Abstract
We describe here an ∼40-kDa Plasmodium vivax tryptophan-rich antigen (PvTRAg40) which contains 321 amino acids and 11.4% tryptophan residues. This protein shows 65% homology (35% identity) with the previously described PvTRAg, besides sharing 23 of 27 positionally conserved tryptophan residues and similar genomic organization. The nucleotide sequence of the entire tryptophan-rich domain of PvTRAg40 was identical among 35 P. vivax clinical isolates. The protein is expressed by ring, trophozoite, and schizont stages of the parasite. The cDNA covering exon 2 of PvTRAg40 was cloned and expressed in the pPROEXHTa vector, and recombinant protein was purified. A high humoral immune response (90.7% seropositivity; n = 43) against this recombinant protein was detected in humans during the course of natural P. vivax infection. Eighty percent of the total of 20 P. vivax-exposed individuals exhibited lymphoproliferative responses against this antigen. The T cells of these individuals produced larger amounts of interleukin-12 (IL-12), IL-4, and IL-10 than gamma interferon and tumor necrosis factor alpha cytokines in response to the recombinant protein. Production of Th2-biased cytokines, conserved T- and B-cell epitopes, and an enhanced humoral immune response indicate that PvTRAg40 could possibly induce antibody-mediated immune protection against infection.
Malaria remains the most prevalent and devastating parasitic disease worldwide (7). Its prevalence is likely to increase because of growing drug resistance and the absence of an effective malaria vaccine (23). Effective control measures are urgently required to contain the disease (28). Therefore, the foremost aim of immunological research on malaria for more than 20 years has been vaccine development (3). A malaria vaccine would be a valuable tool to reduce both the morbidity and mortality of the disease. Two lines of evidence suggest that a malaria vaccine is attainable. Firstly, immunity can be acquired as a result of natural exposure to infection, and secondly, various immunization strategies can enhance protection against infection (21). However, separate vaccines would be required to target Plasmodium vivax and P. falciparum malarial infections, as the two species are phylogenetically and antigenically quite distinct (13). The ability of the parasite to undergo rapid antigenic switching presents an important challenge to vaccine development (16). An effective malaria vaccine must be able to target the exoerythrocytic- and blood-stage forms of the parasite, generate both humoral and cellular immune responses, overcome genetic restriction, and stimulate memory cells (10).
The development of a successful multivalent malaria vaccine therefore requires identification of newer potential vaccine antigens. Furthermore, humoral and cellular immune responses generated against these parasite molecules during natural sensitization against malaria are required at an epidemiological level (20). It is generally believed that identification of T-cell epitopes capable of eliciting an immune response in individuals of different genetic backgrounds would be necessary for designing a subunit vaccine (20). T cells play a central role in the regulation of immune responses and the formation of immunologic memory which can control and eliminate the infection (24). Studies with both mice and humans have repeatedly shown that proinflammatory cytokines, such as interleukin-12 (IL-12), gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α), are essential mediators of protective immunity to erythrocytic malaria; these cytokines can be derived from either the innate or adaptive arm of the immune response (14, 32). It is important to determine the contributions of these cytokines to both immunity and pathology of infection with malaria, since they can support either aspect during a proinflammatory response (3).
In order to examine new parasite molecules and their role in the generation of both humoral and cellular responses, we describe here the cDNA cloning, expression, and immunological characterization of the ∼40-kDa P. vivax tryptophan-rich antigen (PvTRAg40), which shares positionally conserved tryptophan residues with the previously reported PvTRAg protein (19). Similar to PvTRAg and PvTARAg55 (30), this antigen is also expressed by all the asexual blood stages of the parasite, but it shows more conserved B-cell epitopes than the other two antigens do. Humoral responses to this antigen among infected humans are greater than those to PvTARAg55 but similar to those to PvTRAg. Although the cellular immune response to PvTRAg has yet to be determined for the P. vivax-exposed population, it was higher for PvTRAg40 than for PvTARAg55 among the same group of individuals.
MATERIALS AND METHODS
Cloning and expression of PvTRAg40 gene.
Previously isolated P. vivax mRNA (19) was used for the amplification of PvTRAg40 cDNA, using primers FSpeI (5′ GACTAGTTGAAAAGTCAGAAGAAT 3′) and RXbaI (5′ TTTGCTCTAGAAGTGGCTGCATTT 3′). The restriction sites were incorporated into the primer sequences for cloning purposes and are shown in bold. The underlined residues were modified to create these sites. PCR was performed using the high-fidelity proofreading enzyme Platinum Pfx DNA polymerase (Invitrogen Life Technologies, Carlsbad, CA). The parasite DNA was initially denatured at 94°C for 10 min. Thirty-five cycles, consisting of denaturation at 94°C, annealing at 53°C, and extension at 72°C for 1 min each, were carried out. Final extension was carried out for 10 min at 72°C. The PCR product was purified and cloned into the pGEM-T Easy cloning vector (Promega Corp., Madison, WI) and subsequently subcloned into the SpeI and XbaI sites of the pPROEXHTa expression vector (Promega Corp., Madison, WI) and transformed into Escherichia coli BL21 (DE3). A 1 mM final concentration of IPTG (isopropyl-β-d-thiogalactopyranoside) was used to induce the expression of recombinant fusion protein.
Purification of recombinant PvTRAg40.
A recombinant PvTRAg40 protein containing a six-His tag was expressed as inclusion bodies and then refolded by using 3-(cyclohexylamino)-1-propane sulfonic acid (CAPS) and N-lauroylsarcosine at alkaline pH as described earlier (29). The refolded protein was purified by immobilized metal-affinity chromatography (IMAC) on Ni2+-nitrilotriacetic acid (Ni2+-NTA) agarose (Qiagen GmbH, Hilden, Germany) under native conditions, and eventually, bound protein was eluted with elution buffer (50 mM NaH2PO4, 300 mM NaCl, 300 mM imidazole; pH 8.0). One-milliliter fractions were collected and analyzed by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). To further analyze the homogeneity of affinity-purified recombinant PvTRAg40, it was subjected to a C18 reversed-phase chromatography column (Supelco; Sigma-Aldrich, St. Louis, MO) on a high-performance liquid chromatography (HPLC) system (BioLogic dual flow; Bio-Rad Laboratories Inc., Hercules, CA). For column equilibration, 0.05% trifluoroacetic acid was used, and elution was carried out with a gradient (0 to 100%) of 50% acetonitrile and 0.05% trifluoroacetic acid at a flow rate of 1 ml per min. The absorbance was recorded at 280 nm.
Raising polyclonal antisera against PvTRAg40.
Polyclonal sera against PvTRAg40 were raised in rabbits as described earlier for PvTRAg (19). Briefly, 300 μg of purified recombinant PvTRAg40 was emulsified with Freund's complete adjuvant and administered subcutaneously. Before antigenic stimulation, these rabbits were bled for the collection of preimmune serum. To boost the antibody response, 100 μg of antigen was emulsified in incomplete Freund's adjuvant and administered subcutaneously four times at 2-week intervals. Ten days after the last immunization, sera were collected from clotted blood, decomplemented, aliquoted, and stored at −20°C.
