Abstract
Burkholderia pseudomallei is the causative agent of melioidosis. While adaptive immunity has been shown to be important for host resistance to B. pseudomallei, the direct interaction of the bacteria with adaptive immune cells such as T and B cells is not well known. To address this question, we infected Jurkat T cells, as well as human primary CD4+ and CD8+ T cells, with live B. pseudomallei. We found that live bacterial infection could costimulate T cells to produce interleukin-2 (IL-2) and gamma interferon (IFN-γ) in the presence of anti-CD3 cross-linking antibodies. Bacterial supernatant could also costimulate T cells, and this was due to the presence of flagellin in the supernatant. However, T cells infected with bacterial mutants lacking flagellin showed strong impairment in IL-2 but only a slight impairment in IFN-γ production. When cross-linking of CD3 is replaced by IL-2, live bacterial infection was still able to costimulate human primary T cells to produce IFN-γ and flagellin is only a minor ligand contributing to this costimulation. Thus, live B. pseudomallei could potentially costimulate T cells not only in an antigen-specific manner but also in a nonspecific manner through bystander activation via IL-2.
Burkholderia pseudomallei is a gram-negative bacterium responsible for causing melioidosis, an infectious disease endemic in Southeast Asia and Northern Australia. The bacterium can invade and replicate in both cultured phagocytic (18, 28) and nonphagocytic cells (19). The type III secretion system 3 (TTSS3), also known as the Burkholderia secretion apparatus, enables B. pseudomallei to escape from the endosome into the cytoplasm of infected cells (29). Furthermore, the BimA protein found on one pole of the bacteria helps to facilitate intracellular spread by the formation of actin comet tails (30). These characteristics of the bacterium could help it to survive intracellularly. Upon phagocytosis by macrophages, B. pseudomallei is able to activate the suppressor of cytokine signaling 3 (SOCS3) and cytokine-inducible Src homology 2-containing protein, resulting in a decrease in the gamma interferon (IFN-γ) signaling response (9). We had previously shown that B. pseudomallei infection of monocytes, macrophages, and dendritic cells induced caspase-1-dependent cell death (32). The interference with host innate immune cells such as macrophages could have a profound impact on the innate immune response to bacteria, as well as on the development of the adaptive immune response.
The interaction of B. pseudomallei with other immune cells, particularly those from the adaptive immune response such as T and B cells, is not well known. It has been shown that patients surviving melioidosis developed a cell-mediated immune response (20) and, in mice, B. pseudomallei-specific CD4 T cells are important for host resistance in adaptive immunity (16). This means that the ability of the host to develop a robust T-cell response is likely critical in controlling the infection, and there is a possibility that bacteria could interfere with this process through their subversive actions on antigen-presenting cells or directly on T cells. An example would be Yersinia, which has the ability to directly suppress T-cell activation through the virulence factor YopH, a tyrosine phosphatase, which binds to and inhibits signaling intermediates crucial for T-cell antigen receptor (TCR) signaling such as the linker for activation of T cells (LAT) and the 76-kDa SH2-domain-containing leukocyte protein (SLP-76) (2, 13). When we examined the interaction of B. pseudomallei with T cells, not only did we not see a suppression of T-cell responses but we also observed a costimulation effect provided by the bacteria. This could perhaps explain the bystander activation of T cells during the innate immune response to B. pseudomallei described previously (22). In addition, this costimulation effect on T cells is TTSS3 independent but partially attributed to flagellin. Together, our findings could have implications regarding the pathogenesis of B. pseudomallei.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The virulent B. pseudomallei strain KHW (24) was cultured on tryptic soy agar (TSA) or Luria-Bertani (LB) broth at 37°C. To prepare mid-log-phase bacteria, 2 ml of LB broth was inoculated with 100 μl of overnight culture and allowed to grow for 3 h with shaking at 100 rpm in a 37°C incubator. The fliC gene frame-deleted Escherichia coli strain NK9375 (ΔfliC) was kindly provided by Nancy Kleckner (Harvard University).
Cell lines.
Jurkat cell clone E6-1 (ATCC, TIB-152) was cultured in RPMI 1640 (Gibco, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (Gibco), 2 mM l-glutamine (Sigma), 100 U of penicillin (Sigma)/ml, and 0.1 mg of streptomycin (Sigma)/ml. Cells were maintained at 37°C in a humidified 5% CO2 incubator.
Construction of KHWΔfliCKm, KHWfliCpro, and KHWbsaQ mutants.
The KHWΔfliCKm mutant was constructed as described previously (6) and cultured on TSA containing 200 μg of kanamycin/ml. The KHWbsaQ mutant was previously generated by Sun et al. (32). The KHWfliCpro mutant was screened from transposon mutagenesis. Briefly, pOT182 (GI 3282100) maintained in E. coli SM10 was introduced into KHW via filter conjugation (11). Positive clones were selected on TSA containing 100 μg of streptomycin/ml and 50 μg of tetracycline/ml. The genomic DNA of the mutants was digested with BamHI, EcoRI, HindIII, and NotI; self-ligated; and subsequently sequenced with the primer OTF1 (5′-CTG GAA AAC GGG AAA GGT TC). The sequences obtained were subjected to the BLAST against the B. pseudomallei K96243 genome. The site of transposon insertion in KHWfliCpro mutant was identified to be the promoter region of fliC gene.
Isolation of CD4+ and CD8+ T cells from human peripheral blood.
Peripheral blood mononuclear cells were prepared from healthy blood donors by using Histopaque-1077 (Sigma-Aldrich). CD4+ or CD8+ T cells were isolated from peripheral blood mononuclear cells by magnetic cell sorting using human CD4+ or CD8+ microbeads (Miltenyi Biotec, Germany) according to the manufacturer's instructions. The purity of CD4+ or CD8+ T cells was >95%, as determined via flow cytometry by staining with CD4-PE or CD8-PE (BD Pharmingen).
