Abstract
Helicobacter pylori-produced cytotoxin VacA induces intracellular vacuolation. The VacA-induced vacuole is assumed to represent the pathological status of intracellular trafficking. The fusion mechanism of the endosomes requires the formation of a tight complex between the Q-SNAREs and the R-SNAREs. We recently reported that syntaxin 7, a family member of the Q-SNARE protein, is involved in VacA-induced vacuole formation. In order to further elucidate the molecular mechanism, we identified the participation of vesicle-associated membrane protein 7 (VAMP7) as a partner of syntaxin 7. Immunocytochemistry revealed endogenous VAMP7 to be localized to the vacuoles induced by VacA. A Northern blotting study demonstrated that VacA intoxication increased VAMP7 mRNA in a time-dependent manner. VAMP7 was coimmunoprecipitated with syntaxin 7, and the amounts of endogenous VAMP7 and syntaxin 7 bound to syntaxin 7 and VAMP7, respectively, increased in response to VacA. The down-regulation of VAMP7 using small interfering RNA inhibited VacA-induced vacuolation, and the transient transfection of dominant-negative mutant VAMP7, the N-terminal domain of VAMP7, also inhibited the vacuolation. We therefore conclude that R-SNARE VAMP7 plays an important role in VacA-induced vacuolation as a partner of Q-SNARE syntaxin 7.
Helicobacter pylori, a gram-negative bacterial species which selectively colonizes gastric mucosa, is a major cause of chronic gastritis and gastroduodenal ulcers. Chronic gastric inflammation confers a significant increased risk of developing gastric cancer (24). H. pylori produces several virulence factors such as the cytotoxin-associated gene A product (CagA) and vacuolating cytotoxin A (VacA). The vacA gene is present in virtually all of the H. pylori strains that have been isolated from humans, which thus suggests that the production of VacA plays an important role in the colonization or persistence of H. pylori in the human stomach (6). Previous studies reported that the intragastric administration of VacA into mice induced erosions of the gastric epithelia (33). When added to epithelial cells in vitro, VacA induces both structural and functional alterations in the cells, the most prominent being the formation of cytoplasmic vacuolations (15). These vacuoles induced by VacA contain both a late endosomal marker, rab7, and a lysosomal marker, lgp110, but they do not contain any markers for early endocytic compartments, thus suggesting that they are hybrids of late endosomes and lysosomes (18, 22). A current model of vacuole formation is that VacA binds to the plasma membrane, is internalized by cells, and forms anion-selective membrane channels, and the vacuoles then arise due to swelling of the endosomal compartments (6, 8). Papini et al. previously reported that Rab7, a low-molecular-weight GTP-binding protein that regulates late endosomal trafficking, plays an essential role in VacA-induced vacuolation (23). We previously reported that dynamin, a high-molecular-weight GTP-binding protein functioning as a mechanochemical enzyme in vesicle formation, is also involved in VacA-induced vacuolation and that the VacA cytopathic effect on intoxicated cells was attenuated by inhibiting the dynamin function (32), although VacA internalization was mediated mainly by clathrin-independent endocytosis (26, 32). Those results suggest that VacA-induced vacuolation is a result of the toxin-induced alteration of intracellular vesicle trafficking and that the VacA cytopathic effect can be prevented by inhibiting VacA-induced vacuolation. As a result, elucidating the molecular mechanism of VacA-induced vacuolation is therefore expected to contribute to the development of a novel therapeutic strategy for H. pylori-related diseases.
The SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) hypothesis has provided a model for the fusion events of intracellular trafficking (28). SNARE proteins are small proteins (18 to 42 kDa) containing cytoplasmic coiled-coil domains, referred to as SNARE motifs, which interact with other SNAREs and are present on cellular membranous organelles including transport vesicles (11). SNARE proteins are classified as either Q- or R-SNARE according to whether they contain a glutamate or an arginine in the central region of their helical bundles, respectively (11). Alternatively, they are known as t- or v-SNARE, depending on their localization on either the target (t) or vesicle (v) membrane, respectively (27). SNARE-mediated fusion typically results from the formation of a complex consisting of one R-SNARE and three Q-SNARE motifs (25). Q-SNAREs can be subdivided into Qa-SNAREs (syntaxins), Qb-SNAREs (25-kDa synaptosome-associated protein [SNAP-25] N-terminal SNARE motif), or Qc-SNAREs (SNAP-25 C-terminal SNARE motif). R-SNAREs can be also subdivided into short vesicle-associated membrane proteins (VAMPs) and long VAMPs (25).
A number of VAMPs (classified as v-SNAREs) with their specific localizations and functions have been identified. VAMP1 and VAMP2 (also called synaptobrevins 1 and 2, respectively) are expressed in neurons and endocrine cells; are localized to synaptic vesicles, secretory granules, and recycling endosomes; and play their roles in regulated exocytosis (12). VAMP3, also called cellubrevin, is a ubiquitously expressed VAMP isoform that may confer both regulated and constitutive exocytosis (12). VAMP4, which is present on the trans-Golgi network (TGN) and in immature secretory granules, binds with syntaxin 6, thus suggesting that it may play a role in TGN-to-endosome transport (29). VAMP5, also called myobrevin, is preferentially expressed in the skeletal muscle and heart. VAMP5, which is present in the plasma membrane and perinuclear and peripheral vesicular structures of myotubes, may be involved in myotube formation during myogenesis (38). VAMP7, also called tetanus-insensitive VAMP (TiVAMP), is ubiquitously expressed and is localized to the late endosome, the lysosome, and the TGN (11). VAMP7 is involved in heterotypic endosome-lysosome and homotypic lysosome fusion, neurite extension, and apical exocytosis in polarized epithelial cells (1, 5, 14, 25, 36). Another ubiquitously expressed VAMP homolog, known as endobrevin (VAMP8), is present in early endosomes, late endosomes, and recycling endosomes in polarized epithelia. VAMP8 has been shown to be involved in homotypic early endosome and late endosome fusion (3, 4, 20, 25, 30).
