Abstract
Streptococcus sanguinis is one of the pioneers in the bacterial colonization of teeth and is one of the most abundant species in the oral biofilm called dental plaque. S. sanguinis is also the most common viridans group streptococcal species implicated in infective endocarditis. To investigate the association of biofilm and endocarditis, we established a biofilm assay and examined biofilm formation with a signature-tagged mutagenesis library of S. sanguinis. Four genes that have not previously been associated with biofilm formation in any other bacterium, purB, purL, thrB, and pyrE, were putatively identified as contributing to in vitro biofilm formation in S. sanguinis. By examining 800 mutants for attenuation in the rabbit endocarditis model and for reduction in biofilm formation in vitro, we found some mutants that were both biofilm defective and attenuated for endocarditis. However, we also identified mutants with only reduced biofilm formation or with only attenuation in the endocarditis model. This result indicates that the ability to form biofilms in vitro is not associated with endocarditis virulence in vivo in S. sanguinis.
Streptococcus sanguinis is a normal inhabitant of the human oral cavity. Like most oral streptococci, it produces green or alpha-hemolysis on blood agar and is therefore categorized as one of the viridans group streptococci. In one current classification scheme, the viridans group streptococci are divided into five groups, with S. sanguinis, Streptococcus gordonii, and Streptococcus parasanguinis comprising one of these (26). S. sanguinis has no known direct role in oral disease but is often implicated in infective endocarditis. The viridans group streptococci are the most common cause of native-valve infective endocarditis, and among these bacteria, S. sanguinis is the most common (25, 48). Endocarditis is an infection of heart valves or the endocardium. In native-valve endocarditis, infecting bacteria adhere to “vegetations” composed of host components such as fibrin and platelets that form at sites with preexisting injuries (47). Even in the present era of antibiotic availability, endocarditis causes substantial morbidity and mortality. Recent retrospective studies have reported endocarditis mortality rates ranging from 12 to 46% (76). In addition, infective endocarditis is the fourth leading cause of life-threatening infectious disease syndromes (6).
S. sanguinis is also known as a pioneer colonizer in the formation of the oral biofilm known as dental plaque. Biofilms are formed by microorganisms in well-organized and cooperative communities covering biotic or abiotic surfaces in nature. The initiation of oral biofilm development is caused by the adhesion of primary microorganisms, such as streptococci, to a salivary glycoprotein-coated surface (33). The pioneer organisms provide a new surface and appropriate metabolic or other signals for the attachment of succeeding organisms in the biofilms (33).
The association of biofilm formation in vitro and endocarditis has been investigated in several laboratories. Some studies have suggested a lack of association. It was reported that most viridans group streptococci isolated from patients with endocarditis or with neutropenic bloodstream infections did not form biofilms in vitro (10 of 18 and 16 of 22, respectively) (64). Moreover, three strains of S. gordonii with mutations in comD, fruK, and pbp2b that did not form biofilms on fibronectin-coated plates in CDEN medium plus 0.015% yeast extract were found not to be attenuated for virulence in endocarditis (12). On the other hand, endocarditis involves the growth of colonies of bacteria within layers of extracellular matrix and therefore has properties of a biofilm infection (57). Also, biofilm formation has been proposed to be important for endocarditis in other studies. For example, a study showed that endocarditis isolates of Enterococcus faecalis produced significantly more biofilms than nonendocarditis isolates (46). In E. faecalis, a biofilm-defective ebp mutant was significantly attenuated in an endocarditis model (51). Therefore, the association of biofilm and endocarditis virulence remains in doubt. To determine whether the ability to form biofilms in vitro is associated with endocarditis virulence, we have examined biofilm formation with a signature-tagged mutagenesis (STM) library of S. sanguinis strain SK36. We present here the identification of several new genetic loci that are required for biofilm formation in S. sanguinis. We found some mutants that were both biofilm defective and reduced in virulence for endocarditis in a rabbit model. We also found additional mutants with biofilm deficiency but normal virulence. This suggests that biofilm formation is not associated with endocarditis virulence in S. sanguinis.
MATERIALS AND METHODS
Bacterial strain and medium.
S. sanguinis strain SK36, obtained from Mogens Kilian, was isolated from human dental plaque (31, 34). The complete genome sequence of this strain has been recently reported (77). The strain was routinely grown in an atmosphere of 10% H2, 10% CO2, and 80% N2 at 37°C in brain heart infusion broth (BHI; Difco Inc., Detroit, MI) supplemented with 1.5% (wt/vol) agar as described previously (55). Bacterial growth was determined with a FLUOstar plate reader (BMG Labtech, Offenburg, Germany).
Biofilm formation assay.
The biofilm formation assay used in this study was based on the method of O'Toole (54). Flat-bottom polystyrene microtiter plates (Nunc 269787; Nalge Nunc International, Rochester, NY) containing 100 μl of tested medium per well were inoculated with 1 μl of a culture of S. sanguinis SK36 or its mutants grown overnight in BHI without agitation. After 16 h of incubation at 37°C either aerobically or anaerobically, total bacterial growth was determined by measuring the absorbance at 450 nm of cultures in the wells with a FLUOstar microplate reader. Biofilm formation was quantified in parallel plates as follows. Media were decanted from wells, and the remaining planktonic cells were removed by rinsing with 200 μl of distilled H2O. Fifty microliters of 0.4% (wt/vol) crystal violet (CV) solution was added to each well. After 15 min, wells were rinsed three times with 200 μl of distilled H2O and air dried. The CV was solubilized in 95% ethanol, and the absorbance at 600 nm was measured with a FLUOstar plate reader.
Identification of interrupted genes by AP-PCR.
The arbitrary primed PCR (AP-PCR) technique was used to identify interrupted genes as described previously (20). Briefly, 10 ng of genomic DNA, 0.5 μM arbitrary primer, and 0.2 μM transposon primer were used for a first-round PCR. Cycling conditions were as follows: 95°C for 5 min, followed by six cycles of 95°C for 30 s, 30°C for 30 s, and 72°C for 1.5 min; 30 cycles of 95°C for 30 s, 45°C for 30 s, and 72°C for 2 min; and finally 72°C for 4 min. One-eighth of each purified PCR product was then used for a second-round PCR with the Arb2 primer and a nested transposon primer at 0.2 μM each. Cycling conditions were as follows: 95°C for 1 min, followed by 30 cycles of 95°C for 30 s, 52°C for 30 s, and 72°C for 2 min, and then 72°C for an additional 4 min. Second-round PCR products were sequenced with Arb2 and nested transposon primers.
Assessment of virulence in a rabbit endocarditis model.
Competitive-index (CI) assays were performed as described previously (55), except that the virulent parent strain was a derivative of SK36 marked with erythromycin (ERM) resistance (JFP36; L. S. Turner et al., unpublished data) rather than SK36. Briefly, JFP36 and a mutant strain were grown anaerobically in BHI at 37°C overnight and then coinoculated into 12.6 ml prewarmed BHI or biofilm medium (BM) (40) with 1% (wt/vol) sucrose. Incubation was continued for 3 h. Cells were washed and suspended in phosphate-buffered saline to an optical density at 600 nm of 0.8. Five hundred microliters was inoculated into the peripheral ear veins of rabbits which had catheters placed over the aortic heart valve through the carotid artery as described previously (55). Dilutions of the inocula were plated by a two-layer plating technique, with the top layer containing 20 μg/ml ERM or 10 μg/ml chloramphenicol (CM). The day after inoculation, rabbits were sacrificed and heart valve vegetations were collected, homogenized, serially diluted, and incorporated into layer plates for enumeration as described above. Ratios of mutant to wild-type strain CFU counts were determined for the inoculum and for each vegetation from the geometric mean of colony numbers obtained from three plates each. The CI was determined as the mutant/wild-type ratio of the homogenate divided by the mutant/wild-type ratio of the inoculum. Paired t tests were used to determine whether CI values were significantly different from 1, with α = 0.05. All strains were tested in three or four animals each.