Humoral immune response. (i) Direct binding ELISA.
One-hundred-nanogram portions of HPLC-purified recombinant PvTRAg40 were used to coat a 96-well microtiter plate (BD Biosciences, San Diego, CA) in triplicate. Enzyme-linked immunosorbent assay (ELISA) was performed with serum samples from individuals who had been exposed to P. vivax malaria 8 to 14 weeks prior to study and from those without a history of malaria infection (malaria naïve) who were negative for malaria parasites at the time of blood collection. These individuals were living in the vicinity of Jabalpur, Madhya Pradesh State, Central India, where malaria transmission is seasonal and ranges from moderate to high. Sample collection from both females and males (ratio, 1:1.85) with ages ranging from 4 to 58 years was done during July 2006. Blood samples were collected according to institutional guidelines, and all individuals were provided with information on the research use of specimens. ELISA was performed as described earlier (19, 26), and the optical density (OD) was taken in a microplate ELISA reader from Electronics Corporation of India Limited (Bangalore, India). The final reading of each well was derived by subtracting the background OD. An OD value equal to or more than the mean plus 3 standard deviations (SD) of the healthy controls with no history of malaria and no parasitemia at the time of blood collection was considered to be positive.
For immunoglobulin G (IgG) isotyping, ELISA was performed as described above, with the same amount of HPLC-purified PvTRAg40 (100 ng/well) and with human sera (1:100 dilution) from the group of individuals who recovered from P. vivax malaria, except that the secondary antibody was biotin-labeled monoclonal anti-human IgG1 (1:1,000) or IgG4 (1:15,000), with avidin peroxidase (1:2,000) as the primary substrate (Sigma, St. Louis, MO). The enzyme reaction was developed with O-phenylenediamine dihydrochloride as the chromogen and hydrogen peroxide as the substrate, and the absorbance was taken at 490 nm.
(ii) Western blot analysis.
The immunoblot of purified recombinant PvTRAg40 was developed with the above-mentioned P. vivax-exposed sera, as described earlier (19), except that the serum samples were preadsorbed with pPROEXHTa-containing E. coli, the dilution of horseradish peroxidase-conjugated secondary antibody was 1:10,000, and the color reaction was developed with diaminobenzidine reagent (Sigma-Aldrich, St. Louis, MO). The identity of recombinant PvTRAg40 was confirmed by its recognition by the monoclonal anti-His antibody (Serotec, Oxford, UK) at a 1:3,000 dilution prepared in phosphate-buffered saline (PBS) with 1% bovine serum albumin. The polyclonal serum raised against this PvTRAg40 protein was also used to recognize the purified protein at various antibody dilutions (1:200, 1:500, 1:1,000, 1:2,000, 1:5,000, and 1:10,000).
Cellular immune response.
Peripheral blood samples were collected under medical supervision from 20 confirmed P. vivax-infected patients who had recovered from their last P. vivax malaria episodes about 8 to 14 weeks prior to study. Only parasitologically negative convalescent individuals were selected because the in vitro cellular response to malaria antigens is known to be downregulated during acute infections (27, 35). Five milliliters of blood was collected in a 15-ml sterile heparinized tube, stored on ice, and transported to the lab within 6 h. Blood samples were also collected from 10 malaria-naïve volunteers to determine PvTRAg40 T-cell-stimulated proliferation and cytokine levels in persons not sensitized to the malaria antigen. The slide examination of these volunteers confirmed them to be malaria negative at the time of sample collection. The protocol was approved by the institutional ethics committee, and informed consent was obtained from all human subjects. These subjects were provided with information on the research use of specimens that were collected.
Peripheral blood mononuclear cells (PBMC) were isolated from whole blood diluted 1:1 with incomplete RPMI 1640 (supplemented with 0.2% NaHCO3, 40 μg/ml gentamicin, 100 IU/ml penicillin G, and 0.1 mg/ml streptomycin) by centrifugation on a density gradient using Histopaque-1077 (Sigma, St. Louis, MO). Briefly, 10 ml (diluted 1:1) of blood was overlaid on 3 ml of Histopaque in a 15-ml centrifuge tube. The samples were centrifuged at 300 to 400 × g for 20 min at room temperature. The PBMC at the interphase were collected and washed three times with incomplete RPMI, and the viable cells were counted by trypan blue exclusion test. The PBMC were then resuspended in complete RPMI (incomplete RPMI supplemented with 10% heat-inactivated fetal calf serum; Gibco-Invitrogen, Carlsbad, CA) to a density of 2.5 × 106 cells per ml.
One hundred microliters of cell suspension was distributed in quadruplicate in a 96-well flat-bottomed tissue culture plate (BD Biosciences, San Diego, CA). Cells were stimulated with 100 μl of 10-μg/ml filter-sterilized, HPLC-purified recombinant PvTRAg40 prepared in complete RPMI or phytohemagglutinin (PHA; 5 μg/ml) (Gibco-Invitrogen, Carlsbad, CA). The control wells received an equal volume of RPMI. The cells were cultured for 4 days in a humidified atmosphere with 5% CO2 at 37°C. After 48 and 96 h of stimulation, 100 μl of cell-free supernatant was removed and replaced with 100 μl of complete RPMI. The culture supernatants from each of the quadruplicate and control wells were pooled. After 96 h of culture stimulation, the cells were pulsed with 1 μCi of [3H]thymidine (Board of Radiation and Isotope Technology, Navi Mumbai, India) per well in a total volume of 25 μl. The cells were harvested 16 h later onto glass fiber filter mats (Whatman International Ltd., Maidstone, England) by use of a cell harvester (Nalgene Nunc International, Rochester, NY), and the [3H]thymidine incorporation was determined by a liquid scintillation counter (Wallac 1409DSA; Perkin Elmer, Waltham, MA). For calculation, mean counts per minute of quadruplicate antigen- or mitogen-stimulated cultures or medium alone were taken. Results are expressed as stimulation indexes (SI), and a lymphoproliferative response was considered positive when the SI was >2. PBMC from individuals with no history of exposure to malaria served as normal controls in each set of experiments.
Cytokine assays.
Cytokine assay was done on pooled culture supernatants obtained after 48 h and 96 h of stimulation with PvTRAg40 and PHA. Supernatants were diluted 1:1 with RPMI for cytokine estimation, and later their OD values were normalized. The assay was performed using a human immunoassay kit (OptEIA; BD Biosciences, San Diego, CA) per the manufacturer's instructions, and results were expressed in picograms per milliliter, by interpolation from standard curves based on recombinant lymphokines (22). The IFN-γ/IL-4 and TNF-α/IL-10 ratios were taken as indexes for the Th1/Th2 cytokine response to the PvTRAg40-stimulated lymphocytes (17, 33).
Indirect immunofluorescence assay.
