Abstract
Bacterial biofilm formation on inert surfaces is a significant health and economic problem in a wide range of environmental, industrial, and medical areas. Bacterial adhesion is generally a prerequisite for this colonization process and, thus, represents an attractive target for the development of biofilm-preventive measures. We have previously found that the preconditioning of several different inert materials with an aqueous fish muscle extract, composed primarily of fish muscle α-tropomyosin, significantly discourages bacterial attachment and adhesion to these surfaces. Here, this proteinaceous coating is characterized with regards to its biofilm-reducing properties by using a range of urinary tract infectious isolates with various pathogenic and adhesive properties. The antiadhesive coating significantly reduced or delayed biofilm formation by all these isolates under every condition examined. The biofilm-reducing activity did, however, vary depending on the substratum physicochemical characteristics and the environmental conditions studied. These data illustrate the importance of protein conditioning layers with respect to bacterial biofilm formation and suggest that antiadhesive proteins may offer an attractive measure for reducing or delaying biofilm-associated infections.
Many bacteria live in highly complex sessile communities, referred to as biofilms, as well as or even rather than existing as solitary planktonic cells (10, 18). These microbial consortia are ubiquitous in nature; almost all surfaces that are exposed to naturally occurring fluids will inevitably become subject to microbial colonization (10, 45). As a result, microbial biofilms cause a variety of problems in different medical, environmental, and industrial settings, ranging from the fouling of ship hulls and the blocking of industrial piping to the colonization of artificial medical implants (11, 26). Many chronic or persistent bacterial infections have also been linked to the formation of microbial biofilms (11). Biofilm-associated bacteria are notoriously resistant to removal and often tolerate conditions that would readily eliminate their planktonic counterparts (15, 45). They frequently display increased resistance to hydrodynamic shear forces, phages, and different biocides. Moreover, in the medical field, biofilm-associated bacteria regularly display great tolerance toward antibiotics and host immune defense mechanisms. Consequently, they are often intractable and cause multiple recurrent infections (11, 15).
In the human body, a number of highly efficient defense mechanisms normally counter the buildup of microbial biofilms. Simple anatomical barriers, such as the sloughing off of epithelial cells, e.g., from the bladder epithelium, and the secretion of antiadhesive proteins and mucin, are generally very efficient at preventing the establishment of biofilms on mucosal surfaces (28, 41). Notably, one of the only natural surfaces in the human body that is regularly exposed to bacteria but is not protected by such mechanisms is tooth enamel. The buildup of dental plaque, essentially comprising multispecies biofilms, readily testifies to what can happen in the absence of these defense mechanisms (14, 32).
Artificial implants are also very prone to microbial colonization. Urinary catheters, stents, and contact lenses, for example, are readily colonized by innocuous or pathogenic microorganisms. Implant-associated infections often compromise the function and safety of the devices and regularly do so with devastating consequences (11, 34, 36). Moreover, implant-associated biofilms often serve as a source of recurrent infections. Today, the leading type of hospital-acquired infection is the catheter-associated urinary tract infection (CAUTI) (34, 35, 45). Up to 50% of all patients undergoing short-term catheterization and essentially all patients carrying an indwelling urinary catheter for more than 30 days will develop a urinary tract infection (40, 45). Catheter-associated biofilms are usually extremely difficult to eradicate by conventional antimicrobial treatment. Following a course of treatment, bacteria originating from any residual biofilms will often reinfect the urinary tract (29, 39, 40). Most short-term CAUTIs are caused by a single microbial species, such as Escherichia coli, Proteus mirabilis, or Klebsiella pneumonia, while long-term CAUTIs are often polymicrobial (30, 45, 46). Given that E. coli is one of the most frequently occurring species in implant-associated urinary tract infections, much effort has been focused on understanding and controlling this particular microorganism (30).
Bacterial biofilm formation is a remarkably complex process entailing a range of molecular and physiological events. It is generally considered to proceed through several steps, including adhesion, microcolony formation, and structural maturation (31, 47). While strain- and niche-specific variations largely determine the complex interactions that occur within monoclonal and polyclonal microbial biofilms, the initial requirement for bacterial attachment and adhesion appears to be universal. Without adhesion, the following events leading to biofilm formation will generally not take place (25). Bacterial adhesion itself is governed by a wide range of parameters pertaining to the environmental conditions and the bacterial cell surface properties (1, 23). The capacity of a microorganism to adhere, however, also depends on the nature of the surface and any conditioning (23). When a clean, abiotic surface is exposed to a complex physiological fluid, a conditioning film will form almost instantaneously (33, 37, 38). This conditioning layer may in turn influence the extent of bacterial adhesion (1, 23). Recently, we have found that the preconditioning of inert materials with an aqueous extract of fish muscle proteins (FMPs) can significantly reduce bacterial attachment to these surfaces (5, 43). Based on this finding, we have tested the biofilm-reducing activity of this nontoxic coating against a range of urinary tract infectious (UTI) E. coli and Klebsiella strains.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All bacterial strains used in this study are listed in Table S1 in the supplemental material. Cultures were established (at 30 or 37°C) in either supplemented FAB minimal medium (24), modified Luria-Bertani (LB) medium (43), or sterile human urine. The human urine samples were collected from three to four healthy women who had no prior history of urinary tract infections or antibiotic use within the previous 2 months. The urine samples were pooled, sterile filtered, and stored at 4°C.