An individual inoculation study was performed as for the CI assays, by using the following procedure. Approximately 108 CFU of strain JFP36 and mutant 8-20 were each inoculated into separate rabbits (four each). Heart valve vegetations were homogenized and surface plated on BHI agar containing ERM or CM. The differences in log10 CFU counts were evaluated by t test. All animal studies received Institutional Animal Care and Use Committee approval and complied with all relevant federal guidelines and institutional policies.
Sequence analysis and database search.
The PCR amplicons were sequenced at the Nucleic Acids Research Facilities of Virginia Commonwealth University with a fluorescent dye terminator kit (ABI, Foster City, CA). DNA sequences were analyzed with SeqMan II software (DNASTAR Inc., Madison, WI). Sequences were searched against the completed S. sanguinis genome (77) with BLAST. Putative gene functions were obtained by searching proteins against the annotated genome (www.sanguinis.mic.vcu.edu/).
RESULTS
Establishment of a biofilm formation screening system for S. sanguinis.
Several approaches have been used for the identification of biofilm formation genes. One of the most efficient and fruitful approaches has been to screen for biofilm-defective mutants on abiotic surfaces such as polystyrene microtiter plates. This method can be used to screen large numbers of mutants simultaneously and has been successfully used for the identification of novel biofilm formation genes in Staphylococcus epidermidis (30), Escherichia coli (62), and Pseudomonas fluorescens (53). It has also been used for oral streptococci such as S. mutans (78), S. gordonii (40), and S. parasanguinis (27). We essentially applied to S. sanguinis the biofilm formation conditions used for screening biofilm mutants in S. gordonii Challis and S. parasanguinis FW213 as previously described (27, 40). To establish the optimal experimental system for screening biofilm-defective mutants, the biofilm formation of S. sanguinis was first examined under different growth conditions.
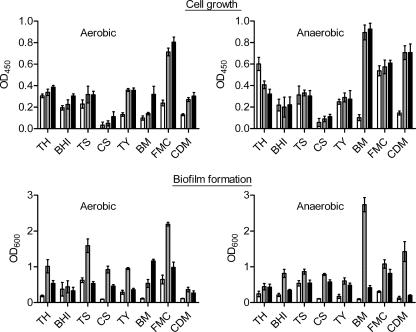
Eight different media that have been used for streptococcal growth or biofilm formation were examined in the S. sanguinis biofilm assay, including BHI (60), BM (40), chemically defined medium (78), complete saliva (63), FMC medium (69), Todd-Hewitt broth (27), Trypticase soy broth (27), and tryptone-yeast extract medium (18). To examine the effect of sucrose or glucose on biofilm formation, all media were supplemented with 1% (wt/vol) glucose or sucrose.
We found that S. sanguinis formed uniform biofilms at the bottom of the microplate wells. Biofilm formation and growth were quantified as described in Materials and Methods (Fig. 1). The results indicated that S. sanguinis formed biofilms under several of the conditions tested. Biofilm formation was greatest, however, in BM supplemented with sucrose under the anaerobic condition. This condition was therefore used to screen for biofilm-defective mutants.
FIG. 1.
S. sanguinis biofilm formation in different media. Bacteria were grown in polystyrene microtiter plates containing the indicated media (open bars) or media supplemented with 1% (wt/vol) glucose (black bars) or sucrose (gray bars). Bacterial growth and biofilm formation were measured after a 16-h incubation under aerobic and anaerobic conditions as described in Materials and Methods. All assays were performed in quadruplicate, and standard deviations are shown. OD450 and OD600, optical densities at 450 and 600 nm, respectively. CDM, chemically defined medium; CS, complete saliva; TH, Todd-Hewitt broth; TS, Trypticase soy broth; TY, tryptone-yeast extract medium.
Screening for S. sanguinis biofilm-defective mutants from an STM library.
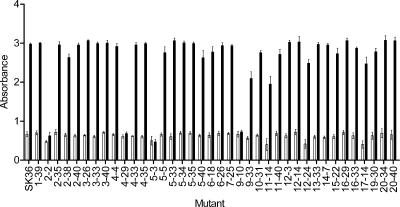
We previously described the creation by in vitro transposition and virulence screening of a library of 800 signature-tagged mutants of SK36 (55). For the present study, we screened this library with the biofilm formation assay. Each mutant, taken from a single colony, was retested three times in the biofilm assay. The signature-tagged mutants were grown in individual wells of microtiter plates under CM selection. About 105 CFU of overnight growing cells were inoculated into 100 μl of BM with sucrose in quadruplicate wells. The biofilms were CV quantified after 16 h of growth under anaerobic conditions, as described in Materials and Methods. We disregarded any mutants that gave inconsistent results in the three biofilm assays or that were significantly impaired for planktonic growth. From a total of 800 signature-tagged mutants, we finally selected 8 mutants that showed consistently significant biofilm reduction and grew normally after three screening cycles. These mutants were 2-2, 4-1, 4-29, 5-3, 5-19, 8-3, 8-20, and 9-10 (Table 1).
TABLE 1.
Biofilm-defective mutants
| Mutant | SSA no. | Gene | Putative function | CI | P value |
|---|---|---|---|---|---|
| 2-2 | SSA_0046 | purB | Adenylosuccinate lyase | NDa | ND |
| 4-1 | Unknown | ND | ND | ||
| 4-29 | SSA_1044 | thrB | Homoserine kinase | 0.034b | <0.01 |
| 5-3 | SSA_0138 | adcA | Metal (Zn)-binding permease | ND | ND |
| 5-19 | SSA_0030 | purL | Phosphoribosylformylglycinamidine synthase | ND | ND |
| 8-3 | SSA_1240 | pyrE | Orotate phosphoribosyltransferase | 0.63 | 0.26 |
| 8-20 | SSA_0030 | purL | Phosphoribosylformylglycinamidine synthase | 1.46 | 0.24 |
| 2.11c | 0.32 | ||||
| 9-10 | SSA_0046 | purB | Adenylosuccinate lyase | 0.0063b | <0.01 |
ND, not determined.
Values are from reference 55 and are in comparison to SK36 rather than JFP36.
Mutant and comparison strains were cultured in BM with 1% (wt/vol) sucrose before inoculation into rabbits.
In our previous STM screen in a rabbit endocarditis model, we obtained 38 putative avirulent mutants from a screen of 800 signature-tagged mutants (55). We noted that four of the eight biofilm-deficient mutants (2-2, 4-29, 5-3, and 9-10) were included among the previously identified avirulent mutants. To compare biofilm formation in all of the attenuated mutants in parallel, we reexamined their biofilm formation in our standard biofilm assay (Fig. 2). The result confirmed that only these four mutants, 2-2, 4-29, 5-3, and 9-10, showed greater-than-twofold reductions in biofilm formation. This result indicated that many mutants that were attenuated for endocarditis formed normal biofilm.