Infection of Jurkat cells with B. pseudomallei and intracellular bacterial replication.
Jurkat cells seeded in 12-well plates at a density of 106 per ml were infected with mid-log-phase KHW at a multiplicity of infection (MOI) of 30:1 at 37°C with 5% CO2. At 2 h after infection, cells were centrifuged at 300 × g for 5 min, and the supernatant was discarded. Cells were washed with phosphate-buffered saline and resuspended in fresh RPMI medium containing 250 μg of kanamycin/ml. At 4 or 24 h after infection, cells were lysed with 0.1% Triton X-100. Serial dilutions of the lysate were plated on TSA plates containing 5 μg of gentamicin/ml. Bacterial CFU was counted after 24 h of incubation at 37°C.
Costimulation and determination of IL-2 and IFN-γ concentration.
Jurkat cells seeded in 12-well plates at a density of 2 × 106 per ml were inoculated with mid-log-phase KHW, KHWbsaQ, KHWΔfliCKm, KHWfliCpro, DH5α, or NK9375ΔfliC at the indicated MOIs. The plates were centrifuged at 1,000 × g for 5 min prior to incubation at 37°C with 5% CO2. At 2 h after infection, cells were collected, washed with phosphate-buffered saline, and resuspended in fresh RPMI medium containing 250 μg of kanamycin/ml or 40 μg of tetracycline/ml. A total of 0.2 × 106 cells were transferred to 96-well MaxiSorp plate (Nunc, Denmark), which was coated overnight with 100 μl of 2.5 μg of purified mouse anti-human CD3 monoclonal antibody (BD Pharmingen)/ml. After 22 h, the supernatant was collected and assayed for interleukin-2 (IL-2) production by using an OptEIA human IL-2 enzyme-linked immunosorbent assay (BD Biosciences, San Diego, CA) according to the manufacturer's instructions. In some experiments, 0.2 × 106 Jurkat cells were incubated with various concentrations of recombinant B. pseudomallei flagellin, which was prepared as previously described (37), or with various volumes of filter-sterilized (0.22-μm pore size) culture supernatant from mid-log-phase bacteria. In other experiments, 0.25 × 106 human primary T cells were inoculated with wild-type B. pseudomallei, the KHWfliCpro mutant, 1 μg of flagellin protein (Flg)/ml, or lipopolysaccharide (LPS) from E. coli O55:B5 (Sigma) and Pam3CSK4 (Sigma), in the presence of recombinant human IL-2 (R&D Systems, Inc.) or in anti-CD3-coated MaxiSorp plates for 2 h, followed by the addition of 250 μg of kanamycin/ml and an additional 22 h of incubation. In some cases, the supernatant was assayed for IFN-γ production by using a Max human IFN-γ ELISA set (Biolegend, San Diego, CA) according to the manufacturer's instructions.
Immunodetection of Flg by Western blotting.
A bacterial pellet from 5 ml of mid-log-phase culture was lysed with bacterial lysis buffer (8 M urea, 0.1 M NaH2PO4, 0.01 M Tris-Cl [pH 8]). Culture supernatant was filtered through a syringe filter (0.22-μm pore size). Proteins in the supernatant were precipitated with 25% trichloroacetic acid. Cell lysate and supernatant precipitates were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel, followed by transfer onto nitrocellulose membrane. The membrane was probed with mouse polyclonal antiserum to B. pseudomallei flagellin (1:100) and detected with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (1:5,000; Sigma).
Real-time PCR determination of IL-2 mRNA transcription and stability.
Jurkat cells were seeded at a cell density of 2 × 106/ml into 12-well plates pretreated with 2.5 μg of anti-CD3 antibody/ml with or without 1 ng of B. pseudomallei flagellin/ml or 1 μg of mouse anti-human CD28 monoclonal antibody (anti-CD28; Biolegend)/ml. After 5 h of stimulation, 0.5 μg of cyclosporine (Cs; Calbiochem, San Diego, CA)/ml was added. At various time points after the addition of Cs, total RNA was isolated from the cells by using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. cDNA was reverse transcribed by using a TaqMan reverse transcriptase kit (Applied Biosystems). Real-time PCR was performed with an Applied Biosystems 7500 real-time PCR system using a Power Sybr green master mix containing AmpliTaq Gold polymerase (Applied Biosystems). The cycling parameters for amplification were as follows: 1 cycle of polymerase activation at 95°C for 10 min and 40 cycles at 95°C for 15 s and 60°C for 1 min. The specific primers used for IL-2 were the forward primer 5′-CTG GAG CAT TTA CTG CTG GAT TT and the reverse primer 5′-TGG TGA GTT TGG GAT TCT TGT AAT T; the primers used for GAPDH were the forward primer 5′-ATG GAA ATC CCA TCA CCA TCT T and the reverse primer 5′-CGC CCC ACT TGA TTT TGG.
Statistical analysis.
A Student t test was performed pairwise to determine whether there was a statistically significant difference (P < 0.05) between KHW and the various mutants.
RESULTS
Live bacterial infection and bacterial supernatant costimulate Jurkat cells.