Preparation of the aqueous extract.
The aqueous fish muscle extracts were prepared as described previously (5, 12). Briefly, pieces of cod fillet were cooked for 5 min in 0.5 liter of water per kg of fillet, and the aqueous solution was passed through a strainer and recooked. The resulting suspension was subsequently filtered through standard coffee filters, and H2KPO4 (0.056 M) and HK2PO4 (0.044 M) were added. The pHs of the final solutions were then adjusted to 6.5, and the solutions were sterilized for 30 min at 100°C. All aqueous extracts were stored at 4°C and used within 1 to 2 months.
Isoelectric fractionation.
Isoelectric fractionation was achieved with a pH adjustment buffer (pH 5) prepared from a 50 mM acetic acid buffer (pH 3; 5 mM NaCl) and a 50 mM MES (morpholineethanesulfonic acid) buffer (pH 6; 5 mM NaCl). The aqueous fish muscle extract was diluted five times in the adjustment buffer, and the pH of the mixture was readjusted to 5. The protein precipitate was then retrieved by centrifugation and resuspended in Tris buffer (10 mM; pH 6.6) according to volume. All fractions were sterile filtered and stored at 4°C prior to use.
SDS-PAGE.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed under denaturing conditions, and the gel was run and stained with Coomassie brilliant blue as described previously (27). All extract samples were diluted five times prior to the loading of the gel.
Microtiter plate biofilm assay.
Biofilm formation was assayed during growth in FAB minimal medium (24) containing 0.2% glucose, 0.2% Casamino Acids, and 1 μg of thiamine ml−1; in LB medium; or in human urine. Briefly, 48-well non-cell-culture-treated polystyrene microtiter plates (Nunc) were preconditioned for 1 h at room temperature and washed twice with 1× phosphate-buffered saline. Aliquots of the cultures (diluted 100-fold in fresh medium) were added to the wells, and the plates were incubated for 16 h at 37°C. Biofilm formation was subsequently quantified by staining with crystal violet (0.1%). The stain was dissolved with ethanol (96%), and the absorbance at 595 nm was measured. When necessary, hydrodynamic conditions were simulated by incubating the plates with agitation (100 rpm).
Inspection of microplate biofilms.
For the microscopic inspection of biofilm formation under static conditions, the biofilms were grown in coated or uncoated sonic seal slide dishes (Nunc), which have removable chambers. Following biofilm growth, each well was washed with phosphate-buffered saline and inspected by microscopy. Pictures were edited using the IMARIS software package (Bitplane).
Cultivation of flow cell biofilms.
All flow chamber biofilms were cultivated (at 30 or 37°C) either in FAB minimal medium (24) supplemented with 0.02% glucose, thiamine (1 μg ml−1), and proline (10 μg ml−1) or in human urine. The flow system was assembled and prepared much as described previously (8), using either glass (Knittel Gläser) or vinyl plastic (Rinzl; Electron Microscope Sciences) coverslips as the substrata. Prior to inoculation, selected channels were preconditioned in situ for 1 h with the extracts and the entire system was washed with medium for 1 to 2 h. The flow chambers were subsequently inoculated with diluted overnight cultures (optical density at 600 nm, 0.05), and adhesion was allowed to take place for 1 h under static conditions. Laminar flow conditions were subsequently established using a Watson Marlow 205S peristaltic pump. Biofilm development was inspected using a Zeiss LSM510 confocal scanning laser microscope, and the pictures were edited using the IMARIS software package (Bitplane).
Biofilm formation on catheters.
Biofilm formation was studied much as described previously by using commercial all-silicone Foley catheters (Coloplast Ltd.) (16). Briefly, small pieces of the catheters (4.5 cm) were connected to the flow system and preconditioned in situ for 1 h at room temperature. The entire system was subsequently washed with prewarmed (37°C) human urine for 1 to 2 h at 37°C. Each segment was inoculated with an overnight culture of E. coli CFT073cfp (diluted 100-fold in fresh urine), and the cells were allowed to adhere for 1 h under static conditions. The biofilms were then grown for 24 h at 37°C. Following biofilm formation, the catheter pieces were disconnected, stained with 0.1% crystal violet, and photographed. Biofilm formation was quantified by dissolving the stain with ethanol (96%) and measuring the absorbance (at 595 nm).
Adhesion assay.
Bacterial adhesion on preconditioned non-cell-culture-treated polystyrene microtiter plates was examined as described previously (43). The bacterial cells (E. coli ZK2686 grown overnight in LB medium) were harvested, washed twice, and resuspended in 1/4-diluted Ringer's solution (3) containing various concentrations of CaCl2 prior to the adhesion assay. The adhesion assay was repeated three times. The relative adhesion values were calculated, based on the uncoated, unsupplemented control, prior to any internal comparisons.
Data analysis.
For the quantification of surface coverage, selected confocal scanning laser microscope pictures were analyzed using the computer software program COMSTAT (21). Surface coverage calculations are based on pictures obtained from a total of four separate channels for each coating and the control (two channels from each of two independent experiments). Five pictures were analyzed for each channel at each time point.
RESULTS
The FMP coatings significantly reduce biofilm formation.