FIG. 2.
Examination of biofilm formation by mutant strains with reduced virulence for endocarditis. SK36 and mutants were grown in BM with 1% (wt/vol) sucrose on polystyrene microtiter plates under anaerobic conditions. Bacterial growth (open bars) and biofilm formation (black bars) were measured after a 16-h incubation. All assays were performed in quadruplicate, and standard deviations are shown.
Identification of the interrupted S. sanguinis genes.
To identify the interrupted S. sanguinis genes in the biofilm-defective mutants, the modified AP-PCR method for rapid characterization of transposon insertion sites was used (20). The sequences of flanking regions were BLAST searched against the completed S. sanguinis SK36 genome (77). Four of eight mutants, 9-10, 2-2, 4-29, and 5-3, had been previously characterized by AP-PCR (55). Three of the four additional biofilm-defective mutants, 5-19, 8-3, and 8-20, were characterized by AP-PCR. However, one mutant (4-1) could not be completely characterized because vector sequences were found on both sides of the inserted minitransposon (Table 1).
Mutants 9-10 and 2-2 contained distinct insertions within the same gene, purB, which encodes adenylosuccinate lyase (SSA_0046). Mutant 4-29 was previously shown to contain an insertion in the thrB gene, which encodes homoserine kinase (SSA_1044), which is involved in threonine biosynthesis (Table 1) (55). The transposon insertion in mutant 5-3 could not be characterized on one side by AP-PCR; the other side was flanked by an interrupted gene which encodes a putative Zn-binding permease (SSA_0138). This gene is a homolog of adcA, one of four genes in the S. sanguinis adc operon. The adc operon also consists of four open reading frames, adcR, adcC, adcB, and adcA, in S. gordonii Challis and S. pneumoniae. This operon was implicated in biofilm formation by S. gordonii Challis and in genetic competence in S. pneumoniae (22, 41), although the expression of these genes is not responsive to competence-signaling peptide in S. gordonii (70). The identification of the adcA gene homolog suggests the comparability of our biofilm gene screen with that performed with S. gordonii Challis (41). Two mutants, 5-19 and 8-20, contained transposon insertions at the exact same location within the purL gene (SSA_0030; Table 1). This gene was annotated as encoding phosphoribosylformylglycinamidine synthase (EC 6.3.5.3) in S. sanguinis (77). The purL gene, involved in the de novo purine biosynthetic pathway, was identified as a virulence factor in three S. pneumoniae STM studies (29, 38, 61). Here, the purL gene was shown to be involved in biofilm formation. One mutant, 8-3, contained an interrupted gene, pyrE (SSA_1240), which encodes orotate phosphoribosyltransferase, one of the enzymes responsible for de novo pyrimidine biosynthesis (75).
Examination of biofilm formation mutants in different media.
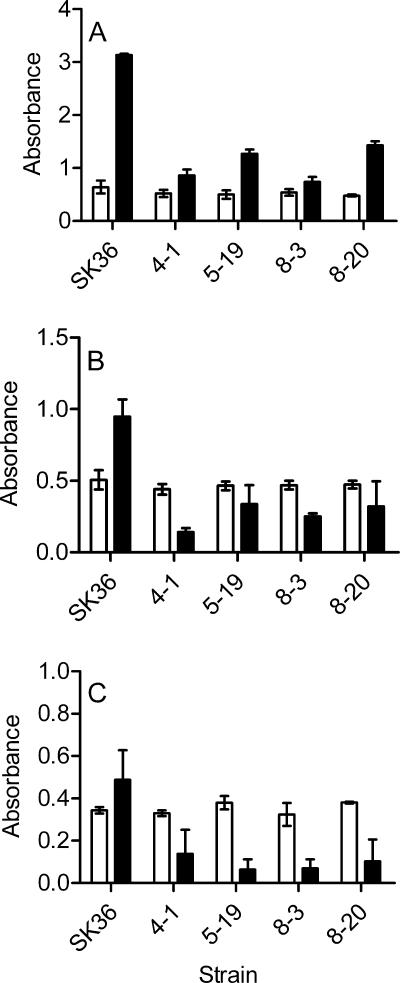
It has been reported that biofilm formation is influenced by the composition of the growth medium (40). We also found that S. sanguinis formed more abundant biofilms in media supplemented with sucrose than in those supplemented with glucose (Fig. 1). The environment of an infected heart valve in vivo is obviously different from the environment of the biofilm formation assay in vitro. Before examining the mutants for virulence in vivo, we wanted to confirm that the biofilm deficiency of the mutants was not confined to the specific medium used. We therefore also compared the biofilm formation of the mutants and the wild type in two additional media, FMC medium supplemented with 1% (wt/vol) glucose and BHI. All four mutants retained their biofilm reductions compared with the wild-type strain, although they all formed less biofilm in FMC medium and BHI than in BM with sucrose (Fig. 3B and C). The biofilm reductions were statistically significant compared with the wild-type strain (P < 0.05). All four mutants showed levels of planktonic growth equivalent to that of the wild-type strain in both FMC and BHI media. This result suggested that the biofilm reductions of the mutants were not dependent on the specific media used. We also examined biofilm formation by SK36 and these four mutants in BM with sucrose after coating the wells with fibrinogen (8). No biofilm formation by any of the strains was observed (data not shown).
FIG. 3.
Biofilm formation by parent and mutant strains in different media. SK36 and selected biofilm-defective mutants were examined for biofilm formation under anaerobic conditions in BM with 1% (wt/vol) sucrose (A), FMC medium supplemented with 1% (wt/vol) glucose (B), and BHI (C). Bacterial growth (open bars) and biofilm formation (black bars) were measured after a 16-h incubation. All assays were performed in quadruplicate, and standard deviations are shown.
Examination of biofilm-defective mutants for endocarditis virulence.
The virulence of all 800 signature-tagged mutants was examined previously in a rabbit endocarditis model (55). In these assays, the mutants were divided into 20 sets of 40 uniquely tagged mutants and each set was inoculated into two or three rabbits that had been previously catheterized to induce the formation of sterile vegetations. All of the mutants in the inoculum pool (input pool) and in the recovered bacteria from the infected vegetations (output pool) were analyzed by dot blotting. A putative decreased-virulence mutant was identified if it exhibited a weak hybridization signal in output blots but a strong signal in input blots (comparable to controls). Mutants producing strong signals in both input and output blots were considered to have virulence indistinguishable from that of the wild-type strain (55). Mutants producing weak signals in all blots were either present at low levels in both the inoculum and infected vegetations or could not be detected by hybridization for unknown reasons (55).
