Abstract
Cryptococcus neoformans is an encapsulated fungal pathogen with a predilection to infect persons with suppressed T-cell function. Cryptococcal mannoproteins (MP) are highly mannosylated antigens which elicit T-cell responses in infected mice and in convalescent patients. Key to the immunogenicity of MP is its capacity to bind to the conserved mannose receptor (MR), CD206, on dendritic cells (DCs). To test the role of the MR in the immune response to C. neoformans, wild-type and MR knockout (MR KO) mice were compared by using in vivo and ex vivo models of cryptococcosis. Following a pulmonary challenge with C. neoformans, MR KO mice died significantly faster than wild-type mice and had higher lung fungal burdens after 4 weeks of infection. Uptake of MP was similar when DCs obtained from wild-type and MR KO mice were compared. Additionally, MP did not upregulate the maturation markers major histocompatibility complex class II, CD86, and CD40 in either wild-type or MR KO DCs. However, MP stimulated lymphoproliferation in CD4+ T cells obtained from the peripheral lymph nodes of infected wild-type but not MR KO mice. These studies demonstrate a nonredundant role for the MR in the development of CD4+ T-cell responses to MP and protection from C. neoformans.
Cryptococcus neoformans is an opportunistic fungal pathogen which preferentially afflicts immunocompromised persons, particularly those with AIDS or lymphoid malignancies and recipients of immunosuppressive treatments for solid organ transplants (2). The primary site of infection is generally the lungs. While exposure is thought to be common, most immunocompetent persons are able to contain the infection. However, in the absence of effective CD4+ T-cell responses, the organism can disseminate and eventually cross the blood-brain barrier to produce cryptococcal meningitis (18).
The inverse correlation between CD4+ T-cell function and the propensity to develop cryptococcosis has prompted investigators to search for antigens which drive the cell-mediated immune response. In this regard, a family of heavily mannosylated proteins, termed mannoproteins (MP), has been identified as immunodominant antigens in mouse models of cryptococcosis and in patients who have recovered from infection (1, 16, 26). MP possess serine- and threonine-rich C-terminal regions which serve as sites for extensive O-linked mannosylation. Additional mannosylation may be provided via N linkages (17). These areas of O-linked and N-linked mannosylation promote the recognition of MP by host receptors for mannose, in particular, the mannose receptor (MR, CD206) and dendritic cell (DC)-specific intercellular adhesion molecule 3 grabbing nonintegrin (CD209) (17, 21). DCs are the main antigen-presenting cells responsible for the uptake, processing, and presentation of MP to CD4+ T cells (21, 22). Blocking receptors for mannose with mannan or deglycosylating MP results in strongly diminished T-cell responses (21, 22, 32).
The MR is a C-type lectin receptor which recognizes terminally mannosylated molecules (24). In addition to MP, a myriad of mannosylated antigens are recognized by the MR, including ManLam from Mycobacterium tuberculosis, the envelope protein gp120 from HIV, the capsular polysaccharide from Streptococcus pneumoniae, and soluble egg antigen from Schistosoma mansoni (21, 37). The MR also recognizes live Pneumocystis carinii and Candida albicans organisms. However, in murine models of infection with C. albicans and P. carinii, there were no differences in survival between infected wild-type and MR KO mice (15, 34). In addition to its role in pathogen recognition, the MR also functions to clear endogenous proteins bearing accessible mannose residues (14). Thus, the MR appears to play dual roles as a pathogen recognition receptor and as a regulator of glycoprotein homeostasis.
Given the in vitro evidence of the importance of the MR in the recognition of MP by DCs and the subsequent stimulation of T-cell responses, we sought to determine if the MR plays a role in protection against C. neoformans in vivo and ex vivo. Accordingly, wild-type and MR KO mice were compared in murine models of experimental cryptococcosis. We found a significant difference in survival between the mouse strains following a pulmonary challenge with C. neoformans. Moreover, wild-type but not MR KO infected mice developed MP-dependent CD4+ T-cell proliferative responses. However, there was no difference in MP uptake or MP-induced induction of maturation marker expression on DCs derived from wild-type and MR KO mice.
MATERIALS AND METHODS
Reagents.