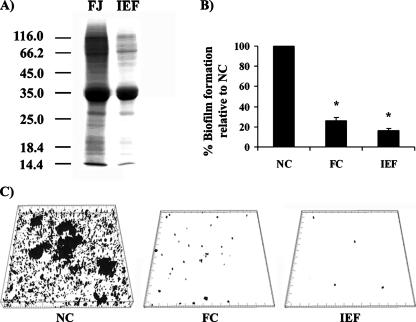
During the preconditioning of various materials with the aqueous fish muscle extract, a significant antiadhesive proteinaceous conditioning film is formed (5, 43). This coating layer was previously found to consist primarily of α-like tropomyosins (43). These fibrous muscle proteins can be crudely isolated from the extract by isoelectric fractionation (at pH 5) (Fig. 1A) and display significant antiadhesive properties (43). To characterize the biofilm-reducing potentials of the aqueous extract and the isoelectric fraction, an E. coli K-12 strain, SAR19 (CSH26cfp), was studied during biofilm growth in a simple crystal violet microtiter plate assay. The polystyrene microtiter plates were preconditioned with either of the two protein extracts and subsequently inoculated. The biofilms were then allowed to develop in minimal medium under static conditions (Fig. 1B). As seen in Fig. 1B, the preconditioning of the polystyrene surfaces with the extracts significantly reduced biofilm formation by SAR19 compared to that on the uncoated controls (paired two-tailed t test; P < 0.001). The inspection of SAR19 during biofilm formation in the coated and uncoated sonic seal slide dishes confirmed this result (Fig. 1C). The biofilm-reducing capacities of the two fractions were of quite similar magnitudes, indicating that the fish tropomyosin proteins contribute significantly to the biofilm-reducing activity. Other proteins may, however, also have contributed to the activity. The repelling effects of the two extracts were not linked to any growth inhibition in itself, and the coatings in fact even appeared to increase the overall bacterial density in the surrounding suspensions. Presumably, this relates to the general complexity and nutritional values of the extracts. In order to account for such variations, all absorbance values were correlated with regards to the overall growth (2).
FIG. 1.
(A) SDS-PAGE analysis of the total aqueous fish extract (FJ) and the isoelectric fraction obtained at pH 5 (IEF). (B) Reduction of biofilm formation by SAR19 by preconditioning with the total aqueous extract or the isoelectric fraction. The biofilms were grown statically in minimal medium at 37°C. The absorbance values relative to that for the uncoated control are shown. Standard deviations of the results obtained from three independent experiments are indicated. Abbreviations: NC, not coated; FC, coated with the aqueous fish extract; IEF, coated with the isoelectric fraction. Asterisk, statistical significance indicated by a P value of <0.001 (paired two-tailed t test). (C) Biofilm formation by SAR19 on uncoated or coated sonic seal slides.
Analysis of FMP-mediated biofilm reduction in flow chambers.
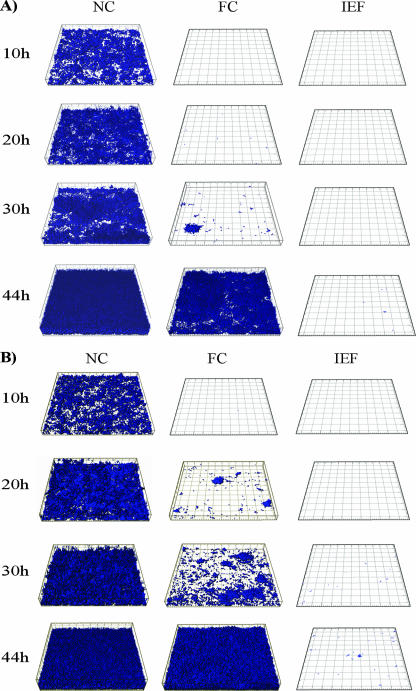
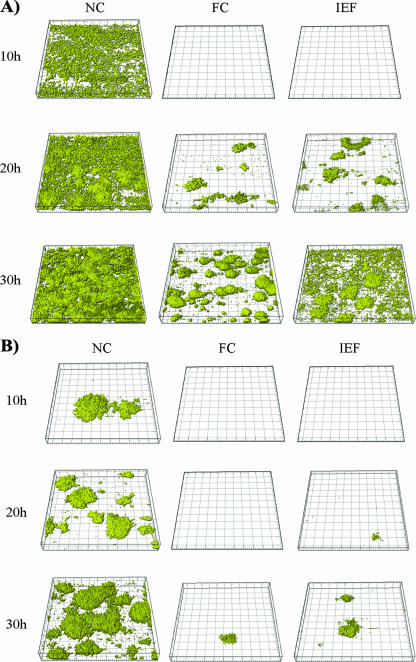
To study the biofilm-reducing activities of the coatings in more detail and to characterize the biofilm-reducing effects under hydrodynamic conditions, SAR19 was grown in flow chamber biofilms. To also examine the significance of the material physicochemical properties in relation to the biofilm-reducing activity, two different substrata were included in the study, specifically, glass and vinyl plastic. When studied under these conditions, the crude extract and the isoelectric fraction significantly reduced or delayed biofilm formation (Fig. 2). The isoelectric fraction, in particular, proved to be very efficient. Even following 76 h of incubation, few if any microcolonies were seen, compared to the near-mature biofilms observed at 20 to 30 h on the uncoated controls. This result most likely reflects the integrity and stability of the coating.
FIG. 2.