Syntaxin 7 is an integral membrane protein in both late endosomes and lysosomes, and it is required for both homotypic late endosome fusion (3) and heterotypic fusion with lysosomes (19, 36). In the former case, the other required SNAREs have been identified as being Vti1b, syntaxin 8, and VAMP8 (2). In the latter case, immunoprecipitated syntaxin 7 complexes have been found to contain a number of different SNAREs: Vti1b, syntaxin 6, VAMP7, and VAMP8 (35). Among them, VAMP7 has been reported to be a key player in the heterotypic fusion events involving lysosomes (25). We recently reported that syntaxin 7 is involved in the intracellular vacuolation induced by VacA and that the VacA cytopathic effect on intoxicated cells was attenuated by inhibiting syntaxin 7 function (31).
To elucidate the molecular mechanism of the final step of VacA-induced vacuolation, which is a hybrid of the late endosome and lysosome, we examined whether VAMP7 is involved in its vacuolation as a partner of Qa-SNARE syntaxin 7. We herein demonstrated that endogenous VAMP7 was localized to the VacA-induced vacuoles and that the expression level of VAMP7 was enhanced in VacA-intoxicated cells. We also showed that the down-regulation of VAMP7 resulted in the inhibition of VacA-induced vacuolation. We therefore concluded that VAMP7 plays an important role in VacA-induced vacuolation.
MATERIALS AND METHODS
Cell culture, purification, and activation of VacA and intoxication of cells.
AGS cells, a human gastric adenocarcinoma cell line, were purchased from the American Type Culture Collection (Manassas, VA) and were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and 100 units/ml of penicillin in a 5% CO2 atmosphere at 37°C. The VacA cytotoxin was purified from toxin-producing H. pylori strain ATCC 49503 according to a procedure reported previously (21) and stored at −20°C. Immediately before use, purified VacA was activated by dropwise acidification with 1 N HCl. For VacA intoxication, the cells were treated with 1 μg/ml activated VacA at 37°C for 24 to 48 h without any addition of ammonium chloride or other bases. VacA was heat inactivated at 95°C for 10 min.
Plasmid and siRNA.
The NH2-terminal green fluorescent protein (GFP)-tagged full-length VAMP7 expression vector (GFP-TiVAMP/VAMP7, from M1 to K220) and the NH2-terminal GFP-tagged NH2-terminal domain of the VAMP7 expression vector (GFP-Nter-TiVAMP/VAMP7, from M1 to N120) were kindly provided by Thierry Galli. The NH2-terminal GFP-tagged syntaxin 7 expression vector was constructed as described in a previous study (14). The pcDNA3.1/NT-GFP vector (Invitrogen) was used as a GFP control plasmid. The small interfering RNA (siRNA) duplexes for VAMP7 (SYBL1-HSS110395 to SYBL1-HSS110397) and the Stealth RNA interference (RNAi) negative control duplexes were purchased from Invitrogen (Carlsbad, CA). siRNA1 (SYBL1-HSS110395) corresponded to nucleotides 240 to 264 of human vamp7 (GenBank accession no. NM_005638), and it was located at the NH2-terminal domain. siRNA2 and siRNA3 corresponded to nucleotides 517 to 541 and 556 to 581, respectively, and they were located in the coiled-coil domain (R-SNARE motif).
Transfection.
The transfection procedures were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instruction. AGS cells were seeded at a density of 1 × 105 cells/cm2 and transfected with constructed plasmids or siRNA duplexes.
Antibodies.
Anti-VAMP7 mouse monoclonal antibody was a generous gift from Thierry Galli. Anti-VAMP8 rabbit polyclonal antibody was purchased from Abcam (Cambridge, United Kingdom) and Covalab (Villeurbanne, France). Anti-VAMP2 rabbit polyclonal antibody was obtained from Chemicon International (Temecula, CA). Anti-syntaxin 7 rabbit polyclonal antibody was kindly provided by Masamitsu Futai (Osaka University, Japan). Anti-syntaxin 4 and anti-syntaxin 6 mouse monoclonal antibodies were purchased from BD Biosciences (San Jose, CA). Anti-GFP rabbit polyclonal antibody and mouse monoclonal antibody were purchased from Clontech (Mountain View, CA) and Medical Biological Laboratories (Nagoya, Japan), respectively. Anti-actin goat polyclonal antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The secondary antibodies (Cy3-conjugated donkey anti-mouse and anti-rabbit immunoglobulin G [IgG], horseradish peroxidase-conjugated donkey anti-mouse and anti-rabbit IgG, and horseradish peroxidase-conjugated donkey anti-goat IgG) were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA), and Alexa 488-conjugated anti-mouse IgG was obtained from Invitrogen.
Immunofluorescence microscopy.
The cells were fixed with 2% formaldehyde in phosphate-buffered saline, treated with Triton X-100 in phosphate-buffered saline for 5 min, and incubated sequentially with Blocking Ace (Snow Brand Milk Products, Sapporo, Japan), first antibodies, and second antibodies. Samples were examined under a Nikon (Tokyo, Japan) E-600 fluorescence microscope. Images were captured and digitized using a Spot charged-coupled-device camera (Diagnostic Instruments, Sterling Heights, MI) and then edited using the Adobe Photoshop CS software program (Adobe Systems Inc., Mountain View, CA).
Northern blotting.
Northern blotting was performed as described previously (17). Briefly, 20 μg of total RNA extracted from AGS cells was denatured and blotted onto a Hybond N+ nylon membrane (Amersham, Arlington, IL). The blots were hybridized with a 32P-labeled cDNA probe and washed for 30 min under high-stringency conditions (0.1× standard saline citrate, 0.1% sodium dodecyl sulfate [SDS]) at 65°C before exposure to X-ray film. To remove the probe, the membrane was incubated with hybridization buffer for 30 min at 60°C. Band intensity was measured using the ImageJ software program.
Western blotting.