Thin smears of P. vivax-infected erythrocytes were prepared, air dried, and fixed with chilled acetone-methanol (1:1) for 20 min at −20°C. The smears were blocked with 1% bovine serum albumin in PBS, washed with PBS, and then incubated with a 1:100 dilution of either preimmune serum or antisera raised in rabbits against HPLC-purified recombinant PvTRAg40. After being washed with PBS, the smears were air dried and incubated with fluorescein isothiocyanate-labeled goat anti-rabbit IgG at a 1:50 dilution (BD Biosciences, San Diego, CA). Slides were viewed under UV and visible light (100× oil immersion objective), using a Nikon Eclipse E600 microscope.
Genetic polymorphism.
The genomic DNAs from P. vivax-infected patients' blood were isolated using an AccuPrep genomic DNA isolation kit (Bioneer Corp., Korea) per the manufacturer's instructions. These DNAs were then used for PCR amplification, using the same primer set as that described above for reverse transcription-PCR. The PCR products were analyzed in a 1.0% agarose gel. The DNA band was excised from the gel and purified using an AccuPrep gel purification kit (Bioneer Corp., Korea) following the manufacturer's instructions. Both the DNA strands were sequenced on an automated DNA sequencer (ABI Prism 310; Perkin Elmer, Applied Biosystems Inc., CA), using the same set of primers as those used for PCR. Sequences thus obtained were aligned together using DNASIS and Lasergene-Seqman software. Homologies with online data bank sequences were determined using the BLAST and FASTA algorithms available at http://www.ncbi.nlm.nih.gov and http://www.plasmodb.org. Multiple sequence alignments using ClustalW (http://www.ebi.ac.uk) were also performed.
Statistical analysis.
All assays were performed in triplicate unless indicated otherwise, and results are reported as means ± SD. The Mann-Whitney nonparametric method was used for comparing two unpaired groups, and two-tailed P values were reported using the software STATA, version 9.0 (StataCorp LP, TX). The Wilcoxon signed-rank test was used to compare the levels of IgG1 and IgG4 antibodies among the individuals in the recovered group. P values of <0.05 were considered statistically significant.
Nucleotide sequence accession numbers.
The nucleotide sequence data reported in this paper are available in the GenBank database under accession numbers EF472686 to EF472720 (from 35 P. vivax clinical isolates obtained in different parts of India) and EF472721 (cDNA sequence).
RESULTS
Sequence analysis of PvTRAg40 gene.
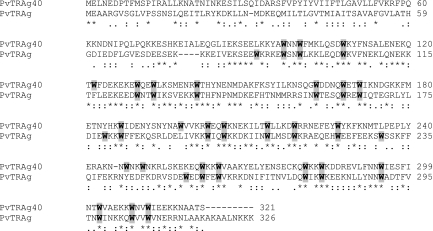
The previously reported PvTRAg sequence (19) was used to find homologies with online data bank sequences (http://www.plasmodb.org and http://www.tigr.org). The homology search revealed the presence of a number of related proteins in P. vivax. Multiple sequence alignments showed the peculiarity of these molecules, as they were not only unusually rich in tryptophans but the latter amino acid residues were also positionally conserved among them. We have analyzed here one of the homologues, named PvTRAg40, containing 321 amino acids (aa) (calculated molecular mass of 39.95 kDa) with an unusually large number of tryptophan residues (11.4%). The PvTRAg40 gene is interrupted by an intron where exon 2 encodes a 261-aa-long polypeptide. All 27 tryptophan residues were present in this exon 2-encoded polypeptide and distributed over its entire length. Twenty-three of the 27 tryptophan residues were positionally conserved between PvTRAg and PvTRAg40. Besides the positionally conserved tryptophan residues and similar genomic organization, PvTRAg and PvTRAg40 shared 65% homology (35% identity) with each other at the amino acid level (Fig. 1).
FIG. 1.
Sequence alignment of PvTRAg40 and the previously reported PvTRAg protein, using the ClustalW program (available online at http://www.ebi.ac.uk). The two sequences share 23 positionally conserved tryptophan residues, which are shown in bold and shaded in gray. Asterisks indicate identical amino acids, gaps are indicated by dashes, and double and single dots indicate conserved and semiconserved substitutions, respectively. Numbers on the right indicate amino acid positions.
Expression and purification of exon 2-encoded polypeptide of PvTRAg40.
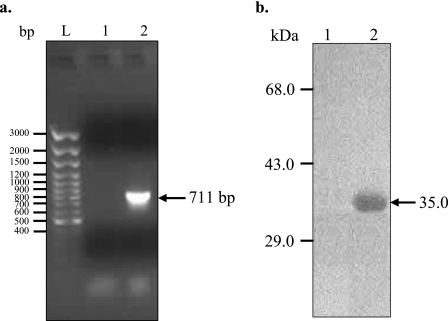
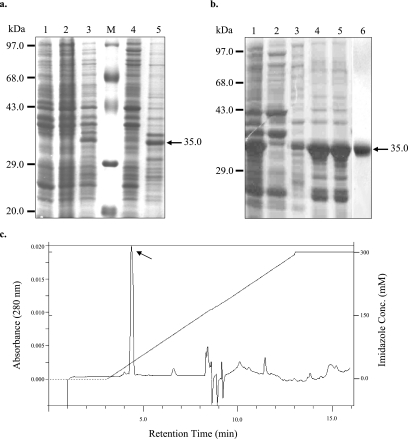
In order to focus on the tryptophan-rich domain of PvTRAg40, we expressed only 237 aa from the exon 2-encoded protein, which excluded 24 aa from the N terminus of the exon 2-encoded protein. This strategy also excluded the exon 1-encoded 60 aa, which would otherwise be present in the whole cDNA-derived protein. Thus, part of the cDNA for exon 2 of PvTRAg40 was amplified and expressed in E. coli to produce a 35.15-kDa fusion protein containing a six-His tag in its N-terminal region (Fig. 2). Most of the expressed protein was present in the insoluble inclusion bodies (Fig. 3a, lane 5), leaving only a minute fraction in the soluble form (Fig. 3a, lane 4). Induction at lower temperatures (as low as 18°C) did not increase the solubility of the protein. Inclusion bodies were therefore used to obtain the purified recombinant protein (Fig. 3b, lanes 4 and 5) under the same conditions as those described earlier (29). The purification of this recombinant protein was achieved by single-step Ni-NTA IMAC (Fig. 3b, lane 6). The protein was purified to a yield of about 18 to 20 mg per liter of induced culture. The purity of this protein preparation was further confirmed by reversed-phase chromatography on an HPLC system, which yielded a very sharp peak (Fig. 3c). The Western blot of this peak with monoclonal anti-six-His antibodies also confirmed the identity of the purified recombinant PvTRAg40 protein (data not shown).
FIG. 2.
