Abstract
Glycerol dialkyl glycerol tetraethers (GDGTs) found in hot springs reflect the abundance and community structure of Archaea in these extreme environments. The relationships between GDGTs, archaeal communities, and physical or geochemical variables are underexamined to date and when reported often result in conflicting interpretations. Here, we examined profiles of GDGTs from pure cultures of Crenarchaeota and from terrestrial geothermal springs representing a wide distribution of locations, including Yellowstone National Park (United States), the Great Basin of Nevada and California (United States), Kamchatka (Russia), Tengchong thermal field (China), and Thailand. These samples had temperatures of 36.5 to 87°C and pH values of 3.0 to 9.2. GDGT abundances also were determined for three soil samples adjacent to some of the hot springs. Principal component analysis identified four factors that accounted for most of the variance among nine individual GDGTs, temperature, and pH. Significant correlations were observed between pH and the GDGTs crenarchaeol and GDGT-4 (four cyclopentane rings, m/z 1,294); pH correlated positively with crenarchaeol and inversely with GDGT-4. Weaker correlations were observed between temperature and the four factors. Three of the four GDGTs used in the marine TEX86 paleotemperature index (GDGT-1 to -3, but not crenarchaeol isomer) were associated with a single factor. No correlation was observed for GDGT-0 (acyclic caldarchaeol): it is effectively its own variable. The biosynthetic mechanisms and exact archaeal community structures leading to these relationships remain unknown. However, the data in general show promise for the continued development of GDGT lipid-based physiochemical proxies for archaeal evolution and for paleo-ecology or paleoclimate studies.
Isoprenoid glycerol dialkyl glycerol tetraethers (GDGTs) are widely used as taxonomic (12, 13, 27, 28) and physicochemical (3, 32, 45, 50, 54, 61; E. S. Boyd, A. Pearson, Y. Pi, W. Li, C. L. Zhang, and G. G. Geesey, unpublished data) biomarkers to study present (1, 3, 24, 35) or ancient (30, 37, 43) communities of Archaea. GDGTs have biphytanyl core lipids with zero to as many as four cyclopentyl rings and zero or one cyclohexyl ring on each side chain (41). Some studies of both pure cultures of Crenarchaeota (11, 16, 50, 51; Boyd et al., unpublished) and of environmental samples (39, 42, 43, 58) show that the number of rings increases with growth temperature, while other studies focusing on different species or environmental settings show that this relationship is weaker or apparently absent (6, 36, 45, 61). Because the increased number of cyclopentyl rings also has been shown experimentally (14, 32; Boyd et al., unpublished) and through surveys (17, 32, 57; Boyd et al., unpublished) to be a response to increased acidity (lower pH), it is likely that multiple variables play significant roles when adjusting archaeal membranes to cope with environmental and energetic stresses (52, 53).
The cyclohexane ring-containing GDGT, crenarchaeol, is widespread in the environment (e.g., see references 18, 24, 31, 36, 39, 41, 46, 56, and 61) and appears to be a biomarker exclusively for Crenarchaeota (42, 46). It originally was proposed to be specific for nonthermophilic species found in marine systems (46). More recently, the environmental niches in which crenarchaeol is found have been expanded to include lacustrine waters (39), soils (31, 56), and terrestrial geothermal environments (36, 61). The formation of a unique cyclohexane ring distinguishes crenarchaeol from the other GDGTs, and it was proposed that this ring reflects physiological and evolutionary adaptation to low-temperature marine environments (30, 46). Thus, the reports of crenarchaeol in geothermal springs at temperatures above 40°C were surprising (36, 61) and have been questioned (45). The recent cultivation, however, of the hyperthermophile “Candidatus Nitrosocaldus yellowstonii” (10) shows that synthesis of crenarchaeol can be abundant in organisms thriving at high temperatures.
Multivariable control of community and lipid compositions results in distributions of environmental GDGTs that can be difficult to interpret. Examination of hot spring data using property-property plots yields inconsistent results among different sets of samples. It was reported that the ring index (weighted number of cyclopentyl rings) does not correlate (36) or only weakly correlates (45, 61) with temperature. In other cases, this index does (36) or does not (61) correlate with alkalinity. Previous data suggest that the abundance of cyclopentyl rings correlates inversely with pH (32, 56), but while this has been shown in pure culture (Boyd et al., unpublished), it has only been examined on a limited basis within an environmental context (32, 45, 61; Boyd et al., unpublished).
For a better understanding of how these competing variables control the distribution of archaeal GDGTs, we integrated our previously published data from terrestrial hot springs (36, 61) with 36 new analyses spanning a wider diversity of geochemical and geographic samples. Principal component analysis (PCA) and cluster analysis are effective methods for analyzing multivariate data to determine the number of significant controlling factors or variables. Here we adopted these methods to study the relationships between GDGTs, temperature, and pH, as well as the relationships among individual GDGTs themselves. Exploration of the internal connections between variables reveals deeper insight into the links between archaeal GDGT profiles and geographic and geochemical parameters, and we speculate that in some cases, these parameters could be universal.
MATERIALS AND METHODS
Source of samples and data.
Forty-three samples of biomass (bacterial mats on sediment surfaces, floating microbial mat communities, and/or mineralized deposits overgrown with biofilm) were collected from hot spring locations in North America and Asia. Seventeen were from the Great Basin region (Nevada and California); 12 were from Yellowstone National Park, United States; 11 were from Kamchatka, Russia; 2 were from Tengchong, China; and one was from Thailand. Relative abundances of GDGTs were measured for each hot spring sample, and in addition, eight pure cultures of Crenarchaeota and three soil samples from locations adjacent to selected hot springs were measured. Temperature and pH were measured for each hot spring at the sampling location. Among the 54 samples discussed here, 18 were reported previously (36, 61), while 36 represent new data (Table 1).
TABLE 1.
HPLC-MS data for GDGT relative abundance, hot spring temperature, and pH
| Sample sourcec | Locationa
|
Temp (°C) | pH | GDGT abundanceb
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| North | West or east | GDGT-0 (m/z 1,302) | GDGT-1 (m/z 1,300) | GDGT-2 (m/z 1,298) | GDGT-3 (m/z 1,296) | GDGT-4 (m/z 1,294) | GDGT-4′ (m/z 1,294) | GDGT-5† (crenarchaeol) | GDGT-5‡ (crenarchaeol isomer) | GDGT-5′ (m/z 1,292) | GDGT-6 (m/z 1,290) | GDGT-6′ (m/z 1,290) | |||
| Yellowstone | |||||||||||||||
