-1B.1.17. In R. Coico, T. Kowalik, J. Quarles, B. Stevenson, and R. Taylor (ed.), Current protocols in microbiology. John Wiley & Sons, Inc., New York, NY. [DOI] [PMC free article] [PubMed]
- 40.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nichols, W. A., S. Clegg, and M. R. Brown. 1990. Characterization of the type 1 fimbrial subunit gene (fimA) of Serratia marcescens. Mol. Microbiol. 4:2119-2126. [DOI] [PubMed] [Google Scholar]
- 42.O'Toole, G. A., K. A. Gibbs, P. W. Hager, P. V. Phibbs, Jr., and R. Kolter. 2000. The global carbon metabolism regulator Crc is a component of a signal transduction pathway required for biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 182:425-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pair, S. D., B. D. Bruton, F. Mitchell, J. Fletcher, A. Wayadande, and U. Melcher. 2004. Overwintering squash bugs harbor and transmit the causal agent of cucurbit yellow vine disease. J. Econ. Entomol. 97:74-78. [DOI] [PubMed] [Google Scholar]
- 44.Patterson, K. L., J. W. Porter, K. B. Ritchie, S. W. Polson, E. Mueller, E. C. Peters, D. L. Santavy, and G. W. Smith. 2002. The etiology of white pox, a lethal disease of the Caribbean elkhorn coral, Acropora palmata. Proc. Natl. Acad. Sci. USA 99:8725-8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peterkofsky, A., and C. Gazdar. 1971. Glucose and the metabolism of adenosine 3′:5′-cyclic monophosphate in Escherichia coli. Proc. Natl. Acad. Sci. USA 68:2794-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pratt, L. A., and R. Kolter. 1998. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 30:285-293. [DOI] [PubMed] [Google Scholar]
- 47.Queck, S. Y., M. Weitere, A. M. Moreno, S. A. Rice, and S. Kjelleberg. 2006. The role of quorum sensing mediated developmental traits in the resistance of Serratia marcescens biofilms against protozoan grazing. Environ. Microbiol. 8:1017-1025. [DOI] [PubMed] [Google Scholar]
- 48.Rajan, N., Q. Cao, B. E. Anderson, D. L. Pruden, J. Sensibar, J. L. Duncan, and A. J. Schaeffer. 1999. Roles of glycoproteins and oligosaccharides found in human vaginal fluid in bacterial adherence. Infect. Immun. 67:5027-5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramey, B. E., M. Koutsoudis, S. B. von Bodman, and C. Fuqua. 2004. Biofilm formation in plant-microbe associations. Curr. Opin. Microbiol. 7:602-609. [DOI] [PubMed] [Google Scholar]
- 50.Reddy, P., and M. Kamireddi. 1998. Modulation of Escherichia coli adenylyl cyclase activity by catalytic-site mutants of protein IIAGlc of the phosphoenolpyruvate:sugar phosphotransferase system. J. Bacteriol. 180:732-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rice, S. A., K. S. Koh, S. Y. Queck, M. Labbate, K. W. Lam, and S. Kjelleberg. 2005. Biofilm formation and sloughing in Serratia marcescens are controlled by quorum sensing and nutrient cues. J. Bacteriol. 187:3477-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richards, M. J., J. R. Edwards, D. H. Culver, and R. P. Gaynes. 2000. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect. Control Hosp. Epidemiol. 21:510-515. [DOI] [PubMed] [Google Scholar]
- 53.Roberts, D. P., L. F. McKenna, X. Hu, S. M. Lohrke, H. S. Kong, J. T. de Souza, C. J. Baker, and J. Lydon. 2007. Mutation in cyaA in Enterobacter cloacae decreases cucumber root colonization. Arch. Microbiol. 187:101-115. [DOI] [PubMed] [Google Scholar]