Amplification and expression of exon 2 of PvTRAg40 cDNA. (a) cDNA amplification of 711-bp PvTRAg40 open reading frame (lane 2). The arrow shows the amplified product. PCR amplification was also done on a reverse transcriptase negative control sample, which did not yield any band (lane 1). The sizes of the DNA ladder marker are shown in lane L. (b) Western blot analysis of PvTRAg40-derived protein with N-terminal six-His fusion in the pPROEXHTa vector. Total E. coli lysate corresponding to 1 ml uninduced (lane 1) or IPTG-induced (lane 2) culture pellet was run on SDS-PAGE, and bands were transferred to a nitrocellulose filter. The filter was allowed to react with a monoclonal anti-six-His mouse antibody. The arrow indicates the His-tagged PvTRAg40 fusion protein (∼35 kDa). Sizes of the protein markers are indicated.
FIG. 3.
Purification of recombinant PvTRAg40. (a) The insoluble inclusion bodies obtained after sonication of the E. coli culture lysate contained a substantial amount of recombinant PvTRAg40 (lane 5), whereas the soluble fraction contained a very small amount (lane 4). Lanes 1, 2, and 3, induced pPROEXHTa vector, an uninduced clone, and an induced clone, respectively; lane M, protein marker. (b) Inclusion bodies (lane 1) containing PvTRAg40 were solubilized (lane 4) and subsequently refolded (lane 5). Lanes 2 and 3 show wash steps 1 and 2, respectively, during the solubilization procedure. The purification of refolded six-His-tagged PvTRAg40 fusion protein was achieved by single-step IMAC on a Ni-NTA column (lane 6). The size of the PvTRAg40 band is marked by the arrow, and molecular size markers are indicated on the left. (c) HPLC profile of the Ni-NTA-purified protein on a C18 reverse-phase column.
Human immune responses against exon 2-encoded polypeptide of PvTRAg40. (i) Humoral response.
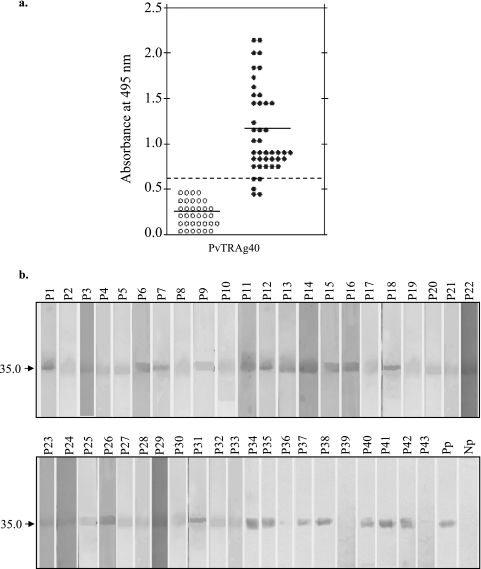
Antibody responses to PvTRAg40 were analyzed in 43 serum samples from P. vivax-exposed individuals and 34 serum samples from healthy volunteers who had no history of malaria infection in the recent past (malaria-naïve individuals). ELISA and Western blot assays were used to assess the humoral response to HPLC-purified recombinant protein derived from exon 2 of PvTRAg40. The serum dilution used for ELISAs was 1:400, while that used for Western blotting experiments was 1:100. In ELISA, the positive responders were defined as those who exhibited an OD495 of >0.62 (mean + 3 SD of control sera). Thirty-nine of the 43 (90.7%) serum samples from the P. vivax-exposed individuals showed ELISA ODs above 0.62 and thus were considered seropositive, while all 34 sera from healthy controls showed lower ODs (Fig. 4a). The means ± SD for P. vivax-exposed and control individuals were 1.15 ± 0.43 and 0.24 ± 0.13, respectively. Different levels of antibodies against this antigen were produced by the individuals (Fig. 4b). The reactivity of each serum sample to the recombinant antigen on the Western blot assay almost complemented the ELISA OD values. PvTRAg40 seemed to be highly immunogenic, as indicated by the production of high-titer (>102,400) antibodies in rabbits. The results thus show the natural immunogenicity and antigenicity of PvTRAg40.
FIG. 4.
Antibody responses to PvTRAg40 in P. vivax patients. (a) ELISA was performed with 43 sera from P. vivax-exposed patients and 34 sera from malaria-naïve individuals (both at a 1:400 dilution), using the HPLC-purified recombinant protein derived from exon 2 of PvTRAg40. The solid horizontal lines indicate the mean OD values, while the broken horizontal line indicates the cutoff value. (b) Western blot analysis of the above-mentioned HPLC-purified recombinant protein and serum samples. Purified protein was resolved by SDS-PAGE and then transferred to a nitrocellulose membrane. The nitrocellulose filter for each lane was cut and incubated with individual P. vivax-exposed and control sera (1:100 dilution). Pp, blot developed with pooled patient sera; Np, pooled malaria-naïve control sera. The arrow indicates the position of the PvTRAg40 band.
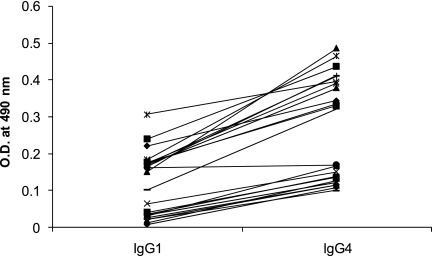
We also determined the levels of IgG1 and IgG4 antibodies against PvTRAg40 among 24 serum samples from the individuals who were exposed to P. vivax malaria 8 to 14 weeks prior to their blood collection (Fig. 5). The mean OD values (± SD) for IgG1 and IgG4 were 0.112 ± 0.086 (median value, 0.125; range, 0.006 to 0.305) and 0.260 ± 0.139 (median value, 0.245; range, 0.099 to 0.485), respectively. The IgG4 values were significantly higher than the IgG1 values (P < 0.05).
FIG. 5.
Levels of IgG1 and IgG4 antibodies against PvTRAg40 in a group of recovered patients. ELISA was performed on serum samples (n = 24) from individuals who had been exposed to P. vivax infection 8 to 14 weeks prior to sample collection, using HPLC-purified PvTRAg40 and biotin-labeled monoclonal anti-human IgG1 or IgG4. The levels of IgG4 antibodies were significantly higher than those of IgG1 (P < 0.05).
(ii) T-cell response and cytokine production.
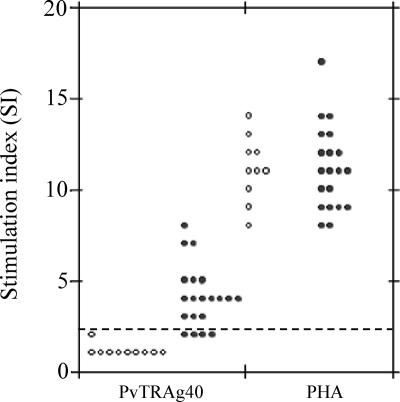
The proliferative responses to recombinant PvTRAg40 of human T lymphocytes from the 20 P. vivax-exposed and 10 malaria-naïve individuals are shown in Fig. 6. The lymphoproliferative responses were considered positive if the individuals showed SI of >2. All individuals, whether exposed or unexposed to P. vivax malaria, showed a positive response to PHA. However, 80% (n = 20) of the P. vivax-exposed individuals showed a positive lymphoproliferative response against PvTRAg40 (mean SI, 3.9; range, 1.79 to 8.2), while none of the samples from the malaria control group showed a positive response to this antigen.