Confocal scanning micrographs of biofilm formation by E. coli CSH26cfp (SAR19) on uncoated and coated glass (A) and vinyl plastic (B) surfaces. The biofilms were grown in flow cells in minimal medium at 30°C. The experiment was repeated at least three times, using different batches of the aqueous extract. Abbreviations: NC, not coated; FC, coated with the aqueous fish extract; IEF, coated with the isoelectric fraction.
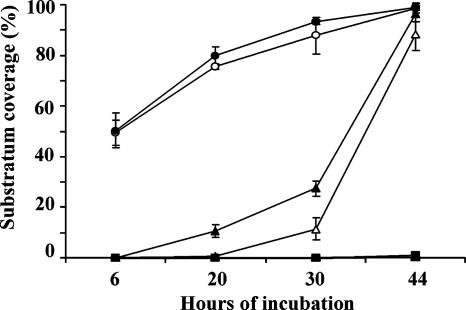
The biofilm-repelling effect also showed some substratum-dependent differences. To quantify these differences, the levels of bacterial coverage of the different surfaces were calculated using the COMSTAT software (Fig. 3) (21). These data clearly showed the delay in the initiation or onset of biofilm formation and the efficiency of the isoelectric fraction coating. The substratum-dependent differences were also apparent. Overall, the biofilm-reducing activity appeared to be more stable on glass than on vinyl plastic. While this finding may relate to differences in protein adsorption patterns, previous studies had not revealed any major compositional or quantitative differences in the conditioning layers formed on these materials (43). Other differences, therefore, such as substratum-dependent conformational changes, seem to account for the effect. The significance of protein conformational states with regards to the biofilm-reducing activity, however, remains elusive. Moreover, glass is in itself often less favorable to bacterial adhesion (data not shown) and may thus contribute indirectly to the activity.
FIG. 3.
Levels of substratum coverage by E. coli SAR19 biofilms formed on glass (open symbols) or vinyl plastic (filled symbols) substrata. The surfaces were either preconditioned with the total extract (triangles) or the isoelectric fraction (squares) or left uncoated (circles). The biofilms were grown in minimal medium in flow cells at 30°C. Standard deviations for four distinct channels are indicated.
The inspection of the biofilms formed on the coated and uncoated surfaces revealed very few, if any, morphological and behavioral differences, indicating that once the bacteria did establish on the coated surfaces, biofilm formation proceeded as normal. This observation correlates well with previous data (from atomic force microscopy and surface topography analyses) which indicated that the coating layer is typically incomplete (5). Arguably, bare sites may become loci for bacterial adhesion and subsequent microcolony formation.
FMPs significantly reduce biofilm formation by several urinary tract isolates.
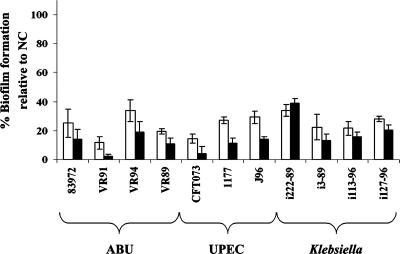
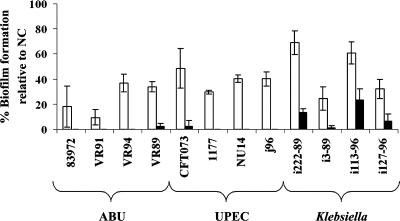
To characterize the biofilm-reducing properties of the protein coatings against various wild-type bacteria, a set of UTI isolates was selected. These bacterial isolates display a wide range of biofilm-forming and virulence properties; some are highly virulent, while others are generally considered avirulent (see Table S1 in the supplemental material). To study the biofilm-reducing activities against these isolates, the efficiencies of the coatings were initially assayed in minimal medium. As seen in Fig. 4, the proteinaceous coatings significantly reduced biofilm formation by all the UTI isolates (paired two-tailed t test; P < 0.001). Under these conditions, the activities of the two coatings appeared to be of quite similar magnitudes.
FIG. 4.
Reduction of biofilm formation by a range of pathogenic and asymptomatic-infection-causing UTI isolates by preconditioning of the polystyrene microtiter plates with the total extract (white bars) or the isoelectric extract (black bars). The biofilms were grown statically in minimal medium for 16 h at 37°C. The absorbance values indicated were calculated relative to that for the uncoated control (NC). Standard deviations of the results obtained from at least three independent experiments are indicated. ABU, asymptomatic-bacteriuria strains.
Having shown that the tropomyosin coating significantly reduces biofilm formation in a simple lab minimal medium, we subsequently examined the activity in a more realistic context. The UTI isolates studied here normally grow in a complex and highly hydrodynamic environment; the urinary tract is usually subjected to very strong hydrodynamic shear forces (an average adult human produces 2 liters of urine per day). To mimic these in vivo conditions, the activity was studied during the growth of bacteria in sterile filtered human urine. This setup takes into account the preconditioning which occurs in complex physiological fluids. To simulate the hydrodynamic flow conditions normally encountered in the urinary tract, the polystyrene microtiter plates were also incubated under shaking conditions. The same set of strains (except strain SAR19, which grows quite poorly in urine) and another uropathogenic E. coli (UPEC) strain, NU14, were studied. As seen in Fig. 5, both of the two protein coatings displayed considerable biofilm-reducing capacities (paired two-tailed t test; P < 0.01). Under these conditions, however, the isoelectric fraction proved to be far superior.
FIG. 5.