The protein extracts from AGS cells were prepared for immunoblotting as described previously (13). For electrophoresis, 20 μg of protein from each sample per lane was loaded onto SDS-polyacrylamide gels and run at 200 V. The proteins were then transferred onto the nitrocellulose membranes at 50 V for 3 h. For Western blotting, the membranes were incubated sequentially with Blocking Ace (Snow Brand Milk Products, Sapporo, Japan), first antibodies, and second antibodies using an enhanced chemiluminescence Western blotting detection reagent (Amersham Biosciences, Piscataway, NJ) to visualize the secondary antibody. To remove the probe, the membranes were incubated with stripping buffer containing 62.5 mM Tris-HCl (pH 6.7), 2% SDS, and 0.1 M 2-mercaptoethanol for 30 min at 50°C.
Immunoprecipitation.
Affinity matrices were prepared using anti-GFP polyclonal antibody to protein A-Sepharose beads (Pierce). The Triton X-100-soluble membrane extracts prepared from the AGS cells were incubated with antibody-coated beads for 4 h at 4°C in the presence of 100 mM NaCl and 20 mM Tris-HCl (pH 7.5). The beads were washed several times in the same buffer, and the elution of bound proteins was achieved by boiling for 10 min in Laemmli sample buffer.
Analysis of mRNA by RT-PCR.
For semiquantitative reverse transcription (RT)-PCR, first-stranded cDNA was synthesized by using the Superscript first-stranded synthesis system for RT-PCR (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The oligonucleotide primers used were 5′-TCAAGAGCACAGACAGCACTTCC-3′ (nucleotides 388 to 410) and 5′-GCCATGTAAATCCACCACAGAGAG-3′ (nucleotides 757 to 734) for human vamp7 (370-bp PCR product) (GenBank accession no. NM_005638), 5′-GAAAGCCAAACTCAACCTCAAGTG-3′ (nucleotides 515 to 538) and 5′-ATGATGCACAGGGTTTTTCTGG-3′ (nucleotides 801 to 780) for human syntaxin 7 (287-bp PCR product) (GenBank accession no. NM_003569), 5′-ATCGTGTGCGGAACCTGCAAAG-3′ (nucleotides 85 to 106) and 5′-ACAGGAAAGGAGACCCTCTTGG-3′ (nucleotides 438 to 417) for human vamp8 (354-bp PCR product) (GenBank accession no. NM_003761), and 5′-CATTAAGGAGAAGCTGTGCTACGTC-3′ (nucleotides 706 to 730) and 5′-GCTGATCCACATCTGCTGGAAG-3′ (nucleotides 1147 to 1126) for human β-actin (442-bp PCR product) (GenBank accession no. NM_001101). Twenty-five PCR cycles were used.
Assay of vacuolating activity.
AGS cells were seeded into 24-well plates and cultured for 18 h. The cells were then transfected with siRNA or control RNAi duplexes. After 8 h of transfection, the cells were treated with VacA and incubated for a further 24 h. To quantify the vacuolating activity, the uptake of neutral red dye into the vacuoles in VacA-treated cells was determined by measuring the absorbance at 540 nm (A540) (37). The vacuolating activity was determined by subtracting the mean A540 of the cells incubated without VacA from the A540 of VacA-treated cells and was shown as a percentage of neutral red accumulation to the mock-transfected cells.
Statistical analysis.
Statistical analysis was performed using analysis of variance. A P value of <0.05 was considered to indicate a significant difference.
RESULTS
VAMP7 is localized to the VacA-induced vacuole.
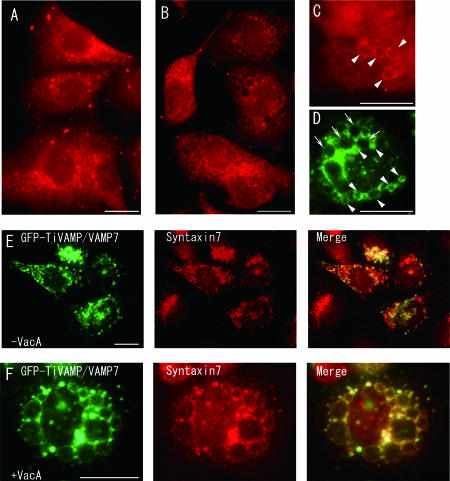
VAMP7 has been shown to be involved in heterotypic late endosome-lysosome fusion events, and immunoprecipitation of VAMP7 demonstrated the colocalization of syntaxin 7, a family member of the Q-SNARE protein (25). Our previous study indicated that syntaxin 7 was involved in VacA-induced vacuolar formation (31). Therefore, these data suggest that VAMP7 is a potential partner for syntaxin 7 in VacA-induced vacuole formation. In order to gain insight into VacA-induced vacuolar formation, we first analyzed the intracellular localization of VAMP7 using anti-VAMP7 antibody in AGS cells. As shown in Fig. 1A, endogenous VAMP7 was localized to the perinuclear region of the untreated cells, consistent with the previous observation that VAMP7 is present on the late endosome, the lysosome, and the TGN (11). In VacA-treated AGS cells, part of endogenous VAMP7 changed its localization to the vacuoles induced by VacA (Fig. 1B to D, arrowheads). However, there were also some vacuoles to which VAMP7 was not localized (Fig. 1D, arrows). We next evaluated the colocalization of VAMP7 and syntaxin 7. We induced the overexpression of GFP-tagged VAMP7 using the GFP-TiVAMP/VAMP7 expression vector with or without VacA and examined the colocalization of GFP and endogenous syntaxin 7. Although a complete overlap between VAMP7 and syntaxin 7 is not expected because both antigens are present on several intracellular organelles, the localizations almost entirely overlapped, especially in the intoxicated cells (Fig. 1E and F).
FIG. 1.
Immunocytochemical localization of VAMP7 to VacA-induced vacuoles in AGS cells and colocalization with syntaxin 7. (A to D) Naïve AGS cells (A) and VacA-treated AGS cells (24 h) (B to D) were fixed, stained with anti-VAMP7 antibody, and visualized with Cy3-conjugated anti-mouse IgG (A to C) or Alexa 488-conjugated anti-mouse IgG antibody (D). (A) Perinuclear distribution of endogenous VAMP7 in naïve AGS cells. (B to D) Localization of endogenous VAMP7 on VacA-induced vacuoles in intoxicated AGS cells (arrowheads). There were also some vacuoles to which VAMP7 did not localize (arrows). AGS cells were transfected with the GFP- TiVAMP/VAMP7 expression vector, cultured for 24 h with (F) or without (E) VacA, and evaluated for the colocalization of GFP and endogenous syntaxin 7. The expressions of VAMP7 and syntaxin 7 almost entirely overlapped, especially in intoxicated cells (F). Bars, 25 μm.