FIG. 6.
T-cell response to PvTRAg40. SI are given for PBMC from subjects exposed to P. vivax or from malaria-naïve controls. The cells were stimulated with HPLC-purified recombinant PvTRAg40 protein, with the mitogen PHA as a control, or with medium alone as a background control. For calculation, mean scintillation counts per minute were calculated. Open circles represent SI values for control individuals who had no history of malaria exposure (n = 10), and solid circles represent SI values for subjects recently exposed to P. vivax who had recovered 8 to 14 weeks prior to study (n = 20). SI values of >2.0 were considered positive (the cutoff value is shown by the dashed line).
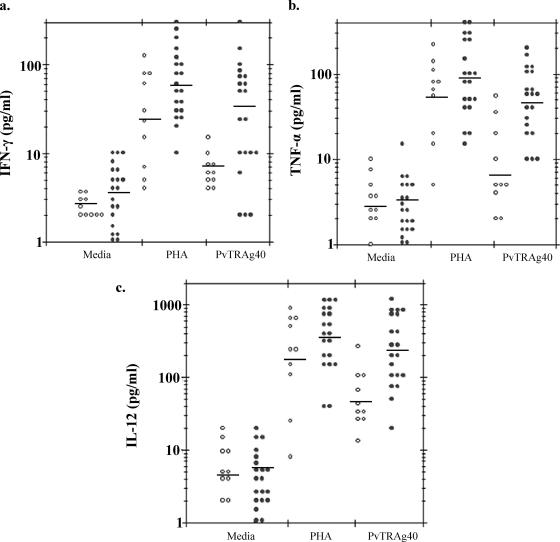
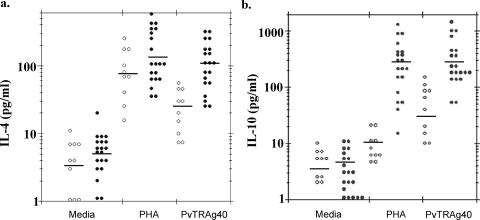
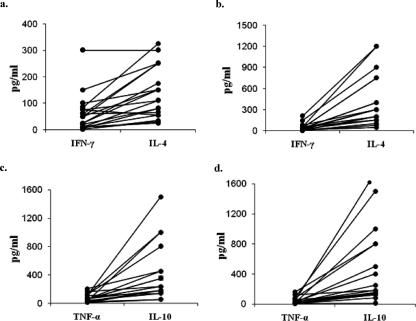
The profiles of the major Th1 (IFN-γ, TNF-α, and IL-12) and Th2 (IL-4 and IL-10) cytokines elicited by PBMC in response to recombinant PvTRAg40 at 48 h are shown in Fig. 7 and 8. PvTRAg40-stimulated IFN-γ production in P. vivax-exposed subjects (geometric mean = 34.43 pg/ml) was significantly higher (P < 0.05) than that in malaria-naïve controls (geometric mean = 7.67 pg/ml) (Fig. 7a), whereas the difference in PHA-stimulated IFN-γ production between test and control populations was insignificant (P > 0.05). The production of the TNF-α cytokine in P. vivax-exposed subjects (geometric mean = 46.81 pg/ml) was also observed to be significantly higher (P < 0.05) than that in malaria-naïve controls (geometric mean = 6.25 pg/ml) (Fig. 7b), whereas the difference in PHA-stimulated TNF-α production between test and control populations was insignificant (P > 0.05). Similarly, production of IL-12, IL-4, and IL-10 was significantly higher (P < 0.05) in the P. vivax-exposed subjects (geometric means = 237.35, 105.56, and 264.79 pg/ml, respectively) (Fig. 7c and 8a and b) than in malaria-naïve controls (geometric means = 48.07, 24.07, and 29.85 pg/ml, respectively). However, differences in PHA-stimulated production of these cytokines in the P. vivax-exposed subjects (geometric means = 345.93, 125.26, and 270.01 pg/ml, respectively) and control groups (geometric means = 183.35, 69.89, and 10.12 pg/ml, respectively) were insignificant (P > 0.05) for IL-12 and IL-4 and significant only for IL-10, while those produced by medium alone were insignificant for all the cytokines.
FIG. 7.
PvTRAg40- and PHA-induced Th1-type cytokine responses in P. vivax-exposed human subjects and malaria-naïve individuals. Individual supernatants from quadruplicate cultures of each sample were collected after 48 h of cell stimulation, pooled, diluted 1:1 with culture medium, and assayed by sandwich ELISA. Cytokine secretion in response to PHA and medium alone was also estimated and plotted. The graphs represent the secretion of IFN-γ (a), TNF-α (b), and IL-12 (c). Open circles represent cytokine values for control individuals who had no history of malaria exposure (n = 10), and solid circles represent values for subjects recently exposed to P. vivax who had recovered 8 to 14 weeks prior to study (n = 20). The geometric mean is shown by a horizontal line for each group.
FIG. 8.
PvTRAg40- and PHA-induced Th2-type cytokine responses in P. vivax-exposed human subjects and malaria-naïve donors. For analysis of the Th2-type subset, IL-4 (a) and IL-10 (b) cytokines were assayed as described in the legend to Fig. 7. Open circles represent cytokine values for control individuals who had no history of malaria exposure (n = 10), and solid circles represent values for subjects recently exposed to P. vivax who had recovered 8 to 14 weeks prior to study (n = 20). The geometric mean is shown by a horizontal line for each group.
The mean difference in cytokines produced at 48 h and 96 h (Table 1) was significantly lower (P < 0.05) for IFN-γ and TNF-α and higher for IL-4, but no significant change in IL-12 and IL-10 production was observed. The geometric mean ratios of IFN-γ to IL-4 and TNF-α to IL-10 at 48 h and 96 h are shown in Table 1. The results show that the production of PvTRAg40-induced Th2 cytokines (IL-4 and IL-10) is more pronounced than that of Th1 cytokines (IFN-γ and TNF-α), suggesting a cytokine response bias toward the Th2 type. Similarly, analysis of the relative production of IFN-γ/IL-4 and TNF-α/IL-10 in each subject at 48 h and 96 h showed a further increase in the production of IL-4 and IL-10 at 96 h, while production of IFN-γ and TNF-α remained almost the same (Fig. 9).
TABLE 1.