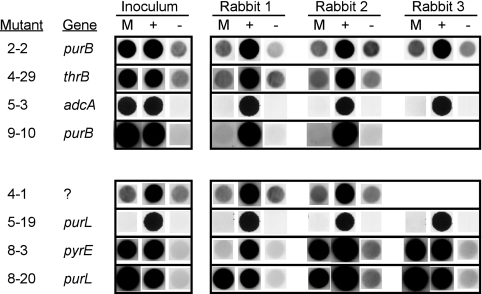
The published and unpublished results from the previous STM screen of the above eight biofilm-defective mutants were reexamined (Fig. 4). Four of eight biofilm-defective mutants, 2-2, 4-29, 5-3, and 9-10, were judged to be attenuated in the original STM screen. The top panel of Fig. 4 confirms that all four strains produced strong signals in the inoculum blot (suggesting that each tagged mutant was well represented in the inoculum pool) but weak signals comparable to the negative control in samples obtained from infected rabbits (suggesting low recovery from the infected vegetations). Two of the strains, 4-29 and 9-10, were further characterized previously with CI assays, which confirmed their attenuation in this model (55). Mutant 2-2 contained the same interrupted purB gene as 9-10. Mutant 5-3, containing the interrupted adcA gene, appeared significantly attenuated by STM. We found that two mutants, 4-1 and 5-19, could not be assessed for virulence in the STM assay because the strains produced weak or no signals in all blots (Fig. 4, bottom panel). However, we confirmed that mutant 8-20 exhibited strong signals in both input and output blots in three rabbits (Fig. 4). The strong signals in this biofilm-defective mutant suggested that its virulence was not reduced significantly compared with that of the wild-type strain. Mutant 8-3 also appeared to have normal virulence in two rabbits, although it produced no signal in one rabbit (Fig. 4).
FIG. 4.
STM analysis of biofilm-defective mutants. Selected results from the STM screen of signature-tagged SK36 mutants performed previously (55) are shown. The identity of each mutant tested and the gene identified as disrupted in the mutant are indicated to the left. Hybridization results from the input inoculum blot and from the cells recovered from two or three rabbit output blots are shown. M, signal of the mutant strain listed to the left; +, signal from a mutant strain that produced a strong signal in the inoculum blot and every output blot, chosen as a positive control; −, signal from the negative control spot, which contained a signature-tagged transposon not used in the creation of any mutants (55). (Top) Mutants originally identified as reduced in virulence and found to be biofilm defective in the present study. (Bottom) Mutants originally identified as possessing normal or unknown virulence and identified as biofilm defective in the present study. All spots from a given blot were taken from the same image without adjustment of image settings. The positive and negative control spots are the same for the 4-1 and 4-29 blots, the 5-3 and 5-19 blots, and the 8-3 and 8-20 blots since each of these strain pairs was tested together in the same pool.
The results of STM suggested that biofilm-defective mutants could be either virulent or attenuated for endocarditis. To confirm the endocarditis virulence of mutants 8-20 and 8-3 (with disrupted purL and pyrE genes, respectively), CI assays were performed to directly compare 8-20 or 8-3 with a derivative of the wild-type strain, JFP36. JFP36 contains an ERM resistance gene in the SSA_0169 locus. This strain has been shown to have virulence equivalent to that of SK36 in CI assays and biofilm formation equivalent to that of SK36 in the biofilm assay (Turner et al., unpublished). In the CI assay, similar numbers of cells of a biofilm-defective mutant and JFP36 were coinoculated into each of three or four rabbits. The ratio of mutant to JFP36 CFU counts in the inoculum and in the vegetations recovered from each rabbit was determined by incorporating serial dilutions into plates containing CM to select for the mutant or ERM to select for JFP36, as described in Materials and Methods. The CI is calculated as the mutant-to-control output ratio divided by the input ratio. A value of <1 indicates reduced virulence or competitiveness of the mutant, whereas a value of >1 indicates increased competitiveness. Mutant 8-20 CI values ranged from 1.00 to 2.11 and were not significantly different from 1 (P > 0.24). This result was consistent with the dot blot results (Fig. 4) and suggested no significant virulence reduction in this mutant. Similarly, the CI ratio of 8-3 was not significantly different from 1 (CIs of 0.37 to 1.08 [P > 0.26]). To exclude the possibility that the wild-type strain was providing the mutant with some potential missing product in the CI assay, individual inoculation of rabbits with JFP36 or mutant 8-20 was also performed. After similar cell numbers of JFP36 (log10 CFU count = 7.94) and mutant 8-20 (log10 CFU count = 7.92) were each inoculated into separate rabbits, the bacterial recovery from vegetations inoculated with mutant 8-20 (mean log10 CFU count = 7.63; range = 7.34 to 8.09) was not significantly different from that of those inoculated with JFP36 (mean log10 CFU count = 7.81; range = 7.39 to 8.30 [P > 0.55]). The retention of virulence in the biofilm-defective mutants suggested that the ability to form biofilms in vitro is not required for the virulence of S. sanguinis in the rabbit endocarditis model.
In our established endocarditis model (55), prior to inoculation into rabbits, S. sanguinis is cultured in BHI—a medium that supports very little biofilm formation (Fig. 3). It seemed possible that growth of cells under conditions that support biofilm formation in vitro might increase endocarditis virulence in vivo. If so, growth under these conditions might reveal decreased competitiveness of biofilm-deficient mutants relative to that of biofilm-producing control strain JFP36. To address this possibility, cells of mutant 8-20 and strain JFP36 cultured overnight were preinoculated into BM supplemented with sucrose for 3 h before the cells were mixed and coinoculated into three rabbits for a CI assay. The CI values from the three rabbits were not significantly different from 1 (CIs of 0.51 to 6.33 [P > 0.32]). Furthermore, the total number of colonies recovered from each infected vegetation (105 to 107) was less than or equal to the number typically recovered after the inoculation of an equivalent number of BHI-grown cells. This suggests that increased biofilm production prior to animal infection does not increase the endocarditis virulence of S. sanguinis.
DISCUSSION
We established a screening system for S. sanguinis to study biofilm formation in vitro. This system will promote biofilm studies of S. sanguinis for understanding the biological function of this organism in the oral environment. Several oral streptococci have been screened for their biofilm genes, including S. gordonii, S. mutans, and S. parasanguinis (27, 40, 78). From our genome comparison, we found that S. sanguinis strain SK36 is significantly different from other streptococci whose genomes are publicly available. For example, the GC content of the S. sanguinis genome is 43.9%, higher than that of other streptococcal genomes, which contain less than 40% GC. It has a larger genome and codes for more proteins than the other 26 sequenced streptococcal genomes (77). As one of the viridans group streptococci, S. sanguinis has long been recognized as a pioneer colonizer of teeth, along with other viridans group streptococci such as S. gordonii, S. parasanguinis, and S. mitis. Although S. sanguinis is closely related to S. gordonii and S. parasanguinis in phylogenetic analyses and they have many biological traits in common, there are undoubtedly significant differences in gene content among them. For example, we found more than 350 genes that are present in S. sanguinis strain SK36 but absent in S. gordonii Challis on the basis of a BLAST search (data not shown). It is likely that S. sanguinis possesses biofilm genes that may vary in S. gordonii or S. mutans.
