Abstract
We investigated the effect of an extract from a helminth (Ascaris suum) in zymosan-induced arthritis (ZYA) or collagen-induced arthritis (CIA). Rats and mice, respectively, received 1 mg and 0.1 mg zymosan intra-articularly (i.a.). Test groups received an A. suum extract either per os (p.o.) or intraperitoneally (i.p.) 30 min prior to i.a. zymosan. Controls received saline. Hypernociception was measured using the articular incapacitation test. Cell influx, nitrite, and cytokine levels were assessed in joint exudates. The synovia and distal femoral extremities were used for histopathology. Cartilage damage was assessed through determining glycosaminoglycan (GAG) content. DBA/1J mice were subjected to CIA. The test group received A. suum extract i.p. 1 day after CIA became clinically detectable. Clinical severity and hypernociception were assessed daily. Neutrophil influx was determined using myeloperoxidase activity. The A. suum extract, either i.p. or p.o., significantly and dose-dependently inhibited cell influx and hypernociception in ZYA in addition to reducing GAG loss and ameliorating synovitis. The A. suum extract reduced i.a. levels of NO, interleukin-1β (IL-1β), and IL-10 but not tumor necrosis factor alpha (TNF-α) in rats subjected to ZYA while reducing i.a. IL-10, but not IL-1β or TNF-α, levels in mice. Clinically, mice subjected to CIA treated with the A. suum extract had less severe arthritis. Hypernociception, myeloperoxidase activity, and synovitis severity were significantly reduced. These data show that a helminth extract given p.o. protects from arthritis severity in two classical arthritis models. This A. suum effect is species independent and functions orally and parenterally. The results show clinical and structural benefits when A. suum extract is given either prophylactically or therapeutically.
Genetic influences have been linked to disease susceptibility and/or severity in autoimmunity (18). However, the relatively low concordance rate in the incidence of these diseases among twins suggests that environmental stimuli play a role in these mechanisms (18). In this context, microorganisms have been linked to the development of arthritis. One possible mechanism involves bacterium-derived antigens. After being processed in the gut and released into the circulation, these antigens would be entrapped in joints, where, after the activation of resident cells, an inflammatory response is triggered (33).
An increased incidence of allergic and autoimmune diseases in highly industrialized countries has been associated with a lower prevalence of infections (2). Less exposure to house dust, high ingestion of industrialized food, diminished breast feeding, and increased vaccination would decrease stimulation to “real-life” germs. Also, the specific protection offered by vaccines would not appropriately prime the immune system, altering the T-cell repertoire (26). Less exposure to helminths, secondary to these lifestyle changes, has been linked to increased incidence of immune-mediated diseases (13). It was proposed that a shift of the balance from a T-helper 1 (Th1) into a Th2 response provoked by fewer infections could explain the increased prevalence of atopy and autoimmunity in wealthy populations (2). More recent data showing that helminths or their products are able to alter both the function and formation of regulatory T cells added to the complexity of this issue (19).
It has been shown that delay in diagnosis, difficulties in access to a specialist, poor compliance with treatment, and failure or delay in the indication of disease-modifying compounds impact the prevalence and severity of autoimmune diseases (24). Therefore, patients from low-income countries should display increased morbidity and/or mortality. However, the prevalence of rheumatoid arthritis is higher in developed countries (1) than that in populations living in tropical climates (3, 17, 27, 38). Regarding severity, a recent report has shown that systemic lupus erythematosus patients from the northeast part of Brazil, where intestinal helminth infections are highly prevalent, had similar organ damage to data reported from developed countries (38), despite the difficulties in access to appropriate treatment.
A role for helminths in the modulation of inflammation has long been proposed (12). For example, Schistosoma worms or their components ameliorated experimental allergic encephalomyelitis, diabetes, and thyroiditis (12). Protozoa may also be similarly involved, since viable Trypanosoma brucei decreased inflammation in collagen-induced arthritis (CIA) (20). In addition, ES-62, a glycoprotein derived from a filarial nematode, was shown to suppress disease severity in the CIA model in mice (21).
Considering these epidemiological data and the fact that helminths may modulate immunoinflammatory reactions, we investigated the effect of an Ascaris suum extract in experimental arthritis. This extract was shown to display in vitro anti-inflammatory properties (29). Our present data reveal that the administration of the A. suum extract provides a remarkable amelioration in clinical and structural parameters in two arthritis models, associated with decreased release of the inflammatory mediators nitric oxide (NO), interleukin-1 (IL-1), and IL-10 into the joints of rats. The extract was equally effective in both rats and mice when given intraperitoneally (i.p.) or per os (p.o.), both prophylactically and therapeutically. These data are an experimental proof-of-concept study that oral stimulation by helminths influences the pathogenesis of autoimmune arthritis.
MATERIALS AND METHODS
Animals.
Male Wistar rats (180 to 200 g) or Swiss mice (25 to 30 g) from our own animal facilities were used throughout for the zymosan-induced arthritis (ZYA) experiments. CIA was induced in DBA/1J male mice. The experimental protocols were approved by the ethical review boards on animal experimentation at the Faculty of Medicine of the Federal University of Ceará and the Faculty of Medicine of Ribeirão Preto, University of São Paulo—Ribeirão Preto, São Paulo, Brazil. For all experiments, we used six animals per group.
Induction of ZYA.
Rats or mice were subjected to intra-articular (i.a.) injection of either 1 mg (50 μl total volume) or 0.1 mg (25 μl total volume) of zymosan (Sigma, St. Louis, MO), respectively, dissolved in sterile saline, into the right knee joints. Control groups received only saline i.a.
Induction and assessment of CIA.
Male DBA/1J mice (12 to 14 weeks old) received 200 μg of bovine type II collagen (CII) intradermally in complete Freund's adjuvant (day 0). A second injection of CII (200 μg in phosphate-buffered saline [PBS]) was given i.p. on day 21. Mice were monitored daily for signs of arthritis, according to the following severity scores: 0, normal; 1, erythema; 2, erythema plus swelling; and 3, extension/loss of function. The total score was the sum of four limbs. The number of arthritic paws was evaluated daily, and mechanical hypernociception was assessed using the paw pressure test (9). Disease onset characterized by erythema and/or paw swelling was typically seen between days 25 and 35 after CII injection. Paw thickness was measured with a plethysmometer (Ugo Basile, Italy) before the CIA induction (Vo) and daily starting after the beginning of the disease (VT). The difference between VT and Vo was taken as the edema value, expressed in mm3.