Reduction of biofilm formation by the UTI isolates during growth in human urine on polystyrene microtiter plates preconditioned with the total extract (white bars) or the isoelectric fraction (black bars). The biofilms were grown with agitation for 16 h at 37°C. The absorbance values indicated were calculated relative to that for the uncoated control (NC). Standard deviations of the results obtained from at least three independent experiments are indicated. ABU, asymptomatic-bacteriuria strains.
Analysis of FMP-mediated biofilm reduction in flow chambers during growth in urine.
To characterize the biofilm-reducing effect further, two of the UTI E. coli strains, 83972yfp and CFT073yfp, were studied in flow cell chambers during growth in urine. These two prototypic strains have previously been found to show very different biofilm characteristics (16, 20). Preconditioning with either of the two fractions significantly delayed the initiation of biofilm formation by these two isolates (Fig. 6). Interestingly, under these conditions, the activity of the total aqueous extract coating appeared to be somewhat superior to that of the isoelectric fraction.
FIG. 6.
Confocal scanning micrographs of E. coli CFT073yfp (A) and ABU83972yfp (B) biofilms formed on vinyl plastic coverslips in flow cells during growth (at 37°C) in human urine. Abbreviations: NC, not coated; FC, coated with the aqueous fish extract; IEF, coated with the isoelectric fraction.
FMPs significantly reduce biofilm formation by CFT073 on urinary catheters.
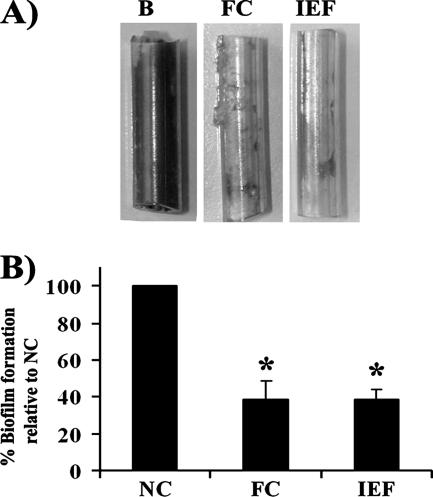
Some of the most commonly used urinary catheters are all-silicone Foley catheters. Silicone materials display a range of desirable properties, including great flexibility and reduced immunological reactivity (4). However, silicone catheters are not impervious to bacterial colonization and may in some cases even select for UPEC strains (16). To probe the biofilm-reducing activities of the extracts on this type of material, small urinary catheter segments were coupled to the flow system and preconditioned with the two extracts. The biofilm-reducing activities against CFT073yfp during growth in sterile filtered human urine were then assayed. This highly pathogenic strain was previously found to be an excellent biofilm former on several types of commercial silicone catheters (16). When studied under these conditions, biofilm formation by CFT073 was significantly reduced by either of the two coatings compared to the formation on the uncoated control (paired two-tailed t test; P < 0.001) (Fig. 7).
FIG. 7.
Reduction of biofilm formation by the prototypic UPEC strain CFT073 on coated urinary catheters during growth in human urine. The biofilms were grown under laminar conditions for 24 h at 37°C. (A) Representative catheter segments after crystal violet staining. Abbreviations: B, buffer; FC, coated with the aqueous fish extract; IEF, coated with the isoelectric fraction. (B) Absorbance values (A595) standardized according to the uncoated control (NC). Standard deviations of the results obtained from at least three independent experiments using different batches of urine are indicated. Asterisk, statistical significance as indicated by a P value of <0.001 (paired two-tailed t test).
Taken together, these data suggest that the ability of UTI E. coli and Klebsiella strains to form biofilms on inert surfaces can be efficiently impeded by fish muscle proteinaceous coatings.
Probing the biofilm-reducing activity.
In order to probe the biofilm-reducing activity further, a range of strains and mutants were tested for their abilities to establish on the coated surfaces. These strains either overexpressed or carried deletions in various well-defined surface structures known to influence adhesion and biofilm formation (e.g., type I fimbriae, conjugative pili, curli, PGA [poly-β-1,6-N-acetyl-d-glucosamine], lipopolysaccharide, and capsule). While some of these mutants, notably, E. coli strains overexpressing type I fimbriae (MS18), conjugative pili (SAR19R1drd19), curli (MG1655ompR234), and PGA adhesin (MG1655csrA::kan) and the E. coli or Klebsiella strains carrying deletions relating to lipopolysaccharide (UTI536rfaH waaG manB) or capsule production (UTI536 ΔkpsK15 and Klebsiella pneumoniae C105NCV), did display increased buildup on coated surfaces compared to the wild types (in LB), they also established better on the uncoated control surfaces (data not shown). This result, therefore, presumably relates to a general increase in biofilm formation rather than to any specific binding. In an effort to identify other potential adhesins, two transposon mutant libraries were also enriched and screened for mutants displaying increased adhesion to the preconditioned surfaces. Two bacterial strains were tested: the UPEC strain CFT073, which is predicted to encode several uncharacterized adhesins, and an environmental isolate, Pseudomonas fluorescens AH2, previously studied in relation to fish probiotics (19). This enrichment scheme (in which 300 mutants from each enrichment were screened) did not, however, identify any mutants showing increased or even equivalent biofilm formation on the coated surfaces compared to the formation on the uncoated controls (data not shown). Rather, this screening also identified mainly mutants with overall increased adhesion and biofilm formation in general.
