Abstract
LipL32 is the major leptospiral outer membrane lipoprotein expressed during infection and is the immunodominant antigen recognized during the humoral immune response to leptospirosis in humans. In this study, we investigated novel aspects of LipL32. In order to define the immunodominant domains(s) of the molecule, subfragments corresponding to the N-terminal, intermediate, and C-terminal portions of the LipL32 gene were cloned and the proteins were expressed and purified by metal affinity chromatography. Our immunoblot results indicate that the C-terminal and intermediate domains of LipL32 are recognized by sera of patients with laboratory-confirmed leptospirosis. An immunoglobulin M response was detected exclusively against the LipL32 C-terminal fragment in both the acute and convalescent phases of illness. We also evaluated the capacity of LipL32 to interact with extracellular matrix (ECM) components. Dose-dependent, specific binding of LipL32 to collagen type IV and plasma fibronectin was observed, and the binding capacity could be attributed to the C-terminal portion of this molecule. Both heparin and gelatin could inhibit LipL32 binding to fibronectin in a concentration-dependent manner, indicating that the 30-kDa heparin-binding and 45-kDa gelatin-binding domains of fibronectin are involved in this interaction. Taken together, our results provide evidence that the LipL32 C terminus is recognized early in the course of infection and is the domain responsible for mediating interaction with ECM proteins.
Leptospirosis, caused by spirochetes of the genus Leptospira, is a widespread zoonosis that remains a public health concern, notably in tropical and subtropical regions. Humans become infected through contact with water, food, or soil containing urine from infected animals. Clinical manifestations range from mild (subclinical infection) to severe and potentially lethal forms characterized by high fever, intense jaundice, bleeding, renal and pulmonary dysfunction, neurologic alterations, and cardiovascular collapse. Pulmonary hemorrhage has been reported to be increasing in recent years, affecting up to 70% of the patients, and has been considered a serious life-threatening concern, becoming the main cause of death due to leptospirosis in some countries (3, 17, 26, 35).
Within the last few years, considerable research has been conducted on outer membrane proteins expressed by Leptospira spp. during infection, prompted by the necessity of developing subunit vaccines or characterizing antigens suitable for early immunodiagnosis of the disease. In this context, putative virulence factors presumed to have a role in adhesion to host tissues, such as the Lig proteins (11) and the leptospiral endostatin-like (Len) outer membrane proteins (1, 37), as well as in complement evasion (LenA/LenB) (37, 38), constitute attractive vaccine candidates.
The most abundant antigen found in the leptospiral total protein profile is LipL32 (40), a lipoprotein displaying a calculated molecular mass of 26.7 kDa but an observed electrophoretic mobility of approximately 32 kDa (22). LipL32 is highly conserved among pathogenic Leptospira species (22) but has no orthologs in the saprophyte Leptospira biflexa (32). It has been shown to enhance hemolysis mediated by sphingomyelinase SphH, and for this reason, the protein was also identified as hemolysis-associated protein Hap-1 (25). Expressed at high levels both during cultivation and during natural infection, LipL32 was shown to be surface exposed and highly immunogenic (14, 15, 21, 22). It has been evaluated as an antigen for immunodiagnosis (4, 16, 19) and as a vaccine antigen, showing protection against Leptospira interrogans challenge in animals immunized with recombinant adenovirus (6), DNA vaccine (7), or recombinant Mycobacterium bovis BCG (36).
In this work, we investigated novel aspects of LipL32. First, we aimed to define the immunogenic portions of the molecule. Our data indicate that both the C terminus and the intermediate portion of LipL32 are recognized by human sera, with the C terminus being detected earlier in the course of infection. We also wondered whether LipL32, as a major leptospiral outer membrane lipoprotein expressed during infection, could contribute to tissue invasion and colonization by interacting with extracellular matrix (ECM). LipL32 interacted with collagen type IV and also with plasma fibronectin in a dose-dependent manner. These interactions were mediated by the LipL32 C terminus.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Leptospiral strains (L. interrogans serovars Canicola, Pyrogenes, Pomona, Autumnalis, Hardjo, Bratislava, Copenhageni, and Icterohaemorrhagiae; L. biflexa serovar Patoc; and L. kirchneri serovar Grippotyphosa) were grown at 29°C under aerobic conditions in liquid EMJH medium (Difco) with 10% rabbit serum, enriched with l-asparagine (wt/vol, 0.015%), sodium pyruvate (wt/vol, 0.001%), calcium chloride (wt/vol, 0.001%), magnesium chloride (wt/vol, 0.001%), peptone (wt/vol, 0.03%), and meat extract (wt/vol, 0.02%). Escherichia coli DH5α was used as the cloning host strain, and E. coli BL21 Star(DE3)pLysS (Novagen) or E. coli BL21 SI (Invitrogen) was used as the host strain for the expression of the recombinant LipL32 or LipL32 subfragment with the T7 promoter-based expression plasmid pAE (33). E. coli cells were grown in 2YT or 2YT ON medium supplemented with specific antibiotics (ampicillin and/or chloramphenicol).
Patients.
Sera from patients with leptospirosis were obtained from the Instituto Adolfo Lutz collection, São Paulo, Brazil. Two serum samples, corresponding to the acute and convalescent phases of illness, were obtained from each of the 12 patients. The criteria for a diagnosis of leptospirosis were a MAT (microscopic agglutination test) with a fourfold antibody titer increase or a conversion from seronegativity to a titer of 1/200 or greater. All patients were hospitalized with symptoms of leptospirosis. Data concerning MAT titers, onset of disease, and infecting serovars are shown in Table 1.
TABLE 1.