Evaluation of joint hypernociception.
We used the rat knee joint incapacitation test, as described previously (25). Briefly, after zymosan injection, rats were made to walk on a steel rotary drum that rotates at 3 rpm. Specially designed metal gaiters were wrapped around both hind paws. After placement of the gaiters, the animals were allowed to walk freely for habituation. The right paw was connected to a microcomputer data input/output port. The paw elevation time (PET) is the time during a 60-s period that the inflamed hind paw is not in contact with the cylinder. This is assumed to reflect joint hypernociception. The PET was measured at baseline and then hourly, until sacrifice, at 6 h. Results (s/1 min) are reported as the maximal PET between 3 and 4 h after injection of the zymosan.
Collection of synovial exudates and analysis of cell influx, cytokines, and NO levels in the joint cavity.
At 6 h after injection of the zymosan, the animals were anesthetized with chloral hydrate (400 mg/kg i.p.), killed by cervical dislocation, and exsanguinated. The synovial cavity of the knee joints was then washed with 0.4 ml (rats) or 0.1 ml (mice) saline containing 10 mM EDTA. The synovial exudates were collected by aspiration, and total cell counts were performed using a Neubauer chamber. After centrifugation (500 g/10 min), the supernatants were stored at −70°C and used for determination of cytokine release. The concentrations of tumor necrosis factor alpha (TNF-α), IL-1β, and IL-10 in synovial exudates obtained 6 h after zymosan injection into rats and mice were measured by enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well microtiter plates (Nunc Immunoplates) were coated overnight at 4°C with immunoaffinity-purified polyclonal antibodies against the respective cytokines. These antibodies were provided by Steve Poole (National Institute for Biological Standards and Control, United Kingdom). After blocking the plates (1% albumin for 1 h), concentrations of cytokines and samples were loaded in duplicate for 2 h (22°C). A secondary rabbit biotinylated immunoaffinity-purified antibody was added, followed by incubation for 1 h (22°C). Finally, 100 μl of avidin-horseradish peroxidase (1:5,000 dilution; DAKO A/S, Denmark) was added to each well; after 30 min, the plates were washed and the color reagent o-phenylenediamine (40 μg/well) was added. After 15 min, the reaction was stopped with 1 M H2SO4 and the optical density was measured at 490 nm. Cytokine concentration was expressed as pg/ml. Total NO2−/NO3−, as a measure of NO levels, was determined using a commercially available kit (Cayman Chemical Co.). Briefly, the NO3− in the synovial exudate supernatants (0.08 ml) is converted to NO2− by incubation with 0.01 ml nitrate reductase from Aspergillus species (1 U/ml) and 0.01 ml NADPH (1 mM) for 30 min at 37°C. NO2− levels were determined spectrophotometrically at 540 nm by comparing the absorbance of 0.1 ml sample after adding 0.1 ml Griess reagent [1% (wt/vol) sulfanilic acid and 0.1% (wt/vol) N-(1-naphythyl) ethylenediamine in 5% phosphoric acid] to that of an NaNO2 standard.
Determination of neutrophil accumulation in the hind paws.
In CIA, myeloperoxidase (MPO) activity was used as an index of neutrophil accumulation in joint tissues, as previously described (6). Briefly, the mouse paws were collected in 50 mM K2HPO4 buffer (pH 6.0) containing 0.5% hexadecyl trimethylammonium bromide and kept at −80°C until use. Samples were homogenized using a Polytron (PT3100) and centrifuged at 16,100 × g for 4 min, and the resulting supernatant was assayed spectrophotometrically for MPO activity determination at 450 nm (Spectra max), with three readings in 1 min. The MPO activity of samples was compared to a standard curve with the MPO activity obtained from a known number of neutrophils collected from the peripheral blood of mice. Ten microliters of sample was mixed with 200 μl of 50 mM phosphate buffer (pH 6.0) containing 0.167 mg/ml O-dianisidine dihydrochloride and 0.0005% hydrogen peroxide. The results were expressed as the MPO activity that reflects the number of neutrophils/mg tissue.
Synovial histopathology.
After sacrifice, the synovia were excised, fixed in 10% buffered formaldehyde, and processed for routine hematoxylin-eosin (HE) staining. Semiquantitative histopathological grading was performed by an independent pathologist blinded to group allocation according to the presence of edema, synovial proliferation, cell infiltration, and necrosis, ranging from 0 to 3 (0, absent; 1, mild; 2, moderate; 3, severe), for each parameter. The maximal total score was 12. Results are expressed as the median (variation) value for each group of six animals.
Assessment of articular cartilage damage.
The glycosaminoglycan (GAG) content of the articular cartilage was determined as follows. The cartilage of the distal femoral extremities was excised. The samples were weighed after drying overnight at 80°C. The material was subjected to proteolysis using Prolav 750 (Prozyn, SP, Brazil) and further precipitation in absolute ethanol, followed by dilution in distilled water. After this protocol, digestion with specific chondroitinases identified chondroitin sulfate as the sole GAG present in the samples. This material was separated by 0.6% agarose gel electrophoresis. After staining with 0.1% toluidine blue, quantitation was made by densitometry (525 nm). For comparison, standards of chondroitin 4-sulfate, chondroitin 6-sulfate, and heparan sulfate were subjected to the same protocol. Data were expressed as μg GAG/mg of dried cartilage.
Preparation of the A. suum extract.