Cytokine production of PvTRAg40-stimulated lymphocyte cultures obtained from peripheral blood from P. vivax-exposed individuals (n = 20)
| Cytokine(s) | Concn (pg/ml) or ratio
|
P valuea | |||
|---|---|---|---|---|---|
| 48 h
|
96 h
|
||||
| Geometric mean | Range | Geometric mean | Range | ||
| IFN-γ | 34.43 | 2-300 | 28.16 | 1.2-200 | 0.0329 |
| TNF-α | 46.81 | 10-205 | 26.18 | 4-160 | 0.0006 |
| IL-12 | 237.35 | 20-1,200 | 218.27 | 18-2,000 | 0.125 |
| IL-4 | 105.56 | 25-325 | 227.431 | 45-1,200 | 0.0004 |
| IL-10 | 264.79 | 55-1,500 | 251.18 | 8-1,750 | 0.291 |
| IFN-γ/IL-4 | 0.274 | 0.057-1.36 | 0.092 | 0.015-0.33 | 0.0003 |
| TNF-α/IL-10 | 0.177 | 0.025-0.785 | 0.089 | 0.012-0.694 | 0.005 |
P values of <0.05 were considered significant.
FIG. 9.
Th2 response dominance, shown by individual values for IFN-γ versus IL-4 after 48 h (a) and 96 h (b) and for TNF-α versus IL-10 after 48 h (c) and 96 h (d), in PvTRAg40-stimulated lymphocyte cultures obtained from peripheral blood from P. vivax-exposed individuals (n = 20). The differences between the ratios of IFN-γ to IL-4 and TNF-α to IL-10 at 48 h and 96 h were statistically significant (P < 0.05). Note that the scales for panels a and b are different from that for panels c and d.
In vivo expression of PvTRAg40.
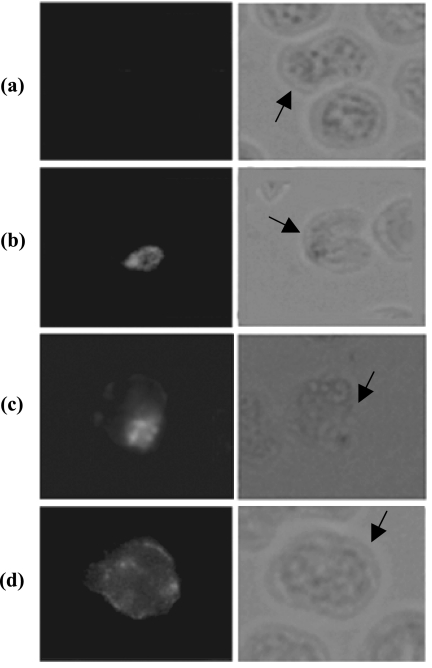
The PvTRAg40 gene is transcriptionally active, as demonstrated by the amplification of cDNA. Similar to the P. falciparum protein PfTryThrA (36) and previously reported PvTRAg (19), which are expressed by all asexual blood stages of the parasite, indirect immunofluorescence assay of P. vivax-infected thin blood smears developed by polyclonal sera raised against PvTRAg40 demonstrated that fluorescence is associated mainly with all the erythrocytic stages of P. vivax (ring, trophozoite, and schizont) (Fig. 10b to d). Smears incubated with preimmune rabbit serum did not show any fluorescence on P. vivax-infected erythrocytes (Fig. 10a). The fluorescence signal for PvTRAg40 increased as asexual blood stages matured from ring to trophozoite but decreased at the schizont stage.
FIG. 10.
Indirect immunofluorescence assay. Thin smears of washed P. vivax-infected erythrocytes were prepared and incubated with preimmune rabbit serum as a negative control on the early schizont stage of the parasite (a) and with polyclonal antibody raised against PvTRAg40 (b, c, and d [ring, trophozoite, and schizont stages of the parasite, respectively]), followed by a fluorescein isothiocyanate-conjugated secondary antibody. Parasitized red blood cells are indicated by arrows. The same fields of cells were examined by fluorescence (left panels) and phase-contrast (right panels) microscopy, at a magnification of ×100.
PvTRAg40 is highly conserved among P. vivax isolates.
Since the tryptophan residues are present only in the exon 2-encoded polypeptide of the PvTRAg40 protein, this region was PCR amplified and sequenced from 35 clinical isolates of P. vivax which were collected from different geographical regions of India. All 35 isolates were found to contain identical nucleotide sequences. This indicates that there was no sequence variation in the exon 2-encoded polypeptide among the isolates, irrespective of their geographical origin. This also indicates that not only are all the tryptophan residues positionally conserved among regional isolates but there is also no antigenic variation in the exon 2-encoded polypeptide of PvTRAg40.
DISCUSSION
We describe here the identification and immunological characterization of PvTRAg40, which shows homology to the previously known P. vivax molecule called PvTRAg (19). Both the antigens are expressed during the erythrocytic stages of the parasite, are translocated to the red cell membrane via caveola vesicle complex, and have the same genomic organization and positionally conserved tryptophan residues. These tryptophan-rich molecules are members of the P. vivax multigene family, which are common in Plasmodium species (25). Multigene families are usually a collection of related genes that probably share a common ancestor and might have originated from each other by gene recombination (25). Since the similar P. yoelii tryptophan-rich proteins have shown protection in the murine model and hence have been proposed as potential vaccine candidates (8, 9), we analyzed PvTRAg40 for its immunological characterization. The majority of P. vivax-exposed individuals (90.7%; n = 43) were found to produce antibodies against this antigen, which also resulted in high-titer antibodies in rabbits. Similarly, 80% (n = 20) of P. vivax-exposed individuals showed lymphoproliferation against PvTRAg40. This indicates that the exon 2-encoded polypeptide of PvTRAg40 contains B- and T-cell epitopes which are recognized by the majority of P. vivax patients.
Since cell-mediated immunity is induced and regulated by cytokines, we also studied the profiles of the major cytokines elicited in vitro by PBMC after stimulation with PvTRAg40. All subjects generated significant levels of PvTRAg40-driven Th1 and Th2 cytokines, with a Th2-type response being more pronounced (Fig. 7 and 8). We detected higher levels of IL-4 and IL-10 produced by PvTRAg40-stimulated T cells, which is indicative of a Th2 profile. This Th2 bias was further confirmed by the presence of significantly higher levels of IgG4 than IgG1 (P < 0.05) in the sera of the group of recovered individuals (Fig. 5). Moreover, we found that the PBMC of P. vivax-exposed individuals produced higher levels of IL-10 in response to PHA and PvTRAg40 than did those of healthy controls. Similar plasma in vitro IL-4 levels have also been reported for P. falciparum-infected patients (4). Furthermore, the geometric mean ratios of IFN-γ to IL-4 and TNF-α to IL-10 at 48 and 96 h and their relative production in the individual subjects also support the prominence of the Th2 response (Table 1 and Fig. 9). This is because a certain lymphokine pattern (IFN-γ/IL-4 ratio) is generally used as representative of a Th1 or Th2 profile (33). Also, smaller values of these ratios are an indication of an anti-inflammatory profile. The further decrease in the ratio of these cytokines after 96 h of stimulation indicates a shift toward the anti-inflammatory response as the time of exposure to the antigen increases. However, the levels of IL-12 were also higher than those of IFN-γ and TNF-α. It may be noted here that IL-12 is required for antibody-mediated protection and that its role in the early immune response is critical, as it links innate and acquired immunity (11). Thus, PvTRAg40 is able to stimulate T cells to produce larger amounts of IL-12, which could be involved in antibody-mediated parasite killing.