| Beowulf-ED1 | 44°43′53.4″ | 110°42′40.9″ (west) | 67 | 3.1 | 0.11 | 0.12 | 0.22 | 0.16 | 0.37 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 |
| Beowulf-ED2 | 44°43′53.4″ | 110°42′ 40.9″ (west) | 62 | 3.1 | 0.12 | 0.12 | 0.21 | 0.17 | 0.31 | 0.00 | 0.00 | 0.00 | 0.05 | 0.02 | 0.00 |
| Beowulf-WD | 44°43′53.4″ | 110°42′41.1″ (west) | 60 | 3.0 | 0.07 | 0.10 | 0.19 | 0.23 | 0.39 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 |
| Calcite Spring-B | 44°54′29.1″ | 110°24′2.42″ (west) | 74 | 8.2 | 0.35 | 0.19 | 0.13 | 0.09 | 0.10 | 0.00 | 0.00 | 0.00 | 0.06 | 0.08 | 0.00 |
| Calcite Spring-C | 44°54′29.1″ | 110°24′2.42″ (west) | 75 | 7.8 | 0.43 | 0.20 | 0.14 | 0.10 | 0.09 | 0.00 | 0.00 | 0.00 | 0.02 | 0.02 | 0.00 |
| Cistern Spring | 44°43′23.1″ | 110°42′14.5″ (west) | 82 | 5.3 | 0.24 | 0.18 | 0.14 | 0.18 | 0.22 | 0.00 | 0.00 | 0.00 | 0.02 | 0.02 | 0.0 |
| Echinus Geyser | 44°43′19.4″ | 110°42′07.6″ (west) | 75 | 3.4 | 0.02 | 0.04 | 0.10 | 0.23 | 0.52 | 0.00 | 0.00 | 0.00 | 0.07 | 0.02 | 0.00 |
| Joseph's Coat | 44°44′21.4″ | 110°19′28.2″ (west) | 82 | 6.2 | 0.14 | 0.20 | 0.21 | 0.23 | 0.15 | 0.00 | 0.00 | 0.00 | 0.03 | 0.04 | 0.00 |
| Monarch Geyser | 44°43′27.5″ | 110°42′20.2″ (west) | 85 | 4.4 | 0.00 | 0.00 | 0.03 | 0.20 | 0.77 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Porcelain Basin | 44°43′43.9″ | 110°42′12.0″ (west) | 75 | 3.5 | 0.00 | 0.00 | 0.04 | 0.12 | 0.39 | 0.00 | 0.00 | 0.00 | 0.27 | 0.18 | 0.00 |
| Perpetual Spouter-B | 44°43′36.0″ | 110°42′29.8″ (west) | 82 | 7.0 | 0.07 | 0.10 | 0.13 | 0.22 | 0.43 | 0.02 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 |
| Whirligig Geyser | 44°43′43.3″ | 110°42′11.3″ (west) | 66 | 3.4 | 0.02 | 0.03 | 0.06 | 0.29 | 0.53 | 0.00 | 0.02 | 0.00 | 0.05 | 0.00 | 0.00 |
| Kamchatka, Russia | |||||||||||||||
| Yellow Blue | 51°29′53.1″ | 160°00′52.6″ (east) | 55 | 6.3 | 0.20 | 0.10 | 0.20 | 0.20 | 0.30 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Zavarzin | NA | NA | 57 | 6.3 | 0.21 | 0.09 | 0.16 | 0.21 | 0.33 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Vent 1, North (K11) | 51°30′40.5″ | 160°00′19.2″ (east) | 64 | 5.8 | 0.27 | 0.11 | 0.17 | 0.18 | 0.23 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | 0.00 |
| Rubber Mat (CR03022) | 51°29′56.3″ | 160°00′55.9″ (east) | 66 | 5.6 | 0.20 | 0.10 | 0.20 | 0.20 | 0.30 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Rubber Mat (JK04078) | 51°29′56.3′ | 160°00′55.9″ (east) | 66 | 6.1 | 0.16 | 0.10 | 0.18 | 0.22 | 0.34 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Arkashin (K1) | 51°30′00.0″ | 160°00′20.0″ (east) | 69 | 4.9 | 0.05 | 0.05 | 0.13 | 0.27 | 0.45 | 0.00 | 0.00 | 0.00 | 0.05 | 0.00 | 0.00 |
| Bubbler (K4) | 51°30′02.4″ | 160°00′27.2″ (east) | 69 | 5.8 | 0.32 | 0.12 | 0.16 | 0.11 | 0.27 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 |
| Blue pool (K7) | 51°30′39.7″ | 160°00′18.7″ (east) | 74 | 5.0 | 0.27 | 0.10 | 0.16 | 0.17 | 0.25 | 0.00 | 0.00 | 0.00 | 0.05 | 0.00 | 0.00 |
| Vent 2, North | 51°30′39.7″ | 160°00′18.7″ (east) | 75 | 5.9 | 0.30 | 0.10 | 0.10 | 0.20 | 0.30 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Vent 2, Jen's site (K3) | 51°30′02.5″ | 160°00′27.3″ (east) | 75 | 5.8 | 0.22 | 0.11 | 0.15 | 0.17 | 0.32 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 |
| Green mat (K2) | 51°30′02.4″ | 160°00′27.2″ (east) | 75 | 5.8 | 0.13 | 0.10 | 0.15 | 0.24 | 0.24 | 0.00 | 0.00 | 0.00 | 0.14 | 0.00 | 0.00 |
| Tengchong, China | |||||||||||||||
| Tengchong-A | NA | NA | 80 | 6.6 | 0.13 | 0.24 | 0.25 | 0.25 | 0.13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Tengchong-C | NA | NA | 80 | 6.6 | 0.13 | 0.11 | 0.16 | 0.25 | 0.30 | 0.00 | 0.00 | 0.00 | 0.05 | 0.00 | 0.00 |
| Pure cultures | |||||||||||||||
| Acidilobus aceticus 1904 | 82 | 3.5 | 0.20 | 0.00 | 0.00 | 0.00 | 0.34 | 0.00 | 0.00 | 0.00 | 0.46 | 0.00 | 0.00 | ||
| Acidilobus saccharovorans 345-15 | 82 | 3.5 | 0.00 | 0.00 | 0.00 | 0.01 | 0.14 | 0.02 | 0.00 | 0.00 | 0.27 | 0.54 | 0.02 | ||
| Metallosphaera sedulad | 75 | 4.5 | 0.07 | 0.15 | 0.23 | 0.25 | 0.26 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | 0.00 | ||
| Sulfolobus solfataricuse | 85 | 4.5 | 0.00 | 0.01 | 0.04 | 0.14 | 0.31 | 0.08 | 0.00 | 0.00 | 0.27 | 0.15 | 0.00 | ||
| Vulcanisaeta moutnovskia 768-28 | 85 | 4.5 | 0.04 | 0.03 | 0.06 | 0.14 | 0.54 | 0.00 | 0.00 | 0.00 | 0.15 | 0.02 | 0.02 | ||
| Thermoproteus uzoniensis Z-605 | 85 | 5.5 | 0.05 | 0.02 | 0.06 | 0.08 | 0.62 | 0.00 | 0.00 | 0.00 | 0.15 | 0.02 | 0.00 | ||
| Desulfurococcus fermentans 1312 | 85 | 6.0 | 0.09 | 0.10 | 0.19 | 0.30 | 0.32 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| Desulfurococcus amylolyticus Z-533 | 85 | 6.5 | 0.08 | 0.06 | 0.11 | 0.20 | 0.55 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| Nevada soils | |||||||||||||||
| Surprise Valley | NA | NA | 0.38 | 0.07 | 0.10 | 0.03 | 0.02 | 0.00 | 0.37 | 0.03 | 0.00 | 0.00 | 0.00 | ||
| Eagleville | NA | NA | 0.20 | 0.20 | 0.10 | 0.05 | 0.00 | 0.00 | 0.39 | 0.06 | 0.00 | 0.00 | 0.00 | ||
| Rick's Hot Creek | NA | NA | 0.36 | 0.07 | 0.09 | 0.00 | 0.00 | 0.00 | 0.37 | 0.12 | 0.00 | 0.00 | 0.00 | ||
NA, not available.
GDGT-5†, chrenarchaeol; GDGT-5‡, crenarchaeol isomer; GDGT-5′, GDGT with five cyclopentyl rings.
Archaeal lipid extraction and HPLC-MS analysis.
Approximately 10 g of each sample was extracted following a modified Bligh-Dyer (2) lipid extraction procedure. Samples were sonicated in phosphate buffer (pH 7.5), CH3OH, and CHCl3 (0.8:1:1) and partitioned into the organic phase by further addition of H2O and CHCl3 to a final ratio of 1.8:1:2. The resulting total lipid extract (TLE) was dried under N2 (gas phase), and 2 ml of 95:5 (vol/vol) methanol and hydrochloric acid was used for transesterification and hydrolysis (70°C, 2 h) to cleave acid-labile polar head groups of GDGTs and phospholipid fatty acids. Transesterified TLE was partitioned by addition of 1:1 H2O-CH2Cl2, and the reconcentrated TLE was passed through a C18 solid-phase extraction column to yield three fractions (polar, GDGT-rich, and nonpolar). The GDGT fraction was used for subsequent high-performance liquid chromatography-mass spectrometry (HPLC-MS) analysis.
The intact GDGTs were identified with an Agilent 1100 series high-performance liquid chromatograph with atmospheric pressure chemical ionization-MS, using published methods (19, 20, 23, 36) and a Prevail Cyano column (2.1 by 150 mm, 3-μm particle size). Conditions for atmospheric pressure chemical ionization-MS were nebulizer pressure of 60 lb/in2, drying gas flow of 6.0 liters/min at 350°C, vaporizer temperature of 375°C, voltage of 3 kV, and corona of 5 μA. Spectra were scanned over the mass range from 1,000 to 1,350 (for branched index of bacterial tetraethers [BIT] plus GDGT) or from 1,250 to 1,350 (for GDGT only). Relative abundances of GDGTs were determined by integration of m/z 1,302, 1,300, 1,298, 1,296, 1,294, 1,292, and 1,290 single-ion chromatograms (SIC) and compared to values from integrated total-ion chromatograms (TIC) when possible (23). Crenarchaeol (4 cyclopentanes plus 1 cyclohexane; m/z 1,292) and GDGT-4 (4 cyclopentanes; m/z 1,294) coelute under these conditions; their respective abundances were decoupled by mass spectral ion fragment balance as described previously (61).