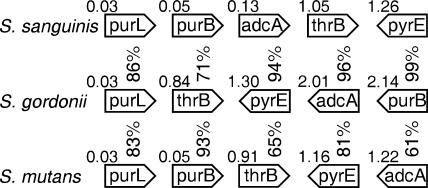
A number of biofilm genes in oral streptococci have been identified and studied intensively because biofilm formation is important in the oral environment. These studies have resulted in the identification of a plethora of genes required for biofilm development. These genes have been associated with many different biological processes, including polymicrobial interactions, surface protein adhesion, cell wall and envelope membrane structures, signal transduction and quorum sensing, carbohydrate metabolism, and exopolysaccharide biosynthesis (1, 4, 10, 11, 13, 14, 27, 32, 36, 37, 39, 40, 56, 58, 68, 71-73, 78, 79). We report here the identification of five genes required for biofilm formation in S. sanguinis: adcA, pyrE, purB, thrB, and purL. To our knowledge, adcA is the only one of these to have been previously reported as required for biofilm formation in any bacterium (45). Although these results should be confirmed by complementation analyses, the isolation of two strains each with mutations in the same genes (purB and purL) and the shared involvement of these two genes and pyrE in nucleotide biosynthesis lend support to the involvement of these genes in biofilm formation. Several genes which encode components of the pyrimidine nucleotide biosynthetic pathway have been reported to be induced in biofilms when comparing gene expression in Staphylococcus aureus biofilm and planktonic cells (7). The pyrE gene has also been reported to be differently expressed in biofilm and tissue communities of Streptococcus pyogenes (16). Three oral streptococcal genomes are now available in public databases: S. mutans UA159 (2), S. sanguinis SK36 (77), and S. gordonii Challis CH1 (70). We compared the distributions of the five putative biofilm genes among the three oral streptococci (Fig. 5). All five genes were present in all three oral streptococci, although they had different degrees of sequence conservation and were in different genomic locations.
FIG. 5.
Organization of the putative biofilm genes among oral streptococcal genomes. The locations and orientations of five putative biofilm genes were identified among three oral streptococci—S. sanguinis SK36 (CP000387), S. gordonii Challis CH1 (CP000725), and S. mutans UA159 (AE014133). The coordinate (in megabases) of the starting point of each gene is shown. Each percentage indicates the identity of each protein relative to its ortholog in S. sanguinis. Arrows indicate gene direction.
The purL and purB genes are especially interesting. Both are involved in purine biosynthesis. The purB gene product, adenylosuccinate lyase (EC 4.3.2.2), catalyzes two distinct reactions in the biosynthesis of purines, with the latter step producing AMP from adenylosuccinate. The purL gene product (EC 6.3.5.3) catalyzes an earlier step in the same pathway. The two genes are separated by a DNA segment more than 14 kb in length and containing 14 genes, suggesting that neither mutation affects biofilm formation simply by affecting the expression of the other gene. If both mutations affect biofilm formation by reducing AMP synthesis, we would expect the mutation of a number of other genes in the same pathway to have the same effect. In that case, it would be surprising that this pathway has not been identified in any of the previous screens for biofilm genes in other viridans group streptococci. Purine synthesis has previously been speculated to play a crucial role in biofilm formation in nonstreptococcal species (21, 42). This role is suggested to be indirect in the regulation of the ica operon, the best-understood operon in staphylococcal biofilm formation (52). It is also interesting that the S. sanguinis purB mutant is attenuated in both biofilm formation and virulence for endocarditis whereas the purL mutant exhibits only biofilm reduction. Both the purB gene and the purL gene have been shown to be important for virulence in studies performed with S. pneumoniae (38) and with Bacillus anthracis (65). Given these results and the overall similarity of the S. pneumoniae and S. sanguinis purine pathways (P. Xu, unpublished observations), it is more surprising that purL is not required for virulence in S. sanguinis than that purB is. Indeed, it has been suggested that not only purB and purL but also pyrE and other nucleotide biosynthetic genes are required for survival in blood by both gram-negative and gram-positive species (65). Our finding that the purL and pyrE mutants retained normal virulence suggests that this is not universally true.
Biofilms are usually defined as microorganisms attached to a solid surface via a polymeric matrix that is largely microbial in origin (17). Dental plaque is a well-known example of such a biofilm. For viridans group streptococci, at least two sources of extracellular matrix have been identified—glucans and DNA. Several oral streptococci produce abundant biofilms when grown in the presence of sucrose (3, 15, 27, 28, 40, 71, 74), as is true for S. sanguinis (Fig. 1). Glucan production by extracellular glucosyltransferase enzymes found in most streptococci is likely largely responsible for this phenotype (5). In the absence of sucrose, such glucan production does not occur (67). In this case, less biofilm formation, which has been shown in one instance to depend upon the binding of cells to extracellular DNA, may still occur (59).
Prosthetic valve endocarditis caused by microorganisms such as coagulase-negative staphylococci and Candida albicans has long been recognized as dependent upon biofilms, as slime or capsule production is important for these microorganisms to initiate and maintain attachment to the prosthetic valve (17, 23, 57). Moreover, biofilm formation in vitro can be correlated with virulence in vivo (43, 66). Native-valve endocarditis caused by viridans group streptococci may not fit the strict definition of a biofilm. The vegetation in which the bacteria are embedded has been shown to be composed principally of platelets and fibrin (24), which are of host rather than bacterial origin. Some evidence has been presented, however, to suggest that streptococci produce an extracellular matrix within the vegetation. Mills et al. (44) used microscopy in combination with histochemical staining to identify an extracellular matrix termed “glycocalyx” produced by various species of viridans group streptococci in rabbit vegetations. Furthermore, the amount of glycocalyx detected in vegetations could be correlated with the production of glycocalyx in vitro in a medium lacking sucrose. This suggests that some viridans group streptococci produce a sucrose-independent extracellular matrix within the vegetation. Further, Dall and Herndon (19) reported that glycocalyx production in vitro correlated strongly with the ability to cause endocarditis among clinical isolates—20 of 24 isolates from patients with endocarditis were positive for glycocalyx production, while only one of 24 isolates from nonendocarditis patients were positive. This suggests that glycocalyx production is advantageous for causing endocarditis, if not absolutely required. In Dall's study, glycocalyx production was assayed with pooled rabbit serum as the carbon source, indicating sucrose independence of glycocalyx production.
More recent studies have also suggested a link between biofilm formation and streptococcal endocarditis (27, 35, 50). Indeed, two of the four genes identified in our previous STM screen as contributing significantly to virulence for endocarditis (purB and thrB) (55) were also identified in the present study as being required for biofilm formation. An additional mutant that was identified as reduced in virulence (5-3; Fig. 4) was also found to be biofilm defective (Fig. 2). This mutant contained a defective adcA gene, one of four genes in the reported biofilm operon, which encodes a component of a metal uptake system (41). However, we identified two mutants, 8-20 and 8-3, with mutations in the purL and pyrE genes, respectively, that were defective for biofilm formation yet retained virulence for endocarditis. As is true for most studies that have examined the linkage between biofilm formation and endocarditis (12, 46, 51, 64), we assayed biofilm formation in vitro in a microtiter plate assay. Clearly, this assay does not model the conditions found in a heart valve vegetation. We therefore performed the assay with a variety of growth media but found none that allowed biofilm formation by any of the mutants tested. Further, to produce a surface more similar to that of a vegetation, we attempted to assess biofilm formation by wild-type and mutant strains after coating microtiter wells with fibrinogen. This eliminated biofilm formation in the parent strain, as well as in the mutants (data not shown). Thus, we can find no condition under which these mutants produce biofilms and no association of biofilm formation with endocarditis virulence.
