Abstract
In gram-negative bacteria, transporters belonging to the RND family are the transporters most relevant for resistance to antimicrobial compounds. In Pseudomonas aeruginosa, a clinically important pathogen, the RND-type pump MexAB-OprM has been recognized as one of the major multidrug efflux systems. Here, homologues of MexAB-OprM in the plant pathogens Pseudomonas syringae pv. phaseolicola 1448A, P. syringae pv. syringae B728a, and P. syringae pv. tomato DC3000 were identified, and mexAB-oprM-deficient mutants were generated. Determination of MICs revealed that mutation of MexAB-OprM dramatically reduced the tolerance to a broad range of antimicrobials. Moreover, the ability of the mexAB-oprM-deficient mutants to multiply in planta was reduced. RNA dot blot hybridization revealed growth-dependent regulation of the mexAB-oprM operon in P. syringae; the expression of this operon was maximal in early exponential phase and decreased gradually during further growth.
Bacteria have developed various ways to resist the toxic effects of antimicrobial compounds, such as enzymatic inactivation, alteration of the target structure, and reduced uptake (39). Another mechanism involves membrane-associated transport systems (23). Some transporters are specific and mediate the extrusion of a given drug or class of drugs. In contrast, so-called multidrug efflux (MDE) transporters can transport a wide range of structurally unrelated compounds.
The important role of efflux systems in multidrug resistance has been well documented for clinically relevant species. Some important bacterial pathogens have efflux mechanisms for all major classes of clinically available antibiotics (30, 38). However, the role of efflux pumps in clinically relevant bacterial pathogens is poorly understood in terms of physiological relevance. Genome sequence analysis revealed the highest numbers of MDE transporters in soil- or plant-associated bacteria (29). Plants are known to produce a wide range of structurally diverse secondary metabolites with antimicrobial activity (8, 40). Thus, it is tempting to speculate that MDE systems may confer resistance to plant antimicrobials and may contribute to the ability of the bacteria to colonize plants and cause disease. Consistent with this, inhibitors of MDE pumps have been used to increase the efficacy of plant antimicrobials up to 2,000-fold (36).
Only a limited number of transporters conferring multidrug resistance in phytopathogenic bacteria have been characterized. The RND-type transporter IfeAB has been identified in Agrobacterium tumefaciens. Palumbo et al. (28) showed that this transporter is able to export isoflavonoids, which are antimicrobial plant metabolites. The MFS-type efflux pump RmrAB in the nitrogen-fixing bean symbiont Rhizobium etli mediated resistance to phytoalexins and was demonstrated to be involved in nodule formation (13). The Xanthomonas oryzae pigment xanthomonadin is transported by a putative RND-type transporter (12). The role of two RND-type and three MFS-type efflux systems in the pathogenesis of Erwinia chrysanthemi was analyzed by Maggiorani Valecillos et al. (24). Mutants with mutations in genes coding for these systems were differentially affected in terms of their virulence in different hosts and in terms of their susceptibility to antimicrobials.
Recently, two transporters belonging to the RND and MFS families were shown to be involved in secretion of phytotoxins and in self-protection. Kang and Gross (20) identified the RND-type PseABC system in the syr-syp genomic island of P. syringae pv. syringae required for biosynthesis of syringomycin and syringopeptin. Disruption of the pseC gene resulted in substantial reductions in phytotoxin secretion and in the virulence of the mutant. Bostock et al. (2) identified an MFS transporter, AlbF, involved in self-protection in an albicidin-producing Xanthomonas albilineans strain.
Previously, we demonstrated that the RND-type AcrAB efflux system plays an important role in virulence of Erwinia amylovora, the causal agent of fire blight in various members of the family Rosaceae, and that it is required for resistance to antimicrobial plant metabolites and for successful colonization of the host plant (4). Moreover, we identified NorM, a multidrug transporter belonging to the MATE family, which is involved in competition of E. amylovora with other bacteria. We demonstrated that NorM confers resistance to a toxin produced by Pantoea agglomerans, an epiphytic bacterium frequently found on apple flowers (5).
While the existence and role of RND-type pumps in many bacterial pathogens have been established, the occurrence and role of these efflux systems in MDE in P. syringae have not been conclusively demonstrated. P. syringae is an important agricultural pathogen that infects a tremendous diversity of important crop species. P. syringae strains are classified into approximately 50 pathogenic varieties or pathovars based on the host plants from which they were originally isolated (17). In this study, we investigated the role of a putative MDE system, MexAB-OprM, in resistance to antimicrobials and in the virulence of three strains, P. syringae pv. phaseolicola 1448A (19), P. syringae pv. syringae B728a (10), and P. syringae pv. tomato DC3000 (3). The host ranges of these three strains are different; 1448A and B728a cause disease in beans, and DC3000 is pathogenic for tomato and Arabidopsis. The RND-type transporter of the P. syringae efflux pumps shared a high level of homology with MexB from Pseudomonas aeruginosa. In P. aeruginosa, MexAB-OprM has been recognized as one of the major MDE systems (30).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
P. syringae strains were routinely maintained on mannitol-glutamate (MG) medium (21) at 28°C. For liquid cultures, bacteria were grown in HSC medium (27) or King's B (KB) medium (22). Escherichia coli strain DH5α (33) cells were grown at 37°C on Luria-Bertani medium.
Drug susceptibility tests.
The MICs of drugs for P. syringae strains were determined by a twofold dilution assay using Mueller-Hinton broth. All tests were done in triplicate by following the Clinical and Laboratory Standards Institute recommendations (7). Growth of bacteria at 28°C was examined by using visual inspection after 24 h of incubation.