Preliminary experiments using the total extract also indicated that the extract did not contain any antibiofilm compounds per se (data not shown). The biofilm-reducing activity of the coating, therefore, appears to be linked primarily to the bacterium-repelling properties of the FMPs, which presumably in turn relate to the physicochemical properties of α-tropomyosin. α-Tropomyosin is a highly charged protein (predominantly negative), which may increase the electrostatic repulsion of negatively charged bacteria (17). In line with this idea, we did find that adhesion to the coated surfaces increased slightly with increasing ionic strength (40 mM CaCl2 increased the relative adhesion of ZK2686 to the aqueous fish extract- and isoelectric fraction-coated surfaces by 8.8% ± 1.9% and 5.8% ± 2.6%, respectively, compared to the relative adhesion in the presence of the unsupplemented buffer, while slightly reducing adhesion [by 7.8% ± 0.6%] to the uncoated control). The influence of protein conformational changes cannot, however, be excluded. The coating has also previously been shown to increase the wettability of the surfaces, suggesting a reduction in surface hydrophobicity (5). These characteristics suggest that the biofilm-reducing activity of the coating is probably linked to changes in electrostatic repulsion and surface hydrophobicity, as also suggested previously for other proteins (42).
DISCUSSION
Bacterial biofilms are a growing concern in the medical field due to the increasing use of artificial medical devices for therapeutic and restorative purposes. CAUTIs are a notable example of this and illustrate perfectly the significant economic and medical problems associated with the use of such devices (34). These problems have also spurred a growing interest in the field of microbial biofilms and in the search for biofilm-preventive measures. Various strategies to reduce the accumulation of microbial biofilms have been studied or suggested over the years, with various degrees of success. In different industrial settings, a range of biocides and toxic metals (e.g., tin and copper) have been used for antifouling coatings and sanitizing purposes (7, 9). These substances are, however, not appropriate in relation to the food industry or medical implants, and other strategies have been sought. In relation to urology, a range of urinary catheters have been developed which incorporate and/or release antimicrobial compounds, e.g., metals (such as silver salts) and antibiotics (13, 22). Nevertheless, the general risk of antibiotic resistance development suggests that these compounds may be of only limited use. Here, we studied the biofilm-reducing properties of a nontoxic proteinaceous coating obtained from an aqueous extract of fish muscle tissue. This cheap, readily available coating, which is composed primarily of tropomyosins, was found to exhibit significant biofilm-reducing or -delaying activity against both UTI E. coli and Klebsiella strains on several different materials. In effect, a 100-fold reduction in the biofilm accumulation was frequently observed. The biofilm-reducing effect was, however, also found to vary depending on the extract, the substratum, the growth medium, and the particular bacterial strain studied. Overall, the biofilm-reducing effect was most significant when assayed in minimal medium. This result is not entirely unexpected, considering that complex physiological fluids such as urine contain a wide range of compounds and macromolecules, some of which are known to affect bacterial adhesion and may also affect protein adsorption and competition as well (6). The biofilm-reducing capacity of the isoelectric fraction was also found to be superior to that of the crude aqueous extract in most cases. However, during growth in urine under continuous-flow conditions, CFT073 and 83972 formed less biofilm on the surfaces coated with the crude aqueous extract than on those coated with the isoelectric fraction. The reason for this discrepancy remains elusive but may relate to differences in the bacterial adhesive capacities or to differences in the general stability of the protein layers with regards to flow and/or competitive adsorption under the different experimental conditions studied. High-molecular-weight proteins are often believed to bind more favorably to surfaces than lower-molecular-weight proteins and may in some cases even displace them (44). Whether the content of high-molecular-weight proteins in the total extract, however, affects the stability of the coating differentially under these conditions remains elusive. Regardless, the results clearly demonstrate the importance of material physicochemical properties and suspension medium, in relation to protein adsorption, and the impact of these parameters on bacterial adhesion and biofilm formation.
Several different experiments were also performed to characterize the biofilm-reducing activity further. These results indicated that the effect was linked primarily to the bacterium-repelling properties of the FMPs and not to any antibiofilm compounds per se. This finding correlates well with the absence of any obvious morphological or behavioral differences in the flow cell biofilms and implies that the biofilm-reducing activity is mainly physicochemical and/or steric. The biofilm-reducing properties of the extract, therefore, seem to be related mainly to the initial stages of biofilm formation and presumably to changes in hydrophobicity and/or electrostatic repulsion. When biofilm formation on the coated surfaces finally does take place, uncoated sites may well act as loci for bacterial establishment. To what extent these physicochemical characteristics correlate with the surface properties of the bacterial strains and mutants studied, in terms of hydrophobicity and zeta potential, remains unknown. The aggregative phenotype of several of the mutants and the incomplete coverage of the protein coating complicate such a correlation.
Competitive protein adsorption may also contribute to the activities of the coatings, at least in the more complex urine medium. While the idea remains purely speculative, we did find that when the flow system was filled with urine prior to preconditioning with the aqueous extract, the subsequent biofilm-reducing activity of the FMP coating was reduced (data not shown). While this result may obviously relate to differences in the general protein adsorption patterns (in the different coating suspensions), it may also relate to the adsorption of urine macromolecules, some of which may directly or indirectly influence bacterial adhesion or biofilm formation. Whether preconditioning with the fish extracts in turn could also reduce urine conditioning film formation, however, remains unknown. Studies are in progress to investigate the influence of the proteinaceous coating with regards to the competitive adsorption of other proteins. Meanwhile, our results indicate that surface-conditioning films are important for the development of bacterial biofilms and that they are largely defined by both environmental conditions and material physicochemical properties. Nevertheless, it is interesting that an efficient nontoxic antibiofilm coating can be obtained by this cheap, low-technology inroad.