VacA increased expression of VAMP7 mRNA.
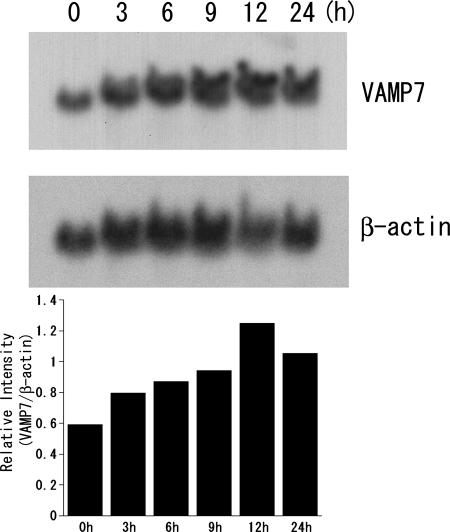
We next examined the expression of the mRNA level of VAMP7 in VacA-treated AGS cells by Northern blotting. As shown in Fig. 2, VacA intoxication enhanced the expression of VAMP7 mRNA in AGS cells in a time-dependent manner. When VacA was heat inactivated (95°C for 10 min), the expression of VAMP7 did not change (data not shown). In a previous study, syntaxin 7 expression in VacA-treated AGS cells was also increased at both mRNA and protein levels in a time-dependent manner (14). These findings thus indicate that VAMP7 may be involved in vacuole formation induced by VacA.
FIG. 2.
Effect of VacA on the expression of VAMP7 mRNA in AGS cells. (Top) The total RNA (20 μg) that was isolated from AGS cells exposed to VacA for the specified times was blotted onto a nylon membrane and probed with 32P-labeled VAMP7 cDNA. (Middle) The blot was reprobed with 32P-labeled β-actin cDNA. (Bottom) Relative intensity of VAMP7 over β-actin. The experiment was repeated three times with similar results, and one representative figure is shown. When VacA was heat inactivated (95°C for 10 min), the expression of VAMP7 did not change (data not shown).
VAMP7 complexes with syntaxin 7 in VacA-induced vacuoles.
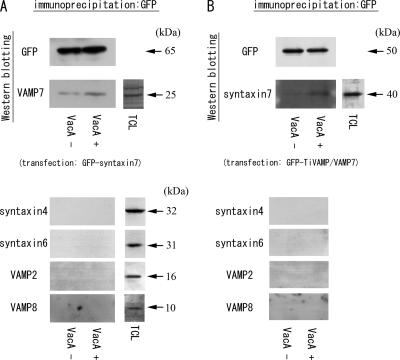
To examine the interaction of VAMP7 and syntaxin 7 using immunoprecipitation experiments, we induced the overexpression of GFP-tagged syntaxin 7 in AGS cells with or without VacA. Overexpressed GFP-tagged wild-type syntaxin 7 changed its intracellular localization from the perinuclear region to the vacuoles after VacA intoxication, which was consistent with the localization of endogenous syntaxin 7 to VacA-induced vacuoles (31). Syntaxin 7 was immunoprecipitated from a Triton X-100-soluble fraction prepared from AGS cells using an anti-GFP polyclonal antibody, and the samples were then immunoblotted for VAMP7 (Fig. 3A). VAMP7 was coimmunoprecipitated with syntaxin 7, and the band in the VacA-treated AGS cells showed a stronger intensity. Conversely, we induced the overexpression of GFP-tagged TiVAMP/VAMP7 in AGS cells, immunoprecipitated them with an anti-GFP polyclonal antibody, and immunoblotted for syntaxin 7. The overexpressed GFP-tagged VAMP7 also changed its cellular distribution from the perinuclear region to the VacA-induced vacuoles (Fig. 1E and F and see Fig. 5A and B). Syntaxin 7 was also coimmunoprecipitated with VAMP7, and a larger amount of endogenous syntaxin 7 was bound to VAMP7 in the VacA-intoxicated cells (Fig. 3B). Since SNARE proteins can be sticky, we examined whether other SNARE proteins might be bound to syntaxin 7 and/or VAMP7. The membranes were immunoblotted for syntaxin 4, syntaxin 6, VAMP2, and VAMP8, and we could not detect any signals for these proteins. These results suggest that the interaction between VAMP7 and syntaxin 7 was not nonspecific and that VAMP7 was involved in VacA-induced vacuolation as a partner of syntaxin 7.
FIG. 3.
Immunoprecipitation of endogenous syntaxin 7 and VAMP7 from VacA-treated or nontreated AGS cells followed by immunoblotting. (A) AGS cells were transfected with the GFP-tagged syntaxin 7 vector and incubated for 24 h with or without VacA. The cellular extracts were immunoprecipitated using an anti-GFP polyclonal antibody, and immunoblotting was performed using an anti-GFP monoclonal antibody and anti-VAMP7 antibody. Since SNARE proteins can be sticky, the membranes were also immunoblotted for syntaxin 4, syntaxin 6, VAMP2, and VAMP8 to prove that the interaction between VAMP7 and syntaxin 7 was not nonspecific. (B) AGS cells were transfected with the GFP-TiVAMP/VAMP7 vector and incubated for 24 h with or without VacA. The cellular extracts were immunoprecipitated using an anti-GFP polyclonal antibody, and immunoblotting was performed. The total cell lysate (TCL) of AGS cells was used as a control for the bands. When the samples of immunoprecipitation from cells not expressing GFP were immunoblotted for VAMP7 and syntaxin 7, we could not detect the specific bands (data not shown). The experiments were repeated three times independently, and representative figures are shown.
FIG. 5.
