Abstract
In this study, we demonstrated that the 40-kDa outer membrane protein of Porphyromonas gingivalis (40-kDa OMP) nasally administered with a nontoxic chimeric adjuvant that combines the A subunit of mutant cholera toxin E112K with the pentameric B subunit of heat-labile enterotoxin from enterotoxigenic Escherichia coli (mCTA/LTB) elicited a long-term protective immune response. Immunization with the 40-kDa OMP and mCTA/LTB induced high levels of 40-kDa-OMP-specific immunoglobulin G (IgG) and IgA antibodies (Abs) in sera and elicited a significant IgA anti-40-kDa OMP Ab response in saliva. These Ab responses were maintained for at least 1 year after the immunization. Although using adjuvant mCTA/LTB gave Ab responses in the saliva comparable to those obtained using native cholera toxin (nCT) as the adjuvant, the levels of total IgE and 40-kDa-OMP-specific IgE Abs as well as interleukin-4 levels induced by the immunization with mCTA/LTB were lower than those induced by the immunization with nCT. Importantly, IgG Abs generated by nasal immunization with the 40-kDa OMP plus mCTA/LTB inhibited the coaggregation and hemagglutinin activities of P. gingivalis. Furthermore, the mice given nasal 40-kDa OMP plus mCTA/LTB showed a significant reduction of alveolar bone loss caused by oral infection with P. gingivalis even 1 year after the immunization compared to the loss in unimmunized mice. Because mCTA/LTB is nontoxic, nasally administered 40-kDa OMP together with mCTA/LTB should be an effective and safe mucosal vaccine against P. gingivalis infection in humans and may be an important tool for the prevention of chronic periodontitis.
Chronic periodontitis is a common oral inflammatory disease that causes the breakdown of periodontal tissue, including the resorption of alveolar bone, and as a consequence, tooth loss (8). Furthermore, recent studies have suggested that chronic periodontitis influences systemic conditions such as cardiovascular diseases, diabetes, and osteoporosis (5, 10, 19, 28, 39, 41, 45). Hence, the prevention of periodontitis is important for both oral and systemic health.
Porphyromonas gingivalis, a gram-negative anaerobic bacterium, has been shown previously to be one of the major pathogens in chronic periodontitis. The colonization of gingival tissues by this bacterium is considered to be the first step in the pathogenic process of periodontal disease resulting in tissue destruction (24, 37). Molecules such as fimbriae, hemagglutinins, aggregation factors, and lipopolysaccharides responsible for colonization have been identified previously as virulence factors (24, 37). An outer membrane protein having a molecular mass of 40 kDa produced by P. gingivalis (40-kDa OMP) is a key virulence factor involved in the coaggregation activity of P. gingivalis (23). Furthermore, this OMP has been shown previously to be a hemin-binding protein (49). The 40-kDa OMP resides both on the cell surface and in extracellular vesicles and is found on many strains of P. gingivalis (1, 22, 23, 47).
Previous studies have demonstrated that monoclonal antibodies (Abs) against the 40-kDa OMP provide an inhibitory effect on the coaggregation activity of P. gingivalis and possess complement-mediated bactericidal activity against P. gingivalis (23, 25, 46). Furthermore, human monoclonal Abs against the 40-kDa OMP provide protection against bone loss caused by P. gingivalis in rats (21). These studies suggest that the induction of 40-kDa-OMP-specific Abs in the oral mucosa is a logical approach for the prevention of P. gingivalis infection.
Cholera toxin (CT) and heat-labile enterotoxin (LT) from enterotoxigenic Escherichia coli are structurally similar, and both toxins act as adjuvants for the enhancement of mucosal and systemic Ab responses to coadministered protein antigen (Ag) given by mucosal routes (51, 58). Indeed, one of our previous studies demonstrated that the nasal administration of the 40-kDa OMP plus CT elicits 40-kDa-OMP-specific secretory immunoglobulin A (IgA) Abs in the saliva, as well as IgG Abs in serum, that inhibit the coaggregation activity of P. gingivalis (43). However, though the combination of the 40-kDa OMP with the adjuvant CT was shown to be effective, CT and LT cause severe diarrhea and thus are unsuitable for use in humans. Hence, nontoxic mutant derivatives of CT or LT have been generated previously for the development of safe adjuvants (9, 11, 13, 15, 18, 35).
We generated mutant CTs (mCTs) by replacing a single amino acid in the ADP-ribosyltransferase active center of the A subunit. These newly created mCTs (S61F and E112K) do not induce enzymatic activity and cyclic AMP formation but still retain adjuvanticity (59, 60). Furthermore, we constructed a novel nontoxic chimeric mucosal adjuvant that combines the nontoxic A subunit of mCT E112K with the pentameric B subunit of LT from enterotoxigenic E. coli (mCTA/LTB). Interestingly, mCTA/LTB acts as a mucosal adjuvant and provides effective support for protective Ab responses against bacterial toxins and influenza virus infection (33).
In this study, we assessed the potential of a combined intranasal vaccine, the 40-kDa OMP with mCTA/LTB, to prevent oral infection with P. gingivalis. The results suggest that nasal 40-kDa OMP plus mCTA/LTB is a practical and effective vaccine candidate for the induction of protective immunity against alveolar bone loss caused by P. gingivalis infection.
MATERIALS AND METHODS
Mice.
Female BALB/c Cr Slc (BALB/c) mice were purchased from Sankyo Laboratories and were maintained under specific-pathogen-free conditions at the experimental facility of the Nihon University School of Dentistry at Matsudo, Chiba, Japan. Mice received sterile food and water and were 8 to 12 weeks old when used for experiments. All animals were maintained and used in accordance with the guidelines for the care and use of laboratory animals of the Nihon University School of Dentistry at Matsudo.
Ag and adjuvants.
Plasmid pMD125 expressing the 40-kDa OMP was kindly provided by Yoshimitsu Abiko (Nihon University). The 40-kDa OMP was purified to homogeneity from a suspension of E. coli K-12 cells harboring pMD125, as described previously (26). The purity of the 40-kDa OMP was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and no contaminating protein bands were noted. Furthermore, the possible presence of residual endotoxin in the preparation was assessed with a Limulus amoebocyte lysate pyrochrome kit (Associates of Cape Cod Inc., Woods Hole, MA). The 40-kDa-OMP preparation contained as little as 0.4 pg of endotoxin.