MP was purified from culture supernatants of C. neoformans acapsular strain Cap 67 (ATCC 52817) by concanavalin A (ConA) affinity chromatography, as previously described (23), and then passed over a polymyxin B column. The endotoxin content of the preparation, as detected by the Limulus amebocyte lysate assay (Associates of Cape Cod, East Falmouth, MA), was 0.020 endotoxin U/μg. All media were obtained from Invitrogen (Carlsbad, CA), chemical reagents were from Sigma (St. Louis, MO), and other materials were from Fischer Scientific (Pittsburgh, PA), unless otherwise noted. R10 medium was defined as RPMI 1640 medium supplemented with 10% fetal bovine serum (Tissue Culture Biologicals, Tulare, CA), 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and 50 μM 2-mercaptoethanol. Tissue culture incubations were done at 37°C in humidified air supplemented with 5% CO2.
Mice.
C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). MR (CD206) knockout (MR KO) mice were a gift from M. C. Nussenzweig (The Rockefeller University, New York, NY) (14) and were backcrossed to a C57BL/6 background for at least 10 generations. Loss of the MR was confirmed by genotyping. Mice used for survival studies and lymphoproliferation experiments were maintained by the Boston University Medical Center Laboratory Animal Sciences Center. Mice used for antigen binding studies and maturation marker expression studies were maintained by the University of Massachusetts Medical School Department of Animal Medicine. Mice were specific pathogen free, and the studies were approved by the respective Institutional Animal Care and Use Committees.
Pulmonary challenge with C. neoformans.
Encapsulated serotype A strain 145 (ATCC 62070) and serotype D strain 52 (ATCC 24067) were grown on Sabouraud dextrose agar at 30°C for 72 h. Strain 145 was used for all of the studies, except where noted otherwise. Colonies were then isolated and grown in liquid culture (yeast extract-peptone-dextrose plus 2% glucose) overnight. The culture was washed twice with phosphate-buffered saline (PBS), counted on a hemocytometer, and resuspended at the desired concentration. Following anesthesia with halothane (Samuel Perkins, Quincy, MA), mice (6 to 10 weeks old) were inoculated with the desired concentration of fungi in 50 μl of sterile PBS by holding the animal upright and placing the suspension on the nares until aspiration occurred (38). For the mortality studies, infected mice were examined twice daily and sacrificed when moribund as in prior studies (23).
Lung CFU assessment.
Lungs were harvested, weighed, and placed in 14-ml sterile polypropylene tubes containing 5 ml of cold PBS. The organs were homogenized with a PowerGen model 700 tissue homogenizer for 10 s. Serial dilutions of the homogenate were made in PBS and then plated on Sabouraud dextrose agar to determine the number of CFU per gram of tissue (40).
BMDC isolation.
Murine bone marrow-derived DCs (BMDCs) were generated by the protocol of Lutz et al., with slight modifications, as previously described (10, 20, 38). Briefly, cells obtained from the femurs and tibias of mice were cultured at 2 × 106 per petri dish (100 by 15 mm) in 10 ml of R10 medium supplemented with 10% supernatant obtained from the granulocyte-macrophage colony-stimulating factor-producing J558L cell line. Media were replenished on days 3, 6, and 8. On day 9, nonadherent cells were harvested and the DCs were purified by two passages over an LS column (Miltenyi Biotec, Auburn, CA) with CD11c+ magnetic beads (Miltenyi Biotec, Auburn, CA). The DCs were >90% CD11c+ CD11b+ B220−, as determined by flow cytometry.
Antigen uptake.
MP was labeled with Oregon green (OG) in sodium bicarbonate buffer (pH 8.0) by following the manufacturer's protocol (Molecular Probes, Carlsbad, CA). Excess OG was removed with a G10 Sephadex column (Sigma Aldrich). MP-OG was then dialyzed overnight against water. MP-OG was incubated for 15 min at 37°C with CD11c+-purified BMDCs (1 × 106) in R10 medium. Cells were then washed three times with fluorescence-activated cell sorter buffer (2% fetal bovine serum in PBS). Flow cytometric data were acquired with an LSR flow cytometer (BD Biosciences) and analyzed with WinMDI software (Scripps Research Institute). Antigen uptake is defined as total cell-associated fluorescence and thus includes both surface-bound and internalized MP-OG. To distinguish surface-bound and internalized MP-OG, confocal microscopy was performed with a Leica SP2 AOBS confocal microscope (Leica Microsystems) with 35-mm glass bottom dishes (MatTek) as previously described (4).