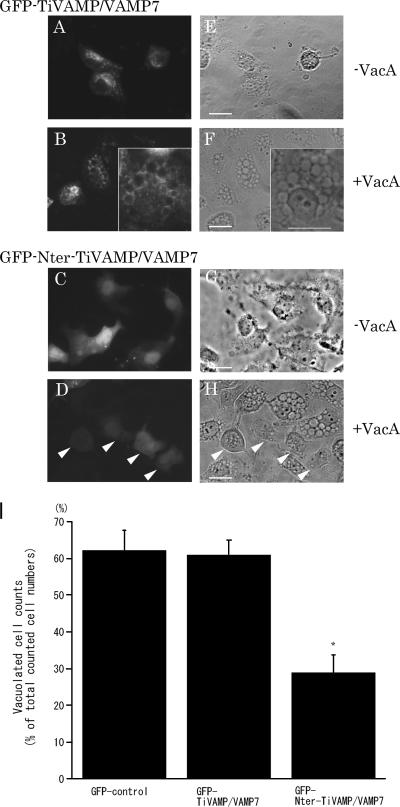
Inhibition of VacA-induced vacuolation in AGS cells by transient transfection of Nter-TiVAMP/VAMP7. (A to D) Images of fluorescence microscopy of transiently transfected GFP-tagged wild-type TiVAMP/VAMP7 (A and B) and GFP-tagged Nter-TiVAMP/VAMP7 (C and D) in the AGS cells treated (B and D) or not treated (A and C) with VacA for 24 h. (E to H) Phase-contrast images of the same fields above (A to D, respectively). VacA failed to induce cytoplasmic vacuolation in AGS cells transfected with Nter-TiVAMP/VAMP7 (D and H, arrowheads). In contrast, VacA induced multiple cytoplasmic vacuoles in AGS cells transfected with GFP-tagged wild-type TiVAMP/VAMP7 (B and F). Bars, 25 μm. (I) Numbers of vacuolated cells in AGS cells transiently transfected with GFP-tagged control, wild-type, and dominant-negative VAMP7 and cultured for 24 h with VacA. The results are expressed as percentages of vacuolated cells. Values are the means ± standard errors (n = 5) for three independent experiments in which more than 100 cells were counted. The number of VacA-induced vacuolated cells in dominant-negative VAMP7-transfected AGS cells was significantly lower than that in the cells transfected with control and wild-type VAMP7 plasmids. *, P < 0.01.
Effect of down-regulation of VAMP7 on VacA-induced vacuolation.
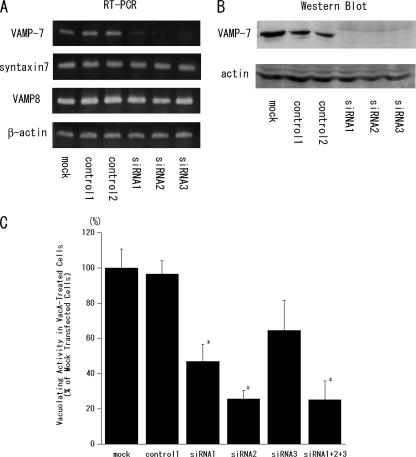
To determine whether the expression level of VAMP7 might affect VacA-induced vacuolation, we first tried to generate an AGS cell line stably transfected with GFP-TiVAMP/VAMP7 or GFP-Nter-TiVAMP/VAMP7 lacking the coiled-coil domain (R-SNARE motif) and the transmembrane domain of VAMP7. However, the transfected cells died within a few weeks. We then decreased the expression level of VAMP7 using siRNA. As shown in Fig. 4A and B, the expression level of VAMP7 was markedly blocked in mRNA and the protein levels with the transfection of siRNA1 to siRNA3 compared to the controls. The down-regulation of VAMP7 did not affect the expression levels of syntaxin 7 and VAMP8. Neutral red dye has been used as a quantitative marker for VacA-induced vacuolation since it is predominantly taken up by the acidic lumen of the vacuoles (31, 37). As shown in Fig. 4C, the down-regulation of the VAMP7 expression inhibited neutral red dye uptake into the VacA-treated cells compared to the controls. When we compared the vacuolation ratio (number of vacuolated cells/number of total counted cells) by microscopy, siRNA treatment also resulted in a reduction of VacA-induced vacuolation (data not shown). These results indicate the direct involvement of VAMP7 in the VacA-induced vacuolation mechanism.
FIG. 4.
Effect of the down-regulation of VAMP7 on neutral red dye uptake into VacA-treated AGS cells. AGS cells were transfected with siRNA1 to siRNA3 or control RNAi duplexes 1 and 2, and the down-regulation of VAMP7 was confirmed by RT-PCR (A) and Western blotting (B). (A) The effect on the expression of syntaxin 7 and VAMP8 was also studied by RT-PCR. (B) AGS cells were transfected with the GFP-TiVAMP/VAMP7 vector 18 h before the transfection of siRNA or control RNAi duplexes, and Western blotting was performed using an anti-GFP monoclonal antibody. (C) For the assay of vacuolating activity, the cells were seeded into a 24-well plate and cultured for 18 h. The cells were then transfected with siRNA or control RNAi duplexes. After 8 h, the cells were treated with VacA and incubated for an additional 24 h. The vacuolating activity was determined as a percentage of neutral red dye uptake into the VacA-treated cells with respect to mock-transfected cells. Values are the means ± standard errors (n = 4) for three independent experiments. The down-regulation of VAMP7 significantly inhibited neutral red dye uptake in comparison to the control. *, P < 0.05.
Transient transfection of the N-terminal domain of VAMP7 inhibits VacA-induced vacuolation in AGS cells.