Cluster analysis of GDGTs.
Statistical analyses were performed in the R program (freeware; http://cran.r-project.org/) (33), following the approach of Turich et al. (49). Relative abundance data were imported into R. The function vegdist using the Bray-Curtis distance measure in the vegan package was performed to compute the dissimilarity matrix, and the agnes function in the cluster package was run using a flexible beta method to generate the agglomerative hierarchical clustering tree. Bias in clustering can be caused by the artificial conclusion that a particular GDGT is absent from a sample, when in fact it is merely below detection limits. Here we attempted to minimize any such bias by limiting the analysis to samples in which a minimum of five (and usually six or seven) of the nine GDGTs could be identified, with one exception (Monarch Geyser; just three GDGTs). The same criterion was not applied to the cultured samples, as we believe the limited GDGT distributions observed in these samples are real (6, 11, 16, 32, 50, 51; Boyd et al., unpublished).
PCA.
For PCA, the eigenvectors and eigenvalues were extracted from a covariance matrix calculated from standardized data. Communality (h2), or the percentage of variance explained by summing the factors, was calculated, and application of Joliffe's modified Kaiser criterion (25), as well as Cattell's scree test (9), suggested that four significant factors be retained. Varimax rotation was applied to maximize the variable loadings onto the four designated factors (26). Standardized, rotated factor loadings (r values) for each of the 11 variables on the four, factors, A to D, were computed (Table 2). The factor scores for all individual samples as mapped onto the four factors also were determined. All calculations were performed in Matlab; code for Varimax rotation was obtained from http://w3eos.whoi.edu/12.747/.
TABLE 2.
Total variance loading for each of the 11 variables as represented by factors A to D
| Variablea | Result for factorb:
|
h2 = Σ(r2) | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| Temp (°C) | 0.64 | 0.23 | 0.34 | 0.34 | 0.70 |
| pH | −0.80 | 0.16 | 0.22 | 0.04 | 0.72 |
| GDGT-0 | −0.13 | 0.91 | 0.13 | 0.25 | 0.92 |
| GDGT-1 | 0.03 | 0.40 | 0.04 | 0.88 | 0.94 |
| GDGT-2 | 0.29 | 0.14 | 0.20 | 0.87 | 0.89 |
| GDGT-3 | 0.59 | −0.30 | 0.25 | 0.64 | 0.91 |
| GDGT-4 | 0.86 | −0.38 | 0.18 | −0.03 | 0.92 |
| GDGT-5† | −0.86 | −0.04 | 0.16 | −0.43 | 0.95 |
| GDGT-5‡ | −0.82 | −0.26 | 0.08 | −0.18 | 0.78 |
| GDGT-5′ | 0.49 | 0.05 | −0.60 | −0.49 | 0.84 |
| GDGT-6 | 0.07 | −0.12 | −0.92 | −0.13 | 0.89 |
GDGT-5†, crenarchaeol; GDGT-5‡, crenarchaeol isomer; GDGT-5′, GDGT with five cyclopentyl rings.
The best correlations between variables and factors are in boldface, while those among them that are significant (r2 > 0.5) are underlined.
RESULTS
GDGTs in hot springs and surrounding soils.
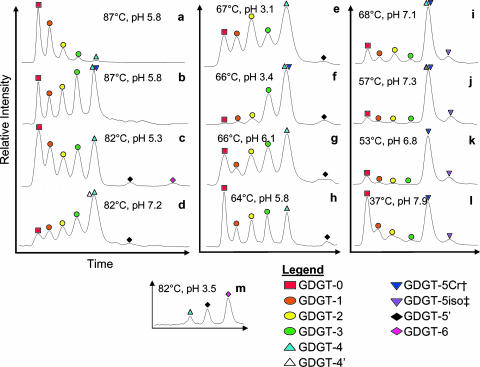
GDGTs measured in hot springs and pure culture samples showed many different patterns (Fig. 1 and Table 1). The greatest variety among GDGT profiles was seen at the highest temperatures (Fig. 1a to d). Samples from >80°C could be observed to have relatively less (Fig. 1a) or relatively more (Fig. 1d) of each GDGT with successively more rings. The sample in Fig. 1d geographically was from Yellowstone, while the one in Fig. 1a was from the Great Basin (61). Microbial communities from high temperatures also sometimes were observed to have a “fingers” profile (Fig. 1b and c), in which the five most common GDGTs (GDGT-0 to -4; m/z 1,302, 1,300, 1,298, 1,296, and 1,294) all were present at similar concentrations. The sample in Fig. 1c was from Yellowstone, while the sample in Fig. 1b was from the same hot spring (Surprise Valley) as the sample in Fig. 1a. These two 87°C Surprise Valley samples were collected within a meter of each other. Figure 1a represents a “streamer” community, while Fig. 1b represents the 0- to 1-cm horizon of microbial mat sitting on the sediment surface at the bottom of the spring.
FIG. 1.
Representative chromatograms for GDGTs from selected hot springs, with temperature and pH values. (a to d) Samples at high temperatures (>80°C): (a) Surprise Valley white streamers (55); (b) Surprise Valley mat community (beneath white streamers) (61); (c) Cistern Spring, Yellowstone; (d) Perpetual Spouter-B, Yellowstone. (e to h) Samples near 65°C: (e) Beowulf ED-1, Yellowstone; (f) Whirligig Geyser, Yellowstone; (g) Rubber Mat, Kamchatka; (h) Vent 1 North, Kamchatka. (i to l) Samples rich in crenarchaeol: (i) Buffalo Valley-2, Nevada (61); (j) Rick's Hot Creek (61); (k) Paradise Valley (36); (l) Seven Devils (61). (m) Pure culture of Acidilobus saccharovorans 345-15.
The “fingers” pattern also was the most common GDGT profile seen in Kamchatka hot springs (e.g., Fig. 1g and h), occurring in all but one (Zavarzin) of these samples. The Kamchatka samples cover a narrower range of temperatures (55 to 75°C) and generally have lower pH values (4.9 to 6.3). The minimum-pH Kamchatka sample (4.9) also was the one from Zavarzin with the unique (among Kamchatka) GDGT profile.
This “fingers” pattern, however, was not universal among middle-temperature (55 to 75°C) hot springs (e.g., Fig. 1f and i to j). Examples include Whirligig Geyser (Fig. 1f), which had relatively more of each GDGT with successively more rings up to m/z 1,294 (four cyclopentyl rings). This is a low-pH hot spring from Yellowstone and is unlike the mid-pH springs from Kamchatka. Other examples of non-“fingers” profiles from 55 to 75°C springs included two Great Basin samples that were rich in crenarchaeol (Fig. 1i and j). These are Buffalo Valley and Rick's Hot Creek, both of which are above pH 7 (61). Among the whole data set, lower-temperature hot springs (<55°C) were fewer in number. All were from the Great Basin (e.g., Fig. 1k and l; abundance data reported in reference 61). These springs tended to have pH values of >7 and to be rich in crenarchaeol.
Several samples had GDGT distributions similar to pure cultures of Crenarchaeota. The data from Porcelain Basin (Yellowstone; 75°C, pH 3.5) were similar to the results from Sulfolobus solfataricus (85°C, pH 4.5). GDGTs from Arkashin and many of the Yellowstone springs (Beowulf, Perpetual Spouter, Whirligig, Echinus Geyser, and Monarch Geyser) were similar to the profiles of Thermoproteus uzoniensis and Vulcanisaeta moutnovskia. However, patterns of GDGTs similar to Acidilobus saccharovorans (Fig. 1m) and Acidilobus aceticus, which had primarily had GDGTs with four to six rings (up to m/z 1,290), were not found among the natural hot spring samples. None of the hot spring samples had five-ring or six-ring GDGTs as the most abundant component, unless the five-ring GDGT was crenarchaeol.