Cell surface marker expression.
The antibodies, including isotype controls, used in this analysis were obtained from eBioscience. CD11c+-purified DCs (1 × 106) were incubated with the indicated stimulus in R10 medium. Polymyxin B (10 μg/ml) was added to all of the incubations, except those with lipopolysaccharide (LPS). After 24 h, cells were harvested with EDTA. Cells (1 × 106) were then resuspended in fluorescence-activated cell sorter buffer and incubated with fluorescently labeled antibodies reactive with CD11c, major histocompatibility complex class II (MHCII), CD86, and CD40 at concentrations recommended by the manufacturer. Cell-associated fluorescence was then determined by flow cytometry as described above. Mean fluorescence intensities were determined after gating on CD11c+ cells.
CD4+ T-cell isolation.
Mice were euthanized by CO2 asphyxiation. Peripheral lymph nodes (inguinal, brachial, axial, cervical, mesenteric, and lumbar) were isolated from infected mice. Lymph nodes were disrupted by pushing them through a sterile wire mesh into a single-cell suspension. CD4+ T cells were then enriched with L3T4+ magnetic beads for positive selection (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions.
Lymphoproliferation studies.
DCs were enriched by positive selection with CD11c+ magnetic beads. Mitomycin (50 μg/ml)-treated DCs from wild-type mice were plated at 1 × 104 per well in 96-well round-bottom plates (19, 39). CD4+-purified T cells (1 × 105) were then added to each well. Cells were incubated with antigen and pulsed on day 3 with 1 μCi [3H]thymidine (Perkin-Elmer, Wellesley, MA). After an additional 18 to 20 h, the cells were freeze-thawed and harvested. Proliferation was measured with a beta scintillation counter. The stimulation index was calculated by dividing the number of counts per minute in the stimulated group by the number of counts per minute in the unstimulated group.
Statistics.
GraphPad Prism Software was used for statistical analyses. Kaplan-Meier survival curves were compared by using the log rank test. All other statistical analyses used the unpaired Student t test for comparisons between two groups and the one-way analysis of variance when three or more groups were compared. Significance was defined as P < 0.05 after Bonferroni's correction.
RESULTS
Effect of deletion of the MR on survival and lung CFU counts following intranasal infection with C. neoformans.
Wild-type and MR KO mice were infected with either 5 × 104 (Fig. 1A) or 1 × 106 (Fig. 1B) C. neoformans organisms. In order to mimic the natural route of infection, the fungal challenge was administered intranasally under conditions promoting pulmonary aspiration. At the lower inoculum, MR KO mice succumbed significantly faster to the infection than did the wild-type mice. Similar results were obtained with the higher inoculum, although the difference did not reach statistical significance (P = 0.08). As the lungs are the initial site of infection, we next determined if there was a difference between the lung fungal burdens of wild-type and MR KO mice. Lungs were examined at 14 and 28 days postinfection (Fig. 2). While there were no significant differences in the CFU counts at the 2-week time point, at 28 days there were significantly more CFU in the lungs of the KO mice.
FIG. 1.
Survival of C57BL/6 wild-type and MR KO mice following intranasal infection with C. neoformans. (A) Wild-type (n = 9) and MR KO (n = 7) mice were infected intranasally with 5 × 104 organisms. P = 0.001 comparing the survival curves of the two groups. (B) Wild-type (n = 8) and MR KO (n = 7) mice were infected intranasally with 1 × 106 organisms. P = 0.08 comparing the survival curves of the two groups.
FIG. 2.
Fungal lung burdens in C57BL/6 wild-type and MR KO mice following a pulmonary challenge with C. neoformans. Wild-type and MR KO mice were infected intranasally with 1 × 106 organisms and sacrificed either 14 (n = 4 in each group) or 28 (n = 3 or 4 in each group) days later. Lungs were then excised and homogenized, and CFU counts were determined. Horizontal lines represent means, whereas circles and triangles represent values for individual mice. P = 0.007 comparing the CFU counts from wild-type and MR mice at 28 days postinfection (p.i.).