Bizzini et al. recently reached a similar conclusion about S. gordonii (12). Using the rat endocarditis model, they found no reduction in the density within vegetations of three biofilm-defective mutant bacteria compared to the wild-type parent strain. Our study complements theirs in several ways. First, we examined S. sanguinis rather than S. gordonii. Although these two species are closely related, the virulence mechanisms of the two species may differ. Second, we used the rabbit model rather than the rat. We have found that SK36 infects rabbits far more reliably than rats (55), suggesting subtle differences in the two models. Third, we used a CI assay in which the wild-type and mutant strains competed for colonization and growth in the same animal. The CI assay is often more sensitive to small changes in colonization efficiency, and fewer animals are required (9). However, the advantage of the individual inoculation approach is that the wild-type strain is separated from the mutant and cannot provide any potential missing products. Therefore, we also examined the virulence of biofilm-defective mutant 8-20 by this approach. We found no difference in bacterial recovery from rabbits inoculated with mutant 8-20 compared to those inoculated with virulent strain JFP36. Fourth, we examined a collection of nearly 1,000 randomly generated mutants for both the abilities to cause endocarditis and form biofilm, rather than selecting a few biofilm-defective mutants for examination in an endocarditis model. In this screen, we identified not only mutants that were deficient in either biofilm formation or endocarditis virulence but also mutants that were deficient in both. Finally, the Bizzini study allowed only for the possibility of biofilm formation within the host animal. We wanted to examine, in addition, the possibility that biofilm formation prior to inoculation increases endocarditis virulence. Viridans group streptococci, including S. sanguinis and S. gordonii, are prominent components of dental plaque. In human infections, these bacteria may enter the bloodstream encased within the dental plaque biofilm, and human plaque samples have been shown to cause endocarditis in an animal model (49). Thus, the contribution of biofilm formation could be prior to entry into the bloodstream. We found, however, that the growth of mutant and wild-type strains under conditions that promote abundant biofilm formation neither decreased the competitiveness of biofilm-defective mutants nor increased the recovery of the biofilm-proficient control strain. Thus, there is no evidence that biofilm formation either during or prior to inoculation enhances the infectivity of S. sanguinis.
Acknowledgments
This work was supported by grants J743 from the Jeffress Memorial Trust, 6-46514 from the AD Williams Fund, and R01DE18138 from the National Institutes of Health to P.X. and grants R01AI47841 and K02AI054908 from the National Institutes of Health to T.K.
We thank Takeshi Unoki and Nicai Zollar for assistance with animal experiments and Lauren Turner and Sankar Das for helpful discussions concerning mutant characterization and the biofilm assay.
Editor: A. Camilli
Footnotes
Published ahead of print on 7 April 2008.
REFERENCES
- 1.Abranches, J., M. M. Candella, Z. T. Wen, H. V. Baker, and R. A. Burne. 2006. Different roles of EIIABMan and EIIGlc in regulation of energy metabolism, biofilm development, and competence in Streptococcus mutans. J. Bacteriol. 1883748-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajdić, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 9914434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banas, J. A., T. L. Fountain, J. E. Mazurkiewicz, K. Sun, and V. M. Margaret. 2007. Streptococcus mutans glucan-binding protein-A affects Streptococcus gordonii biofilm architecture. FEMS Microbiol. Lett. 26780-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banas, J. A., K. R. Hazlett, and J. E. Mazurkiewicz. 2001. An in vitro model for studying the contributions of the Streptococcus mutans glucan-binding protein A to biofilm structure. Methods Enzymol. 337425-433. [DOI] [PubMed] [Google Scholar]
- 5.Banas, J. A., and M. M. Vickerman. 2003. Glucan-binding proteins of the oral streptococci. Crit. Rev. Oral Biol. Med. 1489-99. [DOI] [PubMed] [Google Scholar]
- 6.Bayer, A. S., A. F. Bolger, K. A. Taubert, W. Wilson, J. Steckelberg, A. W. Karchmer, M. Levison, H. F. Chambers, A. S. Dajani, M. H. Gewitz, J. W. Newburger, M. A. Gerber, S. T. Shulman, T. J. Pallasch, T. W. Gage, and P. Ferrieri. 1998. Diagnosis and management of infective endocarditis and its complications. Circulation 982936-2948. [DOI] [PubMed] [Google Scholar]
- 7.Beenken, K. E., P. M. Dunman, F. McAleese, D. Macapagal, E. Murphy, S. J. Projan, J. S. Blevins, and M. S. Smeltzer. 2004. Global gene expression in Staphylococcus aureus biofilms. J. Bacteriol. 1864665-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beg, A. M., M. N. Jones, T. Miller-Torbert, and R. G. Holt. 2002. Binding of Streptococcus mutans to extracellular matrix molecules and fibrinogen. Biochem. Biophys. Res. Commun. 29875-79. [DOI] [PubMed] [Google Scholar]
- 9.Beuzón, C. R., and D. W. Holden. 2001. Use of mixed infections with Salmonella strains to study virulence genes and their interactions in vivo. Microbes Infect. 31345-1352. [DOI] [PubMed] [Google Scholar]
- 10.Bhagwat, S. P., J. Nary, and R. A. Burne. 2001. Effects of mutating putative two-component systems on biofilm formation by Streptococcus mutans UA159. FEMS Microbiol. Lett. 205225-230. [DOI] [PubMed] [Google Scholar]
- 11.Biswas, S., and I. Biswas. 2005. Role of HtrA in surface protein expression and biofilm formation by Streptococcus mutans. Infect. Immun. 736923-6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bizzini, A., S. Beggah-Moller, P. Moreillon, and J. M. Entenza. 2006. Lack of in vitro biofilm formation does not attenuate the virulence of Streptococcus gordonii in experimental endocarditis. FEMS Immunol. Med. Microbiol. 48419-423. [DOI] [PubMed] [Google Scholar]
- 13.Brown, T. A., Jr., S. J. Ahn, R. N. Frank, Y. Y. Chen, J. A. Lemos, and R. A. Burne. 2005. A hypothetical protein of Streptococcus mutans is critical for biofilm formation. Infect. Immun. 733147-3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capestany, C. A., M. Kuboniwa, I. Y. Jung, Y. Park, G. D. Tribble, and R. J. Lamont. 2006. Role of the Porphyromonas gingivalis InlJ protein in homotypic and heterotypic biofilm development. Infect. Immun. 743002-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaudhuri, B., J. Rojek, M. M. Vickerman, J. M. Tanzer, and F. A. Scannapieco. 2007. Interaction of salivary α-amylase and amylase-binding-protein A (AbpA) of Streptococcus gordonii with glucosyltransferase of S. gordonii and Streptococcus mutans. BMC Microbiol. 760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho, K. H., and M. G. Caparon. 2005. Patterns of virulence gene expression differ between biofilm and tissue communities of Streptococcus pyogenes. Mol. Microbiol. 571545-1556. [DOI] [PubMed] [Google Scholar]
- 17.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 2841318-1322. [DOI] [PubMed] [Google Scholar]