MAT titers, onset of the disease, infecting serovar, and detection of antibodies in serum samples from 12 patients with leptospirosisa
| Patient | Sera with IgM reactivity
|
Sera with IgG reactivity
|
Reciprocal MAT titer | No. of days after initiation of symptoms
|
Leptospira serovar reactivity | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute phase
|
Convalescent phase
|
Acute phase
|
Convalescent phase
|
|||||||||||||||||
| L | N | I | C | L | N | I | C | L | N | I | C | L | N | I | C | MAT− | MAT+ | |||
| 1 | + | − | − | + | + | − | − | + | + | − | − | − | + | − | + | − | 6,400 | Unknown | Unknown | Icterohaemorrhagiae, Copenhageni |
| 2 | + | − | − | + | + | − | − | + | + | − | − | + | + | − | + | − | 25,600 | 6 | 23 | Autumnalis |
| 3 | + | − | − | + | + | − | − | + | + | − | − | + | + | − | + | + | 3,200 | 4 | 30 | Icterohaemorrhagiae |
| 4 | + | − | − | + | + | − | − | + | + | − | − | − | + | − | + | + | 25,600 | 1 | 17 | Cynopteri |
| 5 | + | − | − | + | + | − | − | + | + | − | − | − | + | − | + | − | 3,200 | 2 | 9 | Cynopteri |
| 6 | + | − | − | + | + | − | − | + | + | − | − | − | + | − | + | − | 1,600 | 11 | 17 | Many serovars, inconclusive |
| 7 | + | − | − | + | + | − | − | + | + | − | − | − | + | − | + | − | 3,200 | 13 | 21 | Icterohaemorrhagiae |
| 8 | + | − | − | + | + | − | − | + | + | − | − | + | + | − | − | − | 3,200 | 5 | 11 | Icterohaemorrhagiae, Copenhageni |
| 9 | + | − | − | + | + | − | − | + | + | − | − | + | + | − | + | + | 1,600 | 6 | 14 | Copenhageni |
| 10 | + | − | − | + | + | − | − | + | + | − | + | + | + | − | + | + | 1,600 | 4 | 9 | Copenhageni |
| 11 | + | − | − | + | + | − | − | + | + | − | − | − | + | − | − | − | 6,400 | 4 | 17 | Autumnalis |
| 12 | + | − | − | + | + | − | − | + | + | − | − | − | + | − | + | + | 6,400 | 6 | 31 | Icterohaemorrhagiae, Copenhageni |
L, intact LipL32; N, LipL32 N terminus; I, LipL32 intermediate subfragment; C, LipL32 C terminus.
Cloning, expression, and purification of LipL32 and LipL32 subfragments in E. coli.
LipL32 was cloned as previously described (20), and LipL32 subfragments (N terminus, intermediate, and C terminus) were amplified by PCR from total L. interrogans serovar Copenhageni genomic DNA (strain Fiocruz L1-130) with the following primers: N-terminus_F, 5′-CTCGAGCATATGGGTGCTTTCGGTGGTCTG-3′; N-terminus_R, 5′-AAGCTTTTAAGCGATTACGGCAGGAAT-3′; intermediate_F, 5′-CTCGGATGGAAATGGGAGTTCGTATG-3′; intermediate_R, 5′-AGCTTTTAGATTCTAGTAAGAGAGTTGT-3′; C-terminus_F, 5′-CTCGAGATGAAGATCCCTAATCCTCCA-3′; C-terminus_R, 5′-AAGCTTACTTAGTCGCGTCAGAAGC-3′. Underlined nucleotides indicate restriction sites (XhoI/HindIII); nucleotides in bold represent an alternative restriction site (NdeI) in the N terminus forward primer. The amplified products were cloned into the pGEM-T Easy vector (Promega) and subcloned into the pAE expression vector (33). This vector allows the expression of recombinant proteins with a minimal His6 tag at the N terminus. The C terminus and LipL32 constructs were transformed into E. coli BL21 SI. The N terminus and intermediate constructs were transformed into E. coli BL21 Star(DE3)pLysS, since low yields had been obtained with E. coli BL21 SI. E. coli strains containing the recombinant plasmids were grown in 1 liter of 2YT with ampicillin or chloramphenicol or 2YT ON with ampicillin until the culture optical density at 600 nm reached 0.6. The expression of the N-terminal and intermediate fragments was induced by incubation with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h at 37°C. LipL32 and C terminus expression was induced by incubation with 300 mM NaCl for 3 h at 30°C in E. coli strain BL21 SI by the action of T7 DNA polymerase under the control of the osmotically induced proU promoter (2). Cells were collected by centrifugation, resuspended in 100 ml of 150 mM Tris-HCl (pH 8.0) or in phosphate-buffered saline (PBS), and lysed in a French press (Thermo Spectronic). The soluble and insoluble fractions were isolated by centrifugation at 8,400 × g for 10 min. Purification of recombinant proteins proceeded as follows. A column (1-cm diameter) filled with 5 ml of Ni2+-charged chelating Sepharose (GE Healthcare) was equilibrated with 150 mM Tris-HCl, pH 8.0, with or without 8 M urea or PBS. After adsorption of LipL32 subfragments or LipL32, the resin was washed with 10 column volumes of 150 mM Tris-HCl, pH 8.0 (intermediate and C-terminal fragments); 150 mM Tris-HCl-8 M urea, pH 8.0 (N-terminal fragment); or PBS (LipL32) containing 5, 20, 40, and 60 mM imidazole. Proteins were eluted with 5 volumes of the solutions described above containing 1 M imidazole, and the eluted fractions were analyzed by 18 or 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were dialyzed in three steps with 2 liters of 150 mM Tris-HCl, pH 8.0, or PBS. The N-terminal fragment was purified and kept under denaturing conditions to avoid protein precipitation (8 M urea).
Antisera against LipL32 and LipL32 subfragments.
Five- to 8-week-old female BALB/C mice were immunized intraperitoneally with 10 μg of purified LipL32 subfragments (N terminus, intermediate, and C terminus) or intact LipL32 in Al(OH)3. Immunizations were performed over a period of 4 weeks, with booster doses every week. Mice were bled via the retro-orbital plexus 1 week after the last immunization, and the blood was incubated for 30 min at 37°C. The clot was removed by centrifugation, and sera were collected from supernatants and stored at −20°C.
Immunoblot analyses.
Leptospira extracts were fractionated by 18% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were incubated overnight with 10% (wt/vol) nonfat dried milk in PBS-0.05% Tween 20 (PBST), and after three washes with PBS-T, they were incubated with mouse anti-N terminus, anti-intermediate, anti-C terminus, or anti-LipL32 serum in 5% nonfat dried milk-PBS-T for 1 h. After three washes with PBS-T, the membranes were incubated with a 1:10,000 dilution of goat anti-mouse immunoglobulin G (IgG)-peroxidase conjugate (Sigma) in 5% nonfat dried milk-PBS-T, washed, and developed with ECL reagent (GE Healthcare). Alternatively, recombinant LipL32 subfragments and LipL32 were fractionated by SDS-PAGE, transferred to nitrocellulose membranes, blocked with 5% (wt/vol) nonfat dried milk and 2.5% bovine serum albumin in PBS-T, and incubated with serum from mice immunized with recombinant LipL32 or with sera from patients diagnosed with leptospirosis (acute and convalescent phases). To minimize the background, patients' sera were preabsorbed with 25% E. coli BL21 Star(DE3)pLysS extract for 30 min at room temperature with agitation. Blots were developed with goat anti-human IgM (μ chain specific)- or IgG (γ chain specific)-peroxidase conjugate (Sigma) as described above.