One possible explanation for the increased CFU count and mortality in the MR KO mice is that the nasal or tracheobronchial passages of these mice are wider or less mucoid than those of wild-type mice, thus allowing more of the inoculum to reach the lungs. To examine this possibility, wild-type and MR KO mice (n = 4 in each group) were euthanized 10 min after intranasal inoculation with C. neoformans and lung CFU counts were determined (8). There were no significant differences between the fungal burdens of wild-type and MR KO mice (data not shown).
Comparison of MP uptake and induction of maturation markers by wild-type and MR KO BMDCs.
DCs are the primary antigen-presenting cells responsible for initiating a cell-mediated immune response to MP (1). Therefore, we next examined whether DCs isolated from wild-type and MR KO mice differed in the capacity to bind and internalize MP. After a 15-min incubation with OG-labeled MP, there was no significant difference in cell-associated fluorescence between wild-type and MR KO DCs (Fig. 3A). Similar results were found if the incubation was extended to 30 min (data not shown). As the flow cytometry studies did not distinguish between surface-bound and internalized MP, confocal microscopy was performed. The vast majority of the MP was found to be internalized by the DCs (Fig. 3B).
FIG. 3.
MP uptake by BMDCs from C57BL/6 wild-type and MR KO mice as assessed by flow cytometry and confocal microscopy. (A) Wild-type and MR KO CD11c+ BMDCs were incubated with OG-labeled MP (10 μg/ml) for 15 min, washed, and then analyzed by flow cytometry. Histograms are representative of three independent experiments, each with similar results. The filled gray histograms illustrate unstimulated BMDCs from wild-type mice incubated without MP. (B) Same as panel A, except that cells were analyzed by confocal microscopy. Phase-contrast, fluorescence, and merged images are shown. Photomicrographs are representative of five independent experiments. Scale bar represents 11.9 μm.
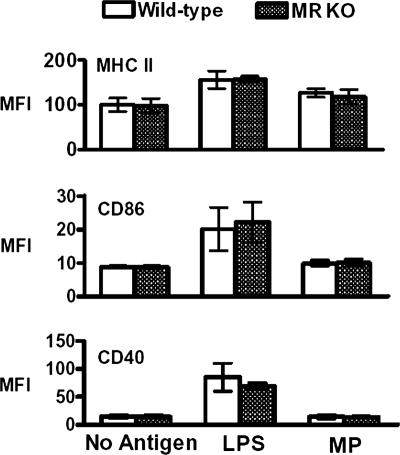
As DC maturation is important for effective antigen presentation to T cells, we next examined the expression of maturation markers in BMDCs obtained from wild-type and MR KO mice stimulated with MP (33). Baseline expression levels of MHCII, CD40, and CD86 were similar on unstimulated DCs derived from both wild-type and MR KO mice. A 24-h incubation with MP did not result in a significant upregulation of the expression of these markers on DCs derived from either strain of mice (Fig. 4). However, incubation of BMDCs with the positive control, LPS, did induce upregulation of MHCII, CD86, and CD40 expression on wild-type and MR KO DCs (Fig. 4).
FIG. 4.
Expression of maturation markers following incubation of BMDCs from C57BL/6 wild-type and MR KO mice with MP. Wild-type and MR KO BMDCs were left unstimulated or incubated with MP (10 μg/ml) or LPS (1 μg/ml) for 24 h. Cells were harvested and analyzed for cell marker surface expression of MHCII, CD86, and CD40 by flow cytometry. Data are means ± standard errors of two independent experiments for MHCII and four independent experiments for CD86 and CD40. Mean fluorescence intensities (MFI) were not significantly different when wild-type and MR KO DCs were compared. In addition, MP did not significantly upregulate maturation marker expression on DCs derived from either strain of mice.
Ex vivo MP-specific proliferation of CD4+ T cells from infected wild-type and MR KO mice.