A predominant Th2 profile is involved in an immune response characterized principally by the secretion of antibodies (33), which supports antibody-mediated immunity. It is possible that cytokines secreted by T cells after activation qualitatively influence the nature of the immune response. As in P. chabaudi (31, 34), the immune response to blood-stage infection could involve sequential activation of Th1 and Th2 cytokine responses during the acute and chronic stages of infection, respectively. There is a general belief that Th2 cytokines predominating during the chronic stage of infection are important for antibody-mediated antimalarial immunity (15). The SI values obtained in this study by and large correlated with the values for cytokine production in a majority of subjects. This is similar to the observation made by various workers for several other Plasmodium antigens (5, 6, 12, 18). Most of the P. vivax-exposed donors in our study who showed positive cellular responses also exhibited a positive antibody response, thereby implying a correlation between B- and T-cell responses and cytokine secretion. Furthermore, these B- and T-cell epitopes of PvTRAg40 are highly conserved among P. vivax populations. This is because the sequences of the exon 2-encoded polypeptide covering the entire tryptophan-rich domain of PvTRAg40 were identical among clinical isolates of different geographical locations. The absence of antigenic polymorphism is highly desirable for a candidate malaria vaccine antigen. The previously reported proteins PvTRAg and PvTARAg55 had also shown conserved sequences, but to a lesser extent than that reported here for PvTRAg40 (19, 30). Nevertheless, these isolates have shown extensive polymorphisms in other genes, e.g., the PvDHFR coding region as well as its flanking microsatellites (1, 2), PvTARAg55 (30), and PvAMA-1 (A. Thakur et al., unpublished data). In light of these observations, the highly conserved nature of PvTRAg40 becomes quite significant. Furthermore, PvTRAg40 results in greater humoral and cellular immune responses than does PvTARAg55 among the same group of P. vivax-exposed individuals (30).
Apart from sharing the positionally conserved tryptophan residues, homologues of PvTRAg present in the database show a high degree of variation, which might represent variation in B- and T-cell determinants. This can be substantiated clearly by the fact that high-titer polyclonal antibodies raised separately against PvTRAg and PvTRAg40 did not show cross-reactivity (data not shown). Although both PvTRAg and PvTRAg40 caused similar humoral immune responses among P. vivax-exposed individuals, the latter antigen shows more conserved B-cell epitopes than does PvTRAg. While the T-cell response against PvTRAg remains to be elucidated, the augmented cellular immune response and Th2 type of cytokine production, together with conserved T-cell epitopes, make PvTRAg40 an attractive vaccine candidate antigen, as it can provide antibody-mediated protection against P. vivax infection to the host.
Acknowledgments
Financial support to Y.D.S. came from the Department of Biotechnology (DBT) and the Indian Council of Medical Research (ICMR). A senior research fellowship to A.A.S. was awarded by ICMR.
We thank H. K. Prasad, S. N. Das, T. K. Das, Nutan Nanda, S. N. Dwivedi, and S. Biswas for their technical help and critical evaluations of the manuscript. We also acknowledge the facility of Biotechnology Information System (BTIS) for analyzing the sequence data. We thank Arun Kumar for his help with sample collection in the field. We are indebted to all of the patients and healthy volunteers who participated in this study.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 24 March 2008.
REFERENCES
- 1.Alam, M. T., R. Agarwal, and Y. D. Sharma. 2007. Extensive heterozygosity at four microsatellite loci flanking Plasmodium vivax dihydrofolate reductase gene. Mol. Biochem. Parasitol. 153178-185. [DOI] [PubMed] [Google Scholar]
- 2.Alam, M. T., H. Bora, P. K. Bharti, M. A. Saifi, M. K. Das, V. Dev, A. Kumar, N. Singh, A. P. Dash, B. Das, Wajihullah, and Y. D. Sharma. 2007. Similar trends of pyrimethamine resistance-associated mutations in Plasmodium vivax and P. falciparum. Antimicrob. Agents Chemother. 51857-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Artavanis-Tsakonas, K., and E. M. Riley. 2002. Innate immune response to malaria: rapid induction of IFN-gamma from human NK cells by live Plasmodium falciparum-infected erythrocytes. J. Immunol. 1692956-2963. [DOI] [PubMed] [Google Scholar]
- 4.Aubouy, A., P. Deloron, and F. Migot-Nabias. 2002. Plasma and in vitro levels of cytokines during and after a Plasmodium falciparum malaria attack in Gabon. Acta Trop. 83195-203. [DOI] [PubMed] [Google Scholar]
- 5.Bharadwaj, A., P. Sharma, S. K. Joshi, B. Singh, and V. S. Chauhan. 1998. Induction of protective immune responses by immunization with linear multiepitope peptides based on conserved sequences from Plasmodium falciparum antigens. Infect. Immun. 663232-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bottius, E., L. BenMohamed, K. Brahimi, H. Gras, J. P. Lepers, L. Raharimalala, M. Aikawa, J. Meis, B. Slierendregt, A. Tartar, A. Thomas, and P. Druilhe. 1996. A novel Plasmodium falciparum sporozoite and liver stage antigen (SALSA) defines major B, T helper, and CTL epitopes. J. Immunol. 1562874-2884. [PubMed] [Google Scholar]
- 7.Breman, J. G. 2001. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am. J. Trop. Med. Hyg. 641-11. [DOI] [PubMed] [Google Scholar]
- 8.Burns, J. M., Jr., E. K. Adeeku, and P. D. Dunn. 1999. Protective immunization with a novel membrane protein of Plasmodium yoelii-infected erythrocytes. Infect. Immun. 67675-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns, J. M., Jr., P. D. Dunn, and D. M. Russo. 1997. Protective immunity against Plasmodium yoelii malaria induced by immunization with particulate blood-stage antigens. Infect. Immun. 653138-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caro-Aguilar, I., A. Rodriguez, J. M. Calvo-Calle, F. Guzman, P. De la Vega, M. E. Patarroyo, M. R. Galinski, and A. Moreno. 2002. Plasmodium vivax promiscuous T-helper epitopes defined and evaluated as linear peptide chimera immunogens. Infect. Immun. 703479-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chehimi, J., and G. Trinchieri. 1994. Interleukin-12: a bridge between innate resistance and adaptive immunity with a role in infection and acquired immunodeficiency. J. Clin. Immunol. 14149-161. [DOI] [PubMed] [Google Scholar]