Binding of LipL32 to ECM components.
All macromolecules, including fetuin, were purchased from Sigma Chemical Co. Laminin 1 and collagen type IV were derived from the basement membrane of Engelbreth-Holm-Swarm mouse sarcoma, cellular fibronectin was derived from human foreskin fibroblasts, plasma fibronectin was isolated from human plasma, collagen type I was isolated from rat tail, and the plasma fibronectin proteolytic fragments of 30 kDa (heparin-binding domain) and 45 kDa (gelatin-binding domain) were from human plasma. Protein attachment to individual macromolecules of the ECM was analyzed according to a previously published protocol (1). Three independent experiments were performed, each one in triplicate. Briefly, enzyme-linked immunosorbent assay (ELISA) plate wells (Nunc-Immuno Plate MaxiSorp Surface) were coated with 1 μg of laminin, collagen type I, collagen type IV, cellular fibronectin, plasma fibronectin, and fetuin (highly glycosylated attachment-negative control protein) in 100 μl of PBS and incubated for 2 h at 37°C. The wells were washed three times with PBST and then blocked with 200 μl of 1% bovine serum albumin for 1 h at 37°C, followed by an overnight incubation at 4°C. One microgram of recombinant protein was added per well in 100 μl of PBS, and the protein was allowed to attach to the different substrates for 90 min at 37°C. After washing six times with PBST, bound protein was detected by adding 100 μl of a 1:10,000 dilution of mouse anti-LipL32 serum in PBS. Incubation proceeded for 1 h, and after three washes with PBST, 100 μl of a 1:5,000 dilution of horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) in PBS was added per well and the plate was incubated for 1 h. All incubations took place at 37°C. The wells were washed three times, and o-phenylenediamine (0.04%) in citrate phosphate buffer (pH 5.0) plus 0.01% (wt/vol) H2O2 was added. The reaction was allowed to proceed for 15 min and was then interrupted by the addition of 50 μl of 8 M H2SO4. The absorbance at 492 nm was determined in a microplate reader (Labsystems Uniscience Multiskan EX). For statistical analyses, the attachment of LipL32 to ECM macromolecules was compared to its binding to fetuin by Student's two-tailed t test.
For determination of dose-dependent attachment of LipL32, as well as its intermediate and C-terminal fragments, to collagen type IV and plasma fibronectin (intact molecule and F30 and F45 domains), protein concentrations varying from 0 to 4 μM in PBS were used. Competitive binding assays involving F30, F45, and collagen type IV were performed with increasing concentrations of the C-terminal or intermediate subfragment (0 to 7 μM) in the presence of 1.5 μM LipL32. The capacity of heparin (Liquemine; Roche) and gelatin (Sigma) to compete for the binding of the LipL32 C-terminal domain to F30 and F45, respectively, was assayed by incubating the recombinant proteins at 2 μM in the presence of heparin (0 to 500 IU) or gelatin (0 to 50 μg). Bound proteins were detected as described for the protein-binding assay.
RESULTS
Expression and purification of LipL32 and LipL32 fragments.
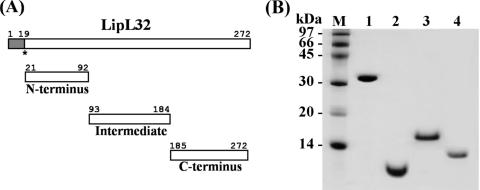
Fragments corresponding to the N-terminal, intermediate, and C-terminal portions of the LipL32 gene (Fig. 1A) were amplified by PCR from genomic Leptospira DNA and cloned into the pAE expression vector. Recombinant LipL32 (30.7 kDa) and the C-terminal subfragment (10.5 kDa) were expressed in E. coli BL21 SI, both in the soluble fraction. The N-terminal (8.5 kDa) and intermediate (11.5 kDa) subfragments were expressed in E. coli BL21 Star(DE3)pLysS in the insoluble and soluble fractions, respectively. The soluble and insoluble proteins were purified by Ni2+-charged chelating Sepharose in single-step chromatography. The purified proteins appeared as single major bands in SDS-PAGE, indicating that most of the contaminants had been removed (Fig. 1B). The observed mobility of the intermediate subfragment did not correspond to its calculated molecular mass. Such a discrepancy has previously been reported for the intact LipL32 molecule (22).
FIG. 1.
(A) Schematic representation of LipL32 protein. Shown are the signal peptide (amino acids 1 to 19), N-terminal domain (amino acids 21 to 92), intermediate domain (amino acids 93 to 184), and C-terminal domain (amino acids 185 to 272). The asterisk indicates the cysteine to be lipidated. (B) Purification of recombinant proteins. SDS-PAGE (15%) of purified recombinant proteins obtained by metal affinity chromatography. Lane 1, LipL32; lane 2, N-terminal fragment; lane 3, intermediate fragment; lane 4, C-terminal fragment; lane M, molecular mass marker.
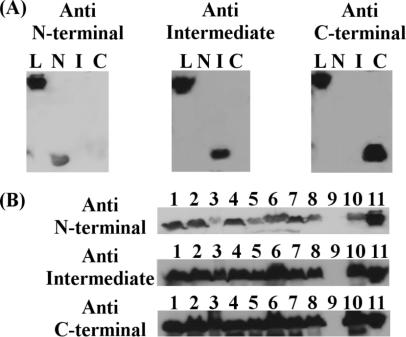
Specificity of antisera against LipL32 subfragments.
Antiserum against each recombinant LipL32 subfragment was raised in mice and reacted specifically with its correspondent fragment (Fig. 2A). Besides recognizing its homologous fragment, each antiserum reacted with the intact recombinant LipL32 molecule (Fig. 2A). In addition, immunoblot analyses were performed on a panel of Leptospira serovars. Antisera against the N-terminal, intermediate, and C-terminal subfragments reacted with a band showing a molecular mass of 32 kDa in all of the pathogenic serovar extracts (L. interrogans serovars Icterohaemorrhagiae, Copenhageni, Bratislava, Hardjo, Autumnalis, Pomona, Pyrogenes, and Canicola and L. kirchneri serovar Grippotyphosa) (Fig. 2B). Sera also recognized the recombinant protein (Fig. 2B, lane 11). No reaction was observed with nonpathogenic saprophytic L. biflexa serovar Patoc (Fig. 2B, lane 9).