Generation of ΔmexAB-oprM mutants.
P. syringae unmarked ΔmexAB-oprM mutants were generated using the broad-host-range Flp-FRT recombination system (18). Two fragments, one located in the mexA gene and one located in the oprM gene, were PCR amplified using primer pairs mexR_fwd1 (5′-GCGTTGTCCTGGCGCTGA-3′)-mexA_rev1_KpnI (5′-TATGGTACCAGTCGCTGCCCACGGTGC-3′) and oprM_fwd2_KpnI (5′-TATGGTACCAATGCCTACATGACCTGGC-3′)-oprM_rev2 (5′-GGAGATGGTTTCGGTCTGC-3′). These primers were designed to bind at conserved sequences found in all three P. syringae strains. PCR products were cloned into pGEM-T Easy (Promega, Mannheim, Germany), yielding plasmids pGEM.mex and pGEM.opr. A 0.9-kb KpnI-SpeI fragment cut from pGEM.opr was ligated into KpnI-SpeI-digested pGEM.mex, yielding plasmid pGEM.mex-opr. A 1,825-bp KpnI FRT cassette fragment cut from pPS858-HW was ligated into KpnI-digested pGEM.mex-opr, yielding plasmid pGEM.mex-Gm-opr. The unique EcoRI site in the FRT cassette of pPS858 was eliminated by digesting the plasmid with EcoRI, blunt ending it, and reclosing it to form pPS858-HW. A 4.3-kb EcoRI fragment cut from pGEM.mex-Gm-opr was ligated into EcoRI-digested pEX18Ap, yielding the final replacement plasmid pEX18Ap.mex-Gm-opr. These plasmids were mobilized into P. syringae strains by triparental mating with helper plasmid pRK2013 (11). Putative mutants were screened for homologous recombination events by checking their antibiotic resistance (18). The FRT cassette was finally excised using plasmid pFLP2 (18). Mutants that were generated were verified by PCR using primers mexA-fwd (5′-GACATGCTCAAGCTGCGC-3′) and oprM_rev (5′-CATCAGCCTGACGGCCAG-3′), which yielded an 800-bp fragment for the mutants.
Plant material and inoculation procedures.
Bean plants (Phaseolus vulgaris cv. Red Kidney) were grown on shelves equipped with fluorescent lamps at 22 to 25°C with 60% humidity and with supplemental light using a 14-h photoperiod (350 microeinsteins m−2 s−1). Arabidopsis thaliana (L.) Heynh. ecotype Columbia plants were grown in a controlled-environment chamber at 22°C with 80% humidity and a photoperiod with 8 h of light (170 microeinsteins m−2 s−1; cold white fluorescent light).
P. syringae strains, grown on MG agar for 24 h at 28°C, were suspended in distilled water, the concentration was adjusted to 1.0 × 107 CFU per ml, and the strains were applied to leaves of the host plants with an airbrush (∼8 lb/in2) until the leaf surface was uniformly wet. Growth of bacterial strains was monitored by removing random leaf samples at 1 to 21 days postinoculation. Leaves were macerated in 10 ml isotonic NaCl per g (fresh weight). Bacterial counts (CFU per g [fresh weight]) were determined by plating dilutions of leaf homogenates onto MG agar.
EtBr efflux assay.
Bacteria were grown on KB agar plates and suspended to an optical density at 600 nm of 0.1 in 100 mM NaCl-50 mM sodium phosphate buffer (pH 7.0) containing 0.05% glycerol (26). Fluorescence was measured at room temperature using a final volume of 1 ml. Ethidium bromide (EtBr) was added at a concentration of 1.6 μg/ml (4 μM), and carbonyl cyanide m-chlorophenylhydrazone (CCCP) was added at a concentration of 20 μg/ml (100 μM). Fluorescence was measured with an SFM25 spectrofluorometer (Kontron Instruments, Zürich, Switzerland).
RNA dot blot analysis.
Total RNA was isolated by acid phenol-chloroform extraction (35). Aliquots of total RNA (200 ng per dot) were transferred to positively charged nylon membranes using the Minifold I spot blot system (Schleicher & Schuell BioScience, Dassel, Germany). The RNA hybridization probes were generated by in vitro transcription of PCR products. The mexA-specific primers mexA-RNA-fwd (5′-CTGCTCAGTGGCTGTAGC-3′) and mexA-revT7 (5′-TAATACGACTCACTATAGGGAGGTGCGCAGCTTGAGCATGTC-3′) were used to amplify PCR products from genomic DNA of 1448A, B728a, and DC3000. Digoxigenin-labeled RNA probes were synthesized by using digoxigenin-11-UTP and a Strip-EZ RNA probe synthesis and removal kit (Ambion Europe, Cambridgeshire, United Kingdom). Hybridization and signal quantification using an FLA-3000 phosphorimager (Raytest, Straubenhardt, Germany) were carried out by using our recently described method (35).
RESULTS AND DISCUSSION
Identification of the mexAB-oprM locus in P. syringae.
A search with the BLASTP program of the National Center for Biotechnology Information using the amino acid sequence of MexB from P. aeruginosa PAO1 (accession number AAG03815) as the query identified homologous sequences in the genomes of P. syringae pv. phaseolicola 1448A (gene number PSPPH_4014), P. syringae pv. syringae B728a (gene number Psyr_4008), and P. syringae pv. tomato DC3000 (gene number PSPTO_4304). These homologues shared 79% amino acid sequence identity with MexB from P. aeruginosa PAO1 and 96% identity with each other. Upstream and downstream analyses of the sequences surrounding the mexB homologues revealed the associated genes (mexA and oprM homologues). We assumed that mexA, mexB, and oprM form an operon in P. syringae, based on the significant sequence homology and the genetic organization, which is similar to that of the mexAB-oprM genes in P. aeruginosa (31).