A plasmid containing both E112K mCTA and LTB genes (pNCMO2-LTB-mCTA) was constructed as described previously (33). E. coli JM109 (Takara Bio Inc., Shiga, Japan) was used as a cloning host, and Brevibacillus choshinensis HPD31 was used as the host for the production of the recombinant protein (42). The mCTA/LTB chimeric protein was purified from culture supernatants of transformants containing pNCMO2-LTB-mCTA. Purity was examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The protein concentration was determined by using MicroBCA protein assay reagent (Pierce). CT was obtained from List Biologic Laboratories (Campbell, CA).
Immunization and sample collection.
Mice were immunized nasally on days 0, 7, and 14 with 20-μl aliquots (10 μl per nostril) of phosphate-buffered saline (PBS) containing 10 μg of the 40-kDa OMP alone or combined with 10 μg of mCTA/LTB or 1 μg of native CT (nCT). Serum and saliva samples were collected from each group, as described elsewhere (56), in order to examine 40-kDa-OMP-specific Ab responses.
Detection of Ag-specific Ab response.
Ab titers in serum and saliva samples were determined by an enzyme-linked immunosorbent assay (ELISA) (38, 53). Briefly, plates were coated with the 40-kDa OMP (5 μg/ml) and blocked with 1% bovine serum albumin (BSA), and analyses were performed in duplicate. After the plates were blocked, serial dilutions of serum or saliva samples were added in duplicate. Starting dilutions of serum and saliva samples were 1:25 and 1:22, respectively. Following incubation, plates were washed and peroxidase-labeled goat anti-mouse γ- or α-heavy-chain-specific Abs (Southern Biotechnology Associates, Birmingham, AL) were added to appropriate wells. Finally, 2,2′-azino-bis(3-ethylbenz-thiazoline-6-sulfonic acid) with H2O2 (Moss, Inc, Pasadena, MD) was added for color development. End point titers were expressed as the log2 reciprocal of the last dilution giving an optical density at 414 nm of 0.1 greater than the background level after 15 min of incubation.
Analysis of 40-kDa-OMP-specific IgE Abs in sera.
For the detection of 40-kDa-OMP-specific IgE Ab levels in sera, immunoplates (Nunc, Inc., Naperville, IL) were coated with purified rat anti-mouse IgE monoclonal Ab (BD Biosciences, San Diego, CA) and incubated overnight at 4°C, as described previously (32). After blocking of the plates with 3% BSA in PBS, serial dilutions of serum samples were added and the plates were incubated for 4 h at room temperature. Following extensive washing, 40-kDa OMP that had been biotinylated by using a biotin-labeling kit (Boehringer Ingelheim) was added. After the plates were incubated overnight at 4°C, peroxidase-labeled antibiotin monoclonal Abs (Vector Laboratories, Burlingame, CA) were added. After the plates were washed, the color reaction was developed with 3,3′,5,5′-tetramethyl-benzidine (Moss) and stopped with 0.5 N HCl. End point titers of 40-kDa-OMP-specific IgE were expressed as the log2 reciprocal as described above.
Analysis of IL-4 response in 40-kDa-OMP-specific CD4+ T cells.
CD4+ T cells from spleens were purified using a magnet-activated cell sorter system (Miltenyi Biotec, Auburn, CA), as described elsewhere (56). Purified CD4+ T cells (2.5 × 106 cells/ml) were cultured with 2 μg of the 40-kDa OMP/ml in the presence of T-cell-depleted, mitomycin-treated splenic feeder cells (2.5 × 106 cells) in RPMI 1640 medium (GIBCO BRL, Rockville, MD) containing 10% fetal bovine serum, 50 μM 2-mercaptoethanol, 15 mM HEPES, 2 mM l-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 10 U of recombinant IL-2 (Genzyme, Cambridge, MA)/ml. Cultures were incubated for 5 days at 37°C under 5% CO2 in air. Levels of interleukin-4 (IL-4) in culture supernatants were determined by an IL-4-specific ELISA, as described previously (53). Briefly, 96-well plates (Nunc) were coated with a monoclonal anti-IL-4 Ab (BD Biosciences). After the plates were blocked with PBS containing 1% BSA, samples were added to duplicate wells and the plates were incubated overnight at 4°C. Wells were washed and incubated with biotinylated monoclonal anti-IL-4 Ab (BD Biosciences). After incubation, horseradish peroxidase-labeled antibiotin Ab (Vector Laboratories) was added and the reaction was developed with 3,3′,5,5′-tetramethyl-benzidine (Moss) and stopped with 0.5 N HCl. Standard curves were generated using mouse recombinant IL-4 (Endogen, Boston, MA).
Coaggregation assay.
IgG Abs in sera from immunized mice were purified using a HiTrap protein G HP column (Amersham Biosciences, Piscataway, MJ). P. gingivalis 381 and Streptococcus gordonii Challis were grown in a brain heart infusion (BBL Microbiology Systems, Cockeysville, MD) containing yeast extract (0.25%), hemin (10 μg/ml), and vitamin K (1 μg/ml). Bacterial cells were incubated at 37°C in an anaerobic chamber containing N2 (80%), H2 (10%), and CO2 (10%). Coaggregation was determined by the visual assay method, as described previously (14, 36). Briefly, P. gingivalis 381 cells (approximately 1010 cells/ml) were preincubated with purified IgG Abs at 37°C for 1 h. A P. gingivalis suspension (100 μl) was then mixed with an equal volume of an S. gordonii suspension (approximately 1010 cells/ml) on the flocculation slide. This mixture was subsequently incubated at 37°C for 10 min with rotation.
Hemagglutination assay.
The hemagglutinating activity of P. gingivalis was assayed as described previously (50) by using mice erythrocytes. Briefly, P. gingivalis 381 cells were preincubated with purified IgG Abs at 37°C for 1 h. Fifty microliters of a P. gingivalis cell suspension (0.5 μg) in PBS was transferred into microtiter wells, and 50 μl of 1% mice erythrocytes was added. After incubation for 1 h at 37°C with humidity, the hemagglutinating activity of P. gingivalis was observed.
Oral infection.
Mice were orally infected with P. gingivalis as described previously (3, 17, 34) with minor modifications. Briefly, mice were given sulfamethoxazole-trimethoprim (Sulfatrim; Goldline Laboratories, Ft. Lauderdale, FL) at 10 ml per pint in deionized water ad libitum for 10 days. This treatment was followed by a 3-day antibiotic-free period. Mice were then given 109 CFU of P. gingivalis suspended in 100 μl of PBS with 2% carboxymethylcellulose via oral topical application over 3 weeks for a total 15 inoculations. Control groups included mock-infected mice, which received the antibiotic pretreatment and carboxymethylcellulose without P. gingivalis. Forty-seven days after the first gavage, mice were euthanized using CO2.