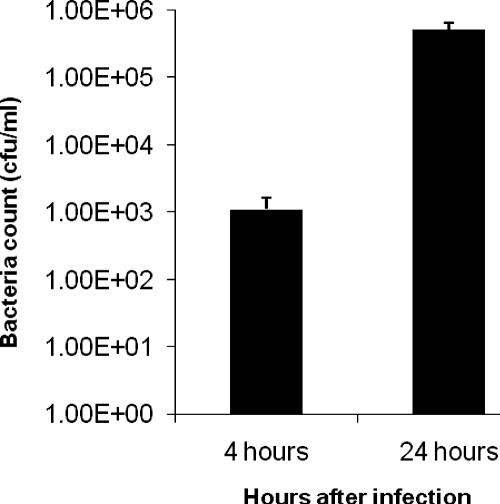
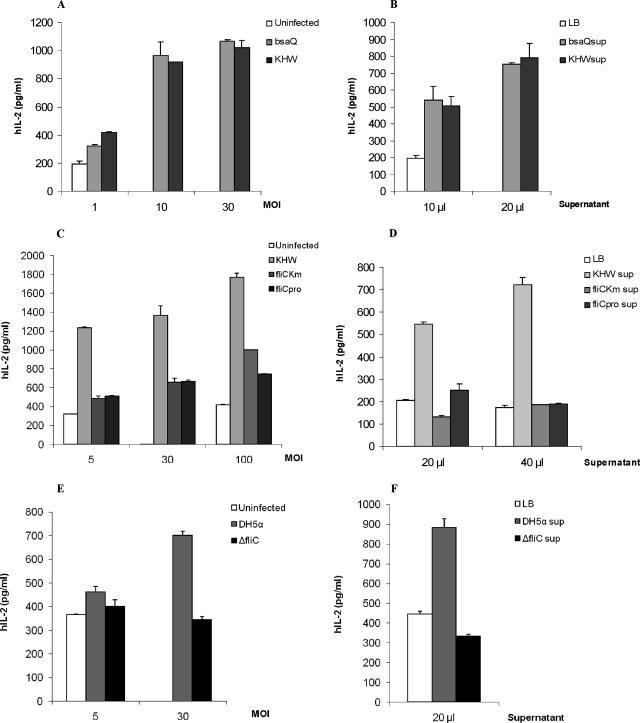
To investigate the interaction of B. pseudomallei with T cells, we first determined whether B. pseudomallei can invade and survive in human T cells. We examined intracellular bacteria 4 or 24 h postinfection. We found that B. pseudomallei could enter Jurkat cells and replicate intracellularly with a 100-fold increase after 20 h (Fig. 1). In contrast to macrophages and dendritic cells, B. pseudomallei infection of Jurkat cells does not result in rapid cell death, and the Jurkat cells in our assay remained viable throughout the duration of our assays (data not shown). Several studies have shown that PAMPs from bacteria such as flagellin (5, 7) and lipoprotein BLP (25, 33) could provide costimulation to T cells through the stimulation of the Toll-like receptors (TLRs). We found that live bacterial infection of anti-CD3 antibody stimulated Jurkat cells at various MOIs could also costimulate Jurkat cells to make significant levels of IL-2 (Fig. 2A). B. pseudomallei infection alone did not induce any IL-2 secretion (data not shown), whereas anti-CD3 treatment alone induced only a low level of IL-2 secretion. To determine whether the factor providing the costimulation is secreted, Jurkat cells were stimulated with plate-coated anti-CD3 antibody and bacterial supernatant. We found that bacterial supernatant could also costimulate T cells to make IL-2 (Fig. 2B).
FIG. 1.
Invasion and replication of B. pseudomallei in Jurkat cells. A total of 106 Jurkat cells were infected with B. pseudomallei strain KHW at an MOI of 30:1. At 4 and 24 h after infection, the intracellular bacterial CFU was determined. The results from one representative experiment out of three are shown, and error bars represent the standard deviation of three values.
FIG. 2.
IL-2 secretion by Jurkat cells infected with wild-type B. pseudomallei, bsaQ and fliC B. pseudomallei mutants, E. coli DH5α, or E. coli ΔfliC mutant or incubated with bacterial culture supernatant. A total of 2 × 106 Jurkat cells were uninfected or infected with KHW or KHWbsaQ mutant at MOIs of 1, 10, and 30 (A) or with KHW, KHWfliCKm, or KHWfliCpro at MOIs of 5, 30, and 100 (C) or with DH5α or ΔfliC mutant (E) for 2 h, followed by 22 h of exposure to 2.5 μg of plate-bound anti-CD3 antibody/ml. In some experiments, 0.2 × 106 Jurkat cells were incubated in anti-CD3 coated plate for 22 h, together with LB medium or log-phase bacterial culture supernatants of KHW, KHWbsaQ (B), KHWfliCKm and KHWfliCpro (D), or E. coli DH5α or ΔfliC mutant (F), which were filter sterilized. The results from one representative experiment out of three are shown, and error bars represent the standard deviation of three values.
Since TTSS3 of B. pseudomallei is an important virulence factor (32, 35), we investigated the role of TTSS3 in the costimulation using the bsaQ mutant, which does not have a functional TTSS3. bsaQ mutant was able to costimulate Jurkat cells to an extent similar to that of wild-type bacteria at the various MOIs tested (Fig. 2A), and its culture supernatant at various concentrations tested was able to costimulate T cells (Fig. 2B).
fliC mutants lose the ability to costimulate Jurkat cells.
Since Jurkat cells only express TLR5 and not TLR2 or TLR4 (37), we tested the contribution of flagellin to the costimulation seen in both live bacterial infection and from bacterial supernatants by using two fliC mutants—KHWΔfliCKm and KHWfliCpro (see Materials and Methods). In live bacterial infection, we centrifuged the plate at 1,000 × g for 5 min to minimize possible differences in the interaction with host cells between the wild type and the fliC mutants due to mobility. At a low MOI of 5:1, both mutants lost their ability to costimulate Jurkat cells (Fig. 2C). At higher MOIs of 30:1 and 100:1, costimulation was increased, although it was still lower than that occurring with wild-type bacteria. This suggests that at higher MOIs, bacterial components other than flagellin accumulating at a higher concentration could also provide T-cell costimulation. On the contrary, costimulation provided by bacterial supernatant from the mutants was completely abolished at various concentrations of supernatant tested (Fig. 2D). The poorer costimulation by fliC mutants was not due to their defective replication in Jurkat T cells since they could replicate to numbers similar to those for wild-type bacteria (data not shown). We further investigated whether other bacteria could costimulate T cells through TLR5 during infection. We found that E. coli DH5α could stimulate Jurkat cells to secrete IL-2 in the presence of anti-CD3 antibody. However, unlike B. pseudomallei, the costimulation by E. coli was abrogated by using the fliC mutant strain at the MOI tested (Fig. 2E). The bacterial supernatant from DH5α was also able to costimulate Jurkat cells but not the supernatant from the flagellin mutant (Fig. 2F). In addition, B. pseudomallei was found to be more potent in the costimulation of Jurkat cells than E. coli when the same MOI was used. To ascertain that our B. pseudomallei fliC mutants do not express the flagellin protein, we examined the bacterial lysate and supernatant for the presence of the flagellin protein. Flagellin was only detected in the bacterial lysate and culture supernatant of wild-type B. pseudomallei but not in either of the fliC mutants (Fig. 3).