Given the importance of CD4+ T cells to host defenses against cryptococcosis, in the last set of experiments we compared MP-specific CD4+ T-cell responses in wild-type and MR KO DCs. A disseminated infection results from intranasal challenge with 145A in C57BL/6 mice (7). Thus, at 2 weeks following a pulmonary challenge with C. neoformans, CD4+ T cells isolated from peripheral lymph nodes were analyzed for their proliferative response to MP. BMDCs were then incubated with CD4+ T cells from either WT or KO mice in the presence of no antigen, MP at 1 or 10 μg/ml, or ConA as a positive control. The BMDCs used in these experiments were derived from wild-type mice so that differences in responses were due solely to the source of T cells. CD4+ T cells from wild-type mice, but not the MR KO mice, proliferated in response to both concentrations of MP (Fig. 5). These experiments used strains representing two different serotypes of C. neoformans, with similar results. In a single experiment with four mice in each group, similar results were obtained when the CD4+ T cells were obtained from the lymph nodes 4 weeks following fungal challenge (data not shown).
FIG. 5.
Ex vivo MP-specific proliferation of CD4+ T cells isolated from infected C57BL/6 wild-type and MR KO mice. CD4+ T cells were isolated from lymph nodes of wild-type and MR KO mice at 2 weeks following a pulmonary challenge with 1 × 106 C. neoformans organisms. The T cells (1 × 105) were then incubated with mitomycin-treated CD11c+ BMDCs (1 × 104) from wild-type mice in the presence of either MP (1 or 10 μg/ml) or ConA (1 μg/ml). Cells were pulsed with [3H]thymidine on day 3 and harvested on day 4. Data are means ± standard errors of three independent experiments, each of which had at least three wild-type and MR KO mice. Two experiments were done with C. neoformans strain 145, and one experiment was done with strain 52. P = 0.007 and P = 0.003 comparing the stimulation indices of wild-type and MR KO mice at MP concentrations of 1 and 10 μg/ml, respectively.
DISCUSSION
The data presented here demonstrate that the MR plays a role in protecting against cryptococcosis. Compared with wild-type mice, MR KO mice died sooner and developed higher lung fungal burdens following a pulmonary challenge with a lethal inoculum of C. neoformans. These results stand in contrast to the findings in two other models of fungal infections, candidiasis and pneumocystosis, in which no differences in mortality between wild-type and MR KO mice were seen (15, 34). While the MR recognizes mannosylated ligands on the surface of C. albicans and P. carinii, exposed mannose groups are not found on the surface of C. neoformans because of masking by the capsule (10, 35). Therefore, it is likely that the major role of the MR in defenses against cryptococcosis is recognition of soluble MP released from the fungus. In support of this interpretation, MR KO mice did not develop a measurable CD4+ T-cell response to MP.
The MR internalizes antigen via clathrin-coated pits into endocytic vesicles (5). The cytoplasmic tail has a conserved tyrosine motif which is required for endocytosis and a dihydrophobic motif which is important for recycling back to the plasma membrane (31). Once internalized, the MR-containing vesicle can either recycle back to the plasma membrane or traffic to late endosomes for subsequent processing of antigen. Additionally, MR has been shown to deliver antigens into MHCII-containing vesicles in DCs (36). While the latter property presumably accounts for the lack of a CD4+ T-cell response to MP in the MR KO mice, a role for the MR in lymphocyte binding to lymphatic endothelium has been suggested (9).
There are no known intracellular signaling motifs in the MR (37). Nevertheless, binding of ligand to the MR has been reported to inhibit interleukin-12 (IL-12) production, stimulate IL-10 production, and activate NF-κB (3, 27, 41). Alternatively activated macrophages, which are induced by IL-4 and IL-13, have high levels of expression of the MR (25). Of note is that IL-12 production is associated with protection and IL-10, IL-4, and IL-13 are associated with deleterious outcomes in murine cryptococcosis (11, 25). It is possible, then, that the MR has both beneficial and harmful effects on host defenses to cryptococcosis, a factor that could account for the lack of a more pronounced difference in survival between the wild-type and MR KO mice infected with C. neoformans.