We next investigated the participation of VAMP7 in VacA-induced vacuolation by a different approach. The N-terminal domain of VAMP7 acts as a dominant-negative VAMP7 in previous studies (16, 25). We then overexpressed the GFP-TiVAMP/VAMP7 and GFP-Nter-TiVAMP/VAMP7 vectors in AGS cells with or without VacA and examined the morphological changes. As shown in Fig. 5, the overexpressed GFP-tagged wild-type VAMP7 changed its intracellular localization from the perinuclear region (Fig. 5A and E) to the vacuoles (B and F) after VacA intoxication. In contrast, the overexpressed GFP-tagged N-terminal domain of VAMP7 was localized to the cytoplasm in both the VacA-treated (D) and untreated (C) cells. The overexpression of the N-terminal domain of VAMP7 inhibited VacA-induced vacuolation in AGS cells (D and H, arrowheads), whereas the surrounding untransfected cells showed vacuolations in response to VacA. Overexpressed wild-type VAMP7 did not affect VacA-induced vacuolation in AGS cells (B and F). For quantitative estimations, we compared the numbers of vacuolated cells under each experimental condition. As depicted in Fig. 5I, the inhibitory effect of dominant-negative VAMP7 on VacA-induced vacuolations was statistically significant in comparison to those of the control and wild-type VAMP7. These data indicate that the N-terminal domain of VAMP7 specifically inhibited VacA-induced vacuolation, thus suggesting the involvement of VAMP7 in the molecular machinery of VacA-induced vacuolation.
DISCUSSION
In the present study, we have shown that VAMP7 directly participated in VacA-induced vacuolation in AGS cells. We first showed that endogenous wild-type VAMP7 was localized to the vacuoles induced by VacA. Moreover, VacA increased the mRNA level of VAMP7. VAMP7 was coimmunoprecipitated with syntaxin 7. The amounts of endogenous VAMP7 and syntaxin 7, which were coimmunoprecipitated with GFP-tagged syntaxin 7 and VAMP7, respectively, were increased in VacA intoxication. The down-regulation of endogenous VAMP7 inhibited VacA-induced vacuolation. From these lines of evidence, we concluded that VAMP7 directly participated in VacA-induced vacuolation in AGS cells as a partner of Q-SNARE syntaxin 7.
The VacA-induced vacuole has been assumed to be a hybrid of the late endosome and the lysosome (18, 22). We have reported that syntaxin 7 was directly involved in intracellular vacuolation induced by VacA (31). We then examined the involvement of VAMP7 in VacA-induced vacuolation and the interaction with syntaxin 7. VAMP8 has been shown to be involved in homotypic early endosome and late endosome fusion (3, 4, 20, 25, 30). Pryor et al. previously reported that VAMP8 and syntaxin 7 were coimmunoprecipitated using detergent-solubilized rat liver membrane fractions (25). We could not exclude the possibility that the cytopathic effect of VacA might alter the function of VAMP8 to cause a heterotypic fusion of the late endosome and lysosome. However, as depicted in Fig. 3, immunoprecipitation studies showed that VAMP7, but not VAMP8, demonstrated specific binding to syntaxin 7 specifically. Moreover, the amounts of endogenous VAMP7 and syntaxin 7 bound to GFP-tagged syntaxin 7 and VAMP7, respectively, were increased in response to VacA intoxication. Since SNARE proteins can be sticky, we also examined whether other SNARE proteins had a tendency to bind to syntaxin 7 and/or VAMP7. Syntaxin 4 and VAMP2 play a role in regulated exocytosis. Syntaxin 6 functions in vesicular transport between the TGN and the endosome. We could not detect any interaction of these proteins with either VAMP7 or syntaxin 7.
We previously reported that dominant-negative mutant syntaxin 7, which lacks a carboxyl-terminal transmembrane domain, inhibits VacA-induced vacuolation. VAMP7 is composed of three domains: an N-terminal domain, the coiled-coil domain (R-SNARE motif), and one comprising the transmembrane domain and a short luminal domain. The N-terminal domain was shown to inhibit the capacity of the R-SNARE motif to form SNARE complexes. Therefore, the N-terminal domain of VAMP7 acts as a dominant-negative form of VAMP7, and conversely, ΔNter-TiVAMP/VAMP7 acts as a constitutively active form as reported previously (16, 25). In AGS cells transiently transfected with the N-terminal domain of VAMP7, VacA-induced vacuolation was considerably inhibited (Fig. 5). When we decreased the expression of VAMP7 using siRNA, vacuolation was also inhibited (Fig. 4). Although the precise mechanism by which VacA affects VAMP7 and syntaxin 7 is still obscure, VacA may interact directly or indirectly with VAMP7 and/or syntaxin 7 on the endosomal membranes.
There have also been several articles reporting that VacA-induced vacuolation can occur without any requirement for SNARE proteins (7, 10). VacA is considered to form anion-selective membrane channels in the membranes of the endocytic compartments after internalization (6, 8), although the mechanisms by which VacA undergoes sorting and trafficking to intracellular sites are poorly understood. The transmembrane VacA channel mediates an influx of anions into endosomes. To compensate for the increased anion concentration, the activity of the vacuolar ATPase proton pump on endosomes increases, thus resulting in their osmotic swelling and transformation into vacuoles (6, 10). In fact, we could see some vacuoles to which VAMP7 had not localized in VacA-treated AGS cells in addition to the vacuoles with the expression of VAMP7 (Fig. 1D, arrows). There may be several processes for VacA-induced vacuole formation. Further precise observations of VAMP7, syntaxin 7, VacA, and the vacuolar ATPase proton pump in living cells may therefore help us to obtain a better understanding of these models.
VacA was also known to affect several cellular signal transduction pathways. When VacA was added to a human adenocarcinoma cell line (AZ-521), two classes of mitogen-activated protein kinases (p38 and extracellular signal-regulated kinase 1/2) and the activating transcription factor 2 (ATF2) signaling pathway were activated (21). In BHK-21 cells expressing receptor tyrosine phosphatase β, tyrosine phosphorylation of G-protein-coupled receptor kinase interactor (Git1) can be detected (9). Therefore, VacA may alter some cellular signal transduction pathway in order to cause vacuolation, although the inhibitor of p38 kinase activity (SB203580) does not block VacA-induced vacuolation (21).