FIG. 3.
Detection of flagellin in KHW cell lysate and supernatant but not in fliC mutants. (A) Western blot analysis of flagellin protein in protein precipitates prepared from filter-sterilized bacterial culture supernatant of KHW (lane 1) and KHWfliCKm (lane 2). Lane 3 contained 1 μg of recombinant B. pseudomallei flagellin protein. (B) Western blot analysis of flagellin protein in KHW (lane 1) and KHWfliCpro (lane 2) lysate.
Flagellin costimulation increases IL-2 mRNA transcription but not stability.
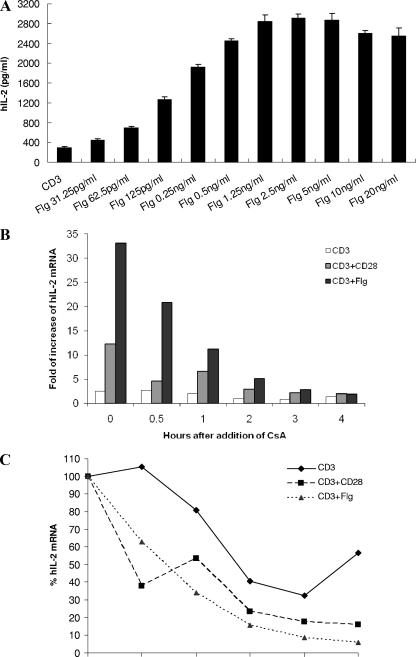
We next used the recombinant flagellin protein from B. pseudomallei to costimulate Jurkat cells. Purified B. pseudomallei flagellin was able to costimulate anti-CD3 stimulated Jurkat cells in a dose-dependent manner (Fig. 4A). IL-2 secretion by Jurkat cells peaked at around 2.5 ng of flagellin/ml. To determine whether the enhanced IL-2 secretion was due to increased expression or stability of the IL-2 mRNA, we examined the mRNA level by real-time PCR. Jurkat cells stimulated with anti-CD3 antibody alone, anti-CD3 plus 1 μg of anti-CD28 antibody/ml, or anti-CD3 plus 1 ng of flagellin/ml for 5 h exhibited 2.5-, 12.3-, or 33.1-fold-higher expression levels, respectively, of IL-2 gene than did the untreated cells (Fig. 4B). However, the IL-2 mRNA level from both anti-CD28- and flagellin-costimulated cells underwent rapid and continuous decline throughout the 4 h after the addition of Cs, which inhibits IL-2 transcription (Fig. 4B and C). This shows that flagellin costimulation enhances IL-2 secretion by Jurkat cells primarily through significant augmentation of mRNA transcription and not through increased stabilization of transcripts over the period of time investigated.
FIG. 4.
IL-2 secretion, mRNA expression, and stability upon costimulation with recombinant B. pseudomallei flagellin. (A) A total of 0.2 × 106 Jurkat cells were stimulated on 2.5-μg/ml anti-CD3 antibody-coated plates alone (CD3) or with the indicated concentrations of B. pseudomallei Flg. A total of 2.0 × 106 Jurkat cells were incubated in the absence of stimulus (untreated), in the presence of 2.5 μg of anti-CD3 antibody/ml alone (CD3), in the presence of anti-CD3 antibody with 1 μg of anti-CD28 antibody/ml (CD3+CD28), or with 1 ng of flagellin/ml (CD3+Flg). After 5 h of stimulation, 0.5 μg of Cs/ml was added to inhibit IL-2 transcription. Total RNA was isolated immediately from cells after 5 h of stimulation (0 h) or 0.5, 1, 2, 3, or 4 h after the addition of Cs. (B) IL-2 mRNA levels in treated samples (CD3, CD3+CD28, and CD3+Flg) at different time points are expressed as the fold increase relative to that of the untreated samples. (C) Within each treatment, the IL-2 mRNA levels in the samples at 0 h are normalized to be 100%; the IL-2 mRNA levels in the samples at 0.5, 1, 2, 3, and 4 h are expressed relative to that at 0 h. The results from one representative experiment out of three are shown.
Bacterial infection costimulates human primary CD4+ and CD8+ T cells.
The T-cell costimulatory effect of B. pseudomallei observed in Jurkat cells was further validated in human primary CD4+ and CD8+ T cells. Infection with live B. pseudomallei alone did not induce human primary T cells to secrete detectable amounts of IL-2 or IFN-γ (data not shown). In the presence of plate-bound anti-CD3 antibody, infection with live bacteria costimulated human CD4+ T cells to produce much more IL-2 (Fig. 5A) and IFN-γ (Fig. 5B) than uninfected cells. Similar to the observation in Jurkat cells, the KHWfliCpro mutant was significantly less capable of costimulating CD4+ T cells to secrete IL-2 than was wild-type B. pseudomallei KHW in all of the donors tested (Fig. 5A). In contrast, the difference between KHW and the KHWfliCpro mutant in costimulating CD4+ cells to produce IFN-γ was not as obvious as for IL-2 (Fig. 5B). Among the donors, CD4+ T cells from donors 2 and 4 made significantly more IFN-γ in response to KHW than the KHWfliCpro mutant, whereas CD4+ T cells from donors 1 and 3 did not show a significant difference. Despite the variation in individual donors, the overall trend shows that flagellin is less prominent as a costimulatory factor involved in optimal production of IFN-γ by CD4+ T cells but is more important in enhancing IL-2 production.