Although previous studies with transfected cell lines established that the MR is a receptor for MP (22), uptake of MP by DCs from wild-type and MR KO mice was similar, as measured by flow cytometry and confocal microscopy. In addition to the MR, murine DCs have other C-type lectin receptors that recognize mannose residues, including DC-specific intercellular adhesion molecule 3 grabbing nonintegrin homologs, langerin and dectin-2 (28). Thus, receptor redundancy and macropinocytosis could explain the lack of difference in MP uptake between the wild-type and MR KO DCs (12, 13). After antigen uptake, subsequent processing and presenting of the antigen are necessary for an adaptive immune response. MP traffics to MHCII-containing compartments of wild-type DCs, which may be critical for stimulating optimal CD4+ T-cell responses (21). Yet, in our studies, MP at a concentration of 10 μg/ml failed to upregulate the expression of maturation markers on either wild-type or MR KO DCs. Our results differ from those of Pietrella et al., who observed that similar concentrations of MP stimulated human DC maturation marker induction and that the stimulation could be inhibited with monoclonal antibodies to the MR (29). These disparate findings could be due to differences in the DC populations studied or in the methods of MP preparation used.
Despite the presence of multiple receptors for mannose, CD4+ T cells harvested from infected MR KO mice did not proliferate in response to MP. This suggests that the MR plays a nonredundant role in priming MP-specific CD4+ T-cell responses in vivo. However, once a T-cell response is established, the MR may be less important as ovalbumin-reactive CD4+ T cells proliferated to similar extents when incubated with wild-type or MR KO DCs that had been pulsed with recombinant mannosylated ovalbumin (13). While the wild-type mice did develop a CD4+ T-cell response against MP, it was relatively weak. This may help explain why the wild-type mice eventually succumbed to cryptococcosis. C57BL/6 mice are relatively susceptible to cryptococcosis (6), and a virulent encapsulated strain of C. neoformans was used for the mortality studies. Moreover, glucuronoxylomannan, the major component of the cryptococcal capsule, directly inhibits T-cell responses (30, 39). Thus, while MR-dependent immune responses to MP contribute to host defenses against cryptococcosis, they do not mediate fully protective responses in the mouse model. Current studies are focused upon methods to boost the immune response by stimulating Toll-like receptors in conjunction with stimulation of the MR by MP (4).
Acknowledgments
This work was supported by National Institutes of Health grants RO1 AI25780 and RO1 AI37532 to S.M.L.
We thank Michele Nussenzweig for the knockout mice and Jianmin Chen for technical help with the experiments.
Editor: A. Casadevall
Footnotes
Published ahead of print on 7 April 2008.
REFERENCES
- 1.Bauman, S. K., K. L. Nichols, and J. W. Murphy. 2000. Dendritic cells in the induction of protective and nonprotective anticryptococcal cell-mediated immune responses. J. Immunol. 165158-167. [DOI] [PubMed] [Google Scholar]
- 2.Bicanic, T., and T. S. Harrison. 2004. Cryptococcal meningitis. Br. Med. Bull. 7299-118. [DOI] [PubMed] [Google Scholar]
- 3.Chieppa, M., G. Bianchi, A. Doni, A. Del Prete, M. Sironi, G. Laskarin, P. Monti, L. Piemonti, A. Biondi, A. Mantovani, M. Introna, and P. Allavena. 2003. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J. Immunol. 1714552-4560. [DOI] [PubMed] [Google Scholar]
- 4.Dan, J. M., J. P. Wang, C. K. Lee, and S. M. Levitz. Cooperative stimulation of dendritic cells by Cryptococcus neoformans mannoproteins and CpG oligodeoxynucleotides. PLoS ONE, in press. [DOI] [PMC free article] [PubMed]
- 5.East, L., and C. M. Isacke. 2002. The mannose receptor family. Biochim. Biophys. Acta 1572364-386. [DOI] [PubMed] [Google Scholar]
- 6.Hoag, K. A., N. E. Street, G. B. Huffnagle, and M. F. Lipscomb. 1995. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. Am. J. Respir. Cell Mol. Biol. 13487-495. [DOI] [PubMed] [Google Scholar]