Adult A. suum worms were obtained from the small intestine of pigs from a local abattoir, Frigorífico FISA, Itapecerica da Serra, São Paulo, Brazil, and prepared as described previously (31). Briefly, worms were washed with saline, minced, and homogenized in PBS (pH 7.2). This material was then centrifuged (12,000 × g for 90 min) at 10°C, and the supernatant was discarded. The precipitate was resuspended again in PBS, under agitation, for 18 h at 4°C. This material was centrifuged (12,000 × g for 90 min) at 10°C, and the supernatant was dialyzed for 48 h against distilled water. The material was then aliquoted, lyophilized, and stored at 4°C, until use.
Characterization of components in the A. suum extract.
As an initial attempt to identify proteins present in the A. suum extract, dilutions were run on a silver-stained gradient (8 to 25%) polyacrylamide gel electrophoresis. The presence of charged carbohydrates was investigated by separating the A. suum extract by 0.6% (wt/vol) agarose gel electrophoresis in diaminopropane acetate buffer (50 mM [pH 9.0]). This gel was stained with a 0.1% (wt/vol) toluidine blue solution (5). For comparison, high- and low-molecular-weight standards (Pharmacia Biotech, United Kingdom) as well as standard chondroitin 4-sulfate and chondroitin 6-sulfate (Sigma, St. Louis, MO) were subjected to the same protocols, as indicated.
Treatments.
Test groups received the A. suum extract, dissolved in sterile saline, either i.p. or p.o. 30 min prior to the i.a. injection of zymosan. The amount of extract to be administered was based on protein content, using the Bradford method. It has been shown that injection of 1 mg of A. suum extract was able to suppress immune responses in mice (30). After obtaining a dose-response curve in rats, the concentration of 1 mg of protein was chosen to use for both rats and mice. The experiments done in the ZYA model were considered as prophylactic interventions, whereas in the CIA, DBA/1J mice were treated with the A. suum extract i.p. or vehicle 1 day after CIA became clinically detectable. The latter group was considered as therapeutic. In order to test for the safety of the administration of the A. suum extract, a group of animals received only the extract. The groups treated with the extract were compared to other groups that received saline either i.p. or p.o. followed by the zymosan i.a. In order to rule out the effect of an irrelevant protein, a group of animals subjected to the ZYA model received 1 mg bovine serum albumin (BSA), either i.p. or p.o.
Statistical analysis.
Unless otherwise stated, results are presented as means ± standard errors of the mean (SEM) or for histology as medians (range). Differences between groups were analyzed using one-way analysis of variance followed by Tukey′s test or Mann-Whitney test, as appropriate. In CIA experiments, two-way analysis of variance was performed. P < 0.05 was considered significant.
RESULTS
A. suum extract reduced cell influx and hypernociception in acute ZYA in rats.
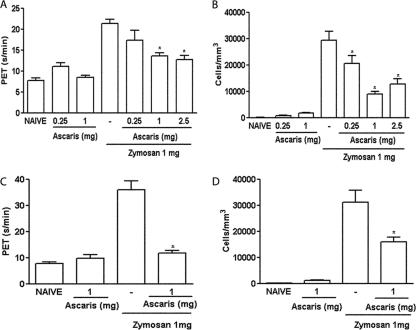
Control rats with ZYA displayed hypernociception and intense cell influx into the joint cavity that was most prominent 6 h after injection of zymosan (25). Both the i.p. and oral administration of the A. suum extract significantly and dose-dependently reduced hypernociception (Fig. 1A and C) as well as the acute (Fig. 1B and D) cell influx, compared to that in vehicle-treated animals. The i.p. injection of 1 mg BSA did not alter these parameters in rats subjected to ZYA (data not shown). The effects achieved following the administration of the A. suum extract were similar whether given p.o. or i.p. Oral administration of the A. suum extract to control (naïve) rats (Fig. 1A to D) did not alter hypernociception or cell influx. Additionally, 1 mg BSA given as an irrelevant protein either p.o. or i.p. did not alter hypernociception or cell influx (data not shown). In order to exclude endotoxin contamination, we should stress that both the extract and BSA were prepared under sterile conditions and filtered before administration. Additionally, incubation of the A. suum extract with polymyxin B did not affect its inhibitory effect on ZYA hypernociception (data not shown). Moreover, the oral activity of the extract per se excludes the possibility of endotoxin contamination being responsible for the effects observed.
FIG. 1.
Effect of the parenteral and oral administration of the A. suum extract (Ascaris) on the hypernociception and cell influx in ZYA in rats. Rats received the A. suum extract or saline (−) either i.p. (A and B) or p.o. (C and D) 30 min prior to 1 mg zymosan i.a. Naive rats received saline i.a. Articular incapacitation was measured hourly over 4 h as the increase in PET using the rat knee joint incapacitation test. Cell infiltration into joint cavities was assessed 6 h after injection of zymosan. Results show means ± SEM of maximal PET between 3 and 4 h of arthritis (A and C) and means ± SEM of total leukocytes (B and D) (n = 6 animals for each group). Experiments were done three times. *, P < 0.05 compared to control (−) rats.
A. suum extract reduced chronic cell influx and prevented cartilage damage in ZYA in rats.
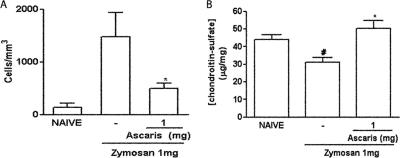
Administration of the A. suum extract significantly reduced the cell influx into the joints of rats, 7 days after injection of the zymosan (Fig. 2A). At this phase, the numbers of cells are significantly lower, with mononuclear cells being predominant (25). Additionally, the A. suum extract prevented the GAG loss that occurs in ZYA (Fig. 2B), as compared to vehicle-treated animals.
FIG. 2.
Effect of the A. suum extract (Ascaris) in the chronic phase of ZYA in rats. Rats received the A. suum extract or saline (−) i.p. 30 min prior to 1 mg zymosan i.a. Naive animals received saline i.a. Cell infiltration into the joint cavities was assessed at 7 days (A). Results represent means ± SEM of total leukocytes. Cartilage damage was assessed by measuring the GAG content, using agarose gel electrophoresis and quantitation by densitometry (B). Results represent means ± SEM of GAG (μg/mg) *, P < 0.05 compared to control (−) rats (n = 6 animals for each group). The experiments were done two times. #, P < 0.05 compared to naive rats.