FIG. 2.
Specificity of antisera against LipL32 fragments. Sera from mice immunized with recombinant LipL32 subfragments recognize the corresponding fragments (N, N terminal; I, intermediate; C, C terminal), as well as the intact protein (L, LipL32) (A), and native LipL32 from whole-cell extracts of L. interrogans serovars Icterohaemorrhagiae (column 1), Copenhageni (column 2), Bratislava (column 3), Hardjo (column 4), Autumnalis (column 5), Pomona (column 6), Pyrogenes (column 7), and Canicola (column 8) and L. kirchneri serovar Grippotyphosa (column 10) (B). Sera also recognized the recombinant protein (column 11) but failed to react with the whole-cell extract of L. biflexa serovar Patoc (column 9).
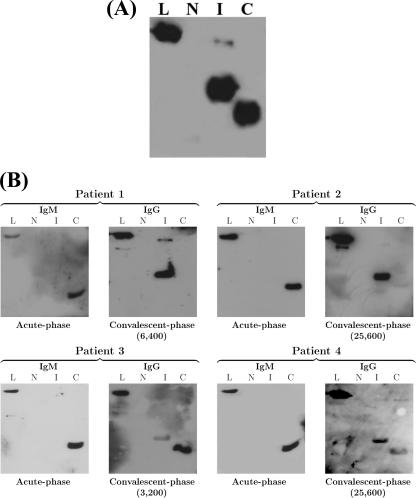
The LipL32 C terminus is detected earlier in the course of infection.
In order to define the immunodominant portion(s) of the LipL32 molecule, we first immunized mice with the intact antigen. Antibodies generated against LipL32 showed that the C-terminal and intermediate subfragments are the major immunoreactive portions of the molecule (Fig. 3A). We extended this study with sera from naturally infected human patients with laboratory-confirmed leptospirosis (Table 1; Fig. 3B). Twelve patient sera were assayed, showing different MAT titers in the convalescent phase of illness (Table 1). In all cases, an IgM response exclusively against the C-terminal subfragment was detected in both the acute and convalescent phases (Table 1; Fig. 3B). Regarding the IgG response in the acute phase, the LipL32 C terminus was recognized by antibodies present in the sera of five patients, and the intermediate portion reacted with IgG antibodies present in a single serum sample (Table 1). During the convalescent phase, IgG antibodies from almost all of the serum samples recognized the intermediate fragment, and antibodies present in 5 of the 12 samples recognized the LipL32 C terminus (Table 1; Fig. 3B). Thus, it seems that reactivity against the C terminus can be detected earlier in the course of infection than that against the intermediate region. The LipL32 N-terminal subfragment was not detected by antibodies present in any serum sample.
FIG. 3.
Recombinant LipL32 protein subfragments and reactivity with sera from mice (A) and leptospirosis patients (B). Immunoblot analyses of N-terminal (N), intermediate (I), C-terminal (C), and intact LipL32 (L) recombinant proteins (2 μg/lane). Membranes were probed with serum from mice immunized with recombinant LipL32 (A) or with sera obtained from leptospirosis patients 1 to 4 (Table 1) during the acute and convalescent phases of illness (B). Blots were developed with goat anti-mouse IgG-peroxidase conjugate (A) or goat anti-human IgM-peroxidase conjugate (acute phase) and goat anti-human IgG-peroxidase conjugate (convalescent phase) (B). MAT titers for each patient are indicated.
LipL32 interacts with collagen IV and plasma fibronectin.
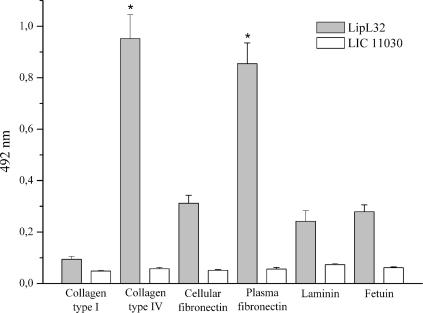
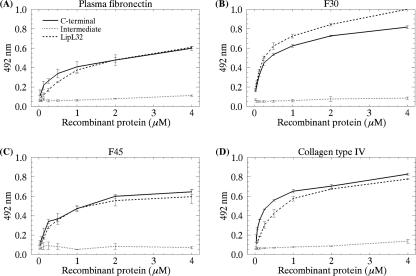
The mechanisms by which pathogenic leptospires invade and colonize the host are poorly understood. During invasion, leptospires interact with the ECM or plasma proteins (1, 11, 27, 30, 31, 37). As a major leptospiral outer membrane lipoprotein expressed during infection, we wondered whether LipL32 could interact with these proteins. Laminin 1, collagen type I, collagen type IV, cellular fibronectin, plasma fibronectin, and the control protein fetuin were immobilized on microdilution wells, and recombinant LipL32 protein attachment was assessed by an ELISA-based method. As shown in Fig. 4, LipL32 exhibited significant binding to collagen IV (P < 0.0001) and plasma fibronectin (P < 0.0001). Binding to the other ECM macromolecules did not differ significantly from that observed with the negative control protein fetuin (Fig. 4). No binding to the target ECM macromolecules was detected with the protein encoded by LIC11030, which was included as a negative control (Fig. 4). This leptospiral recombinant protein was purified from an E. coli lysate in the same way as LipL32. Dose-dependent binding to fibronectin and collagen IV was observed when increasing concentrations of LipL32 recombinant protein (0 to 4 μM) were allowed to adhere to a fixed amount of protein (1 μg) (Fig. 5A and D). Since fibronectin-binding proteins are known to interact with specific domains of fibronectin (11, 18), we examined whether LipL32 would show specific adhesion to purified fibronectin proteolytic fragments F30 (30-kDa heparin-binding domain) and F45 (45-kDa gelatin-binding domain). Our results indicate that LipL32 interacts with both fibronectin domains in a concentration-dependent manner (Fig. 5B and C). In order to map the LipL32 region(s) responsible for ECM-binding activity, assays were also performed with the C-terminal and intermediate subfragments. As the LipL32 N terminus is insoluble in buffers compatible with ELISA, it was not included in the assays. As shown in Fig. 5, the profiles of C-terminal domain binding to plasma fibronectin and collagen type IV resembled those exhibited by the intact LipL32 molecule, suggesting that the binding capacity of LipL32 may be mostly conferred by its C-terminal portion. Increasing the intermediate domain concentration had no effect on its binding to fibronectin or to collagen IV (Fig. 5). The apparent dissociation constants (Kds) for LipL32 and fibronectin or collagen IV binding are shown in Table 2; they ranged from 7.27 to 10.10 μM for plasma fibronectin and the F30 and F45 domains. Similar values were observed for the C-terminal portion of LipL32. Kds ranging from 5.99 to 6.42 were observed for LipL32 and its C-terminal portion for collagen IV. Altogether, these results show that LipL32 binds to the F30 and F45 domains of plasma fibronectin and also interacts with collagen type IV. These interactions seem be mostly mediated by the LipL32 C terminus. Our results are further supported by very recent data implicating the C terminus of LipL32 as the region that binds ECM (23). Despite displaying binding to the above-mentioned ECM macromolecules, LipL32 was not capable of inhibiting Leptospira attachment to ECM proteins (data not shown).