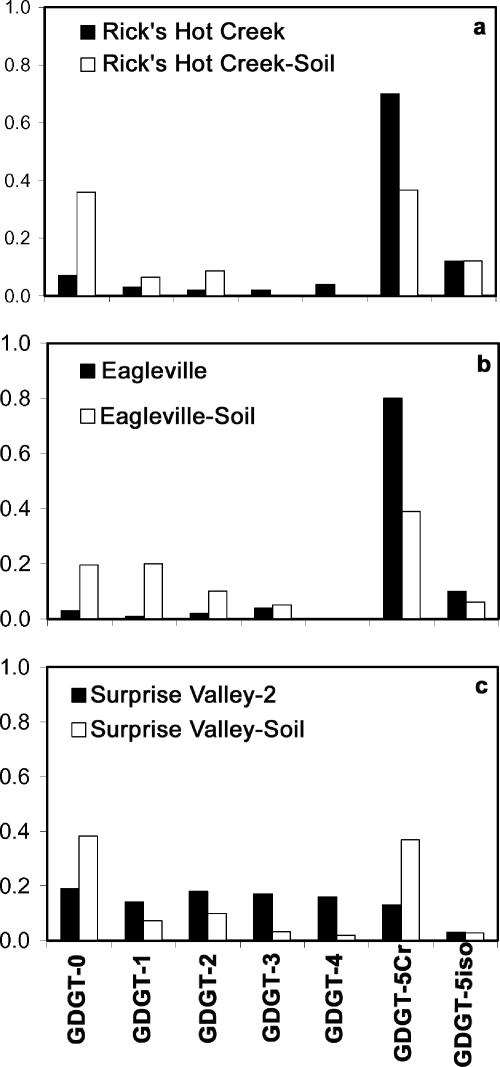
Three soil samples also were collected near Rick's Hot Creek, Eagleville, and Surprise Valley, and GDGT profiles were measured (Table 1). These samples were collected ∼10 m uphill from each spring, to ensure that the soil was not impacted by hydrothermal runoff (21). Crenarchaeol was relatively more abundant in Rick's Hot Creek and Eagleville hot springs than in nearby soils, while GDGT-0, -1, and -2 (m/z 1,302, 1,300, and 1,298) were relatively less abundant in the springs than in the soils (Fig. 2). The crenarchaeol in these two springs could not be exclusively soil derived, unless any concomitant input of GDGT-0, -1, and -2 simultaneously was degraded.
FIG. 2.
Histograms comparing relative abundances of GDGTs from three crenarchaeol-rich hot springs and their surrounding soils. (a) Rick's Hot Creek. (b) Eagleville. (c) Surprise Valley. Cr, crenarcheaeol; iso, crenarchaeol isomer.
In the case of Surprise Valley soil (Fig. 2c), however, the data were more ambiguous. The vent wall sample (56°C) from Surprise Valley contained small enough relative quantities of crenarchaeol (0.13) that the soil end member from Surprise Valley (0.37; Table 1) could be the source. However, as the sample was wall derived (sinter) and not in contact with muds on the floor of the spring, soil remains unlikely. We also cannot disregard the possibility that the trace (<5%) crenarchaeol in samples such as the higher-temperature Surprise Valley samples (from the mud floor of the spring) and Whirligig Geyser, Yellowstone, derived from surrounding soils. The presence of crenarchaeol in all of these springs could reflect archaeal communities that reside near the edges of the springs, either in contact with geothermally impacted soils or at the boundary between the spring and the soil, but the data effectively dispel any idea that geothermally unimpacted soils are significant sources of crenarchaeaol for these hot springs.
We also measured BIT values (20), both from the hot springs and from the soils in the vicinity of hot springs. BIT values were lower for hot springs than for their paired soils. Direct comparison of the hot spring and soil from Eagleville illustrates an example: although Eagleville hot spring BIT (0.58) is relatively high among our hot springs (BIT for all measured hot springs, 0.03 to 0.66), the surrounding soil BIT is 0.81.
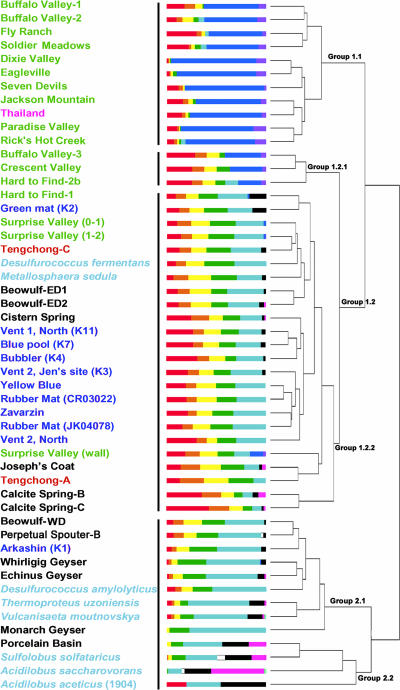
Cluster analysis.
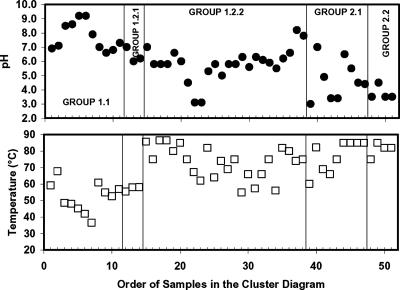
To examine these qualitative patterns, cluster analysis was performed (e.g., see reference 49). The data fell into two major groups of GDGT distributions. Within the first group, three subcategories with different characteristics were adopted (Fig. 3). To aid visualization, a separate figure (Fig. 4) shows the temperature and pH of each sample in descending order from the top to the bottom of the cluster diagram.
FIG. 3.
GDGT cluster tree with sample labels. Labels for sample locations are color coded: Nevada, green; Yellowstone, black; Kamchatka, dark blue; Tengchong, red; Thailand, pink; and pure cultures, light blue. Histograms of GDGTs are colored from left (GDGT-0, red) to right (GDGT-6′, turquoise), in the order of the GDGT columns in Table 1 and the marker symbols in Fig. 1. Group designations are as discussed in the text.
FIG. 4.
Temperature and pH data for samples in the order in which they appear in the cluster diagram (Fig. 3). Sample 1 represents Buffalo Valley-1, while sample 51 represents Acidilobus aceticus 1904.
In Fig. 3, the samples falling within group 1.1 were exclusively from the North American Great Basin and Thailand. They tend to have alkaline or neutral pH and temperatures below the ∼73°C thermal cutoff (7, 15) for photosynthesis (Fig. 4). The pH values range from 6.6 to 9.2, and the average is 7.7. The temperatures range from 36.5°C to 67.8°C, and the average is 52°C. Group 1.1 appears to be defined by samples that have dominant abundances of crenarchaeol.
Reflecting an apparently more widespread GDGT pattern, representatives of nearly all of the geographic sampling locations are found within group 1.2. Group 1.2 includes all of the samples with the “fingers” pattern. A subgroup (group 1.2.1) has measurable, but lower, abundances of crenarchaeol than group 1.1. However, the majority of group 1.2 falls into a second subgroup (1.2.2) which is nearly free of crenarchaeol. On average, group 1.2 hot springs have higher temperatures and a wider range of acidity than group 1.1. The pH values range from 3.1 to 8.2 (average, 5.9). Only 2 of 27 samples in this group are alkaline (pH 7.8 and 8.2). The temperatures range from 55.0°C to 86.5°C, and the average is 71°C.
Group 2.1 shows a dominance of high-temperature samples (range, 60.1 to 85.0°C; average, 77°C) and a wide range of pHs (from 3.0 to 7.0). The average pH (4.7) is much more acidic than group 1. All but one of the environmental samples in this group are from Yellowstone, and the GDGT profiles tend to have high relative abundances of GDGT-4 (m/z 1,294). Cultured Crenarchaeota primarily fall in this group as well, and they contain mostly GDGT-4 to -6. The four samples at the bottom of the figure (group 2.2; Porcelain Basin, Sulfolobus, and Acidilobales spp.) have an average temperature of 81°C and pH of 3.8.
The overall pattern is guided by opposing trends of temperature and pH (Fig. 4). The grouping of archaeal GDGTs appears to be related to both of these variables, although the degree of heterogeneity in group 1.2.1, in particular, is quite large. To examine whether these patterns are statistically significant and to attempt to enumerate the controlling variable(s), the data were examined by PCA.
PCA.
A PCA was run using 11 variables: temperature, pH, and all GDGTs shown in Table 1, except for GDGT-4′ and GDGT-6′, which are rarely occurring, unidentified isomers of GDGT-4 (m/z 1,294) and GDGT-6 (m/z 1,290). After extracting eigenvalues from the normalized data, the data were best explained by four factors (A, B, C, and D). The Varimax-rotated factor loadings (r values) for the 11 variables are shown in Table 2. The factor that yielded the best correlation with each variable is shown in boldface in the table (i.e., one factor highlighted per row). All of the underlined values had individually significant correlation (r2 > 0.5). For some variables, namely, temperature and GDGT-5′ (m/z 1,292′, five cyclopentyl rings), no single factor significantly explained the variance. All others were significantly correlated to a single factor. The factors are arranged in order of the greatest (A) to least (D) fraction of the total cumulative variance that was explained by that factor.