Measurement of alveolar bone loss.
Horizontal bone loss around the maxillary molars was assessed by a morphometric method as described previously (29). Briefly, skulls were unfleshed after 10 min of treatment in boiling water under pressure of 15 lb/in2, immersed overnight in 3% hydrogen peroxide, pulsed for 1 min in bleach, and stained with 1% methylene blue. The distance from the cementoenamel junction to the alveolar bone crest was measured at a total of 14 buccal sites per mouse. Measurements were made under a dissecting microscope (magnification, ×50) fitted with a video image marker measurement system (VHX-100; KEYENCE, Osaka, Japan) standardized to give measurements in micrometers. Bone measurements were performed a total of three times by two evaluators using a random and blind protocol.
Statistics.
Data are expressed as means ± standard errors (SE) and were compared using an unpaired Student t test.
RESULTS
Nasal 40-kDa OMP with mCTA/LTB elicits long-term Ab responses.
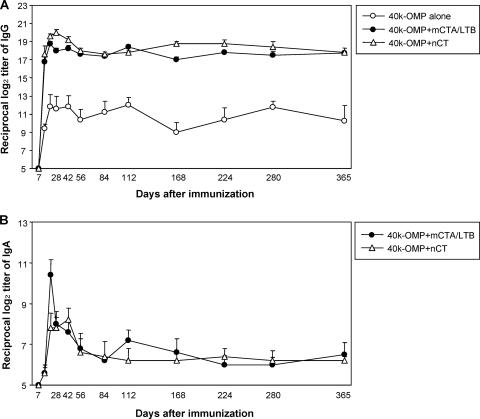
In the initial study, we sought to determine whether the nasal administration of the 40-kDa OMP with mCTA/LTB as a mucosal adjuvant could induce a 40-kDa-OMP-specific Ab response. Mice nasally immunized with the 40-kDa OMP plus mCTA/LTB showed significant 40-kDa-OMP-specific IgG and IgA Ab responses in sera that were comparable to those induced by the 40-kDa OMP plus nCT (Fig. 1). In contrast, only low levels of IgG Abs were induced after immunization with the 40-kDa OMP alone (Fig. 1A). In addition, the nasal administration of the 40-kDa OMP alone failed to elicit a 40-kDa-OMP-specific IgA Ab response detectable in the starting serum dilution (log2 of 5) used in these experiments. Importantly, the serum IgG and IgA Ab responses induced by the 40-kDa OMP plus mCTA/LTB or nCT persisted for more than 1 year (Fig. 1).
FIG. 1.
40-kDa-OMP-specific IgG and IgA Ab responses in sera. Groups of mice were nasally immunized with 10 μg of the 40-kDa OMP alone, 10 μg of the 40-kDa OMP plus 10 μg of mCTA/LTB, or 10 μg of the 40-kDa OMP plus 1 μg of nCT on days 0, 7, and 14. Serum samples were assessed for 40-kDa-OMP-specific IgG (A) and IgA (B) Abs. Results are expressed as the means ± standard errors (SE) for four mice per group and a total of three experiments. IgA anti-40-kDa OMP Abs in sera from mice given the 40-kDa OMP alone were not detectable throughout the experimental period. The P values for IgG and IgA Ab titers obtained with the 40-kDa OMP plus mCTA/LTB or the 40-kDa OMP plus nCT compared to those obtained with the 40-kDa OMP alone at all times except day 7 are <0.05.
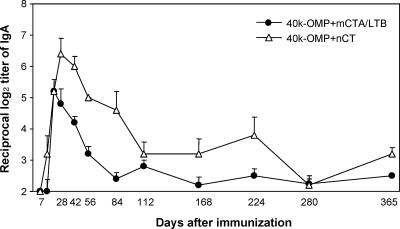
Nasal 40-kDa OMP plus mCTA/LTB induced high levels of 40-kDa-OMP-specific IgA Abs in saliva, and these Abs were maintained for more than 1 year, although the responses gradually decreased from day 28 (Fig. 2). In contrast, no such IgA Ab response was detected in mice given the 40-kDa OMP alone (IgA Ab titers, <log2 of 2). These serum IgG and IgA and salivary IgA Ab titers were confirmed by Ab-forming cell (AFC) responses, which indicated significant numbers of 40-kDa-OMP-specific IgG and IgA AFCs in the spleens, cervical lymph nodes, and submandibular glands of mice given the 40-kDa OMP plus mCTA/LTB or nCT, while low numbers of AFCs were detected in these tissues in mice given the 40-kDa OMP alone (data not shown). These results indicate that a combination of the 40-kDa OMP with mCTA/LTB is a potential nasal vaccine for the induction of Ag-specific mucosal and systemic Ab responses.
FIG. 2.
40-kDa-OMP-specific IgA Ab responses in saliva. Groups of mice were nasally immunized with the 40-kDa OMP plus mCTA/LTB or the 40-kDa OMP plus nCT as described in the legend to Fig. 1. Saliva samples were assessed for 40-kDa-OMP-specific IgA Abs. Results are expressed as the means ± SE for four mice per group and a total of three experiments. IgA anti-40-kDa OMP Abs in mice given the 40-kDa OMP alone were not detectable throughout the experimental period. The P values for Ab titers obtained with the 40-kDa OMP plus mCTA/LTB or the 40-kDa OMP plus nCT compared to those obtained with the 40-kDa OMP alone at all times except days 7 and 14 are <0.05.
40-kDa-OMP-specific IL-4 and IgE Ab responses.
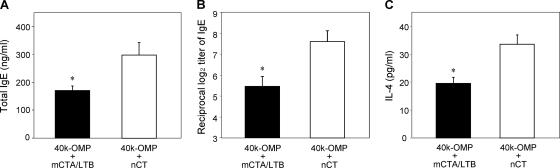
Because mCTA/LTB given nasally has been shown previously to induce a weaker IgE Ab response than nCT (33), it is important to examine the IgE Ab response induced by the 40-kDa OMP together with mCTA/LTB. As may be expected, nasal immunization with the 40-kDa OMP plus nCT induced high-level total and 40-kDa-OMP-specific IgE Ab responses. In contrast, significantly lower levels of total and 40-kDa-OMP-specific IgE Abs in mice given the 40-kDa OMP plus mCTA/LTB than in mice given the 40-kDa OMP plus nCT were noted (Fig. 3A and B). An analysis of the IL-4 response confirmed the IgE Ab titers and showed that CD4+ T cells from the spleens of mice immunized with the 40-kDa OMP plus mCTA/LTB, when restimulated with the 40-kDa OMP in vitro, produced significantly lower levels of IL-4 than those from mice immunized with the 40-kDa OMP plus nCT (Fig. 3B). These findings show that levels of 40-kDa-OMP-specific IL-4 and the subsequent IgE Ab response induced by the 40-kDa OMP plus mCTA/LTB were much lower than those evoked by the 40-kDa OMP plus nCT.