A gene encoding a transcriptional regulator was identified upstream of the mexAB-oprM structural genes, but in the opposite direction, in all three P. syringae genomes. The deduced amino acid sequences showed high degrees of identity to members of the TetR family of transcriptional repressors, including EmhR of Pseudomonas fluorescens (78% identity) (15), TtgR of Pseudomonas putida (70% identity) (37), and AcrR of Erwinia amylovora (46% identity) (4). However, no significant similarity was found to MexR, a member of the MarR family of bacterial transcriptional regulators, which negatively regulates the mexAB-oprM operon in P. aeruginosa (32). Therefore, we suggest renaming the PSPPH_4012, Psyr_4006, and PSPTO_4302 genes encoding a regulator of the P. syringae mexAB-oprM efflux systems pmeR (Pseudomonas multidrug efflux regulator). An intriguing question is why these bacteria use regulators belonging to different families to control the expression of their major MDE systems. The use of these regulators could reflect currently unknown physiological roles of the pumps in the adaptation of the bacteria to different environments.
Generation and genetic analysis of mexAB-oprM-deficient mutants.
To study the influence of the MexAB-OprM efflux pump on antibiotic resistance and the virulence of P. syringae, mexAB-oprM deletion mutants were constructed. Unmarked mutants were generated by gene deletion using the Flp-FRT recombination system (18). Based on genome sequences, primers that amplify regions of the mexA and oprM genes were designed and used for construction of gene replacement vectors, deleting a 3.9-kb fragment in the mexAB-oprM operon and affecting all three open reading frames present in this operon. Generated ΔmexAB-oprM mutants of 1448A, B728a, and DC3000 were verified by PCR analysis, which yielded 800-bp fragments which were absent from the parental strains. Sequencing of these PCR fragments revealed successful deletion of a 3.9-kb region in the mexAB-oprM operon and, as expected, the presence of a short FRT-containing sequence.
EtBr efflux/accumulation assay.
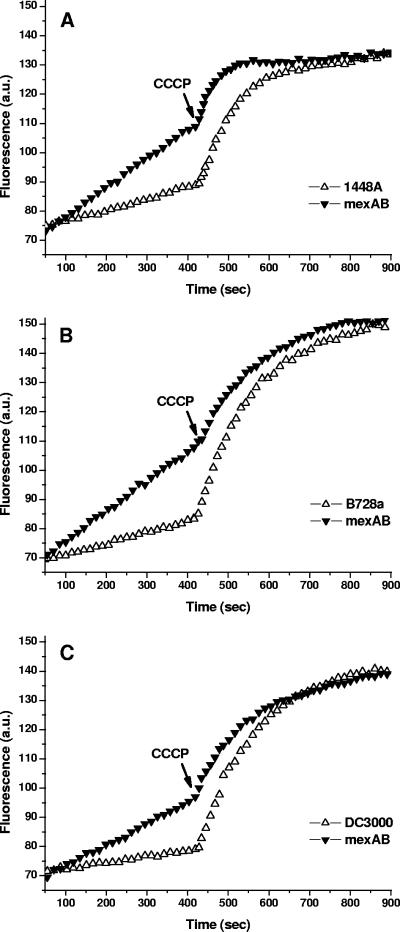
An EtBr accumulation experiment was conducted to demonstrate that MexAB-OprM-mediated extrusion is a proton motive force-driven process. After addition of EtBr, a much lower level of fluorescence was observed in wild-type cells of all three P. syringae strains than in ΔmexAB-oprM cells, indicating that EtBr is a substrate for the MexAB-OprM efflux system (Fig. 1). Addition of CCCP to the assay mixture increased cellular EtBr levels in both types of cells. CCCP is an uncoupler of oxidative phosphorylation and breaks down the proton gradient on the membrane. The final levels of intracellular EtBr after addition of CCCP were similar in wild-type and mutant strains, indicating that the accumulated levels of EtBr in both strains were the same under de-energized conditions (Fig. 1). Importantly, the level of EtBr that accumulated in wild-type cells was much lower than the level in cells of the ΔmexAB-oprM mutant prior to addition of CCCP. This indicated that MexAB-OprM from P. syringae is a proton motive force-dependent EtBr efflux pump. However, addition of CCCP also increased intracellular EtBr accumulation in the ΔmexAB-oprM mutants, suggesting that additional proton motive force-dependent efflux systems are expressed in the absence of MexAB-OprM.
FIG. 1.
Changes in EtBr fluorescence as measured using an excitation wavelength at 500 nm and an emission wavelength at 580 nm. EtBr (4 μM) was added to the assay mixture to initiate the assay, and CCCP (final concentration, 100 μM) was added at the time point indicated by the arrow. (A) P. syringae pv. phaseolicola 1448A (▵) and 1448A.ΔmexAB-oprM (▾). (B) P. syringae pv. syringae B728a (▵) and B728a.ΔmexAB-oprM (▾). (C) P. syringae pv. tomato DC3000 (▵) and DC3000.ΔmexAB-oprM (▾). a.u., arbitrary units.
Substrate specificities of the MexAB-OprM efflux pump.