- 18.Curran, T. M., Y. Ma, G. C. Rutherford, and R. E. Marquis. 1998. Turning on and turning off the arginine deiminase system in oral streptococci. Can. J. Microbiol. 441078-1085. [DOI] [PubMed] [Google Scholar]
- 19.Dall, L. H., and B. L. Herndon. 1990. Association of cell-adherent glycocalyx and endocarditis production by viridans group streptococci. J. Clin. Microbiol. 281698-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das, S., J. C. Noe, S. Paik, and T. Kitten. 2005. An improved arbitrary primed PCR method for rapid characterization of transposon insertion sites. J. Microbiol. Methods 6389-94. [DOI] [PubMed] [Google Scholar]
- 21.De Vriendt, K., S. Theunissen, W. Carpentier, L. De Smet, B. Devreese, and J. Van Beeumen. 2005. Proteomics of Shewanella oneidensis MR-1 biofilm reveals differentially expressed proteins, including AggA and RibB. Proteomics 51308-1316. [DOI] [PubMed] [Google Scholar]
- 22.Dintilhac, A., and J. P. Claverys. 1997. The adc locus, which affects competence for genetic transformation in Streptococcus pneumoniae, encodes an ABC transporter with a putative lipoprotein homologous to a family of streptococcal adhesins. Res. Microbiol. 148119-131. [DOI] [PubMed] [Google Scholar]
- 23.Douglas, L. J. 2003. Candida biofilms and their role in infection. Trends Microbiol. 1130-36. [DOI] [PubMed] [Google Scholar]
- 24.Durack, D. T. 1975. Experimental bacterial endocarditis. IV. Structure and evolution of very early lesions. J. Pathol. 11581-89. [DOI] [PubMed] [Google Scholar]
- 25.Dyson, C., R. A. Barnes, and G. A. Harrison. 1999. Infective endocarditis: an epidemiological review of 128 episodes. J. Infect. 3887-93. [DOI] [PubMed] [Google Scholar]
- 26.Facklam, R. 2002. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin. Microbiol. Rev. 15613-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Froeliger, E. H., and P. Fives-Taylor. 2001. Streptococcus parasanguis fimbria-associated adhesin Fap1 is required for biofilm formation. Infect. Immun. 692512-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilmore, K. S., P. Srinivas, D. R. Akins, K. L. Hatter, and M. S. Gilmore. 2003. Growth, development, and gene expression in a persistent Streptococcus gordonii biofilm. Infect. Immun. 714759-4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hava, D. L., and A. Camilli. 2002. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol. Microbiol. 451389-1406. [PMC free article] [PubMed] [Google Scholar]
- 30.Heilmann, C., C. Gerke, F. Perdreau-Remington, and F. Gotz. 1996. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect. Immun. 64277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu, S. D., J. O. Cisar, A. L. Sandberg, and M. Kilian. 1994. Adhesive properties of viridans group streptococcal species. Microb. Ecol. Health Dis. 7125-137. [Google Scholar]
- 32.Idone, V., S. Brendtro, R. Gillespie, S. Kocaj, E. Peterson, M. Rendi, W. Warren, S. Michalek, K. Krastel, D. Cvitkovitch, and G. Spatafora. 2003. Effect of an orphan response regulator on Streptococcus mutans sucrose-dependent adherence and cariogenesis. Infect. Immun. 714351-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenkinson, H. F., and R. J. Lamont. 2005. Oral microbial communities in sickness and in health. Trends Microbiol. 13589-595. [DOI] [PubMed] [Google Scholar]
- 34.Kilian, M., and K. Holmgren. 1981. Ecology and nature of immunoglobulin A1 protease-producing streptococci in the human oral cavity and pharynx. Infect. Immun. 31868-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiliç, A. O., L. Tao, Y. Zhang, Y. Lei, A. Khammanivong, and M. C. Herzberg. 2004. Involvement of Streptococcus gordonii β-glucoside metabolism systems in adhesion, biofilm formation, and in vivo gene expression. J. Bacteriol. 1864246-4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuboniwa, M., G. D. Tribble, C. E. James, A. O. Kilic, L. Tao, M. C. Herzberg, S. Shizukuishi, and R. J. Lamont. 2006. Streptococcus gordonii utilizes several distinct gene functions to recruit Porphyromonas gingivalis into a mixed community. Mol. Microbiol. 60121-139. [DOI] [PubMed] [Google Scholar]
- 37.Lamont, R. J., A. El Sabaeny, Y. Park, G. S. Cook, J. W. Costerton, and D. R. Demuth. 2002. Role of the Streptococcus gordonii SspB protein in the development of Porphyromonas gingivalis biofilms on streptococcal substrates. Microbiology 1481627-1636. [DOI] [PubMed] [Google Scholar]
- 38.Lau, G. W., S. Haataja, M. Lonetto, S. E. Kensit, A. Marra, A. P. Bryant, D. McDevitt, D. A. Morrison, and D. W. Holden. 2001. A functional genomic analysis of type 3 Streptococcus pneumoniae virulence. Mol. Microbiol. 40555-571. [DOI] [PubMed] [Google Scholar]
- 39.Li, Y. H., N. Tang, M. B. Aspiras, P. C. Lau, J. H. Lee, R. P. Ellen, and D. G. Cvitkovitch. 2002. A quorum-sensing signaling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation. J. Bacteriol. 1842699-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loo, C. Y., D. A. Corliss, and N. Ganeshkumar. 2000. Streptococcus gordonii biofilm formation: identification of genes that code for biofilm phenotypes. J. Bacteriol. 1821374-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loo, C. Y., K. Mitrakul, I. B. Voss, C. V. Hughes, and N. Ganeshkumar. 2003. Involvement of the adc operon and manganese homeostasis in Streptococcus gordonii biofilm formation. J. Bacteriol. 1852887-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mack, D., P. Becker, I. Chatterjee, S. Dobinsky, J. K. Knobloch, G. Peters, H. Rohde, and M. Herrmann. 2004. Mechanisms of biofilm formation in Staphylococcus epidermidis and Staphylococcus aureus: functional molecules, regulatory circuits, and adaptive responses. Int. J. Med. Microbiol. 294203-212. [DOI] [PubMed] [Google Scholar]
- 43.Mateo, M., J. R. Maestre, L. Aguilar, M. J. Gimenez, J. J. Granizo, and J. Prieto. 2008. Strong slime production is a marker of clinical significance in Staphylococcus epidermidis isolated from intravascular catheters. Eur. J. Clin. Microbiol. Infect. Dis. 27311-314. [DOI] [PubMed] [Google Scholar]
- 44.Mills, J., L. Pulliam, L. Dall, J. Marzouk, W. Wilson, and J. W. Costerton. 1984. Exopolysaccharide production by viridans streptococci in experimental endocarditis. Infect. Immun. 43359-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitrakul, K., C. Y. Loo, C. Gyurko, C. V. Hughes, and N. Ganeshkumar. 2005. Mutational analysis of the adcCBA genes in Streptococcus gordonii biofilm formation. Oral Microbiol. Immunol. 20122-127. [DOI] [PubMed] [Google Scholar]
- 46.Mohamed, J. A., W. Huang, S. R. Nallapareddy, F. Teng, and B. E. Murray. 2004. Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by Enterococcus faecalis. Infect. Immun. 723658-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moreillon, P., Y. A. Que, and A. S. Bayer. 2002. Pathogenesis of streptococcal and staphylococcal endocarditis. Infect. Dis. Clin. N. Am. 16297-318. [DOI] [PubMed] [Google Scholar]
- 48.Mylonakis, E., and S. B. Calderwood. 2001. Infective endocarditis in adults. N. Engl. J. Med. 3451318-1330. [DOI] [PubMed] [Google Scholar]
- 49.Nagata, E., H. Okayama, H. O. Ito, I. Semba, M. Inoue, and T. Oho. 2005. Experimental infective endocarditis induced by human supragingival dental plaque in rats. Eur. J. Oral Sci. 113499-504. [DOI] [PubMed] [Google Scholar]