FIG. 4.
Binding of recombinant LipL32 to ECM macromolecules. Wells were coated with 1 μg of laminin, collagen type I, collagen type IV, cellular fibronectin, plasma fibronectin, and the control protein fetuin. Recombinant protein attachment was assessed by an ELISA-based method. One microgram of recombinant protein was added per well. Optical densities were determined at 492 nm. Data represent the mean ± the standard deviation of three independent experiments, each performed in triplicate. For statistical analyses, the attachment of LipL32 to the ECM components was compared to the attachment of the protein to fetuin by the two-tailed t test (*, P < 0.0001).
FIG. 5.
Binding of LipL32, its C-terminal domain, and its intermediate domain to plasma fibronectin (intact molecule and F30 and F45 proteolytic fragments) and to collagen IV as a function of protein concentration. Panels: A, binding to plasma fibronectin; B, binding to F30; C, binding to F45; D, binding to collagen IV. Recombinant protein concentrations ranged from 0 to 4 μM. Each point represents the mean absorbance value at 492 nm ± the standard error of three independent experiments.
TABLE 2.
Plasma fibronectin and collagen type IV binding by intact LipL32 and LipL32 C terminus
| Binding protein | Mean Kda ± SE
|
|||
|---|---|---|---|---|
| Plasma fibronectin | F30 | F45 | Collagen type IV | |
| LipL32 | 10.10 ± 0.30 | 9.38 ± 0.05 | 7.27 ± 0.15 | 5.99 ± 0.12 |
| C terminus | 10.41 ± 0.10 | 9.20 ± 0.05 | 9.90 ± 0.12 | 6.42 ± 0.05 |
Estimated as concentration (micromolar) of binding protein.
C-terminal domain competes for the attachment of LipL32 to collagen IV and fibronectin.
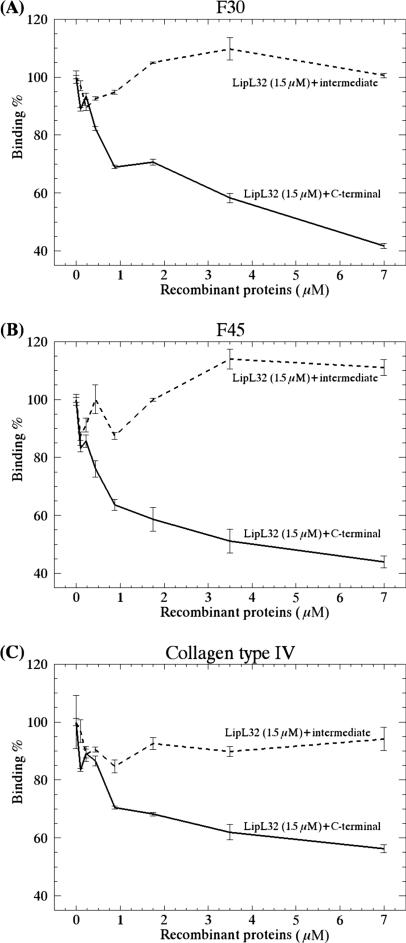
According to the results described above, the C-terminal portion of LipL32 binds to fibronectin, notably to its F30 and F45 domains, and to collagen IV in a dose-dependent manner. In order to further strengthen our results, competitive binding assays were performed with increasing concentrations of the C-terminal or intermediate domain (0 to 7 μM) in the presence of a fixed LipL32 concentration (1.5 μM). Attachment to ECM components was assessed essentially as described above, and the wells were probed with anti-intermediate LipL32 subfragment antibodies (when increasing amounts of C-terminal subfragments were used) or with anti-C-terminal subfragment antibodies (when increasing amounts of intermediate subfragments were used). This approach enabled the detection of bound LipL32 in both cases. A concentration-dependent inhibition of LipL32 binding to collagen IV, F30, and F45 was achieved when increasing amounts of the C-terminal subfragment were used, indicating that this domain competes for the attachment of LipL32 to these ECM components. On the other hand, inhibition did not occur when increasing amounts of the intermediate subfragment were used (Fig. 6). These data provide further evidence that the binding capacity of LipL32 is mediated by its C-terminal domain.
FIG. 6.
Inhibition of LipL32 binding to ECM by the C-terminal fragment. Wells were coated with F30 (A), F45 (B), or collagen IV (C), and increasing concentrations of the C-terminal or intermediate domain (0 to 7 μM) were added in the presence of a fixed LipL32 concentration (1.5 μM). Optical densities were determined at 492 nm. Data represent the mean ± the standard error of three independent experiments, each performed in triplicate.
Heparin and gelatin inhibit LipL32 and C-terminal domain binding to F30 and to F45.
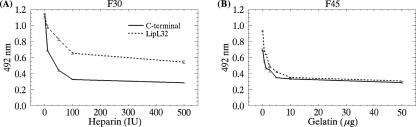
As already mentioned, F30 and F45 are fibronectin proteolytic fragments known to bind to heparin and gelatin, respectively. Therefore, binding of LipL32 and of the C-terminal domain (2 μM) to these immobilized fragments was assayed in the presence of increasing amounts of heparin (0 to 500 IU) or gelatin (0 to 50 μg). Both heparin and gelatin inhibited the attachment of LipL32 and the C-terminal domain to F30 and F45 in a concentration-dependent manner (Fig. 7). Gelatin was able to compete with the intact LipL32 molecule or with its C-terminal portion for F45 binding, fully inhibiting the interaction at relatively low gelatin concentrations (Fig. 7B). However, interaction of intact LipL32 with F30 may also involve other regions of the molecule, since we were unable to totally displace its binding to F30 by heparin, whereas binding of the C terminus to this particular fibronectin domain was successfully abolished (Fig. 7A). In fact, as depicted in Fig. 5B, binding of the intact LipL32 molecule to F30 was significantly higher than that exhibited by the C-terminal subfragment. In conclusion, LipL32 interacts with fibronectin mainly through its C-terminal portion and the binding site substantially overlaps or colocalizes with the heparin- and gelatin-binding sites.