The susceptibilities of wild-type and ΔmexAB-oprM cells to a variety of antimicrobial agents were compared in an attempt to identify substrates of the MexAB-OprM pump. Deletion of MexAB-OprM resulted in increased susceptibility of all three P. syringae strains to almost all antimicrobials, plant-derived antimicrobials, detergents, and dyes tested (Table 1). Only the MICs of the aminoglycosides gentamicin, kanamycin, and streptomycin did not differ from those determined for the wild-type strains. The most striking result was the hypersusceptibility of the ΔmexAB-oprM mutants to the β-lactam antibiotics ampicillin, carbenicillin, and piperacillin. Comparison of the susceptibility profiles revealed that the MexAB-OprM efflux pumps of the three P. syringae strains showed the same substrate specificity. However, slight strain-specific variations in the level of resistance were found, which likely could be attributed to the activity of additional MDE pumps present in these strains. P. syringae pv. phaseolicola 1448A was generally more susceptible than the other two strains, especially to tetraphenylphosphonium chloride and dyes. Apart from this, strain 1448A showed high intrinsic resistance to fluoroquinolones. P. syringae pv. syringae B728a was more resistant to erythromycin than the other P. syringae strains. Moreover, strain B728a showed extremely high resistance to streptomycin. A BLAST search revealed the presence of Tn5393 in the genome of B728a (genes Psyr_2667 to Psyr_2670). Tn5393 harbors the streptomycin resistance genes strA and strB encoding aminoglycoside phosphotransferases (6). This transposon is not present in strains 1448A and DC3000.
TABLE 1.
Antimicrobial susceptibility profiles of P. syringae wild-type strains, ΔmexAB-oprM mutants, and complemented mutants
| Antimicrobial | MIC (μg/ml) for:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1448A | 1448A. ΔmexAB | 1448A.ΔmexAB/pRK.mexAB | B728a | B728a. ΔmexAB | B728a.ΔmexAB/pRK.mexAB | DC3000 | DC3000. ΔmexAB | DC3000.ΔmexAB/pRK.mexAB | |
| Acridine orange | 250 | 31 | 1,000 | 250 | 12.5 | 500 | 250 | 31 | 500 |
| Acriflavin | 7.8 | 0.3 | 15.6 | 31 | 5 | 62.5 | 31 | 2.5 | 62.5 |
| Ampicillin | 125 | 1.5 | 500 | 62.5 | 3.1 | 500 | 62.5 | 0.8 | 500 |
| Benzalkonium chloride | 25 | 3.1 | 50 | 25 | 6.2 | 25 | 25 | 6.2 | 50 |
| Berberine | 1,000 | 15.6 | >1,000 | 1,000 | 62.5 | >1,000 | 1,000 | 125 | >1,000 |
| Carbenicillin | 1,000 | 6.2 | >1,000 | 1,000 | 12.5 | >1,000 | 500 | 3.1 | >1,000 |
| Cefoperazone | 62.5 | 1.5 | 250 | 125 | 12.5 | 250 | 31 | 1.5 | 125 |
| Chloramphenicol | 25 | 1.5 | 250 | 50 | 6.2 | 250 | 31 | 1.5 | 125 |
| Ciprofloxacin | 1.5 | 0.1 | 10 | 0.3 | 0.01 | 0.3 | 0.06 | 0.01 | 0.3 |
| Clindamycin | 1,000 | 125 | >1,000 | 1,000 | 125 | >1,000 | >1,000 | 250 | >1,000 |
| Crystal violet | 25 | 0.8 | 25 | 25 | 3.1 | 25 | 25 | 1.5 | 50 |
| Daunorubicin | 100 | 6.2 | >100 | >100 | 25 | >100 | >100 | 25 | >100 |
| Erythromycin | 25 | 3.1 | 125 | 100 | 25 | 125 | 12.5 | 3.1 | 31 |
| Ethidium bromide | 100 | 1.5 | 250 | 125 | 6.2 | 125 | 125 | 6.2 | 250 |
| Fusaric acid | 1,000 | 31 | >1,000 | 1,000 | 62.5 | 1,000 | 1,000 | 62.5 | >1,000 |
| Fusidic acid | 500 | 62.5 | >1,000 | 1,000 | 125 | >1,000 | 500 | 62.5 | >1,000 |
| Gentamicin | 1.2 | 0.6 | 0.6 | 1.2 | 0.6 | 0.6 | 0.6 | 0.3 | 0.3 |
| Kanamycin | 1.2 | 0.6 | 1.2 | 2.5 | 2.5 | 1.2 | 0.6 | 1.2 | 0.6 |
| Mitomycin C | 3.1 | 0.1 | 10 | 2.5 | 0.2 | 5 | 1.2 | 0.1 | 5 |
| Nalidixic acid | >1,000 | 31 | >1,000 | 50 | 3.1 | 100 | 25 | 3.1 | 100 |
| Naringenin | >1,000 | 250 | >1,000 | >1,000 | 500 | >1,000 | >1,000 | 250 | >1,000 |
| Nitrofurantoin | 500 | 31 | 500 | 500 | 125 | 500 | 500 | 125 | 500 |
| Norfloxacin | 7.8 | 0.6 | 50 | 0.5 | 0.1 | 1.5 | 0.5 | 0.06 | 3.1 |
| Novobiocin | >1,000 | 31 | >1,000 | 1,000 | 31 | >1,000 | >1,000 | 31 | >1,000 |
| Phloretin | >1,000 | 250 | >1,000 | >1,000 | 500 | >1,000 | >1,000 | 250 | >1,000 |
| Piperacillin | 31 | 0.6 | 62.5 | 62.5 | 3.1 | 125 | 15.6 | 0.3 | 31 |
| Puromycin | 125 | 3.1 | 1,000 | 500 | 31 | 1,000 | 125 | 15.6 | 1,000 |
| Rhodamine 6G | 125 | 1.5 | 500 | 250 | 15.6 | 250 | 500 | 31 | 1,000 |
| Streptomycin | >100 | >100 | >100 | 3.1 | 3.1 | 3.1 | 6.2 | 3.1 | 3.1 |
| Tetracycline | 0.5 | 0.03 | 1 | 0.06 | 0.5 | 0.03 | |||
| Tetraphenylphosphonium chloride | 125 | 1.2 | 500 | 1,000 | 125 | 1,000 | 1,000 | 31 | >1,000 |
| Trimethoprim | 500 | 62.5 | 1,000 | 500 | 62.5 | 1,000 | 125 | 31 | 1,000 |
Heterologous complementation of ΔmexAB-oprM mutants.