Supplementary Material
Acknowledgments
This work was financed by a grant from the Danish Food Agency (DFFE 3304-05-66).
Footnotes
Published ahead of print on 18 April 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.An, Y. H., and R. J. Friedman. 1998. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J. Biomed. Mater. Res. 43:338-348. [DOI] [PubMed] [Google Scholar]
- 2.Bagge, D., M. Hjelm, C. Johansen, I. Huber, and L. Gram. 2001. Shewanella putrefaciens adhesion and biofilm formation on food processing surfaces. Appl. Environ. Microbiol. 67:2319-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes, L. M., M. F. Lo, M. R. Adams, and A. H. Chamberlain. 1999. Effect of milk proteins on adhesion of bacteria to stainless steel surfaces. Appl. Environ. Microbiol. 65:4543-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beiko, D. T., B. E. Knudsen, J. D. Watterson, P. A. Dacieux, G. Reid, and J. D. Denstedt. 2004. Urinary tract biomaterials. J. Urol. 171:2438-2444. [DOI] [PubMed] [Google Scholar]
- 5.Bernbom, N., R. L. Jørgensen, Y. Y. Ng, R. L. Meyer, P. Kingshott, R. M. Vejborg, P. Klemm, F. Besenbacher, and L. Gram. 2006. Bacterial adhesion to stainless steel is reduced by aqueous fish extract coatings. Biofilms 3:25-36. [Google Scholar]
- 6.Büeler, M. R., F. Wiederkehr, and D. J. Vonderschmitt. 1995. Electrophoretic, chromatographic and immunological studies of human urinary proteins. Electrophoresis 16:124-134. [DOI] [PubMed] [Google Scholar]
- 7.Chambers, L. D., K. R. Stokes, F. C. Walsh, and R. J. K. Wood. 2006. Modern approaches to marine antifouling coatings. Surf. Coatings Technol. 201:3642-3652. [Google Scholar]
- 8.Christensen, B. B., C. Sternberg, J. B. Andersen, R. J. Palmer, Jr., A. T. Nielsen, M. Givskov, and S. Molin. 1999. Molecular tools for study of biofilm physiology. Methods Enzymol. 310:20-42. [DOI] [PubMed] [Google Scholar]
- 9.Cloete, T. E., L. Jacobs, and V. S. Brözel. 1998. The chemical control of biofouling in industrial water systems. Biodegradation 9:23-37. [DOI] [PubMed] [Google Scholar]
- 10.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 11.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 12.Dalgaard, P. 1995. Qualitative and quantitative characterization of spoilage bacteria from packed fish. Int. J. Food Microbiol. 26:319-333. [DOI] [PubMed] [Google Scholar]
- 13.Darouiche, R. O., J. A. Smith, Jr., H. Hanna, C. B. Dhabuwala, M. S. Steiner, R. J. Babaian, T. B. Boone, P. T. Scardino, J. I. Thornby, and I. I. Raad. 1999. Efficacy of antimicrobial-impregnated bladder catheters in reducing catheter-associated bacteriuria: a prospective, randomized, multicenter clinical trial. Urology 54:976-981. [DOI] [PubMed] [Google Scholar]
- 14.Davey, M. E., and G. A. O'Toole. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64:847-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donlan, R. M., and B. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrieres, L., V. Hancock, and P. Klemm. 2007. Specific selection for virulent urinary tract infectious Escherichia coli strains during catheter-associated biofilm formation. FEMS Immunol. Med. Microbiol. 51:212-219. [DOI] [PubMed] [Google Scholar]
- 17.Gaffin, R. D., K. Gokulan, J. C. Sacchettini, T. Hewett, R. Klevitsky, J. Robbins, and M. Muthuchamy. 2004. Charged residue changes in the carboxy-terminus of alpha-tropomyosin alter mouse cardiac muscle contractility. J. Physiol. 556:531-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geesey, G. G., W. T. Richardson, H. G. Yeomans, R. T. Irvin, and J. W. Costerton. 1977. Microscopic examination of natural sessile bacterial populations from an alpine stream. Can. J. Microbiol. 23:1733-1736. [DOI] [PubMed] [Google Scholar]
- 19.Gram, L., J. Melchiorsen, B. Spanggaard, I. Huber, and T. F. Nielsen. 1999. Inhibition of Vibrio anguillarum by Pseudomonas fluorescens AH2, a possible probiotic treatment of fish. Appl. Environ. Microbiol. 65:969-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hancock, V., L. Ferrières, and P. Klemm. 2007. Biofilm formation by asymptomatic and virulent urinary tract infectious Escherichia coli strains. FEMS Microbiol. Lett. 267:30-37. [DOI] [PubMed] [Google Scholar]
- 21.Heydorn, A., A. T. Nielsen, M. Hentzer, C. Sternberg, M. Givskov, B. K. Ersboll, and S. Molin. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395-2407. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, J. R., M. A. Kuskowski, and T. J. Wilt. 2006. Systematic review: antimicrobial urinary catheters to prevent catheter-associated urinary tract infection in hospitalized patients. Ann. Intern. Med. 144:116-126. [DOI] [PubMed] [Google Scholar]