A. suum extract reduced cell influx in ZYA in mice.
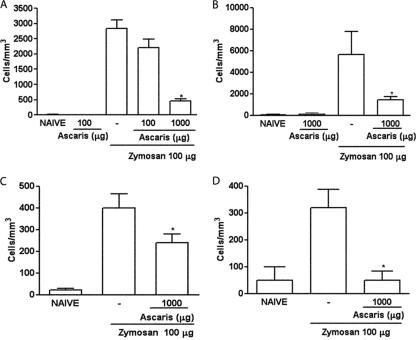
Similar to what was observed in rats, either the i.p. or the oral administration of the A. suum extract dose-dependently reduced cell influx into the joint cavities of mice subjected to ZYA in both the acute and chronic phases (Fig. 3A to D).
FIG. 3.
Effect of the parenteral and oral administration of the A. suum extract (Ascaris) on the acute and chronic cell influx in ZYA in mice. Mice received 1 mg of the A. suum extract or saline (−) either i.p. (A and C) or p.o. (B and D) 30 min prior to 0.1 mg zymosan i.a. Naive mice received saline i.a. Cell infiltration into joint cavities was assessed 6 h (A and B) or 7 days (C and D) after injection of zymosan. Results show means ± SEM of total leukocytes (n = 6 animals for each group). Experiments were done two times. *, P < 0.05 compared to control (−) mice.
A. suum extract reduced the histopathology changes of the synovia in ZYA in mice.
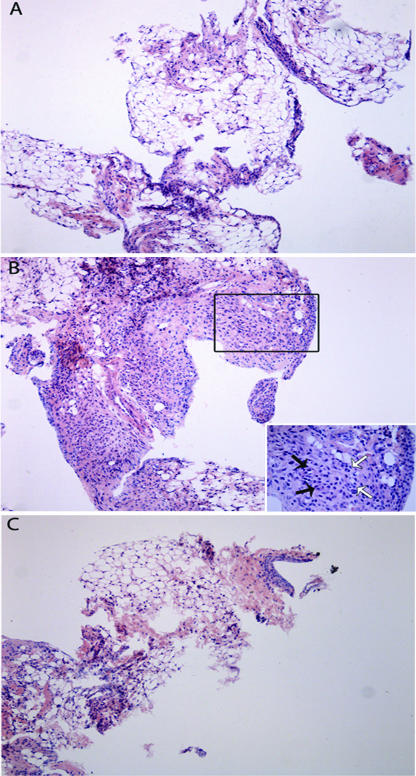
Pretreatment with the extract (Fig. 4C) almost completely abrogated the synovitis in rats subjected to ZYA, provoking a marked decrease in the number of infiltrating cells, as compared to the group that received zymosan and saline i.p. (Fig. 4B). A naïve synovial sample is shown for comparison (Fig. 4A). The semiquantitative analysis of these samples showed a significant reduction (P < 0.05) of the synovitis in the group that received the A. suum extract (median, 2; range, 1 to 2), as compared to saline-treated animals (median, 3.5; range, 2 to 5).
FIG. 4.
Representative illustration of the synovial histopathology of mice subjected to ZYA and treated with the A. suum extract. Mice received 1 mg of A. suum extract (C) or saline (B) given i.p. 30 min prior to 0.1 mg zymosan i.a. Naive mice (A) received saline i.a. All animals were sacrificed at 7 days. The synovia of animals that received just the zymosan display intense and diffuse mononuclear cell infiltration (B) that is clearly reduced in the mice that received the A. suum extract prior to Zy (C). The inset in panel B represents a closer view showing vascular neoformation (black arrows) and macrophage infiltration (white arrows). HE staining. Original magnification, ×100.
A. suum extract alters the release of inflammatory mediators in acute ZYA in rats.
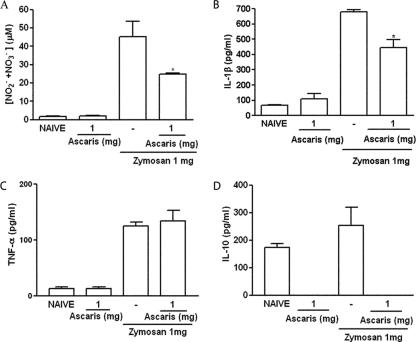
The A. suum extract significantly reduced NO as well as IL-1β levels released into the joints of rats subjected to ZYA (Fig. 5A and B, respectively), whereas levels of TNF-α (Fig. 5C) were not altered. We aimed to evaluate the effect of the A. suum extract on the in vivo levels of IL-10 in the ZYA model. However, levels of IL-10 were similar in the joints of rats subjected to ZYA to those of naïve joints. Surprisingly, treatment with the A. suum extract reduced IL-10 levels to below detection limits both in naïve animals and in those subjected to ZYA (Fig. 5D).
FIG. 5.
Effect of parenteral administration of A. suum extract (Ascaris) on the release of inflammatory mediators into the joint cavity in ZYA in rats. Rats received 1 mg of the A. suum extract or saline (−) i.p. 30 min prior to 1 mg zymosan i.a. Naive rats received saline i.a. or saline i.a. plus A. suum extract i.p. NO (A) was measured as total nitrite/nitrate, whereas IL-1β (B), TNF-α (C), and IL-10 (D) levels were assessed using ELISA (see text for details). Results represent means ± SEM, measured at 6 h (n = 6 animals for each group). Experiments were done two times. *, P < 0.05 compared to control (−) rats.
Effect of the A. suum extract on the release of cytokines in acute ZYA in mice.
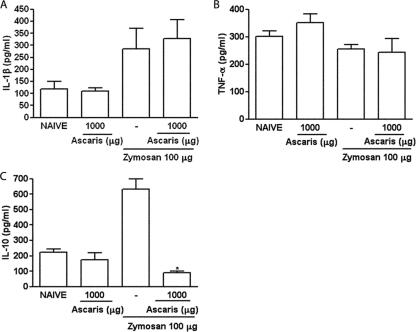
The A. suum extract given p.o. did not alter the levels of either IL-1β or TNF-α released into the joints of mice subjected to ZYA (Fig. 6A and B, respectively). It should be remarked that TNF-α levels were similar in both naïve mice and those subjected to ZYA. Similar to what was seen in rats, IL-10 levels were significantly reduced by pretreating mice subjected to ZYA with the A. suum extract (Fig. 6C).