FIG. 5.
Cytokine production by human primary CD4+ T cells infected with wild-type B. pseudomallei or the fliC mutant. In the anti-CD3-coated plate, 0.25 × 106 CD4+ cells were left uninfected or were infected with KHW or KHWfliCpro at MOIs of 5 and 30 for 2 h before the addition of kanamycin. At 22 h after antibiotic addition, the supernatant was collected and assayed for IL-2 (A) or IFN-γ (B) concentration. Representative results from four different healthy donors are shown. Each bar represents the mean of four values from duplicates, and the error bar indicates the standard deviation. For donors 3 and 4 in panel A, each bar represents the mean of two values. *, P < 0.05; **, P < 0.01.
When exposed to anti-CD3 antibody alone or together with live bacteria, human CD8+ T cells did not produce any detectable IL-2 (data not shown). However, live bacterium-infected CD8+ T cells in the presence of anti-CD3 antibody significantly increased the secretion of IFN-γ versus uninfected cells (Fig. 6A). Except for the infected CD8+ T cells from donor 4, CD8+ cells infected with the KHWfliCpro mutant were costimulated to produce significantly less IFN-γ than the KHW-infected cells. This again reflects a donor-to-donor difference in response to the flagellin mutant and shows that flagellin is dispensable in some donors to costimulate IFN-γ production during live bacterial infection, suggesting that bacterial components other than flagellin are involved.
FIG. 6.
(A) IFN-γ secretion by human primary CD8+ T cells infected with wild-type B. pseudomallei or the fliC mutant. In the anti-CD3-coated plate, 0.25 × 106 CD8+ cells were uninfected or infected with KHW or KHWfliCpro at an MOI of 30 for 2 h before the addition of kanamycin. At 22 h after antibiotic addition, the supernatant was collected and assayed for IFN-γ concentration. The representative results from four healthy donors are shown. Each bar represents the mean of four values from duplicates, and the error bar indicates the standard deviation. (B) IFN-γ secretion by human primary T cells incubated with recombinant human IL-2 (rhIL-2) alone, together with KHW, with recombinant B. pseudomallei flagellin, with LPS, with Pam3CSK4, or with flagellin, LPS, and Pam3CSK4. A total of 0.25 × 106 CD4+ or CD8+ cells were stimulated with 80 U of rhIL-2 alone/ml, with KHW at an MOI of 30 (rhIL-2+KHW), with 1 μg of flagellin/ml (rhIL-2+Flg), with LPS (rhIL-2+LPS), with Pam3CSK4 (rhIL-2+Pam), or with all three ligands (rhIL-2+All) for 2 h, followed by the addition of kanamycin. At 22 h after antibiotic addition, the supernatant was collected and assayed for IFN-γ concentration. The results of one representative experiment out of two from two different donors are shown. The results for rhIL-2+Flg and rhIL-2+KHW were repeated at least in four donors. *, P < 0.05.
Mouse CD8+ T cells had been shown to produce IFN-γ in a bystander activation manner upon B. pseudomallei infection (22). Since the anti-CD3 cross-linking we used is nonphysiological, we sought to determine whether it could be replaced by IL-2. We found that in the presence of recombinant human IL-2 (rhIL-2), human T cells could be costimulated with B. pseudomallei infection to make IFN-γ. IL-2 alone did not stimulate human primary CD4+ or CD8+ T cells to produce detectable IFN-γ (Fig. 6B). Both CD4+ and CD8+ T cells stimulated with B. pseudomallei infection secreted significant amounts of IFN-γ in the presence of IL-2, indicating that B. pseudomallei-infected human T cells could produce IFN-γ in a bystander activation manner if IL-2 is present. Costimulation with B. pseudomallei infection induced much more IFN-γ production than that seen with flagellin, supporting the results in Fig. 5B and 6A that costimulation in CD4+ and CD8+ T cells for IFN-γ production are only slightly dependent on flagellin. However, costimulation with LPS could restore IFN-γ production to the same extent as that induced by live bacteria.
DISCUSSION
In this study, we show that B. pseudomallei costimulates Jurkat T cells. Furthermore, this costimulation to T cells is partially provided by flagellin since the fliC mutants were greatly impaired but not totally deficient in costimulating T cells to produce IL-2. B. pseudomallei is not unique since E. coli could also costimulate Jurkat T cells, although the costimulation is entirely provided by flagellin. This shows that different bacteria could use various pathogen-associated molecular patterns to costimulate T cells. Although bacterial lipoprotein is also able to costimulate T cells (25, 33), Jurkat T cells do not express TLR2 (37). Murine T cells have been reported to be costimulated with CpG DNA (12), but this has not been shown for human T cells yet. Thus, it is likely that B. pseudomallei provides flagellin and other costimulatory signals to Jurkat T cells.