FIG. 7.
Inhibition of LipL32 binding to F30 and F45 by heparin and gelatin. Binding of LipL32 and its C-terminal domain (2 μM) to F30 (A) and F45 (B) was assayed in the presence of increasing amounts of heparin (0 to 500 IU) or gelatin (0 to 50 μg). Optical densities were determined at 492 nm. Data represent the mean ± the standard error of three independent experiments.
DISCUSSION
LipL32 is certainly one of the most studied leptospiral outer membrane proteins. Interest in this particular lipoprotein focuses mainly on its abundance on the surface of the pathogen and on its high degree of conservation among pathogenic leptospires (22). Besides, it has been shown to be highly expressed within renal tubules during mammalian infection (39). Of special interest are the findings indicating that LipL32 is the immunodominant antigen recognized during the humoral immune response to leptospirosis in humans, displaying the greatest sensitivity and specificity in acute- and convalescent-phase sera of patients with the disease (5, 19, 21). In fact, previous immunoblot studies using leptospirosis patient sera from Barbados already pointed to a major immunoreactive antigen with a molecular mass of 32 kDa (10).
One of the aims of the present study was to define the immunodominant portion(s) of the LipL32 molecule. For that purpose, three different domains—N terminal, intermediate, and C terminal—were generated. Our immunoblot results indicate that all of the patients with laboratory-confirmed leptospirosis we tested possessed IgM antibodies to the C terminus and to the intact LipL32 recombinant protein during the acute and convalescent phases of illness. In a previous work (19), an IgM response was detected exclusively in whole-Leptospira ELISAs. Since an IgM response to recombinant LipL32 was not observed, the authors suggested that IgM and IgG antibodies could be directed at different moieties. That may occur, but our data suggest that specific IgM antibodies to the LipL32 C terminus during the acute phase of the disease can also be detected. It is worth mentioning that immunoblot analyses performed by Guerreiro and colleagues failed to detect an IgM antibody response to LipL32 (21). Whole-cell extracts of clinical isolates of Leptospira were used in their assays, in contrast to the recombinant antigens used in this study (Fig. 3). With respect to the IgG response, the LipL32 C terminus was recognized by antibodies present in the sera of five patients during the acute phase of the disease, and the intermediate subfragment was recognized by antibodies present in all but two serum samples during the convalescent phase. Detection of an IgG response to LipL32 during early illness is not surprising, since a number of studies have reported this particular immune response to leptospiral proteins, notably to LipL32, during early illness (4, 5, 9, 10, 19, 21). Our data indicate that LipL32 can be used as a diagnostic antigen for the acute and convalescent phases of patients with leptospirosis. In addition, our results identified the C-terminal domain of LipL32 as the primary immunological target, eliciting an IgM humoral response in all of the patients tested.
Another aspect examined in the present study was the capacity of LipL32 to interact with ECM components. Under normal conditions, ECM is not exposed to bacteria. Pathogens may gain access to the ECM components after tissue trauma following a mechanical or chemical injury or as a consequence of bacterial infection through the activity of toxins and lytic enzymes (28). We wondered whether LipL32, as a major leptospiral surface antigen expressed during infection, could establish interactions with host molecules such as ECM proteins. Specific dose-dependent binding of LipL32 to collagen IV and plasma fibronectin was observed, and the binding capacity seems to be conferred by the C-terminal portion of this molecule. Our findings are in agreement with those recently published by Hoke and colleagues, showing that rLipL32 functions as an ECM-interacting protein via the 72 amino acids of the C terminus (23). We also demonstrated that both heparin and gelatin could inhibit LipL32 binding to fibronectin in a concentration-dependent manner, indicating that the 30-kDa heparin-binding and 45-kDa gelatin-binding domains of fibronectin are involved in this interaction. The Kds for fibronectin and collagen IV binding to LipL32 indicate that this protein interacts with ECM components with lower avidity compared to the well-known Lig adhesins from Leptospira (11, 27). As already mentioned, LipL32 was not capable of inhibiting Leptospira attachment to ECM proteins (data not shown). Besides, in vitro assays indicated that anti-LipL32 serum failed to block Leptospira adhesion to ECM (23). The absence of an inhibitory effect in both cases could be explained by the existence of additional L. interrogans binding proteins that contribute to leptospiral adherence to ECM.
The biological significance of LipL32 binding to ECM remains unclear. There are multiple isoforms of fibronectin. Plasma fibronectin, the isoform that interacts with LipL32, is soluble and circulates in blood and other body fluids, where it is thought to enhance blood clotting, wound healing, and phagocytosis. Recently, Cinco and colleagues demonstrated that leptospires recognize and bind to CR3 integrin, expressed on neutrophils and CHO Mac-1 transfected cells (12). This interaction seems to occur via the CR3 integrin I domain, which is responsible for iC3b, ICAM-1, fibrinogen, and fibronectin recognition. According to their results, adhesion to cells increased when leptospires or cells were pretreated with fibronectin, leading to the suggestion that this molecule may facilitate and enhance the interaction between the microorganism and the CR3 I domain (12). Besides LipL32, a few leptospiral proteins have been shown to bind to plasma fibronectin, i.e., the uncharacterized 36-kDa fibronectin-binding protein (31), the LigA and LigB proteins (11, 27), and the leptospiral endostatin-like (Len) outer membrane proteins (37). Other pathogenic microorganisms have been reported to bear multiple fibronectin-binding proteins (24).
Adhesive glycoproteins, such as fibronectin or laminin, have defined cell- or bacterium-binding domains, while collagens provide multiple interaction sites for cells and pathogens along the triple-helical structures that make up the fibrils or networks (13). Moreover, they frequently form supramolecular aggregates that vary in structure and composition, depending on the association with other ECM molecules such as fibronectin, vitronectin, von Willebrand factor, laminin, nidogen, and proteoglycans. In some cases, interaction with bacteria is not determined exclusively by the collagen component (13). LipL32 exhibited a significant level of binding to collagen type IV, a major component of basement membrane networks. Upon tissue injury, particularly at sites of wound healing or ECM degradation, this macromolecule becomes exposed and interaction with bacterial surface proteins may occur.