FIG. 6.
Effect of oral administration of A. suum extract (Ascaris) on release of cytokines into the joint cavity in ZYA in mice. Mice received 1 mg of the A. suum extract or saline (−) p.o. 30 min prior to 0.1 mg zymosan i.a. Naive mice received only saline i.a. or saline i.a. plus A. suum extract p.o. IL-1β (A), TNF-α (B), and IL-10 (C) levels were assessed using ELISA (see text for details). Results represent means ± SEM, measured at 6 h (n = 6 animals for each group). Experiments were done two times. *, P < 0.05 compared to control (−) mice.
A. suum extract was effective in reducing the severity of CIA.
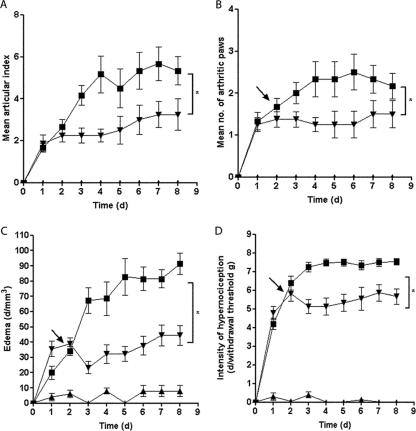
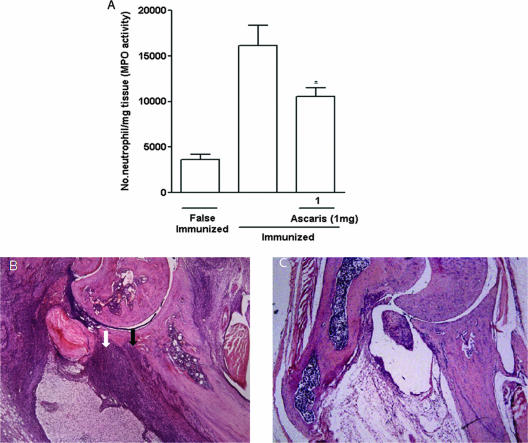
The A. suum extract produced a remarkable and significant amelioration of the inflammatory signs in mice subjected to CIA. There was a significant reduction of the articular index (Fig. 7A), number of inflamed paws (Fig. 7B), paw edema (Fig. 7C), and mechanical hypernociception (Fig. 7D), as compared to immunized animals that received the vehicle. These effects were associated with inhibition of the neutrophil influx, shown by the decrease in the MPO activity in the homogenized hind paws (Fig. 8A). At histopathology, a remarkable and significant (P < 0.05) improvement (65% fewer changes) in total scores was seen in the animals that received the A. suum extract (median, 3; range, 3) compared to mice subjected to CIA (median, 7.5; range, 7 to 8) receiving vehicle. A representative illustration of the synovial histology of an immunized mouse treated with the vehicle or the A. suum extract is shown in Fig. 8B and C, respectively.
FIG. 7.
Effect of A. suum extract on clinical score, number of arthritic paws, edema, and hypernociception in the CIA model. DBA/1J mice were sensitized to type II collagen, and the arthritis was evaluated clinically using a clinical score (A), number of arthritic paws (B), hind paw edema measured by plethysmometry (C), and hypernociception using the paw pressure test (D). Animals received 1 mg of the AS extract i.p. (▾), 1 day after the arthritis was clinically apparent (arrow). Falsely immunized animals (▴) were controls. Results represent means ± SEM (n = 6 animals for each group). Experiments were done two times. *, P < 0.05 compared to immunized mice (▪).
FIG. 8.
Effect of A. suum (Ascaris) extract on neutrophil influx and joint histopathology in the CIA model. DBA/1J mice were sensitized to type II collagen, and neutrophil influx was measured as MPO activity in the homogenized hind paws (A). Falsely immunized animals were controls. Results represent means ± SEM (n = 6 animals for each group). Experiments were done two times. *, P < 0.05 compared to immunized mice. Representative HE-stained sections of the ankle joints of mice subjected to CIA that received the vehicle show severe cell infiltration (white arrow), cartilage thinning, and pannus invasion (black arrow) (B), as compared to that of an animal that received 1 mg of A. suum extract i.p., which shows preservation of the cartilage and marked reduction in cell infiltration (C). Original magnification, ×40.
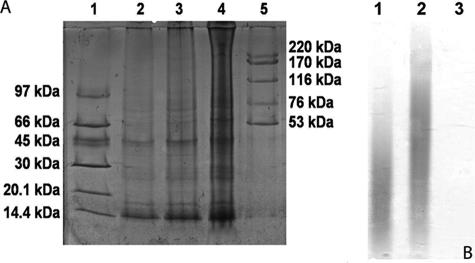
Preliminary characterization of components in the A. suum extract.
Figure 9A and B illustrate serious dilutions of a silver-stained 8 to 25% polyacrylamide gradient electrophoresis gel of the A. suum extract (Fig. 9A) to identify proteins and the results of toluidine blue-stained agarose gel electrophoresis (Fig. 9B) aimed to identify charged carbohydrates. As expected, there were a wide range of both low- and high-molecular-weight proteins shown in the silver-stained gel. Additional purification was needed in order to try to identify active components implicated in the protective effects of the A. suum extract. On the other hand, the absence of charged carbohydrates, as shown in lane 3 of Fig. 9B, virtually excludes such components as being responsible for the results achieved.
FIG. 9.