Heterologous complementation of the ΔmexAB-oprM mutants in trans with plasmid pRK415.mexAB-oprM, which carried the 4.5-kb mexAB-oprM region from P. aeruginosa, restored resistance to all antimicrobials tested (Table 1). This suggested that the efflux pumps have similar substrate profiles. The levels of resistance of the trans-complemented mutants were higher than those of the wild-type strains. The complemented mutants were two- to threefold more resistant to most of the antimicrobials, suggesting that there was a gene copy effect due to the presence of the plasmid or differences in the expression levels due to different regulatory proteins and promoter regions, which share only 34 and 43% nucleotide sequence identity, respectively.
It is noteworthy that expression of MexAB-OprM from P. aeruginosa in the P. syringae mutants resulted in significantly higher levels of resistance to several antibiotics, such as chloramphenicol, ampicillin, and cefoperazone. This result suggested that despite similar substrate specificities, the MexAB-OprM efflux systems of P. aeruginosa and P. syringae have slight but significant differences in potency for extrusion of several substrates.
Transcriptional analysis of mexAB-oprM genes.
To monitor mexAB-oprM transcript abundance in response to different growth media and growth phases, total RNA was prepared from P. syringae cells grown in minimal medium (HSC medium) and complex medium (KB medium), respectively. Cells were harvested at distinct optical densities. The expression of the mexAB-oprM operon was examined by dot blot analysis with strain-specific 580-bp RNA probes directed to mexA.
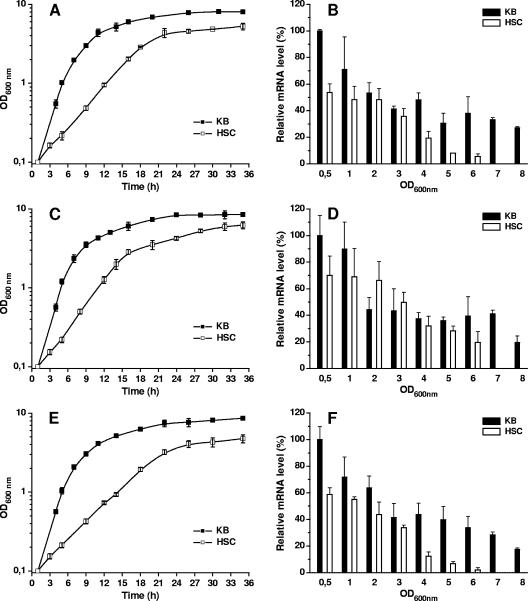
Quantification of signals revealed that there was growth phase-dependent transcription of the mexAB-oprM operon in P. syringae (Fig. 2). The signal intensities for mexA mRNA in all three strains were highest during the early exponential growth phase and gradually decreased during further growth of the cultures. These results are in contrast to the results of reporter gene fusion experiments which showed that the expression of mexAB-oprM from P. aeruginosa is increased in stationary growth phase and enhanced by the quorum-sensing autoinducer N-butyryl-l-homoserine lactone (9, 25, 34). These contradictory findings indicate that the pumps may not have the same physiological function. Moreover, different methods were used to analyze mexAB-oprM expression. The proteins encoded by reporter genes are often very stable and may persist in the cells even after the corresponding promoter is shut down, thereby reflecting the overall activity of the promoter during growth.
FIG. 2.
Bacterial growth and mexAB-oprM transcript abundance in P. syringae pv. phaseolicola 1448A, P. syringae pv. syringae B728a, and P. syringae pv. tomato DC3000. For liquid cultures, bacteria were grown in HSC medium and KB medium. (A) Growth kinetics of 1448A in HSC and KB media. (B) Quantitative RNA dot blot analysis for mexAB-oprM mRNA abundance in 1448A grown in HSC and KB media. (C) Growth kinetics of B728a in HSC and KB media. (D) Quantitative RNA dot blot analysis for mexAB-oprM mRNA abundance in B728a grown in HSC and KB media. (E) Growth kinetics of DC3000 in HSC and KB media. (F) Quantitative RNA dot blot analysis for mexAB-oprM mRNA abundance in DC3000 grown in HSC and KB media. The relative mRNA level was related to the highest mean value determined for a strain, which was defined as 100%. The data show the means and standard errors of two experiments with three replicates. OD600nm, optical density at 600 nm.
Interestingly, a significant effect of the growth medium on mexAB-oprM expression was observed. The expression of the mexAB-oprM operon was higher in complex medium than in minimal medium (Fig. 2). Moreover, the level of mexAB-oprM expression remained high until the late stationary growth phase in complex medium, whereas the level of mexA transcript signals decreased during later stages of bacterial growth in minimal medium. Either these data suggest that there is induction of mexAB-oprM expression by specific components of the complex medium or they may reflect a particular physiological function during growth in complex medium. The bacteria showed significantly increased growth rates in KB medium compared to the growth rates in the minimal HSC medium; thus, MexAB-OprM might play a role in the secretion of metabolites important for fast-growing cells.