Unlike the costimulation provided by live bacterial infection, flagellin is the sole factor contributing to the costimulation by bacterial culture supernatant. Culture supernatant from wild-type B. pseudomallei costimulated Jurkat cells, and this was abrogated in supernatants from fliC mutants. The same is observed for E. coli. The flagellin present in culture supernatant is possibly shed from the degradation of flagella. It is also possible that in live bacterial infection, flagellin could be synthesized de novo and secreted by bacteria interacting with host cells (31). Using purified recombinant B. pseudomallei flagellin, we found that the costimulatory effect of flagellin could be detected at as low as 30 pg/ml and was optimal at 2.5 ng/ml. The potency of flagellin could explain why the amount in the bacterial culture supernatant would be sufficient for stimulation, and it also means that this amount of flagellin is likely present during an in vivo infection. In addition, flagellin substantially enhanced IL-2 mRNA expression by costimulating Jurkat cells. Blocking IL-2 transcription by Cs caused a considerable and continuous fall in IL-2 mRNA in both flagellin- and CD28-costimulated cells. Thus, sustaining IL-2 mRNA level requires the presence of continuous stimulatory signals on transcription. The potent costimulatory effect of flagellin on IL-2 production is largely mediated through upregulating IL-2 expression and not through IL-2 mRNA stabilization.
We also validated the costimulation by B. pseudomallei in human primary CD4+ and CD8+ T cells. Infection with B. pseudomallei could costimulate CD3-cross-linked primary T cells to produce IL-2 and IFN-γ. Similar to what is seen in Jurkat T cells, flagellin provides partial costimulation to CD4+ T cells to produce IL-2 and IFN-γ. However, the situation in primary T cells is more complex than in Jurkat T cells. The flagellin mutant showed a stronger impairment in IL-2 than IFN-γ production in CD4+ T cells. This differential response to the costimulatory signal provided by flagellin in IL-2 and IFN-γ expression could be due to the differences in transcription regulation of these two genes. Although the optimal expression of IL-2 requires transcription factors, including NFAT, NF-κB, and AP-1, the expression of IFN-γ is regulated by NFAT, NF-κB, and STATs (38). Since IFN-γ can be induced through the STAT signaling pathway by cytokines such as IL-12, IL-18, and IL-2, it is possible that IFN-γ production by T cells is partially ascribed to a secondary response to autocrine IL-2. However, this could not explain why CD8+ T cells, which did not produce detectable IL-2, produced IFN-γ. Furthermore, the fliC mutant produced significantly less IL-2, and if IL-2 could compensate for the flagellin signal, there should be less IFN-γ compared to wild-type bacteria. Perhaps signaling to STATs by signals provided by B. pseudomallei could constitute partially for the loss of costimulation provided by flagellin. The weaker dependence on flagellin in IFN-γ than IL-2 production could also be explained by the stability of the interactions between IL-2 promoters and its transcription factors. The sites in IL-2 promoters are individually of low affinity, thus recruitment of multiple transcription factors is facilitated and stabilized by cooperative binding of factors to adjacent binding sites (1). This makes IL-2 expression more sensitive to the integrity and adequateness of transcription factors. Therefore, the lack of transcription factors in flagellin-induced signaling pathway would strongly affect IL-2 but only slightly affect IFN-γ expression.
Previous studies in mice (16, 17) and in humans (3, 14, 20) have shown the importance of adaptive immunity in protection against B. pseudomallei. Our findings on the direct costimulation by live B. pseudomallei could provide a mechanism for the activation of B. pseudomallei-specific T cells. This mechanism may be significant when antigen-presenting cells do not express costimulatory molecules either because the presenting cells are nonprofessionals, such as epithelial cells, or when antigen-presenting cells could be killed by the bacteria (732). Although human memory CD4+ T cells have been described to be costimulated with TLR2 (21) and TLR5, TLR7, and TLR8 ligands (5), we are the first to use a live bacterial infection to demonstrate costimulation and show that human CD8 T cells can be costimulated to make IFN-γ as well. Costimulation of naturally occurring CD4+ CD25+ regulatory T cells (Treg) by TLR2, TLR4, TLR5, and TLR8 agonists has also been reported and, in some instances, costimulation led to enhanced suppressive Treg activity, while in others it led to the inhibition of suppressive activity (7, 8, 23, 25, 26, 33). Since the role of Treg in melioidosis is completely unknown, it is difficult to comment on the implication of the possible costimulation of Treg by B. pseudomallei.
Although our primary human T cells were not further fractionated into memory or naive populations, it is highly likely that the memory T-cell population was costimulated with flagellin and with other costimulatory ligands described by others (5, 21). The different proportions of memory T cells and their level of TLR expression could contribute to the slight donor to donor variation in our results. Due to the ability of B. pseudomallei to costimulate T cells not only with anti-CD3 cross-linking in the mimic of a specific immune response but also in a nonspecific manner with IL-2, this could also induce bystander activation of T cells. Lertmemongkolchai et al. (22) found that murine memory CD8+ T cells were preferentially activated by bacterial infection to produce IFN-γ in an antigen-nonspecific manner. We suggest that the mechanism of bystander activation could be due to bacterial costimulation of the CD8+ memory T cells. Since memory T cells have a lower threshold for activation, the primary stimulus provided through the TCR could be replaced by cytokines such as IL-2. In fact, IL-2 could replace TCR signals (anti-CD3 stimulation) in the costimulation of CD4+ memory T cells by bacterial lipoprotein (21) and flagellin (5). We found that LPS could costimulate T cells to make IFN-γ in the presence of IL-2 to the same extent as bacterial infection in both CD4+ and CD8+ T cells. TLR4 expression on memory T cells has been reported (4) and, although stimulation via LPS alone does not result in proliferation nor cytokine production, T cells are able to respond to LPS via TLR4 by upregulating adherence to fibronectin and SOCS3 (39). Thus, LPS may be able to directly costimulate human T cells during B. pseudomallei infection, although LPS from B. pseudomallei has been described as less stimulatory (34). This non-antigen-specific stimulation could serve to perpetuate and maintain long-term T-cell memory. In the early bystander IFN-γ production by memory CD8+ T cells (22), initial IL-2 may be provided by innate immune cells such as dendritic cells stimulated by bacteria (10, 15). In situations where low-grade inflammation already exists such as in diabetic individuals (27, 36), infection with B. pseudomallei may lead to non-antigen-specific costimulation of memory T cells, leading in turn to greater cytokine production and inflammation. Thus, it remains to be seen whether bystander activation of T cells via bacterial costimulation contributes to innate immune response against the bacteria or whether it accentuates inflammation in the host.