- 50.Nakano, K., K. Fujita, K. Nishimura, R. Nomura, and T. Ooshima. 2005. Contribution of biofilm regulatory protein A of Streptococcus mutans, to systemic virulence. Microbes Infect. 71246-1255. [DOI] [PubMed] [Google Scholar]
- 51.Nallapareddy, S. R., K. V. Singh, J. Sillanpaa, D. A. Garsin, M. Hook, S. L. Erlandsen, and B. E. Murray. 2006. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J. Clin. Investig. 1162799-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Gara, J. P. 2007. ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol. Lett. 270179-188. [DOI] [PubMed] [Google Scholar]
- 53.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30295-304. [DOI] [PubMed] [Google Scholar]
- 54.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28449-461. [DOI] [PubMed] [Google Scholar]
- 55.Paik, S., L. Senty, S. Das, J. C. Noe, C. L. Munro, and T. Kitten. 2005. Identification of virulence determinants for endocarditis in Streptococcus sanguinis by signature-tagged mutagenesis. Infect. Immun. 736064-6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park, Y., M. R. Simionato, K. Sekiya, Y. Murakami, D. James, W. Chen, M. Hackett, F. Yoshimura, D. R. Demuth, and R. J. Lamont. 2005. Short fimbriae of Porphyromonas gingivalis and their role in coadhesion with Streptococcus gordonii. Infect. Immun. 733983-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parsek, M. R., and P. K. Singh. 2003. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 57677-701. [DOI] [PubMed] [Google Scholar]
- 58.Pecharki, D., F. C. Petersen, S. Assev, and A. A. Scheie. 2005. Involvement of antigen I/II surface proteins in Streptococcus mutans and Streptococcus intermedius biofilm formation. Oral Microbiol. Immunol. 20366-371. [DOI] [PubMed] [Google Scholar]
- 59.Petersen, F. C., L. Tao, and A. A. Scheie. 2005. DNA binding-uptake system: a link between cell-to-cell communication and biofilm formation. J. Bacteriol. 1874392-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Phan, T. N., J. S. Reidmiller, and R. E. Marquis. 2000. Sensitization of Actinomyces naeslundii and Streptococcus sanguis in biofilms and suspensions to acid damage by fluoride and other weak acids. Arch. Microbiol. 174248-255. [DOI] [PubMed] [Google Scholar]
- 61.Polissi, A., A. Pontiggia, G. Feger, M. Altieri, H. Mottl, L. Ferrari, and D. Simon. 1998. Large-scale identification of virulence genes from Streptococcus pneumoniae. Infect. Immun. 665620-5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pratt, L. A., and R. Kolter. 1998. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 30285-293. [DOI] [PubMed] [Google Scholar]
- 63.Pratten, J., K. Wills, P. Barnett, and M. Wilson. 1998. In vitro studies of the effect of antiseptic-containing mouthwashes on the formation and viability of Streptococcus sanguis biofilms. J. Appl. Microbiol. 841149-1155. [DOI] [PubMed] [Google Scholar]
- 64.Presterl, E., A. J. Grisold, S. Reichmann, A. M. Hirschl, A. Georgopoulos, and W. Graninger. 2005. Viridans streptococci in endocarditis and neutropenic sepsis: biofilm formation and effects of antibiotics. J. Antimicrob. Chemother. 5545-50. [DOI] [PubMed] [Google Scholar]
- 65.Samant, S., H. Lee, M. Ghassemi, J. Chen, J. L. Cook, A. S. Mankin, and A. A. Neyfakh. 2008. Nucleotide biosynthesis is critical for growth of bacteria in human blood. PLoS Pathog. 4e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shiro, H., E. Muller, N. Gutierrez, S. Boisot, M. Grout, T. D. Tosteson, D. Goldmann, and G. B. Pier. 1994. Transposon mutants of Staphylococcus epidermidis deficient in elaboration of capsular polysaccharide/adhesin and slime are avirulent in a rabbit model of endocarditis. J. Infect. Dis. 1691042-1049. [DOI] [PubMed] [Google Scholar]
- 67.Tanzer, J. M., B. M. Chassy, and M. I. Krichevsky. 1971. Sucrose metabolism by Streptococcus mutans, SL-I. Biochim. Biophys. Acta 261379-387. [DOI] [PubMed] [Google Scholar]
- 68.Tanzer, J. M., L. Grant, A. Thompson, L. Li, J. D. Rogers, E. M. Haase, and F. A. Scannapieco. 2003. Amylase-binding proteins A (AbpA) and B (AbpB) differentially affect colonization of rats' teeth by Streptococcus gordonii. Microbiology 1492653-2660. [DOI] [PubMed] [Google Scholar]
- 69.Terleckyj, B., N. P. Willett, and G. D. Shockman. 1975. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect. Immun. 11649-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vickerman, M. M., S. Iobst, A. M. Jesionowski, and S. R. Gill. 2007. Genome-wide transcriptional changes in Streptococcus gordonii in response to competence signaling peptide. J. Bacteriol. 1897799-7807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang, B., and H. K. Kuramitsu. 2006. A pleiotropic regulator, Frp, affects exopolysaccharide synthesis, biofilm formation, and competence development in Streptococcus mutans. Infect. Immun. 744581-4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wen, Z. T., H. V. Baker, and R. A. Burne. 2006. Influence of BrpA on critical virulence attributes of Streptococcus mutans. J. Bacteriol. 1882983-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wen, Z. T., and R. A. Burne. 2004. LuxS-mediated signaling in Streptococcus mutans is involved in regulation of acid and oxidative stress tolerance and biofilm formation. J. Bacteriol. 1862682-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wen, Z. T., P. Suntharaligham, D. G. Cvitkovitch, and R. A. Burne. 2005. Trigger factor in Streptococcus mutans is involved in stress tolerance, competence development, and biofilm formation. Infect. Immun. 73219-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.West, T. P. 2004. Regulation of pyrimidine nucleotide formation in Pseudomonas taetrolens ATCC 4683. Microbiol. Res. 15929-33. [DOI] [PubMed] [Google Scholar]
- 76.Wilson, W., K. A. Taubert, M. Gewitz, P. B. Lockhart, L. M. Baddour, M. Levison, A. Bolger, C. H. Cabell, M. Takahashi, R. S. Baltimore, J. W. Newburger, B. L. Strom, L. Y. Tani, M. Gerber, R. O. Bonow, T. Pallasch, S. T. Shulman, A. H. Rowley, J. C. Burns, P. Ferrieri, T. Gardner, D. Goff, and D. T. Durack. 2007. Prevention of infective endocarditis: guidelines from the American Heart Association. A guideline from the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. J. Am. Dent. Assoc. 138739-760. [DOI] [PubMed] [Google Scholar]
- 77.Xu, P., J. M. Alves, T. Kitten, A. Brown, Z. Chen, L. S. Ozaki, P. Manque, X. Ge, M. G. Serrano, D. Puiu, S. Hendricks, Y. Wang, M. D. Chaplin, D. Akan, S. Paik, D. L. Peterson, F. L. Macrina, and G. A. Buck. 2007. Genome of the opportunistic pathogen Streptococcus sanguinis. J. Bacteriol. 1893166-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoshida, A., and H. K. Kuramitsu. 2002. Multiple Streptococcus mutans genes are involved in biofilm formation. Appl. Environ. Microbiol. 686283-6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang, Y., Y. Lei, A. Khammanivong, and M. C. Herzberg. 2004. Identification of a novel two-component system in Streptococcus gordonii V288 involved in biofilm formation. Infect. Immun. 723489-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]