Factor A shows that the qualitative relationships previously observed between pH, GDGT-4 (m/z 1,294), and crenarchaeol (m/z 1,292) are significant. The correlation between pH (r = −0.80) and GDGT-4 (r = 0.86) is inverse in direction, reflecting the fact that more GDGT-4 is likely to be found at lower pH (e.g., group 2; Fig. 3). The correlation between pH (r = −0.80) and crenarchaeol (r = −0.86) is in the same direction, reflecting a greater abundance of crenarchaeol at higher pH (e.g., group 1.1; Fig. 3). The most significant amount of the temperature variance also was associated with factor A (r = 0.64), although with relatively poor correlation (r2 = 0.41). It is revealing nonetheless, as it confirms the direction of association (lower temperature with higher pH) while showing that the correlation with GDGT distributions is better for pH than for temperature. Such effects have been observed for other GDGTs (32; Boyd et al., unpublished).
Factor B was associated with only one measured variable, GDGT-0. It also had one of the highest factor scores in the table (0.91), and as the second-rank factor, accounts for the second-highest fraction of the total variance within the samples. This provides strong support for its independence with respect to other GDGTs, as well as from constraints imposed by the physical variables temperature and pH. The uniqueness of GDGT-0 may be due to its expected contribution from Euryarchaeota as well as Crenarchaeota. While both acyclic and cyclopentyl ring-containing GDGTs have been reported in Euryarchaeota (3, 32), the acyclic compound in particular is reported frequently among Euryarchaeota (12, 13, 48).
Factor C groups the variables GDGT-5′ (all cyclopentyl, m/z 1,292) and GDGT-6 (m/z 1,290), which occur primarily in pure cultures but more rarely in environmental samples. GDGTs in this category favor the hottest, most acidic springs (group 2.2; Fig. 3). Factor D shows a significant association between the compounds containing one to three cyclopentyl rings (GDGT-1 to GDGT-3; m/z 1,300, 1,298, and 1,296, respectively). Neither factor C nor D explains a significant amount of the variance associated with the variable pH or temperature.
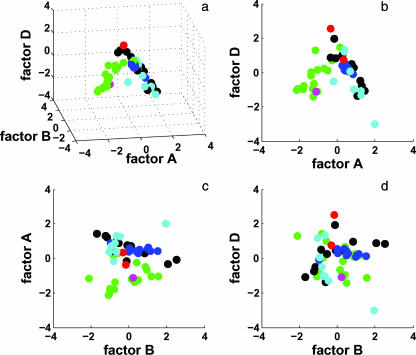
A three-dimensional plot of the factor scores for all samples is shown in Fig. 5a; only A, B, and D are shown, as few samples have high scores for factor C. It is apparent that most of the samples from the Great Basin (and the one from Thailand) are distinct from the other environments, consistent with cluster group 1.1. Samples from Yellowstone, Kamchatka, and China (YKC samples) cluster around an anticorrelated relationship between factors A and D (Fig. 5a and b). The positive relationship between factors A and D for the Great Basin samples is a direct reflection of their high concentrations of crenarchaeol and thus low scores for factor A. Conversely, YKC samples have more GDGT-4 at higher temperatures and lower pH, leading to high scores for factor A. The general lack of overlap between the two major families of GDGT-bearing samples (Great Basin and YKC; loosely equivalent to groups 1 and 2 in Fig. 3) results in a V-like pattern (Fig. 5b). The grouping of crenarchaeal cultures with YKC samples is consistent with the original sources from which these strains were cultivated (4-6, 22, 38, 40, 62).
FIG. 5.
Results from PCA. Samples are colored to match the geographic color codes used in Fig. 3. (a) The relationship between environmental factors A, B, and D shows that the samples form a “tent” or V shape around an apex between factors A and D. (b) Factor D relative to factor A shows the grouping of Yellowstone and Kamchatka samples with pure cultures and Great Basin (Nevada and California) samples with the single Thailand sample. (c and d) There is no pattern in the relationship between factor A or D and factor B, but samples from common geographic origins tend to cluster together.
A plot of factor D versus B (the putative factor for euryarchaeotal influence) shows that there is no obvious relationship between the two (Fig. 5d); the same holds true for all other factors when compared to factor B (Fig. 5c).
DISCUSSION
Positive correlations between ring index (weighted average number of cyclopentyl rings) and temperature (50, 51; Boyd et al., unpublished), and negative correlations with pH (Boyd et al., unpublished), have been observed in pure cultures of thermophilic Archaea. Similar patterns are inconsistently observed in terrestrial geothermal systems within our own work (36, 61) as well as by others (45). Far more reliable is the correlation between marine surface water temperatures and TEX86, an index constructed from the abundances of GDGTs recovered from low-temperature marine and lacustrine environments. The linear correlation between TEX86 and temperature holds over a wide range of aquatic settings (18, 39, 42, 58, 59). However, when values of TEX86 are calculated from GDGTs from cultured Crenarchaeota (50, 51; Boyd et al., unpublished) and are compared to marine environmental samples, the slopes of these temperature relationships are not the same. If universal, the TEX86 relationship would require that GDGT-1 disappear above 36 to 48°C (42, 43), and thus its presence in cultures and geothermal springs above 65°C (Table 1) (36, 61) indicates that other environmental or community variables also influence archaeal GDGT distributions.
The samples as grouped by cluster analysis (Fig. 3) were most strongly distributed according to the compounds GDGT-4 and crenarchaeol. Hot-temperature, low-pH springs (groups 2.1 and 2.2) generally were distinct and never contained crenarchaeol. The representatives of cultured species of Crenarchaeota analyzed here also fell mainly into these groups. In contrast, presumably the newly reported thermophilic, ammonia-oxidizing crenarchaeon “Candidatus Nitrosocaldus yellowstonii” (10) would fall into group 1.1 or group 1.2.1. It grows optimally at 72°C under slightly alkaline conditions and produces abundant crenarchaeol. The division between crenarchaeol-rich and crenarchaeol-poor samples, while dominated by the influence of pH (factor A; Table 2 and Fig. 5), also appears to be related to temperature. Geothermal samples collected at temperatures of >∼75°C generally do not contain large amounts of crenarchaeol, even at high pH. Interestingly, this apparent cutoff temperature is very similar to the upper thermal limit (∼73°C) for photosynthetic communities (7, 15). “Candidatus Nitrosocaldus yellowstonii” may grow naturally within a community that lives at the cusp of this boundary.
In PCA, communality (h2) is analogous to reported R2 values for linear regressions (Table 2). Among these samples, all of the GDGTs have ≥70% of their total communality explained using four orthogonal factors, A, B, C, and D. The identification of four, rather than two, significant factors is consistent with our observations that GDGT profiles are functions of more than just temperature and pH. One such example is shown in Fig. 1a and b. These samples have the same environmental temperature and pH but different GDGT patterns, indicating there must be more than two total factors that control GDGT abundances. Examples of inorganic controls might be salinity, total dissolved solids, total inorganic carbon, alkalinity, NH4+, S2−, H2, CH4, or major cations. Other controls could be biotic, which might be detected as large differences in abundance of particular crenarchaeotal or euryarchaeotal genera. A more complete analysis of the geochemical distributions in these springs, in combination with additional phylogenetic information (21), is needed to provide more insight into these GDGT patterns. The four factors may reflect differences in community structure and/or in metabolic expression (e.g., ammonia oxidation versus hydrogen or sulfur oxidation). Great Basin springs are rich in Na+, Cl−, SiO2, and HCO3− (60); while communities in Yellowstone often live in acidic conditions and often are sustained by the oxidation of H2 (47) and/or sulfur compounds (34).
Temperature also may be an important influence within each of the different factors. This could account for the relatively even splitting of its variance among factors A to D. This also would be consistent with other observations indicating, for example, that multiring tetraethers are not physiological requirements for survival at high temperatures. The extremely thermophilic euryarchaeote Methanopyrus kandleri was observed to produce exclusively or mostly glycerol diethers (29, 48), while in situ measurements indicate that multiringed GDGTs are produced at depth in the open ocean and in the Black Sea, at temperatures below 10°C (8, 24, 55).