FIG. 3.
Total IgE Ab levels (A), 40-kDa-OMP-specific IgE Ab titers (B), and IL-4 levels in splenic CD4+ T cells (C). Groups of mice were nasally immunized with the 40-kDa OMP plus mCTA/LTB or the 40-kDa OMP plus nCT as described in the legend to Fig. 1. Seven days after the final immunization, serum samples were collected and assessed for total and 40-kDa-OMP-specific IgE Abs. For the measurement of IL-4 production, CD4+ T cells obtained from the spleens of immunized mice were restimulated with the 40-kDa OMP. Culture supernatants were harvested, and the levels of secreted cytokines were assessed by a cytokine-specific ELISA. Results are expressed as the means ± SE for four mice per group and a total of three experiments. *, P < 0.05 compared with results for mice given the 40-kDa OMP plus nCT.
Nasally induced 40-kDa-OMP-specific IgG inhibits the coaggregation and hemagglutinin activities of P. gingivalis.
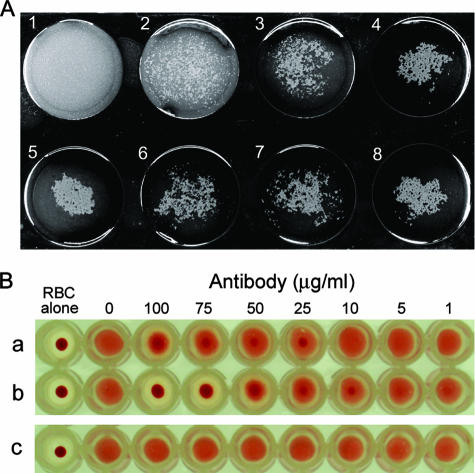
We next determined whether the Abs induced by nasally administered 40-kDa OMP plus mCTA/LTB were capable of inhibiting the coaggregation and hemagglutinin activities of P. gingivalis. A flocculation slide assay showed that the coaggregation activity of P. gingivalis cells with S. gordonii cells was inhibited by IgG Abs from mice given the 40-kDa OMP plus mCTA/LTB in a dose-dependent manner. In contrast, IgG from mice given the 40-kDa OMP alone showed marginal effects against the coaggregation activity of P. gingivalis (Fig. 4A).
FIG. 4.
Effects of 40-kDa-OMP-specific IgG Abs on coaggregation activity and hemagglutinin activity of P. gingivalis. (A) P. gingivalis was preincubated with 40-kDa-OMP-specific IgG and then mixed with S. gordonii on the flocculation slide for the assessment of coaggregation activity. Results of the flocculation slide assay with S. gordonii (panel 1), a mixture of S. gordonii and P. gingivalis with 150-μg/ml IgG Abs derived from the sera of mice given the 40-kDa OMP plus mCTA/LTB (panel 2), a mixture of S. gordonii and P. gingivalis with 100-μg/ml IgG Abs derived from the sera of mice given the 40-kDa OMP plus mCTA/LTB (panel 3), a mixture of S. gordonii and P. gingivalis with 50-μg/ml IgG Abs derived from the sera of mice given the 40-kDa OMP plus mCTA/LTB (panel 4), a mixture of S. gordonii and P. gingivalis (panel 5), a mixture of S. gordonii and P. gingivalis with 150-μg/ml IgG Abs derived from the sera of mice given the 40-kDa OMP alone (panel 6), a mixture of S. gordonii and P. gingivalis with 100-μg/ml IgG Abs derived from the sera of mice given the 40-kDa OMP alone (panel 7), and a mixture of S. gordonii and P. gingivalis with 50-μg/ml IgG Abs derived from the sera of mice given the 40-kDa OMP alone (panel 8) are shown. (B) For the hemagglutination assay, P. gingivalis was preincubated with several concentrations of 40-kDa-OMP-specific IgG Abs and then mixed with erythrocytes. Results of the hemagglutination assay with a mixture of erythrocytes and P. gingivalis with IgG Abs derived from the sera of mice given the 40-kDa OMP alone (row a), a mixture of erythrocytes and P. gingivalis with IgG Abs derived from the sera of mice given the 40-kDa OMP plus mCTA/LTB (row b), and a mixture of erythrocytes and P. gingivalis with IgG Abs derived from the sera of unimmunized mice (row c) are shown. IgG Abs used in this experiment were purified from the pooled sera of five mice per group. The results are representative of three separate experiments. RBC, red blood cells.
In the next study, the inhibitory effects of Abs induced by the 40-kDa OMP plus mCTA/LTB on the hemagglutinin activity of P. gingivalis were determined. IgG Abs purified from mice immunized with the 40-kDa OMP plus mCTA/LTB significantly inhibited the hemagglutinating activity of P. gingivalis in a dose-dependent manner. In contrast, although IgG from mice given the 40-kDa OMP alone showed some inhibition, the effects were much weaker than those of IgG from mice given the 40-kDa OMP with mCTA/LTB. As expected, IgG from unimmunized mice failed to inhibit the hemagglutinating activity of P. gingivalis (Fig. 4B).
Nasal 40-kDa OMP plus mCTA/LTB reduces alveolar bone loss caused by oral infection with P. gingivalis.
As nasal 40-kDa OMP plus mCTA/LTB elicited long-term Ag-specific Ab responses in sera and saliva, we sought to determine whether these Abs were capable of suppressing bone resorption caused by oral infection with P. gingivalis. Thus, mice given the 40-kDa OMP plus mCTA/LTB or the 40-kDa OMP alone were infected orally with P. gingivalis 381 7 days or 1 year after immunization. Mice immunized with the 40-kDa OMP plus mCTA/LTB showed a significant reduction in alveolar bone loss caused by P. gingivalis infection 7 days after immunization compared to the loss in unimmunized mice (Fig. 5A and C). Furthermore, the Ag-specific Ab response induced by nasal 40-kDa OMP plus mCTA/LTB provided significant protection and reduced bone loss caused by P. gingivalis infection, even at 1 year after immunization (Fig. 5B and D). In contrast, mice immunized with the 40-kDa OMP alone did not exhibit reduced bone loss caused by P. gingivalis infection compared to that in unimmunized mice (Fig. 5). These findings indicate that nasal immunization with the 40-kDa OMP plus mCTA/LTB provides long-term protection against oral infection with P. gingivalis.