Acknowledgments
This study is funded by a grant from the Singapore Biomedical Research Council (BMRC 04/1/21/19/329).
Editor: J. B. Bliska
Footnotes
Published ahead of print on 17 March 2008.
REFERENCES
- 1.Agarwal, S., and A. Rao. 1998. Long-range transcriptional regulation of cytokine gene expression. Curr. Opin. Immunol. 10345-352. [DOI] [PubMed] [Google Scholar]
- 2.Alonso, A., N. Bottini, S. Bruckner, S. Rahmouni, S. Williams, S. P. Schoenberger, and T. Mustelin. 2004. Lck dephosphorylation at Tyr-394 and inhibition of T-cell antigen receptor signaling by Yersinia phosphatase YopH. J. Biol. Chem. 2794922-4928. [DOI] [PubMed] [Google Scholar]
- 3.Barnes, J. L., J. Warner, W. Melrose, D. Durrheim, R. Speare, J. C. Reeder, and N. Ketheesan. 2004. Adaptive immunity in melioidosis: a possible role for T cells in determining outcome of infection with Burkholderia pseudomallei. Clin. Immunol. 11322-28. [DOI] [PubMed] [Google Scholar]
- 4.Cairns, B., R. Maile, C. M. Barnes, J. A. Frelinger, and A. A. Meyer. 2006. Increased Toll-like receptor 4 expression on T cells may be a mechanism for enhanced T-cell response late after burn injury. J. Trauma. 61293-298. [DOI] [PubMed] [Google Scholar]
- 5.Caron, G., D. Duluc, I. Fremaux, P. Jeannin, C. David, H. Gascan, and Y. Delneste. 2005. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-γ production by memory CD4+ T cells. J. Immunol. 1751551-1557. [DOI] [PubMed] [Google Scholar]
- 6.Chua, K. L., Y. Y. Chan, and Y. H. Gan. 2003. Flagella are virulence determinants of Burkholderia pseudomallei. Infect. Immun. 711622-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crellin, N. K., R. V. Garcia, O. Hadisfar, S. E. Allan, T. S. Steiner, and M. K. Levings. 2005. Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+CD25+ T regulatory cells. J. Immunol. 1758051-8059. [DOI] [PubMed] [Google Scholar]
- 8.Dai, J., B. Liu, S. M. Ngoi, S. Sun, A. T. Vella, and Z. Li. 2007. TLR4 hyper-responsiveness via cell surface expression of heat shock protein gp96 potentiates suppressive function of regulatory T cells. J. Immunol. 1783219-3225. [DOI] [PubMed] [Google Scholar]
- 9.Ekchariyawat, P., S. Pudla, K. Limposuwan, S. Arjcharoen, S. Sirisinha, and P. Utaisincharoen. 2005. Burkholderia pseudomallei-induced expression of suppressor of cytokine signaling 3 and cytokine-inducible src homology 2-containing protein in mouse macrophages: a possible mechanism for suppression of the response to gamma interferon stimulation. Infect. Immun. 737332-7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feau, S., V. Facchinetti, F. Granucci, S. Citterio, D. Jarrossay, S. Seresini, M. P. Protti, A. Lanzavecchia, and P. Ricciardi-Castagnoli. 2005. Dendritic cell-derived IL-2 production is regulated by IL-15 in humans and in mice. Blood 105697-702. [DOI] [PubMed] [Google Scholar]
- 11.Gan, Y. H., K. L. Chua, H. H. Chua, B. Liu, C. S. Hii, H. L. Chong, and P. Tan. 2002. Characterization of Burkholderia pseudomallei infection and identification of novel virulence factors using a Caenorhabditis elegans host system. Mol. Microbiol. 441185-1197. [DOI] [PubMed] [Google Scholar]
- 12.Gelman, A. E., D. F. LaRosa, J. Zhang, P. T. Walsh, Y. Choi, J. O. Sunyer, and L. A. Turka. 2006. The adaptor molecule MyD88 activates PI-3 kinase signaling in CD4+ T cells and enables CpG oligonucleotide mediated costimulation. Immunity 25783-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerke. C., S. Falkow, and Y. H. Chien. 2005. The adaptor molecules LAT and SLP-76 are specifically targeted by Yersinia to inhibit T-cell activation. J. Exp. Med. 201361-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Govan, B., and N. Ketheesan. 2004. Exposure to Burkholderia pseudomallei induces cell-mediated immunity in healthy individuals. Clin. Microbiol. Infect. 10585-587. [DOI] [PubMed] [Google Scholar]
- 15.Granucci, F., S. Feau, V. Angeli, F. Trottein, and P. Ricciardi-Castagnoli. 2003. Early IL-2 production by mouse dendritic cells is the result of microbial-induced priming. J. Immunol. 1705075-5081. [DOI] [PubMed] [Google Scholar]
- 16.Haque, A., A. Easton, D. Smith, A. O'Garra, N. Van Rooijen, G. Lertmemongkolchai, R. W. Titball, and G. J. Bancroft. 2006. Role of T cells in innate and adaptive immunity against murine Burkholderia pseudomallei infection. J. Infect. Dis. 193370-379. [DOI] [PubMed] [Google Scholar]
- 17.Healey, G. D., S. J. Elvin, M. Morton, and E. D. Williamson. 2005. Humoral and cell-mediated adaptive immune responses are required for protection against Burkholderia pseudomallei challenge and bacterial clearance postinfection. Infect. Immun. 735945-5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones, A. L., T. J. Beveridge, and D. E. Woods. 1996. Intracellular survival of Burkholderia pseudomallei. Infect. Immun. 64782-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kespichayawattana, W., P. Intachote, P. Utaisincharoen, and S. Sirisinha. 2004. Virulent Burkholderia pseudomallei is more efficient than avirulent Burkholderia thailandensis in invasion of and adherence to cultured human epithelial cells. Microb. Pathog. 36287-292. [DOI] [PubMed] [Google Scholar]