Because GDGT-0 does not vary systematically with any of the other variables (factor B), we propose that GDGT-0 is heavily influenced by the presence of euryarchaeota. While euryarchaeota are known to synthesize both GDGT-0 and GDGTs containing cyclopentyl rings (3, 32, 44), the influence of crenarchaeota on the abundance of ring-containing GDGTs appears to be strong enough to decouple these compounds from variations in GDGT-0. Since factor B accounts for the second-largest amount of total variance among the data, seemingly without identifiable pattern (Fig. 5), our results support its continued exclusion from current and future GDGT-derived biogeochemical proxies such as TEX86 (20, 42, 57).
Because the cultured species of hyperthermophilic, acid-tolerant archaea are heavily clustered on factor C, this factor could represent communities rich in thermoacidophiles. Community analysis of hot spring samples weighted onto factor C might reveal a dominance of Sulfolobales and Acidilobales. At least one environmental sample (Porcelain Basin) was rich in GDGT-6. Conversely, if uncultured relatives of these archaeal groups are found at more moderate temperatures, communities growing at these lower temperatures could be found to be heavily loaded on factor C. An example is Beowulf-ED2, which was measured at only 62°C but contained some GDGT-6.
Most of the GDGTs associated with the TEX86 temperature index covary with factor D. The factor loadings indicate that GDGT-1 to -3 show a stronger relationship to each other than they do to temperature. However, despite factor D being a weak function of temperature, it is important to recognize that the result does not preclude systematic variation of these compounds with respect to each other as a function of temperature. This principle forms the basis for the TEX86 proxy (42) and is encouraging for TEX86: it suggests that because GDGT-1, -2, and -3 may be under the control of the same process or community, they are likely to have mutually similar, consistent, and predictable relationships as a function of temperature.
Such analysis also supports the original decision to exclude GDGT-0 and crenarchaeol from the TEX86 formula. As noted originally (42), the variance in these compounds in marine samples appeared to overwhelm the signal from GDGT-1 to -3. Here the association of crenarchaeol and GDGT-0 with the top-ranked (greatest variance) factors, A and B, agrees. The TEX86 formula effectively isolates a set of GDGTs from these large variances and then relates them to temperature. The sensitivities of this relationship (slope of rings versus temperature) certainly are different among the different settings (marine and geothermal), but the community or geochemical driver that causes these compounds to coassociate could be universal.
However, the PCA results also suggest reexamination of the influence of crenarchaeol and its isomer on TEX86. These compounds may show greater variability in nonbuffered systems, due to their high variance with pH. This could explain some of the difficulties of applying the TEX86 index to small lakes (39). Factor A predicts that an increase in pH would cause crenarchaeol and its isomer to increase, raising the apparent temperature. Similarly, low pH could be mistaken for cooler temperatures. pH effects on aquatic samples should be considered along with other explanations such as terrestrially derived GDGTs (18, 39, 56, 57). Some precedent for joint pH-temperature adjustment of bacterial GDGT-based paleoproxies in soils already exists (57). An important caveat is that our argument assumes factor A applies globally, which certainly requires further investigation. Such an interpretation would, however, provide an explanation for why modern marine TEX86 values are so reliable: the ocean is highly buffered, and pH variability is small.
The finding that crenarchaeol and GDGT-4 are stronger functions of pH than temperature is inconsistent with our previous reports (36, 61). However, all of those earlier samples remain as a subset of the data reported here; they are less diverse geographically and have a smaller spread in pH values. The integrated data are consistent with temperature exerting the primary control when the pH range is narrow, but remaining uncertainties argue for continued expansion of the breadth and geography of sampling in the future.
We also find that very few samples have significant quantities of both GDGT-4 and crenarchaeol. Crenarchaeol is present at neutral or alkaline pH, but it has not yet been found under acidic conditions (<pH 6). The data qualitatively support the suggestion that crenarchaeol may be a biomarker for archaeal ammonia oxidation (10), something that will be explored further as more strains of ammonia-oxidizing crenarchaeota are cultivated. At present, the only two characterized strains of archaeal ammonia oxidizers, Nitrosopumilus maritimus and “Candidatus Nitrosocaldus yellowstonii,” both produce crenarchaeol.
Conclusions.
GDGTs have been found widely distributed in aquatic and terrestrial communities. Their relative abundances reflect archaeal community structure and/or the associated geochemical conditions. Because of the potential for metabolic similarity among marine, terrestrial, and hot spring crenarchaeota, studying the factors that affect the distribution of hot spring GDGTs could provide hints to what may be universal controls on GDGT compositions. Based on our results from 54 samples, we find that variance in GDGT profiles primarily involves GDGT-0, GDGT-4, and crenarchaeol. This is consistent with marine and lacustrine settings in which GDGT-0 and crenarchaeol have larger variances than GDGT-1 to -3. We also find that while we cannot identify any predictor for GDGT-0, the covariance of GDGT-4 and crenarchaeol is such that one appears to nearly replace the other under acidic or alkaline conditions, respectively, especially at ≤∼73°C. The metabolic or community mechanism leading to this anticorrelation remains to be determined. The hypothesis that crenarchaeol could be a biomarker for archaeal ammonia oxidation in nonacidic environments (10) is apparently supported by the predominance of crenarchaeol in circumneutral or alkaline hot springs in the Great Basin. The grouping of GDGT-1 to -3 provides theoretical support for the formulation of the TEX86 paleotemperature index, but it also suggests that the role of the crenarchaeol isomer in TEX86 should be reexamined. If its abundance is positively correlated with the abundance of crenarchaeol, then it is likely that this compound is sensitive to variations in pH.
Acknowledgments
We thank David and Sandy Jamieson and Suzy Jackson for their hospitality during sampling in Nevada and California. We also thank Paul Schroeder and Doug Crowe and colleagues from the Microbiology Institute of the Russian Academy of Sciences (Elizaveta Bonch-Osmolavskaya, Nikolai Pimenov, and Tatyana Sokolova) and the Institute of Volcanology (Gennadii A. Karpov) for field support during sampling of Kamchataka hot springs in 2003 and 2004. Soil samples from Nevada were provided by B. Hedlund. Suping Yao provided the two hot spring samples collected from Tengchong, China. We appreciate collaboration with A.-L. Reysenbach regarding samples collected at Calcite Springs, Yellowstone National Park (YNP), and we are grateful to C. Hendrix and T. Olliff (Center for Resources, YNP) for permitting the research conducted with samples collected within YNP. We thank C. Turich for advice on cluster analyses. Three anonymous reviewers provided excellent, detailed comments that greatly improved the manuscript.
C.L.Z. and C.S.R. were supported by NSF MCB 0348180. W.P.I. was supported by NASA NNG04GR46G, NSF MCB-0132022, and NSF RCN-0342269. A.P. was supported by NSF OCE-0241363, NSF EAR-0311937, and the David & Lucille Packard Foundation.
Footnotes
Published ahead of print on 4 April 2008.
REFERENCES
- 1.Biddle, J. F., J. S. Lipp, M. A. Lever, K. G. Lloyd, K. B. Sorensen, R. Anderson, H. K. Fredricks, M. Elvert, T. J. Kelly, D. P. Schrag, M. L. Sogin, J. E. Brenchley, A. Teske, C. H. House, and K. U. Hinrichs. 2006. Heterotrophic Archaea dominate sedimentary subsurface ecosystems off Peru. Proc. Natl. Acad. Sci. USA 103:3846-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bligh, E. G., and W. J. Dyer. 1959. A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 3.Blumenberg, M., R. Seifert, K. Nauhaus, T. Pape, and W. Michaelis. 2005. In vitro study of lipid biosynthesis in an anaerobically methane-oxidizing microbial mat. Appl. Environ. Microbiol. 71:4345-4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonch-Osmolovskaya, E. A., A. I. Slesarev, M. L. Miroshnichenko, T. P. Svetlichnaya, and V. A. Alekseev. 1988. Characteristics of Desulfurococcus amylolyticus n. sp.—a new extremely thermophilic archaebacterium isolated from thermal springs of Kamchatka and Kunashir Island. Mikrobiologiya 57:94-101. (In Russian.) [Google Scholar]
- 5.Bonch-Osmolovskaya, E. A., M. L. Miroshnichenko, N. A. Kostrikina, N. A. Chernyh, and G. A. Zavarzin. 1990. Thermoproteus uzoniensis sp. nov., a new extremely thermophilic archaebacterium from Kamchatka continental hot springs. Arch. Microbiol. 154:556-559. [Google Scholar]
- 6.Boyd, E. S., R. A. Jackson, G. Encarnacion, J. A. Zahn, T. Beard, W. D. Leavitt, Y. Pi, C. L. Zhang, A. Pearson, and G. G. Geesey. 2007. Isolation, characterization, and ecology of sulfur-respiring Crenarchaea inhabiting acid-sulfate-chloride-containing geothermal springs in Yellowstone National Park. Appl. Environ. Microbiol. 73:6669-6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brock, T. D. 1967. Micro-organisms adapted to high temperatures. Nature 214:882-885. [DOI] [PubMed] [Google Scholar]
- 8.Coolen, M. J. L., B. Abbas, J. van Bleijswijk, E. C. Hopmans, M. M. M. Kuypers, S. G. Wakeham, and J. S. Sinninghe Damsté. 2007. Putative ammonia-oxidizing Crenarchaeota in suboxic waters of the Black Sea: a basin-wide ecological study using 16S ribosomal and functional genes and membrane lipids. Environ. Microbiol. 9:1001-1016. [DOI] [PubMed] [Google Scholar]
- 9.Davis, J. C. 2002. Statistics and data analysis in geology, 3rd ed. John Wiley and Sons, New York, NY.