The vacuolated cells exclude trypan blue, which indicates that VacA-induced cell vacuolation is not a cytolethal phenomenon (15). Recently, VacA has been reported to promote the intracellular survival of H. pylori through the retention of Rab7 in the interference with the full maturation of vacuoles (34). In order to examine the effect of VAMP7 on VacA-induced vacuolation, we tried to generate an AGS cell line stably transfected with either GFP-TiVAMP/VAMP7 or GFP-Nter-TiVAMP/VAMP7, but the transfected cells died within a few weeks. This suggests that the appropriate expression of VAMP7 is indispensable for AGS cells to survive.
In conclusion, we have herein shown that VAMP7 is involved in the molecular mechanism of VacA-induced vacuolation. These observations are therefore considered to provide new insights into the molecular pathogenesis of gastroduodenal disease induced by H. pylori.
Acknowledgments
This work was supported in part by grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology (to T.H.).
Editor: S. R. Blanke
Footnotes
Published ahead of print on 24 March 2008.
REFERENCES
- 1.Advani, R. J., B. Yang, R. Prekeris, K. C. Lee, J. Klumperman, and R. H. Scheller. 1999. VAMP-7 mediates vesicular transport from endosomes to lysosomes. J. Cell Biol. 146765-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonin, W., D. Fasshauer, S. Becker, R. Jahn, and T. R. Schneider. 2002. Crystal structure of the endosomal SNARE complex reveals common structural principles of all SNAREs. Nat. Struct. Biol. 9107-111. [DOI] [PubMed] [Google Scholar]
- 3.Antonin, W., C. Holroyd, D. Fasshauer, S. Pabst, G. F. Von Mollard, and R. Jahn. 2000. A SNARE complex mediating fusion of late endosomes defines conserved properties of SNARE structure and function. EMBO J. 196453-6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonin, W., C. Holroyd, R. Tikkanen, S. Honing, and R. Jahn. 2000. The R-SNARE endobrevin/VAMP-8 mediates homotypic fusion of early endosomes and late endosomes. Mol. Biol. Cell 113289-3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coco, S., G. Raposo, S. Martinez, J. J. Fontaine, S. Takamori, A. Zahraoui, R. Jahn, M. Matteoli, D. Louvard, and T. Galli. 1999. Subcellular localization of tetanus neurotoxin-insensitive vesicle-associated membrane protein (VAMP)/VAMP7 in neuronal cells: evidence for a novel membrane compartment. J. Neurosci. 199803-9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cover, T. L., and S. R. Blanke. 2005. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat. Rev. 3320-332. [DOI] [PubMed] [Google Scholar]
- 7.de Bernard, M., M. Moschioni, A. Habermann, G. Griffiths, and C. Montecucco. 2002. Cell vacuolization induced by Helicobacter pylori VacA cytotoxin does not depend on late endosomal SNAREs. Cell. Microbiol. 411-18. [DOI] [PubMed] [Google Scholar]
- 8.Figueiredo, C., J. C. Machado, and Y. Yamaoka. 2005. Pathogenesis of Helicobacter pylori infection. Helicobacter 10(Suppl. 1)14-20. [DOI] [PubMed] [Google Scholar]
- 9.Fujikawa, A., D. Shirasaka, S. Yamamoto, H. Ota, K. Yahiro, M. Fukada, T. Shintani, A. Wada, N. Aoyama, T. Hirayama, H. Fukamachi, and M. Noda. 2003. Mice deficient in protein tyrosine phosphatase receptor type Z are resistant to gastric ulcer induction by VacA of Helicobacter pylori. Nat. Genet. 33375-381. [DOI] [PubMed] [Google Scholar]
- 10.Genisset, C., A. Puhar, F. Calore, M. de Bernard, P. Dell'Antone, and C. Montecucco. 2007. The concerted action of the Helicobacter pylori cytotoxin VacA and of the v-ATPase proton pump induces swelling of isolated endosomes. Cell. Microbiol. 91481-1490. [DOI] [PubMed] [Google Scholar]
- 11.Hay, J. C. 2001. SNARE complex structure and function. Exp. Cell Res. 27110-21. [DOI] [PubMed] [Google Scholar]
- 12.Hay, J. C., and R. H. Scheller. 1997. SNAREs and NSF in targeted membrane fusion. Curr. Opin. Cell Biol. 9505-512. [DOI] [PubMed] [Google Scholar]
- 13.Kanzaki, M., Y. Q. Zhang, H. Mashima, L. Li, H. Shibata, and I. Kojima. 1999. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat. Cell Biol. 1165-170. [DOI] [PubMed] [Google Scholar]
- 14.Lafont, F., P. Verkade, T. Galli, C. Wimmer, D. Louvard, and K. Simons. 1999. Raft association of SNAP receptors acting in apical trafficking in Madin-Darby canine kidney cells. Proc. Natl. Acad. Sci. USA 963734-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leunk, R. D., P. T. Johnson, B. C. David, W. G. Kraft, and D. R. Morgan. 1988. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J. Med. Microbiol. 2693-99. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Arca, S., P. Alberts, A. Zahraoui, D. Louvard, and T. Galli. 2000. Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth. J. Cell Biol. 149889-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mashima, H., S. Yamada, T. Tajima, M. Seno, H. Yamada, J. Takeda, and I. Kojima. 1999. Genes expressed during the differentiation of pancreatic AR42J cells into insulin-secreting cells. Diabetes 48304-309. [DOI] [PubMed] [Google Scholar]
- 18.Molinari, M., C. Galli, N. Norais, J. L. Telford, R. Rappuoli, J. P. Luzio, and C. Montecucco. 1997. Vacuoles induced by Helicobacter pylori toxin contain both late endosomal and lysosomal markers. J. Biol. Chem. 27225339-25344. [DOI] [PubMed] [Google Scholar]