Contribution of MexAB-OprM to the virulence of P. syringae.
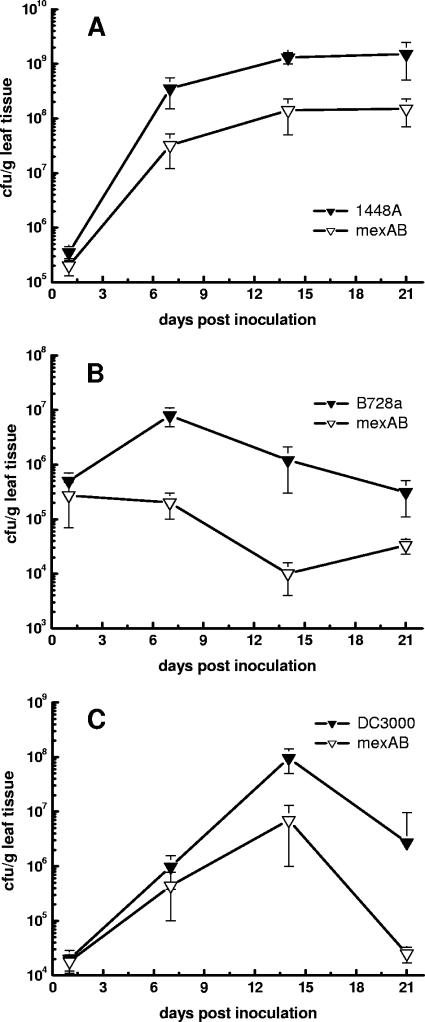
The impact of MexAB-OprM on the virulence of P. syringae pv. phaseolicola 1448A, P. syringae pv. syringae B728a, and P. syringae pv. tomato DC3000 was evaluated by monitoring bacterial populations and by studying the development of disease symptoms in the host plants for 21 days (Fig. 3). The population size of P. syringae pv. phaseolicola 1448A reached a maximum of 1.3 × 109 CFU per g leaf tissue on bean, whereas the population size of the ΔmexAB-oprM mutant of this strain was 10-fold lower throughout the 21-day sampling period. The ΔmexAB-oprM mutant of P. syringae pv. syringae B728a showed an even greater reduction in virulence. The population size of strain B728a reached 1.4 × 107 CFU per g leaf tissue on bean, whereas the maximum population size of its ΔmexAB-oprM mutant was 20-fold lower (1.3 × 105 CFU per g leaf tissue). The growth rates of P. syringae pv. tomato DC3000 and its ΔmexAB-oprM mutant were about the same until day 7. After day 7, the wild type continued to grow until the population size was 1 × 108 CFU/ml, whereas the population size of the ΔmexAB-oprM mutant was 10-fold lower. At day 21, the population sizes of both the wild type and the mutant were decreased, albeit to different degrees. The population size of the wild type (2.7 × 106 CFU per g leaf tissue) remained almost 200-fold greater than that of the mutant (1.4 × 104 CFU per g leaf tissue).
FIG. 3.
Growth of P. syringae wild-type strains and ΔmexAB-oprM mutants in planta. (A) Growth of P. syringae pv. phaseolicola 1448A (▾) and 1448A.ΔmexAB-oprM (▿) on bean leaves. (B) Growth of P. syringae pv. syringae B728a (▾) and B728a.ΔmexAB-oprM (▿) on bean leaves. (C) Growth of P. syringae pv. tomato DC3000 (▾) and DC3000.ΔmexAB-oprM (▿) on leaves of A. thaliana. The symbols indicate the means for three independent leaf samples. The error bars indicate the standard deviations from the means (n = 3; P < 0.005).
The disease symptoms caused by the ΔmexAB-oprM mutants were indistinguishable from those caused by the wild-type strains. However, consistent with the lower populations sizes, the symptoms caused by the ΔmexAB-oprM mutants were less abundant than the symptoms caused by the wild-type strains. Small, water-soaked spots developed on the undersides of the bean leaves inoculated with P. syringae pv. phaseolicola 1448A and its ΔmexAB-oprM mutant within 7 to 9 days after inoculation. These spots soon turned brown, and the tissues surrounding the spots became yellow. P. syringae pv. syringae B728a and B728a.ΔmexAB-oprM caused necrotic brown spots surrounded by narrow chlorotic halos that were visible within 9 to 12 days after inoculation. The disease symptoms caused by P. syringae pv. tomato DC3000 and DC3000.ΔmexAB-oprM consisted of small water-soaked lesions surrounded by large regions of chlorosis.
These results clearly indicated that disruption of MexAB-OprM resulted in reduced abilities of the bacteria to colonize their host plants. Plants synthesize a wide spectrum of secondary metabolites with antimicrobial activity (8, 14, 40), and it has been shown that MDE systems involved in resistance to such plant antimicrobials play a pivotal role in virulence (1, 4, 24). Thus, it is tempting to speculate that MexAB-OprM contributes to in planta growth of the bacteria by protecting them from the toxic effects of host antimicrobial compounds. Another possible explanation is based on the suggestion that MDE systems may play a role in the export of virulence factors. Hirakata et al. (16) demonstrated that the P. aeruginosa MexAB-OprM efflux system exports virulence determinants important for the bacteria to invade or transmigrate across epithelial cells. Whether MexAB-OprM in P. syringae is critical for the efflux of virulence factors in addition to its established role in exporting toxic substances remains to be elucidated.