FIG. 5.
Reduction of P. gingivalis-induced alveolar bone loss by a nasal vaccine containing the 40-kDa OMP plus mCTA/LTB. Groups of mice were immunized nasally with the 40-kDa OMP plus mCTA/LTB, the 40-kDa OMP alone, or PBS as described in the legend to Fig. 1. Seven days (A and C) or 1 year (B and D) after immunization, mice given PBS (unimmunized mice), the 40-kDa OMP alone, or the 40-kDa OMP plus mCTA/LTB were inoculated orally with 109 CFU of P. gingivalis in 2% carboxymethylcellulose, as described in Materials and Methods. Control mice were mock-infected mice inoculated with 2% carboxymethylcellulose only. The distances from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC) at 14 predetermined sites in the unfleshed maxilla were measured and totaled for each mouse. Results are expressed as the means ± SE for six mice per group. *, P < 0.05 compared with results for unimmunized mice.
DISCUSSION
In this study, we demonstrated that the nasal administration of the 40-kDa OMP together with mCTA/LTB induced long-term 40-kDa-OMP-specific IgG and IgA in sera, as well as salivary IgA Abs, that persisted for more than 1 year. Furthermore, these mucosally induced IgG Abs inhibited the coaggregation and hemagglutinin activities of P. gingivalis. Importantly, mice given the 40-kDa OMP plus mCTA/LTB were significantly protected against alveolar bone loss caused by oral infection with P. gingivalis, even 1 year after immunization. In the infection study, bone loss measured 1 year after immunization was greater than that measured 7 days after immunization, even in the absence of infection. In this regard, several studies have demonstrated a positive correlation between aging and alveolar bone loss (2, 4, 40). Another study using senescence-accelerated mice has demonstrated that alveolar bone loss is gradually increased with advancing age, although the mice do not develop chronic periodontitis (48). Thus, bone loss in the aged mice may be related to the aging process and to functional changes in tooth movement associated with aging. Taken together, these findings indicate that the 40-kDa OMP is an effective Ag for the induction of protective immune responses against P. gingivalis infection; however, mucosal immunization with the 40-kDa OMP alone induced only low levels of IgG anti-40-kDa OMP Abs in sera. IgA Abs were not detected in either serum or saliva samples. Indeed, the 40-kDa OMP without an adjuvant is a weak immunogen when given via the nasal route (43). Our previous studies have demonstrated that transcutaneous administration of the 40-kDa OMP without an adjuvant elicits a substantial IgG but not IgA Ab response in the sera and saliva of mice (36) and rats (30). However, transcutaneous immunization with the 40-kDa OMP alone failed to provide protection against bone loss caused by oral infection with P. gingivalis (unpublished observation). The findings of these studies together with our results in the present study indicate that mucosal adjuvants such as mCTA/LTB are required for the induction of the protective immune responses against oral infection with P. gingivalis when the 40-kDa OMP is given by mucosal routes.
CT and LT have been used widely as adjuvants for mucosal immunization, and our results indicate that the nasal administration of nCT with the 40-kDa OMP has an adjuvant effect and induces 40-kDa-OMP-specific mucosal IgA, as well as IgG and IgA Abs in serum (43). However, despite these beneficial attributes, CT and LT are unsuitable for use in humans because they cause severe diarrhea (51). To establish molecules that are nontoxic but retain adjuvant activity, several groups, including ours, have developed nontoxic mutant derivatives of CT or LT that may be suitable for use in humans (9, 11-13, 15, 18, 35, 44, 60). We previously showed that mCTA/LTB does not induce increases in intracellular cyclic AMP and fails to elicit fluid accumulation in ligated ileal loops (33). Furthermore, the nasal administration of an influenza vaccine together with the adjuvant mCTA/LTB elicits significant Ag-specific IgG and IgA Abs in the lung and provides complete protection against mucosal infection with influenza virus (33). The results of these studies demonstrate that mCTA/LTB is an effective adjuvant for nasal immunization and that, when given with the 40-kDa OMP, it facilitates the development of a long-term protective Ab response to the 40-kDa OMP. These results are the first to show that a nontoxic chimeric molecule combining the E112K mCTA and LTB induces a long-term protective Ab response to coadministered 40-kDa OMP in both serum and mucosal secretions.
As a mucosal adjuvant, CT given via mucosal routes induces Th2 cells that secrete high levels of IL-4 (55, 57, 58); however, the IL-4 cytokine provides a helper signal for the induction of IgE Abs that may cause anaphylactic reactions (27, 38). In this regard, our previous study demonstrated that biased Th1 and Th2 responses depend on the presence of the LT B subunit or the CT B subunit and that the CTA/LTB chimera, similar to LT, induces a gamma interferon- and IL-4-independent Th2 response while the chimera comprising the LT A subunit and the CT B subunit, similar to CT, elicits high-level IL-4 production along with an IgE Ab response (6). These results suggest that the B subunits of enterotoxins regulate T helper responses and that mCTA/LTB induces a low-level IL-4 response with subsequently reduced IgE Abs compared to those induced by the LT A subunit/CT B subunit chimera. Indeed, in a previous study, the nasal administration of tetanus toxoid together with chimeric mCTA/LTB induced a marginal IgE Ab response (33). The results of this study also showed that nasal 40-kDa OMP plus mCTA/LTB induced significantly weaker 40-kDa-OMP-specific IL-4 and IgE Ab responses than the 40-kDa OMP plus nCT. Taken together with the present results, these data suggest that the mCTA/LTB chimera has the beneficial features of both enterotoxins and avoids the danger of allergic reactions provoked by IgE Abs.
The immunization protocol used in this study was designed to induce a significant 40-kDa-OMP-specific Ab response in the oral cavity. We selected nasal administration as the delivery route because of its successful record (6, 9, 12, 13, 18, 43, 56-59). However, nasally administered CT accumulates in the olfactory nerves and epithelial regions via GM-1 ganglioside (54). When used as a mucosal adjuvant, CT can influence the trafficking of coadministered protein Ag into these neuronal tissues (54). These findings raise some concerns about nasal administration and the potential threat posed by GM-1-binding molecules targeting neural tissues, including the central nervous system. However, another study has demonstrated that the deposition of CT in the olfactory tissues does not lead to obvious pathological changes in the brain after nasal administration (20). Although the exact effects of a nasally administered enterotoxin adjuvant on the central nervous system remain uncertain, a previous study has demonstrated that, unlike nCT, mCTA/LTB does not influence the trafficking of coadministered protein into the olfactory nerves and epithelium or into olfactory bulbs while mCTA/LTB itself accumulates in olfactory central nervous system regions (31). Taken together, these results suggest that mCTA/LTB is a more promising mucosal adjuvant than nCT.