Identification of proteins and carbohydrates present in A. suum extract. Silver-stained 8 to 25% polyacrylamide gradient electrophoresis gel of A. suum extract (A) and a toluidine blue-stained 0.6% agarose gel for carbohydrate staining (B). Lane 1, low-molecular-mass markers (97-kDa phosphorylase b, 66-kDa albumin, 45-kDa ovalbumin, 30-kDa carbonic anhydrase, 20.1-kDa trypsin inhibitor; 14.4-kDa α-lactoalbumin); lanes 2, 3, and 4, 0.5, 1, and 2 μg of the A. suum extract, respectively; lane 5, high-molecular-mass markers (220-kDa thyroglobulin, 170-kDa ferritin, 116-kDa catalase, 76-kDa lactase dehydrogenase, and 53-kDa albumin). (B) Lane 1, chondroitin-4-sulfate; lane 2, chondroitin-6-sulfate; lane 3, 50 μg A. suum. Experiments were done three times.
DISCUSSION
The present study is the first report that a helminth extract, given p.o., has in vivo anti-inflammatory properties in experimental arthritis. The reduction of hypernociception, number of inflamed paws, and visual inflammatory score, assessed in two arthritis models, indicate clinical benefit. Damage to the articular cartilage using in vivo models can be assessed by directly quantifying its GAG content, which is reduced in ZYA compared to naïve animals (5). Virtual abrogation of the chronic synovitis, seen at histopathology, and prevention of damage to the articular cartilage indicate a protective effect of the A. suum extract also at the structural level. Demonstration of efficacy in different species and models is a crucial step in order to prove the therapeutic value of a compound. The results were dose dependent and not species related, working similarly in the acute and chronic phases and equally effective regardless of oral or parenteral (i.p.) administration.
DBA/1J mice subjected to CIA display less-inflammatory changes when pups are fed shortly postpartum with Streptococcus sanguis (7). Infection of rats with the protozoan responsible for African sleeping sickness (Trypanosoma brucei) partially prevented the development of collagen arthritis (20). More recently, it was shown that a secreted product from the nematode Acanthocheilonema vitae, named ES-62, comprising a phosphorylcholine-containing glycoprotein, provided clinical and histological amelioration of collagen arthritis in the DBA/1J mouse, while reducing the ex vivo production of the proinflammatory cytokines TNF-α, IL-6, as well as gamma interferon (21). Our present data add further information to these previous studies in a number of ways. First, we have demonstrated that the A. suum extract is active when administered orally. Second, the A. suum extract reduced the hypernociception and synovitis both prophylactically and therapeutically, indicating intervention could be effective across a wide clinical spectrum. We may also speculate that phosphorylcholine, present in the A. suum extract, similar to the molecule contained in ES-62 (21), would be responsible for the anti-inflammatory effects observed in the present study.
Multiple mechanisms are probably involved in the protection provided by the A. suum extract in arthritis. The reduction in the acute influx of neutrophils, leading to decreased release of lysosomal enzymes, metalloproteinases, and reactive oxygen/nitrogen species, probably contributes to the joint protection achieved. We detected reduced levels of NO in the joint exudates of animals treated with the A. suum extract. While NO per se appears to be beneficial to joint homeostasis (5, 10, 11), the combination of NO with other reactive species, such as the superoxide anion, may result in peroxynitrite formation and subsequent tissue lesion (23). We have recently demonstrated that neutrophils contribute to the formation of peroxynitrite in ZYA (4). Hence, the reduced formation of reactive oxygen species secondary to the reduced neutrophil cell influx may participate in the benefit provided by the A. suum extract in arthritis.
Down-modulation of synoviocytes and dendritic cells could also be involved in these phenomena. Using mice sensitized to ovalbumin, it has been demonstrated that the A. suum crude extract, prepared as used in the present study, or its affinity-purified high-molecular-weight components are able to down-modulate the antigen-processing ability of dendritic cells (28). In addition, induction of arginase could also explain the reduced levels of NO seen in the animals treated with the A. suum extract. Indeed, nematode-elicited macrophages produce less NO due to increased expression of arginase, leading to less substrate availability to the NO synthases (19).
The joint damage and cell influx observed in ZYA are closely related to the increase in the production of proinflammatory mediators such as TNF-α, IL-1β, and NO (5, 34, 35). In addition, IL-10 has been shown to possess anti-inflammatory properties (22). Measuring the level of these mediators in the synovial fluid rather than in serum or using ex vivo strategies may more appropriately reflect what is happening inside the joint. Trying to unravel a possible shift in the cytokine profile secondary to the A. suum extract administration, we investigated the levels of locally released cytokines. The extract reduced IL-1 levels in rats, whereas the levels of TNF-α were not altered in both species. Actually, TNF-α values were similar in mice subjected to ZYA compared to naive mice. This apparent discrepancy regarding IL-1 and TNF-α levels in rats and mice may at least partially be due to kinetics and species differences. TNF-α has been clearly implicated in joint damage in both experimental and human arthritis. However, most patients need combination therapy (e.g., anti-TNF-α antibodies with methotrexate) to achieve significant clinical benefit (16). Thus, inflammatory mediators other than TNF-α are operative in arthritis pathogenesis. Usually, cytokine production in experimental arthritis is evaluated using in vitro or ex vivo approaches. We cannot rule out that the A. suum extract alters the release of cytokines at other time points. Further studies are needed to explain these consistent immunomodulating effects promoted by the A. suum extract that appear to be independent of IL-1 and TNF-α release in the ZYA model.
Levels of IL-10 were similar in both zymosan-injected and naïve joints in rats, suggesting that this cytokine is not significantly involved in the early phase of ZYA in rats. However, levels of IL-10 were significantly higher in mice subjected to ZYA. Surprisingly, the A. suum extract decreased IL-10 levels to below detection limits in naïve rats as well as in both rats and mice subjected to ZYA. This is intriguing, since high-molecular-weight components of this extract were shown to down-regulate the presentation of antigens by dendritic cells via an IL-10-dependent mechanism (28). The fact that we measured IL-10 levels in vivo, whereas the results from reference 28 were obtained in vitro, may explain this discrepancy. Our data indicate that the A. suum effects are not due to an increase in IL-10.
Down-modulation of the humoral response could also explain the anti-inflammatory properties of the A. suum extract. In fact, we have shown previously that high-molecular-weight components of this extract inhibited ovalbumin-induced antibody (immunoglobulin G1 [IgG1], IgG2a, IgM, and IgE) production in mice (14).