- 23.Katsikogianni, M., and Y. F. Missirlis. 2004. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cells Mater. 8:37-57. [DOI] [PubMed] [Google Scholar]
- 24.Kjaergaard, K., M. A. Schembri, C. Ramos, S. Molin, and P. Klemm. 2000. Antigen 43 facilitates formation of multispecies biofilms. Environ. Microbiol. 2:695-702. [DOI] [PubMed] [Google Scholar]
- 25.Klemm, P., and M. Schembri. 15 November 2004, posting date. Chapter 8.3.2.6, Type 1 fimbriae, curli, and antigen 43: adhesion, colonization, and biofilm formation. In R. Curtiss III et al. (ed.), EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC. http://www.ecosal.org. [DOI] [PubMed]
- 26.Kumar, C. G., and S. K. Anand. 1998. Significance of microbial biofilms in food industry: a review. Int. J. Food Microbiol. 42:9-27. [DOI] [PubMed] [Google Scholar]
- 27.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 28.Mysorekar, I. U., M. A. Mulvey, S. J. Hultgren, and J. I. Gordon. 2002. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J. Biol. Chem. 277:7412-7419. [DOI] [PubMed] [Google Scholar]
- 29.Nickel, J. C., J. W. Costerton, R. J. McLean, and M. Olson. 1994. Bacterial biofilms: influence on the pathogenesis, diagnosis and treatment of urinary tract infections. J. Antimicrob. Chemother. 33:31-41. [DOI] [PubMed] [Google Scholar]
- 30.Nicolle, L. E. 2005. Catheter-related urinary tract infection. Drugs Aging 22:627-639. [DOI] [PubMed] [Google Scholar]
- 31.O'Toole, G., H. B. Kaplan, and R. Kolter. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49-79. [DOI] [PubMed] [Google Scholar]
- 32.Rosan, B., and R. J. Lamont. 2000. Dental plaque formation. Microbes Infect. 2:1599-1607. [DOI] [PubMed] [Google Scholar]
- 33.Santin, M., A. Motta, S. P. Denyer, and M. Cannas. 1999. Effect of the urine conditioning film on ureteral stent encrustation and characterization of its protein composition. Biomaterials 20:1245-1251. [DOI] [PubMed] [Google Scholar]
- 34.Stamm, W. E. 1991. Catheter-associated urinary tract infections: epidemiology, pathogenesis, and prevention. Am. J. Med. 91:65S-71S. [DOI] [PubMed] [Google Scholar]
- 35.Stamm, W. E., and S. R. Norrby. 2001. Urinary tract infections: disease panorama and challenges. J. Infect. Dis. 183(Suppl. 1):S1-S4. [DOI] [PubMed] [Google Scholar]
- 36.Tenke, P., B. Kovacs, M. Jackel, and E. Nagy. 2006. The role of biofilm infection in urology. World J. Urol. 24:13-20. [DOI] [PubMed] [Google Scholar]
- 37.Tieszer, C., G. Reid, and J. Denstedt. 1998. Conditioning film deposition on ureteral stents after implantation. J. Urol. 160:876-881. [DOI] [PubMed] [Google Scholar]
- 38.Tieszer, C., G. Reid, and J. Denstedt. 1998. XPS and SEM detection of surface changes on 64 ureteral stents after human usage. J. Biomed. Mater. Res. 43:321-330. [DOI] [PubMed] [Google Scholar]
- 39.Trautner, B. W., and R. O. Darouiche. 2004. Role of biofilm in catheter-associated urinary tract infection. Am. J. Infect. Control 32:177-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trautner, B. W., and R. O. Darouiche. 2004. Catheter-associated infections: pathogenesis affects prevention. Arch. Intern. Med. 164:842-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uehling, D. T. 1991. Current concepts of the urinary bladder defenses against infection. Int. Urogynecol. J. 2:32-35. [Google Scholar]
- 42.van Loosdrecht, M. C., W. Norde, and A. J. Zehnder. 1990. Physical chemical description of bacterial adhesion. J. Biomater. Appl. 5:91-106. [DOI] [PubMed] [Google Scholar]
- 43.Vejborg, R. M., N. Bernbom, L. Gram, and P. Klemm. 20 February 2008, posting date. Anti-adhesive properties of fish tropomyosins. J. Appl. Microbiol. doi: 10.1111/j.1365-2672.2007.03718.x. [DOI] [PubMed]
- 44.Wahlgren, M., and T. Arnebrant. 1991. Protein adsorption to solid surfaces. Trends Biotechnol. 9:201-208. [DOI] [PubMed] [Google Scholar]
- 45.Warren, J. W. 2001. Catheter-associated urinary tract infections. Int. J. Antimicrob. Agents 17:299-303. [DOI] [PubMed] [Google Scholar]
- 46.Warren, J. W., J. H. Tenney, J. M. Hoopes, H. L. Muncie, and W. C. Anthony. 1982. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J. Infect. Dis. 146:719-723. [DOI] [PubMed] [Google Scholar]
- 47.Watnick, P., and R. Kolter. 2000. Biofilm, city of microbes. J. Bacteriol. 182:2675-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.