- 10.De La Torre, J. R., C. B. Walker, A. E. Ingalls, M. Könneke, and D. A. Stahl. 2008. Cultivation of a thermophilic ammonia oxidizing archaeon synthesizing crenarchaeol. Environ. Microbiol. 10:810-818. [DOI] [PubMed]
- 11.De Rosa, M., E. Esposito, A. Gambacorta, B. Nicolaus, and J. D. Bu'Lock. 1980. Effects of temperature on the lipid composition of Caldariella acidophila. Phytochemistry 19:827-831. [Google Scholar]
- 12.De Rosa, M., A. Gambacorta, and A. Gliozzi. 1986. Structure, biosynthesis, and physicochemical properties of archaebacterial lipids. Microbiol. Rev. 50:70-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Rosa, M., and A. Gambacorta. 1988. The lipids of archaebacteria. Prog. Lipid Res. 27:153-175. [DOI] [PubMed] [Google Scholar]
- 14.Elferink, M. G., J. G. de Wit, A. J. Driessen, and W. N. Konings. 1994. Stability and proton-permeability of liposomes composed of archaeal tetraether lipids. Biochim. Biophys. Acta 1193:247-254. [DOI] [PubMed] [Google Scholar]
- 15.Ferris, M. J., and D. M. Ward. 1997. Seasonal distributions of dominant 16S rRNA-defined populations in a hot spring microbial mat examined by denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 63:1375-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gliozzi, A., G. Paoli, M. DeRosa, and A. Gambacorta. 1983. Effect of isoprenoid cyclization on the transition temperature of lipids in thermophilic archaeabacteria. Biochim. Biophys. Acta 735:234-242. [Google Scholar]
- 17.Golyshina, O. V., and K. N. Timmis. 2005. Ferroplasma and relatives, recently discovered cell wall-lacking archaea making a living in extremely acid, heavy metal-rich environments. Environ. Microbiol. 7:1277-1288. [DOI] [PubMed] [Google Scholar]
- 18.Herfort, L., S. Schouten, J. P. Boon, and J. S. Sinninghe Damsté. 2005. Application of the TEX86 temperature proxy to the southern North Sea. Org. Geochem. 37:1715-1726. [Google Scholar]
- 19.Hopmans, E. C., S. Schouten, R. D. Pancost, M. T. J. van der Meer, and J. S. Sinninghe Damsté. 2000. Analysis of intact tetraether lipids in archaeal cell material and sediments by high performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry. Rapid Commun. Mass Spectrom. 14:585-589. [DOI] [PubMed] [Google Scholar]
- 20.Hopmans, E. C., J. W. H. Weijers, E. Schefu, L. Herfort, J. S. Sinninghe Damsté, and S. Schouten. 2004. A novel proxy for terrestrial organic matter in sediments based on branched and isoprenoid tetraether lipids. Earth Planet. Sci. Lett. 224:107-116. [Google Scholar]
- 21.Huang, Z., B. P. Hedlund, J. Wiegel, J. Zhou, and C. L. Zhang. 2007. Molecular phylogeny of uncultivated Crenarchaeota in Great Basin hot springs of moderately elevated temperature. Geomicrobiol. J. 24:535-542. [Google Scholar]
- 22.Huber, G., C. Spinnler, A. Gambacorta, and K. O. Stetter. 1989. Metallosphaera sedula gen. nov. and sp. nov. represents a new genus of aerobic, metal-mobilizing, thermoacidophilic archaebacteria. Syst. Appl. Microbiol. 12:38-47. [Google Scholar]
- 23.Huguet, C., E. C. Hopmans, W. Febo-Ayala, D. H. Thompson, J. S. Sinninghe Damsté, and S. Schouten. 2006. An improved method to determine the absolute abundance of glycerol dibiphytanyl glycerol tetraether lipids. Org. Geochem. 37:1036-1041. [Google Scholar]
- 24.Ingalls, A. E., S. R. Shah, R. L. Hansman, L. I. Aluwihare, G. M. Santos, E. R. M. Druffel, and A. Pearson. 2006. Quantifying archaeal community autotrophy in the mesopelagic ocean using natural radiocarbon. Proc. Natl. Acad. Sci. USA 103:6442-6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joliffe, I. T. 1986. Principal component analysis. Springer-Verlag, New York, NY.
- 26.Kaiser, H. F. 1958. The varimax criterion for analytic rotation in factor analysis. Psychometrika 23:187-200. [Google Scholar]
- 27.Koga, Y., H. Morii, M. Akagawa-Matsushita, and M. Ohga. 1998. Correlation of polar lipid composition with 16S rRNA phylogeny in methanogens: further analysis of lipid component parts. Biosci. Biotechnol. Biochem. 62:230-236. [DOI] [PubMed] [Google Scholar]
- 28.Koga, Y., and H. Morii. 2005. Recent advances in structural research on ether lipids from archaea including comparative and physiological aspects. Biosci. Biotechnol. Biochem. 69:2019-2034. [DOI] [PubMed] [Google Scholar]
- 29.Kurr, M., R. Huber, H. Konig, H. W. Jannasch, H. Fricke, A. Trincone, J. K. Kristjansson, and K. O. Stetter. 1991. Methanopyrus kandleri, gen. and sp. nov. represents a novel group of hyperthermophilic methanogens, growing at 110°C. Arch. Microbiol. 156:239-247. [Google Scholar]
- 30.Kuypers, M. M. M., P. Blokker, J. Erbacher, H. Kinkel, R. D. Pancost, S. Schouten, and J. S. Sinninghe Damsté. 2001. Massive expansion of marine archaea during a mid-Cretaceous oceanic anoxic event. Science 293:92-94. [DOI] [PubMed] [Google Scholar]
- 31.Leininger, S., T. Urich, M. Schloter, L. Schwark, J. Qi, G. W. Nicol, J. I. Prosser, S. C. Schuster, and C. Schleper. 2006. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806-809. [DOI] [PubMed] [Google Scholar]
- 32.Macalady, J. L., M. M. Vestling, D. Baumler, N. Boekelheide, C. W. Kaspar, and J. F. Banfield. 2004. Tetraether-linked membrane monolayers in Ferroplasma spp.: a key to survival in acid. Extremophiles 8:411-419. [DOI] [PubMed] [Google Scholar]
- 33.Maindonald, J., and J. Braun. 2003. Data analysis and graphics using R—an example-based approach. Cambridge University Press, Cambridge, United Kingdom.