The presence of multiple ECM-binding sites in one individual protein would be of great importance (8). The leptospiral LigA, LigB, and Len proteins were shown to bind to several ECMs (11, 37), as well as the Haemophilus influenzae Hap autotransporter (18). Circulating leptospires bound to plasma fibronectin could target different tissues. In fact, plasma fibronectin was reported to enhance the adherence of Streptococcus sanguis to gelatin (29). We could speculate that fibronectin bound to LipL32 also helps its interaction with collagen IV present in the endothelium ECM. The combination of biochemical and new genetic tools useful in producing mutant leptospires (34) will allow future studies to address the precise role of leptospiral proteins and host component interactions.
Acknowledgments
We express our deep gratitude to Roberta Morozetti Blanco and Débora Andrade Silva for their skilled technical assistance. We also thank A. Leyva for English editing of the manuscript.
This work benefited from grants from FAPESP, CNPq, and Fundação Butantan.
Editor: A. Camilli
Footnotes
Published ahead of print on 7 April 2008.
REFERENCES
- 1.Barbosa, A. S., P. A. E. Abreu, F. O. Neves, M. V. Atzingen, M. M. Watanabe, M. L. Vieira, Z. M. Morais, S. A. Vasconcellos, and A. L. T. O. Nascimento. 2006. A newly identified leptospiral adhesin mediates attachment to laminin. Infect. Immun. 746356-6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhandari, P., and J. Gowrishankar. 1997. An Escherichia coli host strain useful for efficient overproduction of cloned gene products with NaCl as the inducer. J. Bacteriol. 1794403-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharti, A. R., J. E. Nally, J. N. Ricaldi, M. A. Matthias, M. M. Diaz, M. A. Lovett, P. N. Levett, R. H. Gilman, M. R. Willig, E. Gotuzzo, and J. M. Vinetz on behalf of the Peru-United States Leptospirosis Consortium. 2003. Leptospirosis: a zoonotic disease of global importance. Lancet Infect. Dis. 3757-771. [DOI] [PubMed] [Google Scholar]
- 4.Bomfim, M. R. Q., A. I. Ko, and M. C. Koury. 2005. Evaluation of the recombinant LipL32 in enzyme-linked immunosorbent assay for the serodiagnosis of bovine leptospirosis. Vet. Microbiol. 10989-94. [DOI] [PubMed] [Google Scholar]
- 5.Boonyod, D., Y. Poovorawan, P. Bhattarakosol, and C. Chirathaworn. 2005. LipL32, an outer membrane protein of Leptospira, as an antigen in a dipstick assay for diagnosis of leptospirosis. Asian Pac. J. Allergy Immunol. 23133-141. [PubMed] [Google Scholar]
- 6.Branger, C., C. Sonrier, B. Chatrenet, B. Klonjkowski, N. Ruvoen-Clouet, A. Aubert, G. André-Fontaine, and M. Eloit. 2001. Identification of the hemolysis-associated protein 1 as a cross-protective immunogen of Leptospira interrogans by adenovirus-mediated vaccination. Infect. Immun. 696831-6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Branger, C., B. Chatrenet, A. Gauvrit, F. Aviat, A. Aubert, J. M. Bach, and G. Andre-Fontaine. 2005. Protection against Leptospira interrogans sensu lato challenge by DNA immunization with the gene encoding hemolysin-associated protein 1. Infect. Immun. 734062-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron, C. E., E. L. Brown, J. M. Kuroiwa, L. M. Schnapp, and N. L. Brouwer. 2004. Treponema pallidum fibronectin-binding proteins. J. Bacteriol. 1867019-7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman, A. J., B. Adler, and S. Faine. 1988. Antigens recognised by the human immune response to infection with Leptospira interrogans serovar Hardjo. J. Med. Microbiol. 25269-278. [DOI] [PubMed] [Google Scholar]
- 10.Chapman, A. J., C. O. Everard, S. Faine, and B. Adler. 1991. Antigens recognized by the human immune response to severe leptospirosis in Barbados. Epidemiol. Infect. 107143-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choy, H. A., M. M. Kelley, T. L. Chen, A. K. Møller, J. Matsunaga, and D. A. Haake. 2007. Physiological osmotic induction of Leptospira interrogans adhesion: LigA and LigB bind extracellular matrix proteins and fibrinogen. Infect. Immun. 752441-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cinco, M., B. Cini, S. Perticarari, and G. Presani. 2002. Leptospira interrogans binds to CR3 receptor on mammalian cells. Microb. Pathog. 33299-305. [DOI] [PubMed] [Google Scholar]
- 13.Cossart, P., P. Boquet, S. Normark, and R. Rappuoli. 2005. Cellular microbiology, second edition, ASM Press, Washington, DC.