- 7.Huffnagle, G. B., G. H. Chen, J. L. Curtis, R. A. McDonald, R. M. Strieter, and G. B. Toews. 1995. Down-regulation of the afferent phase of T cell-mediated pulmonary inflammation and immunity by a high melanin-producing strain of Cryptococcus neoformans. J. Immunol. 1553507-3516. [PubMed] [Google Scholar]
- 8.Huffnagle, G. B., J. L. Yates, and M. F. Lipscomb. 1991. T cell-mediated immunity in the lung: a Cryptococcus neoformans pulmonary infection model using SCID and athymic nude mice. Infect. Immun. 591423-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irjala, H., E. L. Johansson, R. Grenman, K. Alanen, M. Salmi, and S. Jalkanen. 2001. Mannose receptor is a novel ligand for l-selectin and mediates lymphocyte binding to lymphatic endothelium. J. Exp. Med. 1941033-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly, R. M., J. Chen, L. E. Yauch, and S. M. Levitz. 2005. Opsonic requirements for dendritic cell-mediated responses to Cryptococcus neoformans. Infect. Immun. 73592-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koguchi, Y., and K. Kawakami. 2002. Cryptococcal infection and Th1-Th2 cytokine balance. Int. Rev. Immunol. 21423-438. [DOI] [PubMed] [Google Scholar]
- 12.Lam, J. S., H. Huang, and S. M. Levitz. 2007. Effect of differential N-linked and O-linked mannosylation on recognition of fungal antigens by dendritic cells. PLoS ONE 2e1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam, J. S., M. K. Mansour, C. A. Specht, and S. M. Levitz. 2005. A model vaccine exploiting fungal mannosylation to increase antigen immunogenicity. J. Immunol. 1757496-7503. [DOI] [PubMed] [Google Scholar]
- 14.Lee, S. J., S. Evers, D. Roeder, A. F. Parlow, J. Risteli, L. Risteli, Y. C. Lee, T. Feizi, H. Langen, and M. C. Nussenzweig. 2002. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science 2951898-1901. [DOI] [PubMed] [Google Scholar]
- 15.Lee, S. J., N. Y. Zheng, M. Clavijo, and M. C. Nussenzweig. 2003. Normal host defense during systemic candidiasis in mannose receptor-deficient mice. Infect. Immun. 71437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levitz, S. M., and E. A. North. 1997. Lymphoproliferation and cytokine profiles in human peripheral blood mononuclear cells stimulated by Cryptococcus neoformans. J. Med. Vet. Mycol. 35229-236. [DOI] [PubMed] [Google Scholar]
- 17.Levitz, S. M., and C. A. Specht. 2006. The molecular basis for the immunogenicity of Cryptococcus neoformans mannoproteins. FEMS Yeast Res. 6513-524. [DOI] [PubMed] [Google Scholar]
- 18.Lin, X., and J. Heitman. 2006. The biology of the Cryptococcus neoformans species complex. Annu. Rev. Microbiol. 6069-105. [DOI] [PubMed] [Google Scholar]
- 19.Luong, M., J. S. Lam, J. Chen, and S. M. Levitz. 2007. Effects of fungal N- and O-linked mannosylation on the immunogenicity of model vaccines. Vaccine 254340-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lutz, M. B., N. Kukutsch, A. L. Ogilvie, S. Rossner, F. Koch, N. Romani, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 22377-92. [DOI] [PubMed] [Google Scholar]
- 21.Mansour, M. K., E. Latz, and S. M. Levitz. 2006. Cryptococcus neoformans glycoantigens are captured by multiple lectin receptors and presented by dendritic cells. J. Immunol. 1763053-3061. [DOI] [PubMed] [Google Scholar]
- 22.Mansour, M. K., L. S. Schlesinger, and S. M. Levitz. 2002. Optimal T cell responses to Cryptococcus neoformans mannoprotein are dependent on recognition of conjugated carbohydrates by mannose receptors. J. Immunol. 1682872-2879. [DOI] [PubMed] [Google Scholar]
- 23.Mansour, M. K., L. E. Yauch, J. B. Rottman, and S. M. Levitz. 2004. Protective efficacy of antigenic fractions in mouse models of cryptococcosis. Infect. Immun. 721746-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGreal, E. P., J. L. Miller, and S. Gordon. 2005. Ligand recognition by antigen-presenting cell C-type lectin receptors. Curr. Opin. Immunol. 1718-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Müller, U., W. Stenzel, G. Kohler, C. Werner, T. Polte, G. Hansen, N. Schutze, R. K. Straubinger, M. Blessing, A. N. McKenzie, F. Brombacher, and G. Alber. 2007. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J. Immunol. 1795367-5377. [DOI] [PubMed] [Google Scholar]