Acknowledgments
This study was supported by EU grant MRTN-CT-2005-019335 (Translocation) and by grants from the Deutsche Forschungsgemeinschaft.
We thank Keith Poole for providing plasmid pRK.mexAB-oprM. We are grateful to Mathias Winterhalter, Alexander Schenk, and Annette Wensing for stimulating discussions.
Footnotes
Published ahead of print on 4 April 2008.
REFERENCES
- 1.Barabote, R. D., O. L. Johnson, Z. Eric, S. K. San Francisco, J. A. Fralick, and M. J. D. San Francisco. 2003. Erwinia chrysanthemi tolC is involved in resistance to antimicrobial plant chemicals and is essential for phytopathogenesis. J. Bacteriol. 185:5772-5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bostock, J. M., G. Huang, S. M. Hashimi, L. Zhang, and R. G. Birch. 2006. A DHA14 drug efflux gene from Xanthomonas albilineans confers high-level albicidin antibiotic resistance in Escherichia coli. J. Appl. Microbiol. 101:151-160. [DOI] [PubMed] [Google Scholar]
- 3.Buell, C. R., V. Joardar, M. Lindeberg, J. Selengut, I. T. Paulsen, M. L. Gwinn, R. J. Dodson, R. T. Deboy, A. S. Durkin, J. F. Kolonay, R. Madupu, S. Daugherty, L. Brinkac, M. J. Beanan, D. H. Haft, W. C. Nelson, T. Davidsen, N. Zafar, L. Zhou, J. Liu, Q. Yuan, H. Khouri, N. Fedorova, B. Tran, D. Russell, K. Berry, T. Utterback, S. E. Vanaken, T. V. Feldblyum, M. D'Ascenzo, W. L. Deng, A. R. Ramos, J. R. Alfano, S. Cartinhour, A. K. Chatterjee, T. P. Delaney, S. G. Lazarowitz, G. B. Martin, D. J. Schneider, X. Tang, C. L. Bender, O. White, C. M. Fraser, and A. Collmer. 2003. The complete sequence of the tomato and Arabidopsis pathogen Pseudomonas syringae pv. tomato DC3000. Proc. Natl. Acad. Sci. USA 100:10181-10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burse, A., H. Weingart, and M. S. Ullrich. 2004. The phytoalexin-inducible multidrug efflux pump AcrAB contributes to virulence in the fire blight pathogen, Erwinia amylovora. Mol. Plant-Microbe Interact. 17:43-54. [DOI] [PubMed] [Google Scholar]
- 5.Burse, A., H. Weingart, and M. S. Ullrich. 2004. NorM, an Erwinia amylovora multidrug efflux pump involved in in vitro competition with other epiphytic bacteria. Appl. Environ. Microbiol. 70:693-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiou, C. S., and A. L. Jones. 1993. Nucleotide sequence analysis of a transposon (Tn5393) carrying streptomycin resistance genes in Erwinia amylovora and other gram-negative bacteria. J. Bacteriol. 175:732-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 7th ed. CLSI document M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 8.Dixon, R. A., and N. L. Paiva. 1995. Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans, K., and K. Poole. 1999. The MexA-MexB-OprM multidrug efflux system of Pseudomonas aeruginosa is growth-phase regulated. FEMS Microbiol. Lett. 173:35-39. [DOI] [PubMed] [Google Scholar]
- 10.Feil, H., W. S. Feil, P. Chain, F. Larimer, G. DiBartolo, A. Copeland, A. Lykidis, S. Trong, M. Nolan, E. Goltsman, J. Thiel, S. Malfatti, J. E. Loper, A. Lapidus, J. C. Detter, M. Land, P. M. Richardson, N. C. Kyrpides, N. Ivanova, and S. E. Lindow. 2005. Comparison of the complete genome sequences of Pseudomonas syringae pv. syringae B728a and pv. tomato DC3000. Proc. Natl. Acad. Sci. USA 102:11064-11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goel, A. K., L. Rajagopal, N. Nagesh, and R. V. Sonti. 2002. Genetic locus encoding functions involved in biosynthesis and outer membrane localization of xanthomonadin in Xanthomonas oryzae pv. oryzae. J. Bacteriol. 184:3539-3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzáles-Pasayo, R., and E. Martínez-Romero. 2000. Multiresistance genes of Rhizobium etli CFN42. Mol. Plant-Microbe Interact. 13:572-577. [DOI] [PubMed] [Google Scholar]
- 14.Hammerschmidt, R. 1999. Phytoalexins: what have we learned after 60 years? Annu. Rev. Phytopathol. 37:285-306. [DOI] [PubMed] [Google Scholar]
- 15.Hearn, E. M., J. J. Dennis, M. R. Gray, and J. M. Foght. 2003. Identification and characterization of the emhABC efflux system for polycyclic aromatic hydrocarbons in Pseudomonas fluorescens cLP6a. J. Bacteriol. 185:6233-6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirakata, Y., R. Srikumar, K. Poole, N. Gotoh, T. Suematsu, S. Kohno, S. Kamihira, R. E. Hancock, and D. P. Speert. 2002. Multidrug efflux systems play an important role in the invasiveness of Pseudomonas aeruginosa. J. Exp. Med. 196:109-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirano, S. S., and C. D. Upper. 2000. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae—a pathogen, ice nucleus, and epiphyte. Microbiol. Mol. Biol. Rev. 64:624-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 19.Joardar, V., M. Lindeberg, R. W. Jackson, J. Selengut, R. Dodson, L. M. Brinkac, S. C. Daugherty, R. DeBoy, A. S. Durkin, M. G. Giglio, R. Madupu, W. C. Nelson, M. J. Rosovitz, S. Sullivan, J. Crabtree, T. Creasy, T. Davidsen, D. H. Haft, N. Zafar, L. Zhou, R. Halpin, T. Holley, H. Khouri, T. Feldblyum, O. White, C. M. Fraser, A. K. Chatterjee, S. Cartinhour, D. J. Schneider, J. Mansfield, A. Collmer, and C. R. Buell. 