The present results indicated that 40-kDa-OMP-specific IgG Abs inhibited the coaggregation and hemagglutinin activities of P. gingivalis. In this regard, it is known that immune responses in the oral cavity are derived from both the mucosal and systemic immune systems. The salivary glands, part of the mucosal immune system, are known to produce secretory IgA Abs in saliva. On the other hand, serum-derived IgG Ab-rich crevicular fluid, which continuously flows from the gingival capillaries, is part of the systemic immune system (7). Because P. gingivalis colonizes subgingival and supragingival biofilms (16, 31, 52), the generation of serum-derived IgG Abs in crevicular fluid, in addition to the IgA Ab response in saliva, may be effective in preventing P. gingivalis colonization.
In summary, this study has provided evidence that the nasal administration of the 40-kDa OMP together with nontoxic mCTA/LTB elicits 40-kDa-OMP-specific IgG and IgA in serum, as well as mucosal IgA Ab responses in saliva. These Ab responses persisted for more than 1 year after immunization. Moreover, 40-kDa-OMP-specific immune responses induced by the 40-kDa OMP plus mCTA/LTB provided protective immunity against alveolar bone loss caused by P. gingivalis infection. These findings suggest that nasally administered 40-kDa OMP with mCTA/LTB effectively elicits protective levels of Abs against the 40-kDa OMP and should therefore be considered as a candidate vaccine to immunize humans against P. gingivalis infection.
Acknowledgments
This work was supported by grants-in-aid for scientific research (18592270, 19791624, and 19390537) from the Japan Society for the Promotion of Science and an “Academic Frontier” Project for Private Universities matching-fund subsidy from the Ministry of Education, Culture, Sports, Science and Technology, 2007 to 2011.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 14 April 2008.
REFERENCES
- 1.Abiko, Y., N. Ogura, U. Matsuda, K. Yanagi, and H. Takiguchi. 1997. A human monoclonal antibody which inhibits the coaggregation activity of Porphyromonas gingivalis. Infect. Immun. 653966-3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amstad-Jossi, M., and H. E. Schroeder. 1978. Age-related alterations of periodontal structures around the cemento-enamel junction and of the gingival connective tissue composition in germ-free rats. J. Periodontal Res. 1376-90. [DOI] [PubMed] [Google Scholar]
- 3.Baker, P. J., R. T. Evans, and D. C. Roopenian. 1994. Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Arch. Oral Biol. 391035-1040. [DOI] [PubMed] [Google Scholar]
- 4.Barnett, N. A., and D. J. Rowe. 1986. A comparison of alveolar bone in young and aged mice. J. Periodontol. 57447-452. [DOI] [PubMed] [Google Scholar]
- 5.Beck, J., R. Garcia, G. Heiss, P. S. Vokonas, and S. Offenbacher. 1996. Periodontal disease and cardiovascular disease. J. Periodontol. 671123-1137. [DOI] [PubMed] [Google Scholar]
- 6.Boyaka, P. N., M. Ohmura, K. Fujihashi, T. Koga, M. Yamamoto, M. N. Kweon, Y. Takeda, R. J. Jackson, H. Kiyono, Y. Yuki, and J. R. McGhee. 2003. Chimeras of labile toxin one and cholera toxin retain mucosal adjuvanticity and direct Th cell subsets via their B subunit. J. Immunol. 170454-462. [DOI] [PubMed] [Google Scholar]
- 7.Challacombe, S. J., and P. J. Shirlaw. 1999. Immunity of diseases of the oral cavity, p. 1313-1337. In P. L. Ogra, J. Mestecky, M. E. Lamm, W. Strober, J. Bienenstock, and J. R. McGhee (ed.), Mucosal immunology. Academic Press, San Diego, CA.
- 8.Cutler, C. W., J. R. Kalmar, and C. A. Genco. 1995. Pathogenic strategies of the oral anaerobe, Porphyromonas gingivalis. Trends Microbiol. 345-51. [DOI] [PubMed] [Google Scholar]
- 9.de Haan, L., W. R. Verweij, I. K. Feil, T. H. Lijnema, W. G. Hol, E. Agsteribbe, and J. Wilschut. 1996. Mutants of the Escherichia coli heat-labile enterotoxin with reduced ADP-ribosylation activity or no activity retain the immunogenic properties of the native holotoxin. Infect. Immun. 645413-5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeStefano, F., R. F. Anda, H. S. Kahn, D. F. Williamson, and C. M. Russell. 1993. Dental disease and risk of coronary heart disease and mortality. BMJ 306688-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickinson, B. L., and J. D. Clements. 1995. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect. Immun. 631617-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Tommaso, A., G. Saletti, M. Pizza, R. Rappuoli, G. Dougan, S. Abrignani, G. Douce, and M. T. De Magistris. 1996. Induction of antigen-specific antibodies in vaginal secretions by using a nontoxic mutant of heat-labile enterotoxin as a mucosal adjuvant. Infect. Immun. 64974-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douce, G., C. Turcotte, I. Cropley, M. Roberts, M. Pizza, M. Domenghini, R. Rappuoli, and G. Dougan. 1995. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as nontoxic, mucosal adjuvants. Proc. Natl. Acad. Sci. USA 921644-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellen, R. P., and D. A. Grove. 1989. Bacteroides gingivalis vesicles bind to and aggregate Actinomyces viscosus. Infect. Immun. 571618-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontana, M. R., R. Manetti, V. Giannelli, C. Magagnoli, A. Marchini, R. Olivieri, M. Domenighini, R. Rappuoli, and M. Pizza. 1995. Construction of nontoxic derivatives of cholera toxin and characterization of the immunological response against the A subunit. Infect. Immun. 632356-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibbons, R. J., and M. Nygaard. 1970. Interbacterial aggregation of plaque bacteria. Arch. Oral Biol. 151397-1400. [DOI] [PubMed] [Google Scholar]