Interference with innate immune response may be a relevant mechanism involved in the response to helminth infections. In this regard, lysophosphatidylserine molecules derived from adult Schistosoma mansoni worms were shown to couple to toll-like receptor 2 in dendritic cells (36). Since toll-like receptor 2 activation has been shown to modulate ZYA (15), the possibility that a similar mechanism explains the effects of the A. suum extract merits investigation.
There is some published evidence showing clinical benefit following the administration of either helminths, eggs, or their extracts in inflammatory diseases (12). A recent review focusing on this subject has summarized data showing the association of increased incidence of immune-mediated diseases (e.g., inflammatory bowel disease, multiple sclerosis, type 1 diabetes, and asthma) in areas where people are less exposed to helminths (13). In humans, the administration of viable Trichuris suis ova provided clinical amelioration in inflammatory bowel disease (32). However, sustained benefit required repeated administration, raising concerns about safety (37). Another report showed a decline in the clinical activity of patients with Crohn's disease that were inoculated with viable Necator americanus hookworms (8).
Apart from providing compelling proof-of-concept experimental evidence that helminths' products modulate joint inflammation, these data may have therapeutic implications. The present in vivo study is a proof of concept that a helminth extract provides both clinical and structural benefit in two arthritis models. The mechanisms involved are probably multifactorial, involving decreased IL-1β and NO production. The efficacy of this probably innocuous crude extract, as compared to living organisms, indicates that the mixture of components targets various pathways, the combination of them resulting in positive effects. Simple as it may seem, this may be a more reasonable approach than the strategy of finding a unique cytokine or gene target in these complex diseases. Our data show vividly that in real life the coexistence of helminth infections with a parasite load kept to a subclinical level may positively justify the lower incidence and severity of certain autoimmune rheumatic diseases in low- and middle-income countries. Unraveling these mechanisms may devise strategies for alternative and cost-effective therapeutic options in autoimmune diseases.
Acknowledgments
We are grateful to Jörg Heukelbach (Federal University of Ceará, Fortaleza, Brazil) and Richard Speare (James Cook University, Australia) for critically revising the manuscript. We thank Giuliana Bertozzi for the ELISA work and Fabiola Mestriner for the silver-stained gel.
The work was partially supported by a grant from CNPq and CAPES, Brazil.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 14 April 2008.
REFERENCES
- 1.Alamanos, Y., and A. A. Drosos. 2005. Epidemiology of adult rheumatoid arthritis. Autoimmun. Rev. 4130-136. [DOI] [PubMed] [Google Scholar]
- 2.Bach, J. F. 2002. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 347911-920. [DOI] [PubMed] [Google Scholar]
- 3.Beighton, P., L. Solomon, and H. A. Valkenburg. 1975. Rheumatoid arthritis in a rural South African Negro population. Ann. Rheum. Dis. 34136-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bezerra, M. M., S. D. Brain, V. C. Girao, S. Greenacre, J. Keeble, and F. A. Rocha. 2007. Neutrophils-derived peroxynitrite contributes to acute hyperalgesia and cell influx in zymosan arthritis. Naunyn Schmiedebergs Arch. Pharmacol. 374265-273. [DOI] [PubMed] [Google Scholar]
- 5.Bezerra, M. M., S. D. Brain, S. Greenacre, S. M. B. Jeronimo, L. B. de Melo, J. Keeble, and F. A. da Rocha. 2004. Reactive nitrogen species scavenging, rather than nitric oxide inhibition, protects from articular cartilage damage in rat zymosan-induced arthritis. Br. J. Pharmacol. 141172-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley, P. P., R. D. Christensen, and G. Rothstein. 1982. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood 60618-622. [PubMed] [Google Scholar]
- 7.Costalonga, M., J. S. Hodges, and M. C. Herzberg. 2002. Streptococcus sanguis modulates type II collagen-induced arthritis in DBA/1J mice. J. Immunol. 1692189-2195. [DOI] [PubMed] [Google Scholar]
- 8.Croese, J., J. O′Neil, J. Masson, S. Cook, W. Melrose, D. Pritchard, and R. Speare. 2005. A proof of concept study establishing Necator americanus in Crohn's patients and reservoir donors. Gut 55136-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunha, T. M., W. A. Verri, Jr., G. G. Vivancos, I. F. Moreira, S. Reis, C. A. Parada, F. Q. Cunha, and S. H. Ferreira. 2004. An electronic pressure-meter nociception paw test for mice. Braz. J. Med. Biol. Res. 37401-407. [DOI] [PubMed] [Google Scholar]
- 10.Da Rocha, F. A., and de A. J. Brum-Fernandes. 2002. Evidence that peroxynitrite affects human osteoblast proliferation and differentiation. J. Bone Miner. Res. 17434-442. [DOI] [PubMed] [Google Scholar]
- 11.Del Carlo, M., and R. F. Loeser. 2002. Nitric oxide-mediated chondrocyte cell death requires the generation of additional reactive oxygen species. Arthritis Rheum. 46394-403. [DOI] [PubMed] [Google Scholar]
- 12.Dunne, D. W., and A. Cooke. 2005. A worm's eye view of the immune system: consequences for evolution of human autoimmune diseases. Nat. Rev. Immunol. 5420-426. [DOI] [PubMed] [Google Scholar]
- 13.Elliott, D. E., R. W. Summers, and J. V. Weinstock. 2007. Helminths as governors of immune-mediated inflammation. Int. J. Parasitol. 37457-464. [DOI] [PubMed] [Google Scholar]