- 12.Connelly, M., C. L. King, K. Bucci, S. Walters, B. Genton, M. P. Alpers, M. Hollingdale, and J. W. Kazura. 1997. T-cell immunity to peptide epitopes of liver-stage antigen 1 in an area of Papua New Guinea in which malaria is holoendemic. Infect. Immun. 655082-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escalante, A. A., and F. J. Ayala. 1994. Phylogeny of the malarial genus Plasmodium, derived from rRNA gene sequences. Proc. Natl. Acad. Sci. USA 9111373-11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Favre, N., B. Ryffel, G. Bordmann, and W. Rudin. 1997. The course of Plasmodium chabaudi chabaudi infections in interferon-gamma receptor deficient mice. Parasite Immunol. 19375-383. [DOI] [PubMed] [Google Scholar]
- 15.Fell, A. H., and N. C. Smith. 1998. Immunity to asexual blood stages of Plasmodium: is resistance to acute malaria adaptive or innate? Parasitol. Today 14364-369. [DOI] [PubMed] [Google Scholar]
- 16.Gardiner, D. L., J. S. McCarthy, and K. R. Trenholme. 2005. Malaria in the post-genomics era: light at the end of the tunnel or just another train? Postgrad. Med. J. 81505-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giron-Gonzalez, J. A., F. J. Moral, J. Elvira, D. Garcia-Gil, F. Guerrero, I. Gavilan, and L. Escobar. 2000. Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared with women. Eur. J. Endocrinol. 14331-36. [DOI] [PubMed] [Google Scholar]
- 18.Hollingdale, M. R., M. Aikawa, C. T. Atkinson, W. R. Ballou, G. X. Chen, J. Li, J. F. Meis, B. Sina, C. Wright, and J. D. Zhu. 1990. Non-CS pre-erythrocytic protective antigens. Immunol. Lett. 2571-76. [DOI] [PubMed] [Google Scholar]
- 19.Jalah, R., R. Sarin, N. Sud, M. T. Alam, N. Parikh, T. K. Das, and Y. D. Sharma. 2005. Identification, expression, localization and serological characterization of a tryptophan-rich antigen from the human malaria parasite Plasmodium vivax. Mol. Biochem. Parasitol. 142158-169. [DOI] [PubMed] [Google Scholar]
- 20.Joshi, S. K., A. Bharadwaj, S. Chatterjee, and V. S. Chauhan. 2000. Analysis of immune responses against T- and B-cell epitopes from Plasmodium falciparum liver-stage antigen 1 in rodent malaria models and malaria-exposed human subjects in India. Infect. Immun. 68141-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwiatkowski, D., and K. Marsh. 1997. Development of a malaria vaccine. Lancet 3501696-1701. [DOI] [PubMed] [Google Scholar]
- 22.Malhotra, I., P. Mungai, A. Wamachi, J. Kioko, J. H. Ouma, J. W. Kazura, and C. L. King. 1999. Helminth- and bacillus Calmette-Guerin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J. Immunol. 1626843-6848. [PubMed] [Google Scholar]
- 23.Mendis, K., B. J. Sina, P. Marchesini, and R. Carter. 2001. The neglected burden of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 6497-106. [DOI] [PubMed] [Google Scholar]
- 24.Nardin, E. H., and R. S. Nussenzweig. 1993. T cell responses to pre-erythrocytic stages of malaria: role in protection and vaccine development against pre-erythrocytic stages. Annu. Rev. Immunol. 11687-727. [DOI] [PubMed] [Google Scholar]
- 25.Ntumngia, F. B., N. Bahamontes-Rosa, and J. F. Kun. 2005. Genes coding for tryptophan-rich proteins are transcribed throughout the asexual cycle of Plasmodium falciparum. Parasitol. Res. 96347-353. [DOI] [PubMed] [Google Scholar]
- 26.Ray, P., M. A. Ansari, and Y. D. Sharma. 1994. Plasmodium vivax: immune responses in a cross-section of the population in the Delhi area of India. Am. J. Trop. Med. Hyg. 51436-443. [PubMed] [Google Scholar]
- 27.Riley, E. M., G. Andersson, L. N. Otoo, S. Jepsen, and B. M. Greenwood. 1988. Cellular immune responses to Plasmodium falciparum antigens in Gambian children during and after an acute attack of falciparum malaria. Clin. Exp. Immunol. 7317-22. [PMC free article] [PubMed] [Google Scholar]
- 28.Scheiblhofer, S., R. Weiss, and J. Thalhamer. 2006. Genetic vaccination approaches against malaria based on the circumsporozoite protein. Wien Klin. Wochenschr. 1189-17. [DOI] [PubMed] [Google Scholar]
- 29.Siddiqui, A. A., R. Jalah, and Y. D. Sharma. 2007. Expression and purification of HtpX-like small heat shock integral membrane protease of an unknown organism related to Methylobacillus flagellatus. J. Biochem. Biophys. Methods 70539-546. [DOI] [PubMed] [Google Scholar]
- 30.Siddiqui, A. A., N. Singh, and Y. D. Sharma. 2007. Expression and purification of a Plasmodium vivax tryptophan- and alanine-rich antigen and its immunological responses in human subjects. Vaccine 2696-107. [DOI] [PubMed] [Google Scholar]
- 31.Stevenson, M. M., and M. F. Tam. 1993. Differential induction of helper T cell subsets during blood-stage Plasmodium chabaudi AS infection in resistant and susceptible mice. Clin. Exp. Immunol. 9277-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevenson, M. M., M. F. Tam, S. F. Wolf, and A. Sher. 1995. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-gamma and TNF-alpha and occurs via a nitric oxide-dependent mechanism. J. Immunol. 1552545-2556. [PubMed] [Google Scholar]
- 33.Swain, S. L., L. M. Bradley, M. Croft, S. Tonkonogy, G. Atkins, A. D. Weinberg, D. D. Duncan, S. M. Hedrick, R. W. Dutton, and G. Huston. 1991. Helper T-cell subsets: phenotype, function and the role of lymphokines in regulating their development. Immunol. Rev. 123115-144. [DOI] [PubMed] [Google Scholar]
- 34.Taylor-Robinson, A. W., and R. S. Phillips. 1994. B cells are required for the switch from Th1- to Th2-regulated immune responses to Plasmodium chabaudi chabaudi infection. Infect. Immun. 622490-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Troye-Blomberg, M., P. Romero, M. E. Patarroyo, A. Bjorkman, and P. Perlmann. 1984. Regulation of the immune response in Plasmodium falciparum malaria. III. Proliferative response to antigen in vitro and subset composition of T cells from patients with acute infection or from immune donors. Clin. Exp. Immunol. 58380-387. [PMC free article] [PubMed] [Google Scholar]
- 36.Uhlemann, A. C., R. M. Oguariri, D. J. McColl, R. L. Coppel, P. G. Kremsner, R. F. Anders, and J. F. Kun. 2001. Properties of the Plasmodium falciparum homologue of a protective vaccine candidate of Plasmodium yoelii. Mol. Biochem. Parasitol. 11841-48. [DOI] [PubMed] [Google Scholar]