- 20.Ketheesan, N., J. L. Barnes, G. C. Ulett, H. J. VanGessel, R. E. Norton, R. G. Hirst, and J. T. LaBrooy. 2002. Demonstration of a cell-mediated immune response in melioidosis. J. Infect. Dis. 186286-289. [DOI] [PubMed] [Google Scholar]
- 21.Komai-Koma, M., L. Jones, G. S. Ogg, D. Xu, and F. Y. Liew. 2004. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc. Natl. Acad. Sci. USA 1013029-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lertmemongkolchai, G., G. Cai, C. A. Hunter, and G. J. Bancroft. 2001. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-γ in response to bacterial pathogens. J. Immunol. 1661097-1105. [DOI] [PubMed] [Google Scholar]
- 23.Lewkowicz, P., N. Lewkowicz, A. Sasiak, and H. Tchórzewski. 2006. Lipopolysaccharide-activated CD4+ CD25+ T regulatory cells inhibit neutrophil function and promote their apoptosis and death. J. Immunol. 1777155-7163. [DOI] [PubMed] [Google Scholar]
- 24.Liu, B., G. C. Koo, E. H. Yap, K. L. Chua, and Y. H. Gan. 2002. Model of differential susceptibility to mucosal Burkholderia pseudomallei infection. Infect. Immun. 70504-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, H., M. Komai-Koma, D. Xu, and F. Y. Liew. 2006. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc. Natl. Acad. Sci. USA 1037048-7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng. G., Z. Guo, Y. Kiniwa, K. S. Voo, W. Peng, T. Fu, D. Y. Wang, Y. Li, H. Y. Wang, and R. F. Wang. 2005. Toll-like receptor 8-mediated reversal of CD4+ regulatory T-cell function. Science 3091380-1384. [DOI] [PubMed] [Google Scholar]
- 27.Pickup, J. C. 2004. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 27813-823. [DOI] [PubMed] [Google Scholar]
- 28.Pruksachartvuthi, S., N. Aswapokee, and K. Thankerngpol. 1990. Survival of Pseudomonas pseudomallei in human phagocytes. J. Med. Microbiol. 31109-114. [DOI] [PubMed] [Google Scholar]
- 29.Stevens, M. P., M. W. Wood, L. A. Taylor, P. Monaghan, P. Hawes, P. W. Jones, T. S. Wallis, and E. E. Galyov. 2002. An Inv/Mxi-Spa-like type III protein secretion system in Burkholderia pseudomallei modulates intracellular behavior of the pathogen. Mol. Microbiol. 46649-659. [DOI] [PubMed] [Google Scholar]
- 30.Stevens, M. P., J. M. Stevens, R. L. Jeng, L. A. Taylor, M. W. Wood, P. Hawes, P. Monaghan, M. D. Welch, and E. E. Galyov. 2005. Identification of a bacterial factor required for actin-based motility of Burkholderia pseudomallei. Mol. Microbiol. 5640-53. [DOI] [PubMed] [Google Scholar]
- 31.Subramanian, N., and A. Qadri. 2006. Lysophospholipid sensing triggers secretion of flagellin from pathogenic salmonella. Nat. Immunol. 7583-589. [DOI] [PubMed] [Google Scholar]
- 32.Sun, G. W., J. Lu, S. Pervaiz, W. P. Cao, and Y. H. Gan. 2005. Caspase-1 dependent macrophage death induced by Burkholderia pseudomallei. Cell Microbiol. 71447-1458. [DOI] [PubMed] [Google Scholar]
- 33.Sutmuller, R. P., M. H. den Brok, M. Kramer, E. J. Bennink, L. W. Toonen, B. J. Kullberg, L. A. Joosten, S. Akira, M. G. Netea, and G. J. Adema. 2006. Toll-like receptor 2 controls expansion and function of regulatory T cells. J. Clin. Investig. 116485-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Utaisincharoen, P., N. Anuntagool, S. Arjcharoen, I. Lengwehasatit, K. Limposuwan, P. Chaisuriya, and S. Sirisinha. 2004. Burkholderia pseudomallei stimulates low interleukin-8 production in the human lung epithelial cell line A549. Clin. Exp. Immunol. 13861-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warawa, J., and D. E. Woods. 2005. Type III secretion system cluster 3 is required for maximal virulence of Burkholderia pseudomallei in a hamster infection model. FEMS Microbiol. Lett. 242101-108. [DOI] [PubMed] [Google Scholar]
- 36.Wellen, K. E., and G. S. Hotamisligil. 2005. Inflammation, stress and diabetes. J. Clin. Investig. 1151111-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye, Z., and Y. H. Gan. 2007. Flagellin contamination of recombinant heat shock protein 70 is responsible for its activity on T cells. J. Biol. Chem. 2824479-4484. [DOI] [PubMed] [Google Scholar]
- 38.Young, H. A., and P. Ghosh. 1997. Molecular regulation of cytokine gene expression: interferon-γ as a model system. Prog. Nucleic Acids Res. Mol. Biol. 56109-127. [DOI] [PubMed] [Google Scholar]
- 39.Zanin-Zhorov, A., G. Tal-Lapidot, L. Cahalon, M. Cohen-Sfady, M. Pevsner-Fischer, O. Lider, and I. R. Cohen. 2007. Cutting edge: T cells respond to lipopolysaccharide innately via TLR4 signaling. J. Immunol. 17941-44. [DOI] [PubMed] [Google Scholar]