- 19.Mullock, B. M., C. W. Smith, G. Ihrke, N. A. Bright, M. Lindsay, E. J. Parkinson, D. A. Brooks, R. G. Parton, D. E. James, J. P. Luzio, and R. C. Piper. 2000. Syntaxin 7 is localized to late endosome compartments, associates with Vamp 8, and is required for late endosome-lysosome fusion. Mol. Biol. Cell 113137-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagamatsu, S., Y. Nakamichi, T. Watanabe, S. Matsushima, S. Yamaguchi, J. Ni, E. Itagaki, and H. Ishida. 2001. Localization of cellubrevin-related peptide, endobrevin, in the early endosome in pancreatic beta cells and its physiological function in exo-endocytosis of secretory granules. J. Cell Sci. 114219-227. [DOI] [PubMed] [Google Scholar]
- 21.Nakayama, M., M. Kimura, A. Wada, K. Yahiro, K. Ogushi, T. Niidome, A. Fujikawa, D. Shirasaka, N. Aoyama, H. Kurazono, M. Noda, J. Moss, and T. Hirayama. 2004. Helicobacter pylori VacA activates the p38/activating transcription factor 2-mediated signal pathway in AZ-521 cells. J. Biol. Chem. 2797024-7028. [DOI] [PubMed] [Google Scholar]
- 22.Papini, E., M. de Bernard, E. Milia, M. Bugnoli, M. Zerial, R. Rappuoli, and C. Montecucco. 1994. Cellular vacuoles induced by Helicobacter pylori originate from late endosomal compartments. Proc. Natl. Acad. Sci. USA 919720-9724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papini, E., B. Satin, C. Bucci, M. de Bernard, J. L. Telford, R. Manetti, R. Rappuoli, M. Zerial, and C. Montecucco. 1997. The small GTP binding protein rab7 is essential for cellular vacuolation induced by Helicobacter pylori cytotoxin. EMBO J. 1615-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peek, R. M., Jr., and M. J. Blaser. 2002. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer 228-37. [DOI] [PubMed] [Google Scholar]
- 25.Pryor, P. R., B. M. Mullock, N. A. Bright, M. R. Lindsay, S. R. Gray, S. C. Richardson, A. Stewart, D. E. James, R. C. Piper, and J. P. Luzio. 2004. Combinatorial SNARE complexes with VAMP7 or VAMP8 define different late endocytic fusion events. EMBO Rep. 5590-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricci, V., A. Galmiche, A. Doye, V. Necchi, E. Solcia, and P. Boquet. 2000. High cell sensitivity to Helicobacter pylori VacA toxin depends on a GPI-anchored protein and is not blocked by inhibition of the clathrin-mediated pathway of endocytosis. Mol. Biol. Cell 113897-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothman, J. E. 1994. Mechanisms of intracellular protein transport. Nature 37255-63. [DOI] [PubMed] [Google Scholar]
- 28.Sollner, T., M. K. Bennett, S. W. Whiteheart, R. H. Scheller, and J. E. Rothman. 1993. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 75409-418. [DOI] [PubMed] [Google Scholar]
- 29.Steegmaier, M., J. Klumperman, D. L. Foletti, J. S. Yoo, and R. H. Scheller. 1999. Vesicle-associated membrane protein 4 is implicated in trans-Golgi network vesicle trafficking. Mol. Biol. Cell 101957-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steegmaier, M., K. C. Lee, R. Prekeris, and R. H. Scheller. 2000. SNARE protein trafficking in polarized MDCK cells. Traffic (Copenhagen) 1553-560. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki, J., H. Ohnishi, A. Wada, T. Hirayama, H. Ohno, N. Ueda, H. Yasuda, T. Iiri, Y. Wada, M. Futai, and H. Mashima. 2003. Involvement of syntaxin 7 in human gastric epithelial cell vacuolation induced by the Helicobacter pylori-produced cytotoxin VacA. J. Biol. Chem. 27825585-25590. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki, J., H. Ohnsihi, H. Shibata, A. Wada, T. Hirayama, T. Iiri, N. Ueda, C. Kanamaru, T. Tsuchida, H. Mashima, H. Yasuda, and T. Fujita. 2001. Dynamin is involved in human epithelial cell vacuolation caused by the Helicobacter pylori-produced cytotoxin VacA. J. Clin. Investig. 107363-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Telford, J. L., P. Ghiara, M. Dell'Orco, M. Comanducci, D. Burroni, M. Bugnoli, M. F. Tecce, S. Censini, A. Covacci, Z. Xiang, et al. 1994. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J. Exp. Med. 1791653-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terebiznik, M. R., C. L. Vazquez, K. Torbicki, D. Banks, T. Wang, W. Hong, S. R. Blanke, M. I. Colombo, and N. L. Jones. 2006. Helicobacter pylori VacA toxin promotes bacterial intracellular survival in gastric epithelial cells. Infect. Immun. 746599-6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wade, N., N. J. Bryant, L. M. Connolly, R. J. Simpson, J. P. Luzio, R. C. Piper, and D. E. James. 2001. Syntaxin 7 complexes with mouse Vps10p tail interactor 1b, syntaxin 6, vesicle-associated membrane protein (VAMP)8, and VAMP7 in b16 melanoma cells. J. Biol. Chem. 27619820-19827. [DOI] [PubMed] [Google Scholar]
- 36.Ward, D. M., J. Pevsner, M. A. Scullion, M. Vaughn, and J. Kaplan. 2000. Syntaxin 7 and VAMP-7 are soluble N-ethylmaleimide-sensitive factor attachment protein receptors required for late endosome-lysosome and homotypic lysosome fusion in alveolar macrophages. Mol. Biol. Cell 112327-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yahiro, K., T. Niidome, M. Kimura, T. Hatakeyama, H. Aoyagi, H. Kurazono, K. Imagawa, A. Wada, J. Moss, and T. Hirayama. 1999. Activation of Helicobacter pylori VacA toxin by alkaline or acid conditions increases its binding to a 250-kDa receptor protein-tyrosine phosphatase beta. J. Biol. Chem. 27436693-36699. [DOI] [PubMed] [Google Scholar]
- 38.Zeng, Q., V. N. Subramaniam, S. H. Wong, B. L. Tang, R. G. Parton, S. Rea, D. E. James, and W. Hong. 1998. A novel synaptobrevin/VAMP homologous protein (VAMP5) is increased during in vitro myogenesis and present in the plasma membrane. Mol. Biol. Cell 92423-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]