- 14.Faquim-Mauro, E. L., and M. S. Macedo. 1998. The immunosupressive activity of Ascaris suum is due to high molecular weight components. Clin. Exp. Immunol. 114245-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frasnelli, M. E., D. Tarussio, V. Chobaz-Peclat, N. Busso, and A. So. 2005. TLR2 modulates inflammation in zymosan-induced arthritis in mice. Arthritis Res. Ther. 75R370-R379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furst, D. E., F. C. Breedveld, J. R. Kalden, J. S. Smolen, G. R. Burmester, P. Emery, E. C. Keystone, M. H. Schiff, P. L. Van Riel, M. E. Weinblatt, and M. H. Weisman. 2006. Updated consensus statement on biological agents for treatment of rheumatic diseases. Ann. Rheum. Dis. 65iii2-iii15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenwood, B. M. 1968. Autoimmune disease and parasitic infections in Nigerians. Lancet ii380-382. [DOI] [PubMed] [Google Scholar]
- 18.Gregersen, P. K., and T. W. Behrens. 2006. Genetics of autoimmune diseases—disorders of immune homeostasis. Nat. Rev. Genet. 7917-928. [DOI] [PubMed] [Google Scholar]
- 19.Maizels, R. M., and M. Yazdanbakhsh. 2003. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat. Rev. Immunol. 3733-744. [DOI] [PubMed] [Google Scholar]
- 20.Mattsson, L., P. Larsson, H. Erlandsson-Harris, L. Klareskog, and R. A. Harris. 2000. Parasite-mediated down-regulation of collagen-induced arthritis (CIA) in DA rats. Clin. Exp. Immunol. 122477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McInnes, I. B., B. P. Leung, M. Harnett, J. A. Gracie, F. Y. Liew, and W. Harnett. 2003. A novel therapeutic approach targeting articular inflammation using the filarial nematode-derived phosphorylcholine-containing glycoprotein ES-62. J. Immunol. 1712127-2133. [DOI] [PubMed] [Google Scholar]
- 22.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19683-765. [DOI] [PubMed] [Google Scholar]
- 23.Pryor, W. A., K. N. Houk, C. S. Foote, J. M. Fukuto, L. J. Ignarro, G. L. Squadrito, and K. J. Davies. 2006. Free radical biology and medicine: it's a gas, man! Am. J. Physiol. 291R491-R511. [DOI] [PubMed] [Google Scholar]
- 24.Quinn, M. A., and P. Emery. 2005. Potential for altering rheumatoid arthritis outcome. Rheum. Dis. Clin. North Am. 31763-772. [DOI] [PubMed] [Google Scholar]
- 25.Rocha, F. A., A. G. Aragão, Jr., R. C. Oliveira, M. M. Pompeu, M. R. Vale, and R. A. Ribeiro. 1999. Periarthritis promotes gait disturbance in zymosan-induced arthritis in rats. Inflamm. Res. 48485-490. [DOI] [PubMed] [Google Scholar]
- 26.Rook, G. A., and J. L. Stanford. 1998. Give us this day our daily germs. Immunol. Today 19113-116. [DOI] [PubMed] [Google Scholar]
- 27.Silman, A. J., W. Ollier, S. Holligan, F. Birrell, A. Adebajo, M. C. Asuzu, W. Thomson, and L. Pepper. 1993. Absence of rheumatoid arthritis in a rural Nigerian population. J. Rheumatol. 20618-622. [PubMed] [Google Scholar]
- 28.Silva, S. R., J. F. Jacysyn, M. S. Macedo, and E. L. Faquim-Mauro. 2006. Immunosuppressive components of Ascaris suum down-regulate expression of costimulatory molecules and function of antigen-presenting cells via an IL-10-mediated mechanism. Eur. J. Immunol. 363227-3237. [DOI] [PubMed] [Google Scholar]
- 29.Soares, M. F., I. Mota, and M. S. Macedo. 1992. Isolation of Ascaris suum components which suppress IgE antibody responses. Int. Arch. Allergy Immunol. 9737-43. [DOI] [PubMed] [Google Scholar]
- 30.Souza, V. M. O., E. L. Faquim-Mauro, and M. S. Macedo. 2002. Extracts of Ascaris suum egg and adult worm share similar immunosuppressive properties. Braz. J. Med. Biol. Res. 3581-89. [DOI] [PubMed] [Google Scholar]
- 31.Strejan, G., and D. H. Campbell. 1967. Hypersensitivity to Ascaris antigens. I. Skin-sensitizing activity of serum fractions from guinea pigs sensitized to crude extracts. J. Immunol. 98893-900. [PubMed] [Google Scholar]
- 32.Summers, R. W., Jr. J. F. Urban, D. E. Elliott, R. Thompson, and J. V. Weinstock. 2005. Trichuris suis therapy in Crohn's disease. Gut 5487-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toivanen, P. 2003. Normal intestinal microbiota in the aetiopathogenesis of rheumatoid arthritis. Ann. Rheum. Dis. 62807-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van de Loo, F. A., L. A. B. Joosten, P. L. van Lent, O. J. Arntz, and W. B. van der Berg. 1995. Role of interleukin-1, tumor necrosis factor alpha, and iterleukin-6 in cartilage- and zymosan-induced arthritis. Arthritis Rheum. 38164-172. [DOI] [PubMed] [Google Scholar]
- 35.van de Loo, F. A., O. J. Arntz, F. H. van Enckevort, P. L. van Lent, and W. B. van den Berg. 1998. Reduced cartilage proteoglycan loss during zymosan-induced gonarthritis in NOS2-deficient mice and in anti-interleukin-1-treated wild-type mice with unabated joint inflammation. Arthritis Rheum. 41634-646. [DOI] [PubMed] [Google Scholar]
- 36.van Die, I., S. J. van Vliet, A. K. Nyame, R. D. Cummings, C. M. Bank, B. Appelmelk, T. B. Geijtenbeek, and Y. Van Kooyk. 2003. The dendritic cell specific C-type lectin DC-SING is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis-x. Glycobiology 13471-478. [DOI] [PubMed] [Google Scholar]
- 37.van Kruiningen, H. J., and A. B. West. 2005. Potential danger in the medical use of Trichuris suis for the treatment of inflammatory disease. Inflamm. Bowel Dis. 11515. [DOI] [PubMed] [Google Scholar]
- 38.Vilar, M. J., E. L. Bezerra, and E. L. Sato. 2005. Skin is the most frequently damaged system in recent-onset systemic lupus erythematosus in a tropical region. Clin. Rheumatol. 24377-380. [DOI] [PubMed] [Google Scholar]