2005. Whole-genome sequence analysis of Pseudomonas syringae pv. phaseolicola 1448A reveals divergence among pathovars in genes involved in virulence and transposition. J. Bacteriol. 187:6488-6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang, H., and D. C. Gross. 2005. Characterization of a resistance-nodulation-cell division transporter system associated with the syr-syp genomic island of Pseudomonas syringae pv. syringae. Appl. Environ. Microbiol. 71:5056-5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keane, P. J., A. Kerr, and P. B. New. 1970. Crown gall of stone fruit. II. Identification and nomenclature of Agrobacterium isolates. Aust. J. Biol. Sci. 23:585-595. [Google Scholar]
- 22.King, E. O., M. K. Ward, and D. E. Raney. 1954. Two simple media for the demonstration of pyocyanin and fluorescein. J. Lab. Clin. Med. 44:301-307. [PubMed] [Google Scholar]
- 23.Kumar, A., and H. P. Schweizer. 2005. Bacterial resistance to antibiotics: active efflux and reduced uptake. Adv. Drug Deliv. Rev. 57:1486-1513. [DOI] [PubMed] [Google Scholar]
- 24.Maggiorani Valecillos, A., P. Rodriguez Palenzuela, and E. López-Solanilla. 2006. The role of several multidrug resistance systems in Erwinia chrysanthemi pathogenesis. Mol. Plant-Microbe Interact. 19:607-613. [DOI] [PubMed] [Google Scholar]
- 25.Maseda, H., I. Sawada, K. Saito, H. Uchiyama, T. Nakae, and N. Nomura. 2004. Enhancement of the mexAB-oprM efflux pump expression by a quorum-sensing autoinducer and its cancellation by a regulator, MexT, of the mexEF-oprN efflux pump operon in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 48:1320-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ocaktan, A., H. Yoneyama, and T. Nakae. 1997. Use of fluorescence probes to monitor function of the subunit proteins of the MexA-MexB-OprM drug extrusion machinery in Pseudomonas aeruginosa. J. Biol. Chem. 272:21964-21969. [DOI] [PubMed] [Google Scholar]
- 27.Palmer, D. A., and C. L. Bender. 1993. Effects of environmental and nutritional factors on production of the polyketide phytotoxin coronatine by Pseudomonas syringae pv. glycinea. Appl. Environ. Microbiol. 59:1619-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palumbo, J. D., C. I. Kado, and D. A. Phillips. 1998. An isoflavonoid-inducible efflux pump in Agrobacterium tumefaciens is involved in competitive colonization of roots. J. Bacteriol. 180:3107-3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paulsen, I. T. 2003. Multidrug efflux pumps and resistance: regulation and evolution. Curr. Opin. Microbiol. 6:446-451. [DOI] [PubMed] [Google Scholar]
- 30.Poole, K. 2004. Efflux-mediated multiresistance in gram-negative bacteria. Clin. Microbiol. Infect. 10:12-26. [DOI] [PubMed] [Google Scholar]
- 31.Poole, K., K. Krebes, C. McNally, and S. Neshat. 1993. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J. Bacteriol. 175:7363-7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poole, K., K. Tetro, Q. Zhao, S. Neshat, D. E. Heinrichs, and N. Bianco. 1996. Expression of the multidrug resistance operon mexA-mexB-oprM in Pseudomonas aeruginosa: mexR encodes a regulator of operon expression. Antimicrob. Agents Chemother. 40:2021-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 34.Sawada, I., H. Maseda, T. Nakae, H. Uchiyama, and N. Nomura. 2004. A quorum-sensing autoinducer enhances the mexAB-oprM efflux-pump expression without the MexR-mediated regulation in Pseudomonas aeruginosa. Microbiol. Immunol. 48:435-439. [DOI] [PubMed] [Google Scholar]
- 35.Schenk, A., H. Weingart, and M. S. Ullrich. 2008. Extraction of high-quality bacterial RNA from infected leaf tissue for bacterial in planta gene expression analysis by multiplexed fluorescent Northern hybridization. Mol. Plant Pathol. 9:227-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tegos, G., F. R. Stermitz, O. Lomovskaya, and K. Lewis. 2002. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob. Agents Chemother. 46:3133-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terán, W., A. Felipe, A. Segura, A. Rojas, J.-L. Ramos, and M.-T. Gallegos. 2003. Antibiotic-dependent induction of Pseudomonas putida DOT-T1E TtgABC efflux pump is mediated by the drug binding repressor TtgR. Antimicrob. Agents Chemother. 47:3067-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Bambeke, F., Y. Glupczynski, P. Plésiat, J. C. Pechère, and P. M. Tulkens. 2003. Antibiotic efflux pumps in prokaryotic cells: occurrence, impact on resistance and strategies for the future of antimicrobial therapy. J. Antimicrob. Chemother. 51:1055-1065. [DOI] [PubMed] [Google Scholar]
- 39.Walsh, C. 2000. Molecular mechanisms that confer antibacterial drug resistance. Nature 406:775-781. [DOI] [PubMed] [Google Scholar]
- 40.Wink, M. 2003. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 64:3-19. [DOI] [PubMed] [Google Scholar]