- 17.Gibson, F. C., III, C. Hong, H. H. Chou, H. Yumoto, J. Chen, E. Lien, J. Wong, and C. A. Genco. 2004. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation 1092801-2806. [DOI] [PubMed] [Google Scholar]
- 18.Giuliani, M. M., G. Del Giudice, V. Giannelli, G. Dougan, G. Douce, R. Rappuoli, and M. Pizza. 1998. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knockout of ADP-ribosyltransferase activity. J. Exp. Med. 1871123-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grossi, S. G., and R. J. Genco. 1998. Periodontal disease and diabetes mellitus: a two-way relationship. Ann. Periodontol. 351-61. [DOI] [PubMed] [Google Scholar]
- 20.Hagiwara, Y., T. Iwasaki, H. Asanuma, Y. Sato, T. Sata, C. Aizawa, T. Kurata, and S. Tamura. 2001. Effects of intranasal administration of cholera toxin (or Escherichia coli heat-labile enterotoxin) B subunits supplemented with a trace amount of the holotoxin on the brain. Vaccine 191652-1660. [DOI] [PubMed] [Google Scholar]
- 21.Hamada, N., K. Watanabe, T. Tahara, K. Nakazawa, I. Ishida, Y. Shibata, T. Kobayashi, H. Yoshie, Y. Abiko, and T. Umemoto. 2007. The r40-kDa outer membrane protein human monoclonal antibody protects against Porphyromonas gingivalis-induced bone loss in rats. J. Periodontol. 78933-939. [DOI] [PubMed] [Google Scholar]
- 22.Hamajima, S., M. Maruyama, T. Hijiya, H. Hatta, and Y. Abiko. 2007. Egg yolk-derived immunoglobulin (IgY) against Porphyromonas gingivalis 40-kDa outer membrane protein inhibits coaggregation activity. Arch. Oral Biol. 52697-704. [DOI] [PubMed] [Google Scholar]
- 23.Hiratsuka, K., Y. Abiko, M. Hayakawa, T. Ito, H. Sasahara, and H. Takiguchi. 1992. Role of Porphyromonas gingivalis 40-kDa outer membrane protein in the aggregation of P. gingivalis vesicles and Actinomyces viscosus. Arch. Oral Biol. 37717-724. [DOI] [PubMed] [Google Scholar]
- 24.Holt, S. C., L. Kesavalu, S. Walker, and C. A. Genco. 1999. Virulence factors of Porphyromonas gingivalis. Periodontol. 2000 20168-238. [DOI] [PubMed] [Google Scholar]
- 25.Katoh, M., S. Saito, H. Takiguchi, and Y. Abiko. 2000. Bactericidal activity of a monoclonal antibody against a recombinant 40-kDa outer membrane protein of Porphyromonas gingivalis. J. Periodontol. 71368-375. [DOI] [PubMed] [Google Scholar]
- 26.Kawamoto, Y., M. Hayakawa, and Y. Abiko. 1991. Purification and immunochemical characterization of a recombinant outer membrane protein from Bacteroides gingivalis. Int. J. Biochem. 231053-1061. [DOI] [PubMed] [Google Scholar]
- 27.Kelso, J. M., R. T. Jones, and J. W. Yunginger. 1993. Anaphylaxis to measles, mumps, and rubella vaccine mediated by IgE to gelatin. J. Allergy Clin. Immunol. 91867-872. [DOI] [PubMed] [Google Scholar]
- 28.Kinane, D. F. 1998. Periodontal diseases' contributions to cardiovascular disease: an overview of potential mechanisms. Ann. Periodontol. 3142-150. [DOI] [PubMed] [Google Scholar]
- 29.Klausen, B., R. T. Evans, and C. Sfintescu. 1989. Two complementary methods of assessing periodontal bone level in rats. Scand. J. Dent. Res. 97494-499. [DOI] [PubMed] [Google Scholar]
- 30.Koizumi, Y., T. Kurita-Ochiai, and M. Yamamoto. 2008. Transcutaneous immunization with an outer membrane protein of Porphyromonas gingivalis without adjuvant elicits marked antibody responses. Oral Microbiol. Immunol. 23131-138. [DOI] [PubMed] [Google Scholar]
- 31.Kolenbrander, P. E., and R. N. Andersen. 1984. Cell to cell interactions of Capnocytophaga and Bacteroides species with other oral bacteria and their potential role in development of plaque. J. Periodontal Res. 19564-569. [DOI] [PubMed] [Google Scholar]
- 32.Kweon, M. N., M. Yamamoto, M. Kajiki, I. Takahashi, and H. Kiyono. 2000. Systemically derived large intestinal CD4+ Th2 cells play a central role in STAT6-mediated allergic diarrhea. J. Clin. Investig. 106199-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kweon, M. N., M. Yamamoto, F. Watanabe, S. Tamura, F. W. Van Ginkel, A. Miyauchi, H. Takagi, Y. Takeda, T. Hamabata, K. Fujihashi, J. R. McGhee, and H. Kiyono. 2002. A nontoxic chimeric enterotoxin adjuvant induces protective immunity in both mucosal and systemic compartments with reduced IgE antibodies. J. Infect. Dis. 1861261-1269. [DOI] [PubMed] [Google Scholar]
- 34.Lalla, E., I. B. Lamster, M. A. Hofmann, L. Bucciarelli, A. P. Jerud, S. Tucker, Y. Lu, P. N. Papapanou, and A. M. Schmidt. 2003. Oral infection with a periodontal pathogen accelerates early atherosclerosis in apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 231405-1411. [DOI] [PubMed] [Google Scholar]
- 35.Lycke, N., T. Tsuji, and J. Holmgren. 1992. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur. J. Immunol. 222277-2281. [DOI] [PubMed] [Google Scholar]
- 36.Maeba, S., S. Otake, J. Namikoshi, Y. Shibata, M. Hayakawa, Y. Abiko, and M. Yamamoto. 2005. Transcutaneous immunization with a 40-kDa outer membrane protein of Porphyromonas gingivalis induces specific antibodies which inhibit coaggregation by P. gingivalis. Vaccine 232513-2521. [DOI] [PubMed] [Google Scholar]
- 37.Maiden, M. F., R. J. Carman, M. A. Curtis, I. R. Gillett, G. S. Griffiths, J. A. Sterne, J. M. Wilton, and N. W. Johnson. 1990. Detection of high-risk groups and individuals for periodontal diseases: laboratory markers based on the microbiological analysis of subgingival plaque. J. Clin. Periodontol. 171-13. [DOI] [PubMed] [Google Scholar]