- 14.Cullen, P. A., S. J. Cordwell, D. M. Bulach, D. A. Haake, and B. Adler. 2002. Global analysis of outer membrane proteins from Leptospira interrogans serovar Lai. Infect. Immun. 702311-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cullen, P. A., X. Xu, J. Matsunaga, Y. Sanchez, A. I. Ko, D. A. Haake, and B. Adler. 2005. Surfaceome of Leptospira spp. Infect. Immun. 734853-4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dey, S., C. M. Mohan, T. M. Kumar, P. Ramadass, A. M. Nainar, and K. Nachimuthu. 2004. Recombinant LipL32 antigen-based single serum dilution ELISA for detection of canine leptospirosis. Vet. Microbiol. 10399-106. [DOI] [PubMed] [Google Scholar]
- 17.Dolhnikoff, M., T. Mauad, E. P. Bethlem, and C. R. Carvalho. 2007. Pathology and pathophysiology of pulmonary manifestations in leptospirosis. Braz. J. Infect. Dis. 11142-148. [DOI] [PubMed] [Google Scholar]
- 18.Fink, D. L., B. A. Green, and J. W. St. Geme III. 2002. The Haemophilus influenza Hap autotransporter binds to fibronectin, laminin, and collagen IV. Infect. Immun. 704902-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flannery, B., D. Costa, F. P. Carvalho, H. Guerreiro, J. Matsunaga, E. D. da Silva, A. G. Ferreira, L. W. Riley, M. G. Reis, D. A. Haake, and A. I. Ko. 2001. Evaluation of recombinant Leptospira antigen-based enzyme-linked immunosorbent assays for the serodiagnosis of leptospirosis. J. Clin. Microbiol. 393303-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gamberini, M., R. M. Gómez, M. V. Atzingen, E. A. L. Martins, S. A. Vasconcellos, E. C. Romero, L. C. C. Leite, P. L. Ho, and A. L. T. O. Nascimento. 2005. Whole-genome analysis of Leptospira interrogans to identify potential vaccine candidates against leptospirosis. FEMS Microbiol. Lett. 244305-313. [DOI] [PubMed] [Google Scholar]
- 21.Guerreiro, H., J. Croda, B. Flannery, M. Mazel, J. Matsunaga, M. G. Reis, P. N. Levett, A. I. Ko, and D. A. Haake. 2001. Leptospiral proteins recognized during the humoral immune response to leptospirosis in humans. Infect. Immun. 694958-4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haake, D. A., G. Chao, R. L. Zuerner, J. K. Barnett, D. Barnett, M. Mazel, J. Matsunaga, P. N. Levett, and C. A. Bolin. 2000. The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection. Infect. Immun. 682276-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoke, D. E., S. Egan, P. A. Cullen, and B. Adler. 19 February 2008. LipL32 is an extracellular-matrix-interacting protein of Leptospira and Pseudoalteromonas tunicata. Infect. Immun. [Epub ahead of print.] doi:10.1128/IAI.01643-07. [DOI] [PMC free article] [PubMed]
- 24.Joh, D., E. R. Wann, B. Kreikemeyer, P. Speziale, and M. Höök. 1999. Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biol. 18211-223. [DOI] [PubMed] [Google Scholar]
- 25.Lee, S. H., K. A. Kim, Y. G. Park, I. W. Seong, M. J. Kim, and Y. J. Lee. 2000. Identification and partial characterization of a novel hemolysin from Leptospira interrogans serovar Lai. Gene 25419-28. [DOI] [PubMed] [Google Scholar]
- 26.Levett, P. N. 2001. Leptospirosis. Clin. Microbiol. Rev. 14296-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin, Y.-P., and Y.-F. Chang. 2007. A domain of the Leptospira LigB contributes to high affinity binding of fibronectin. Biochem. Biophys. Res. Commun. 362443-448. [DOI] [PubMed] [Google Scholar]
- 28.Ljungh, A., and T. Wadstrom. 1996. Interactions of bacterial adhesins with the extracellular matrix. Adv. Exp. Med. Biol. 408129-140. [DOI] [PubMed] [Google Scholar]
- 29.Lowrance, J. H., D. L. Hasty, and W. A. Simpson. 1988. Adherence of Streptococcus sanguis to conformationally specific determinants in fibronectin. Infect. Immun. 562279-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meri, T., R. Murgia, P. Stefanel, S. Meri, and M. Cinco. 2005. Regulation of complement activation at the C3-level by serum resistant leptospires. Microb. Pathog. 39139-147. [DOI] [PubMed] [Google Scholar]
- 31.Merien, F., J. Truccolo, G. Baranton, and P. Perolat. 2000. Identification of a 36-kDa fibronectin-binding protein expressed by a virulent variant of Leptospira interrogans serovar Icterohaemorrhagiae. FEMS Microbiol. Lett. 18517-22. [DOI] [PubMed] [Google Scholar]
- 32.Picardeau, M., D. M. Bulach, C. Bouchier, R. L. Zuerner, N. Zidane, P. J. Wilson, S. Creno, E. S. Kuczek, S. Bommezzadri, J. C. Davis, A. McGrath, M. J. Johnson, C. Boursaux-Eude, T. Seemann, Z. Rouy, R. L. Coppel, J. I. Rood, A. Lajus, J. K. Davies, C. Médigue, and B. Adler. 2008. Genome sequence of the saprophyte Leptospira biflexa provides insights into the evolution of Leptospira and the pathogenesis of leptospirosis. PLoS ONE 3e1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramos, C. R. R., P. A. E. Abreu, A. L. T. O. Nascimento, and P. L. Ho. 2004. A high-copy T7 Escherichia coli expression vector for the production of recombinant proteins with a minimal N-terminal His-tagged fusion peptide. Braz. J. Med. Biol. Res. 371103-1109. [DOI] [PubMed] [Google Scholar]
- 34.Ristow, P., P. Bourhy, F. W. McBride, C. P. Figueira, M. Huerre, P. Ave, I. S. Girons, A. I. Ko, and M. Picardeau. 2007. The OmpA-like protein Loa22 is essential for leptospiral virulence. PLoS Pathog. 3e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seijo, A., H. Coto, J. San Juan, J. Videla, B. Deodato, B. Cernigoi, O. G. Messina, O. Collia, D. de Bassadoni, R. Schtirbu, A. Olenchuk, G. D. de Mazzonelli, and A. Parma. 2002. Lethal leptospiral pulmonary hemorrhage: an emerging disease in Buenos Aires, Argentina. Emerg. Infect. Dis. 81004-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seixas, F. K., E. F. da Silva, D. D. Hartwig, G. M. Cerqueira, M. Amaral, M. Q. Fagundes, R. G. Dossa, and O. A. Dellagostin. 2007. Recombinant Mycobacterium bovis BCG expressing the LipL32 antigen of Leptospira interrogans protects hamsters from challenge. Vaccine 2688-95. [DOI] [PubMed] [Google Scholar]
- 37.Stevenson, B., H. A. Choy, M. Pinne, M. L. Rotondi, M. C. Miller, E. Demoll, P. Kraiczy, A. E. Cooley, T. P. Creamer, M. A. Suchard, C. A. Brissette, A. Verma, and D. A. Haake. 2007. Leptospira interrogans endostatin-like outer membrane proteins bind host fibronectin, laminin and regulators of complement. PLoS ONE 2e1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verma, A., J. Hellwage, S. Artiushin, P. F. Zipfel, P. Kraiczy, J. F. Timoney, and B. Stevenson. 2006. LfhA, a novel factor H-binding protein of Leptospira interrogans. Infect. Immun. 742659-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang, C. W., M. S. Wu, M. J. Pan, W. J. Hsieh, A. Vandewalle, and C. C. Huang. 2002. The Leptospira outer membrane protein LipL32 induces tubulointerstitial nephritis-mediated gene expression in mouse proximal tubule cells. J. Am. Soc. Nephrol. 82037-2045. [DOI] [PubMed] [Google Scholar]
- 40.Zuerner, R. L., W. Knudtson, C. A. Bolin, and G. Trueba. 1991. Characterization of outer membrane and secreted proteins of Leptospira interrogans serovar Pomona. Microb. Pathog. 10311-322. [DOI] [PubMed] [Google Scholar]