- 26.Murphy, J. W. 1988. Influence of cryptococcal antigens on cell-mediated immunity. Rev. Infect. Dis. 10(Suppl. 2)S432-S435. [DOI] [PubMed] [Google Scholar]
- 27.Nigou, J., C. Zelle-Rieser, M. Gilleron, M. Thurnher, and G. Puzo. 2001. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J. Immunol. 1667477-7485. [DOI] [PubMed] [Google Scholar]
- 28.Park, C. G., K. Takahara, E. Umemoto, Y. Yashima, K. Matsubara, Y. Matsuda, B. E. Clausen, K. Inaba, and R. M. Steinman. 2001. Five mouse homologues of the human dendritic cell C-type lectin, DC-SIGN. Int. Immunol. 131283-1290. [DOI] [PubMed] [Google Scholar]
- 29.Pietrella, D., C. Corbucci, S. Perito, G. Bistoni, and A. Vecchiarelli. 2005. Mannoproteins from Cryptococcus neoformans promote dendritic cell maturation and activation. Infect. Immun. 73820-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Retini, C., A. Vecchiarelli, C. Monari, F. Bistoni, and T. R. Kozel. 1998. Encapsulation of Cryptococcus neoformans with glucuronoxylomannan inhibits the antigen-presenting capacity of monocytes. Infect. Immun. 66664-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schweizer, A., P. D. Stahl, and J. Rohrer. 2000. A di-aromatic motif in the cytosolic tail of the mannose receptor mediates endosomal sorting. J. Biol. Chem. 27529694-29700. [DOI] [PubMed] [Google Scholar]
- 32.Specht, C. A., S. Nong, J. M. Dan, C. K. Lee, and S. M. Levitz. 2007. Contribution of glycosylation to T cell responses stimulated by recombinant Cryptococcus neoformans mannoprotein. J. Infect. Dis. 196796-800. [DOI] [PubMed] [Google Scholar]
- 33.Steinman, R. M., and J. Banchereau. 2007. Taking dendritic cells into medicine. Nature 449419-426. [DOI] [PubMed] [Google Scholar]
- 34.Swain, S. D., S. J. Lee, M. C. Nussenzweig, and A. G. Harmsen. 2003. Absence of the macrophage mannose receptor in mice does not increase susceptibility to Pneumocystis carinii infection in vivo. Infect. Immun. 716213-6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Syme, R. M., J. C. Spurrell, E. K. Amankwah, F. H. Green, and C. H. Mody. 2002. Primary dendritic cells phagocytose Cryptococcus neoformans via mannose receptors and Fcγ receptor II for presentation to T lymphocytes. Infect. Immun. 705972-5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan, M. C., A. M. Mommaas, J. W. Drijfhout, R. Jordens, J. J. Onderwater, D. Verwoerd, A. A. Mulder, A. N. van der Heiden, D. Scheidegger, L. C. Oomen, T. H. Ottenhoff, A. Tulp, J. J. Neefjes, and F. Koning. 1997. Mannose receptor-mediated uptake of antigens strongly enhances HLA class II-restricted antigen presentation by cultured dendritic cells. Eur. J. Immunol. 272426-2435. [DOI] [PubMed] [Google Scholar]
- 37.Taylor, P. R., S. Gordon, and L. Martinez-Pomares. 2005. The mannose receptor: linking homeostasis and immunity through sugar recognition. Trends Immunol. 26104-110. [DOI] [PubMed] [Google Scholar]
- 38.Wozniak, K. L., J. M. Vyas, and S. M. Levitz. 2006. In vivo role of dendritic cells in a murine model of pulmonary cryptococcosis. Infect. Immun. 743817-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yauch, L. E., J. S. Lam, and S. M. Levitz. 2006. Direct inhibition of T-cell responses by the Cryptococcus capsular polysaccharide glucuronoxylomannan. PLoS Pathog. 2e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yauch, L. E., M. K. Mansour, S. Shoham, J. B. Rottman, and S. M. Levitz. 2004. Involvement of CD14, Toll-like receptors 2 and 4, and MyD88 in the host response to the fungal pathogen Cryptococcus neoformans in vivo. Infect. Immun. 725373-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang, J., J. Zhu, A. Imrich, M. Cushion, T. B. Kinane, and H. Koziel. 2004. Pneumocystis activates human alveolar macrophage NF-κB signaling through mannose receptors. Infect. Immun. 723147-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]