- 54.Rosenzweig, W. D., and G. Stotzky. 1980. Influence of environmental factors on antagonism of fungi by bacteria in soil: nutrient levels. Appl. Environ. Microbiol. 39:354-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rupp, M. E., N. Sloot, H. G. Meyer, J. Han, and S. Gatermann. 1995. Characterization of the hemagglutinin of Staphylococcus epidermidis. J. Infect. Dis. 172:1509-1518. [DOI] [PubMed] [Google Scholar]
- 56.Saier, M. H. 1996. Cyclic AMP-independent catabolite repression in bacteria. FEMS Microbiol. Lett. 138:97-103. [DOI] [PubMed] [Google Scholar]
- 57.Saier, M. H., M. R. Schmidt, and M. Leibowitz. 1978. Cyclic AMP-dependent synthesis of fimbriae in Salmonella typhimurium: effects of cya and pts mutations. J. Bacteriol. 134:356-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shanks, R. M. Q., N. A. Stella, E. J. Kalivoda, M. R. Doe, D. M. O'Dee, K. L. Lathrop, F. L. Guo, and G. J. Nau. 2007. A Serratia marcescens OxyR homolog mediates surface attachment and biofilm formation. J. Bacteriol. 189:7262-7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shanks, R. M. Q., N. C. Caiazza, S. M. Hinsa, C. M. Toutain, and G. A. O'Toole. 2006. Saccharomyces cerevisiae-based molecular tool kit for manipulation of genes from gram-negative bacteria. Appl. Environ. Microbiol. 72:5027-5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skorupski, K., and R. K. Taylor. 1997. Cyclic AMP and its receptor protein negatively regulate the coordinate expression of cholera toxin and toxin-coregulated pilus in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 94:265-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith, H., E. A. Yates, J. A. Cole, and N. J. Parsons. 2001. Lactate stimulation of gonococcal metabolism in media containing glucose: mechanism, impact on pathogenicity, and wider implications for other pathogens. Infect. Immun. 69:6565-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smyth, C. J., M. B. Marron, J. M. Twohig, and S. G. Smith. 1996. Fimbrial adhesins: similarities and variations in structure and biogenesis. FEMS Immunol. Med. Microbiol. 16:127-139. [DOI] [PubMed] [Google Scholar]
- 63.Thormann, K. M., R. M. Saville, S. Shukla, and A. M. Spormann. 2005. Induction of rapid detachment in Shewanella oneidensis MR-1 biofilms. J. Bacteriol. 187:1014-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uchiyama, T., R. Kaneko, J. Yamaguchi, A. Inoue, T. Yanagida, N. Nikaidou, M. Regue, and T. Watanabe. 2003. Uptake of N,N′-diacetylchitobiose [(GlcNAc)2] via the phosphotransferase system is essential for chitinase production by Serratia marcescens 2170. J. Bacteriol. 185:1776-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watnick, P. I., K. J. Fullner, and R. Kolter. 1999. A role for the mannose-sensitive hemagglutinin in biofilm formation by Vibrio cholerae El Tor. J. Bacteriol. 181:3606-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.West, S. E., A. K. Sample, and L. J. Runyen-Janecky. 1994. The vfr gene product, required for Pseudomonas aeruginosa exotoxin A and protease production, belongs to the cyclic AMP receptor protein family. J. Bacteriol. 176:7532-7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winkler, U., H. Scholle, and L. Bohne. 1975. Mutants of Serratia marcescens lacking cyclic nucleotide phosphodiesterase activity and requiring cyclic 3′,5′-AMP for the utilization of various carbohydrates. Arch. Microbiol. 104:189-196. [DOI] [PubMed] [Google Scholar]
- 68.Wolff, J. A., C. H. MacGregor, R. C. Eisenberg, and P. V. Phibbs, Jr. 1991. Isolation and characterization of catabolite repression control mutants of Pseudomonas aeruginosa PAO. J. Bacteriol. 173:4700-4706. [DOI] [PMC free article] [PubMed] [Google Scholar]