- 38.Marinaro, M., H. F. Staats, T. Hiroi, R. J. Jackson, M. Coste, P. N. Boyaka, N. Okahashi, M. Yamamoto, H. Kiyono, H. Bluethmann, et al. 1995. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J. Immunol. 1554621-4629. [PubMed] [Google Scholar]
- 39.Mealey, B. L. 1999. Influence of periodontal infections on systemic health. Periodontol. 2000 21197-209. [DOI] [PubMed] [Google Scholar]
- 40.Messer, H. H. 1980. Alveolar bone loss in a strain of mice. J. Periodontal Res. 15193-205. [DOI] [PubMed] [Google Scholar]
- 41.Meyer, D. H., and P. M. Fives-Taylor. 1998. Oral pathogens: from dental plaque to cardiac disease. Curr. Opin. Microbiol. 188-95. [DOI] [PubMed] [Google Scholar]
- 42.Miyauchi, A., M. Ozawa, M. Mizukami, K. Yashiro, S. Ebisu, T. Tojo, T. Fujii, and H. Takagi. 1999. Structural conversion from non-native to native form of recombinant human epidermal growth factor by Brevibacillus choshinensis. Biosci. Biotechnol. Biochem. 631965-1969. [DOI] [PubMed] [Google Scholar]
- 43.Namikoshi, J., S. Otake, S. Maeba, M. Hayakawa, Y. Abiko, and M. Yamamoto. 2003. Specific antibodies induced by nasally administered 40-kDa outer membrane protein of Porphyromonas gingivalis inhibit coaggregation activity of P. gingivalis. Vaccine 22250-256. [DOI] [PubMed] [Google Scholar]
- 44.Pizza, M., M. R. Fontana, M. M. Giuliani, M. Domenighini, C. Magagnoli, V. Giannelli, D. Nucci, W. Hol, R. Manetti, and R. Rappuoli. 1994. A genetically detoxified derivative of heat-labile Escherichia coli enterotoxin induces neutralizing antibodies against the A subunit. J. Exp. Med. 1802147-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reddy, M. S. 2002. Oral osteoporosis: is there an association between periodontitis and osteoporosis? Compend. Contin. Educ. Dent. 2321-28. [PubMed] [Google Scholar]
- 46.Saito, S., M. Hayakawa, K. Hiratsuka, H. Takiguchi, and Y. Abiko. 1996. Complement-mediated killing of Porphyromonas gingivalis 381 by the immunoglobulin G induced by recombinant 40-kDa outer membrane protein. Biochem. Mol. Med. 58184-191. [DOI] [PubMed] [Google Scholar]
- 47.Saito, S., K. Hiratsuka, M. Hayakawa, H. Takiguchi, and Y. Abiko. 1997. Inhibition of a Porphyromonas gingivalis colonizing factor between Actinomyces viscosus ATCC 19246 by monoclonal antibodies against recombinant 40-kDa outer-membrane protein. Gen. Pharmacol. 28675-680. [DOI] [PubMed] [Google Scholar]
- 48.Sashima, M., M. Satoh, and A. Suzuki. 1990. Alveolar bone loss of senescence-accelerated mouse (SAM). J. Dent. Res. 6982-86. [DOI] [PubMed] [Google Scholar]
- 49.Shibata, Y., K. Hiratsuka, M. Hayakawa, T. Shiroza, H. Takiguchi, Y. Nagatsuka, and Y. Abiko. 2003. A 35-kDa co-aggregation factor is a hemin binding protein in Porphyromonas gingivalis. Biochem. Biophys. Res. Commun. 300351-356. [DOI] [PubMed] [Google Scholar]
- 50.Shibata, Y., Y. Hosogi, M. Hayakawa, N. Hori, M. Kamada, and Y. Abiko. 2005. Construction of novel human monoclonal antibodies neutralizing Porphyromonas gingivalis hemagglutination activity using transgenic mice expressing human Ig loci. Vaccine 233850-3856. [DOI] [PubMed] [Google Scholar]
- 51.Spangler, B. D. 1992. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol. Rev. 56622-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Theilade, E., J. Theilade, and L. Mikkelsen. 1982. Microbiological studies on early dento-gingival plaque on teeth and Mylar strips in humans. J. Periodontal Res. 1712-25. [DOI] [PubMed] [Google Scholar]
- 53.VanCott, J. L., H. F. Staats, D. W. Pascual, M. Roberts, S. N. Chatfield, M. Yamamoto, M. Coste, P. B. Carter, H. Kiyono, and J. R. McGhee. 1996. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. J. Immunol. 1561504-1514. [PubMed] [Google Scholar]
- 54.van Ginkel, F. W., R. J. Jackson, Y. Yuki, and J. R. McGhee. 2000. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J. Immunol. 1654778-4782. [DOI] [PubMed] [Google Scholar]
- 55.Xu-Amano, J., H. Kiyono, R. J. Jackson, H. F. Staats, K. Fujihashi, P. D. Burrows, C. O. Elson, S. Pillai, and J. R. McGhee. 1993. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J. Exp. Med. 1781309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamamoto, M., D. E. Briles, S. Yamamoto, M. Ohmura, H. Kiyono, and J. R. McGhee. 1998. A nontoxic adjuvant for mucosal immunity to pneumococcal surface protein A. J. Immunol. 1614115-4121. [PubMed] [Google Scholar]
- 57.Yamamoto, M., H. Kiyono, M. N. Kweon, S. Yamamoto, K. Fujihashi, H. Kurazono, K. Imaoka, H. Bluethmann, I. Takahashi, Y. Takeda, M. Azuma, and J. R. McGhee. 2000. Enterotoxin adjuvants have direct effects on T cells and antigen-presenting cells that result in either interleukin-4-dependent or -independent immune responses. J. Infect. Dis. 182180-190. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto, M., J. R. McGhee, Y. Hagiwara, S. Otake, and H. Kiyono. 2001. Genetically manipulated bacterial toxin as a new generation mucosal adjuvant. Scand. J. Immunol. 53211-217. [DOI] [PubMed] [Google Scholar]
- 59.Yamamoto, S., H. Kiyono, M. Yamamoto, K. Imaoka, K. Fujihashi, F. W. Van Ginkel, M. Noda, Y. Takeda, and J. R. McGhee. 1997. A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc. Natl. Acad. Sci. USA 945267-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamamoto, S., Y. Takeda, M. Yamamoto, H. Kurazono, K. Imaoka, K. Fujihashi, M. Noda, H. Kiyono, and J. R. McGhee. 1997. Mutants in the ADP-ribosyltransferase cleft of cholera toxin lack diarrheagenicity but retain adjuvanticity. J. Exp. Med. 1851203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]