- 34.Nordstrom, D. K., J. W. Ball, and R. B. McCleskey. 2005. Ground water to surface water: chemistry of thermal outflows in Yellowstone National Park, p. 143-162. In W. P. Inskeep and T. R. McDermott (ed.), Geothermal biology and geochemistry in Yellowstone National Park, vol. 1. Montana State University, Bozeman. [Google Scholar]
- 35.Pancost, R. D., S. Pressley, J. M. Coleman, L. G. Benning, and B. W. Mountain. 2005. Lipid biomolecules in silica sinters: indicators of microbial biodiversity. Environ. Microbiol. 7:66-77. [DOI] [PubMed] [Google Scholar]
- 36.Pearson, A., Z. Huang, A. E. Ingalls, C. S. Romanek, J. Wiegel, K. H. Freeman, R. H. Smittenberg, and C. L. Zhang. 2004. Nonmarine crenarchaeol in Nevada hot springs. Appl. Environ. Microbiol. 70:5229-5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peckmann, J., and V. Thiel. 2004. Carbon cycling at ancient methane-seeps. Chem. Geol. 205:443-467. [Google Scholar]
- 38.Perevalova, A. A., V. A. Svetlichny, I. B. Kublanov, N. A. Chernyh, N. A. Kostrikina, T. P. Tourova, B. B. Kuznetsov, and E. A. Bonch-Osmolovskaya. 2005. Desulfurococcus fermentans sp. nov., a novel hyperthermophilic archaeon from a Kamchatka hot spring, and emended description of the genus Desulfurococcus. Int. J. Syst. Evol. Microbiol. 55:995-999. [DOI] [PubMed] [Google Scholar]
- 39.Powers, L. A., J. P. Werne, T. C. Johnson, E. C. Hopmans, J. S. Sinninghe Damsté, and S. Schouten. 2004. Crenarchaeotal membrane lipids in lake sediments: a new paleotemperature proxy for continental paleoclimate reconstruction? Geology 32:613-616. [Google Scholar]
- 40.Prokofeva, M., M. Miroshnichenko, N. Kostrikina, N. Chernyh, B. Kuznetsov, T. Tourova, and E. Bonch-Osmolovskaya. 2000. Acidilobus aceticus gen. nov., sp. nov., a novel anaerobic thermoacidophilic archaeon from continental hot vents in Kamchatka. Int. J. Syst. Evol. Microbiol. 50:2001-2008. [DOI] [PubMed] [Google Scholar]
- 41.Schouten, S., E. C. Hopmans, R. D. Pancost, and J. S. Sinninghe Damsté. 2000. Widespread occurrence of structurally diverse tetraether membrane lipids: evidence for the ubiquitous presence of low-temperature relatives of hyperthermophiles. Proc. Natl. Acad. Sci. USA 97:14421-14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schouten, S., E. C. Hopmans, E. Schefuß, and J. S. Sinninghe Damsté. 2002. Distributional variations in marine crenarchaeotal membrane lipids: a new tool for reconstructing ancient sea water temperatures? Earth Planet. Sci. Lett. 204:265-274. [Google Scholar]
- 43.Schouten, S., E. C. Hopmans, A. Forster, Y. van Breugel, M. M. M. Kuypers, and J. S. Sinninghe Damsté. 2003. Extremely high sea-surface temperatures at low latitudes during the middle Cretaceous as revealed by archaeal membrane lipids. Geology 31:1069-1072. [Google Scholar]
- 44.Schouten, S., S. G. Wakeham, E. C. Hopmans, and J. S. Sinninghe Damsté. 2003. Biogeochemical evidence that thermophilic archaea mediate the anaerobic oxidation of methane. Appl. Environ. Microbiol. 69:1680-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schouten, S., M. T. J. van der Meer, E. C. Hopmans, W. I. C. Rijpstra, A.-L. Reysenbach, D. M. Ward, and J. S. Sinninghe Damsté. 2007. Archaeal and bacterial glycerol dialkyl glycerol tetraether lipids in hot springs of Yellowstone National Park. Appl. Environ. Microbiol. 73:6181-6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sinninghe Damsté, J. S., E. C. Hopmans, S. Schouten, A. C. T. van Duin, and J. A. J. Geenevasen. 2002. Crenarchaeol: the characteristic glycerol dibiphytanyl glycerol tetraether membrane lipid of cosmopolitan pelagic Crenarchaeota. J. Lipid Res. 43:1641-1651. [DOI] [PubMed] [Google Scholar]
- 47.Spear, J. R., J. J. Walker, T. M. McCollom, and N. R. Pace. 2005. Hydrogen and bioenergetics in the Yellowstone hydrothermal system. Proc. Natl. Acad. Sci. USA 102:2555-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sprott, G. D., B. J. Agnew, and G. B. Patel. 1997. Structural features of ether lipids in the archaeobacterial thermophiles Pyrococcus furiosus, Methanopyrus kandleri, Methanothermus fervidus, and Sulfolobus acidocaldarius. Can. J. Microbiol. 43:467-476. [Google Scholar]
- 49.Turich, C., K. H. Freeman, M. A. Bruns, M. Conte, A. D. Jones, and S. G. Wakeham. 2007. Lipids of marine Archaea: patterns and provenance in the water-column and sediments. Geochim. Cosmochim. Acta 71:3272-3291. [Google Scholar]
- 50.Uda, I., A. Sugai, Y. H. Itoh, and T. Itoh. 2001. Variation on molecular species of polar lipids from Thermoplasma acidophilum depends on growth temperature. Lipids 36:103-105. [DOI] [PubMed] [Google Scholar]
- 51.Uda, I., A. Sugai, Y. H. Itoh, and T. Itoh. 2004. Variation in molecular species of core lipids from the order Thermoplasmales strains depends on growth temperature. J. Oleo Sci. 53:399-404. [Google Scholar]
- 52.Valentine, D. L. 2007. Adaptations to energy stress dictate the ecology and evolution of the Archaea. Nat. Rev. Microbiol. 5:316-323. [DOI] [PubMed] [Google Scholar]
- 53.van de Vossenberg, J. L. C. M., A. J. M. Driessen, and W. N. Konings. 1998. The essence of being extremophilic: the role of the unique archaeal membrane lipids. Extremophiles 2:163-170. [DOI] [PubMed] [Google Scholar]
- 54.van de Vossenberg, J. L. C. M., A. J. M. Driessen, W. Zillig, and W. N. Konings. 1998. Bioenergetics and cytoplasmic membrane stability of the extremely acidophilic, thermophilic archaeon Picrophilus oshimae. Extremophiles 2:67-74. [DOI] [PubMed] [Google Scholar]
- 55.Wakeham, S. G., E. C. Hopmans, S. Schouten, and J. S. Sinninghe Damsté. 2004. Archaeal lipids and anaerobic oxidation of methane in euxinic water columns: a comparative study of the Black Sea and Cariaco Basin. Chem. Geol. 205:427-442. [Google Scholar]
- 56.Weijers, J. W. H., S. Schouten, O. C. Spaargaren, and J. S. Sinninghe Damsté. 2006. Occurrence and distribution of tetraether membrane lipids in soils: implications for the use of the TEX86 proxy and the BIT index. Org. Geochem. 37:1680-1693. [Google Scholar]
- 57.Weijers, J. W. H., S. Schouten, J. C. van den Donker, E. C. Hopmans, and J. S. Sinninghe Damsté. 2007. Environmental controls on bacterial tetraether membrane lipid distribution in soils. Geochim. Cosmochim. Acta 8:648-657. [Google Scholar]
- 58.Wuchter, C., S. Schouten, M. J. L. Coolen, and J. S. Sinninghe Damsté. 2004. Temperature-dependent variation in the distribution of tetraether membrane lipids of marine Crenarchaeota: implications for TEX86 paleothermometry. Paleoceanography 19:PA4028. doi: 10.1029/2004PA001041. [DOI] [Google Scholar]
- 59.Wuchter, C., S. Schouten, S. G. Wakeham, and J. S. Sinninghe Damsté. 2005. Temporal and spatial variation in tetraether membrane lipids of marine Crenarchaeota in particulate organic matter: implications for TEX86 paleothermometry. Paleoceanography 20:PA3013. doi: 10.1029/2004PA001110. [DOI] [Google Scholar]
- 60.Zehner, R. E., M. F. Coolbaugh, and L. Shevenell. 2006. Regional groundwater geochemical trends in the Great Basin: implications for geothermal exploration. GRC Transact. 30:117-124. [Google Scholar]
- 61.Zhang, C. L., A. Pearson, Y. L. Li, G. Mills, and J. Wiegel. 2006. A thermophilic temperature optimum for crenarchaeol and its implication for archaeal evolution. Appl. Environ. Microbiol. 72:4419-4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zillig, W., K. O. Stetter, S. Wunderl, W. Schulz, H. Priess, and I. Scholz. 1980. The Sulfolobus-“Caldariella” group: taxonomy on the basis of the structure of DNA-dependent RNA polymerases. Arch. Mikrobiol. 125:259-269. [Google Scholar]