Abstract
The contribution of Toll-like receptors (TLRs) to phagocytosis of Borrelia burgdorferi has not been extensively studied. We show that bone marrow-derived macrophages (BMDM) from MyD88−/− mice or Raw cells transfected with a dominant-negative MyD88 were unable to efficiently internalize B. burgdorferi. Knockouts of TLR2 and TLR9 or knockdown of TLR5 by small interfering RNA produced no defects in phagocytosis of B. burgdorferi. Production of inflammatory cytokines was greatly diminished in MyD88−/− BMDM but only partially affected in TLR2−/− BMDM or knockdown of TLR5 and unaffected in TLR9−/− BMDM. Cytochalasin D reduced cytokine induction, but not to the level of the MyD88−/− BMDM. Addition of cytochalasin D to TLR2−/− BMDM inhibited inflammatory responses to B. burgdorferi to the level of MyD88−/− BMDM, consistent with a role for TLR2 in both recognition of extracellular products and lysosomal sampling by TLR2 after processing of the organism. Cytochalasin D had no impact on cytokine production in cells undergoing TLR5 knockdown. These results suggest that MyD88, but not TLR2, TLR5, and TLR9, is important for the uptake of B. burgdorferi and that MyD88 affects inflammatory responses through both its effects on phagocytosis and its role in transducing signals from TLR2 and TLR5.
Toll-like receptors (TLRs) play an important role in host innate immune responses to microbial pathogens. Engagement of surface-associated TLRs results in the activation of signaling pathways, which release multiple inflammatory mediators that shape the early host response. TLRs have been shown to be important in dendritic cell maturation and antigen processing and thus also have an important role in determining adaptive immune responses (23, 34, 42). Deficiency of an adapter molecule, myeloid differentiation primary response gene 88 (MyD88), which is a key signaling molecule utilized by most of the TLRs, results in a severe immune impairment of host defenses against many microorganisms (7, 11, 13, 18, 38, 39, 43).
Borrelia burgdorferi is the etiologic agent of Lyme disease. The B. burgdorferi genome encodes over 160 lipoproteins that are expressed during different stages of its life cycle. Borrelial lipoproteins are recognized by TLR1/2 heterodimers and have been shown to activate peripheral blood mononuclear cells to produce inflammatory cytokines and chemokines (21, 36, 40). Studies of mice deficient in either TLR2 or MyD88 have found that loss of either of these proteins results in a severely impaired ability to clear spirochetes from infected mice (5, 9, 28, 49, 51). This impairment does not appear to be due to the effects of TLR signaling on the appropriate development of the adaptive immune response, as mice deficient in MyD88 develop an antibody response that is essentially indistinguishable from that of wild-type mice (28).
One possible reason for the inability of TLR2−/− or MyD88−/− mice to appropriately control infection with B. burgdorferi is that TLR signaling may be important for the early killing of the organism by phagocytes, such as macrophages. The role of TLR signaling in the phagocytosis of bacteria has varied depending upon the organisms studied. For many bacteria, the major phagocytic defect in MyD88−/− cells is in the killing of the organism after it reaches the phagosome. This may be due to a reduction in phagosome maturation or in oxidative killing (8, 26). Blander and Medzhitov have shown that phagocytosis and killing of Staphylococcus aureus, Escherichia coli, and Salmonella enterica serovar Typhimurium is greatly decreased in the absence of TLR2 (for S. aureus), TLR4 (for E. coli and S. enterica serovar Typhimurium), or MyD88 (for all three) (8). This defect appears to be due to a loss of activation of p38 mitogen-activated protein kinase (MAPK), which plays an important role in both phagosome maturation and oxidative killing (8, 26). However, there remains some controversy about this mechanism, as Yates and Russell, using defined microparticles, have suggested that phagosome maturation proceeds independently of TLR2 and TLR4 signaling (52). In their studies, while MyD88−/− cells did exhibit a defect in phagolysosome maturation, it appeared that this defect was not due to the loss of direct activation of TLR signaling pathways by the TLR ligands, but rather, was attributed to baseline differences between the cells. These defects may arise secondarily to the role of TLRs in cellular development and/or the impact of low-level stimulation on the cytokine “milieu” and activation state of the cells.
TLR activation also appears to play a role in the internalization of some, but not all, bacteria by macrophages. Internalization of S. aureus, E. coli, and S. enterica serovar Typhimurium bacteria is decreased in the absence of TLR activation, while loss of TLR activation has no effect on the internalization of either group B streptococcus or Listeria monocytogenes (20, 50).
TLR2 has been known to be a major signaling receptor for B. burgdorferi lipoprotein, outer surface protein A (OspA) (21, 51). While blocking TLR2 results in great reduction of inflammatory signaling in response to purified lipoproteins, antibody blocking of surface-bound TLR2 does not block inflammatory signaling in response to whole organisms (6). There are several possible explanations for this. First, receptors other than TLR2 may recognize products of B. burgdorferi and contribute to the inflammatory response. Second, as it has been shown that TLR2 may be recruited to phagosomes, it is possible that the majority of TLR2 signaling does not occur through ligation of extracellular lipoproteins (14, 24, 33).
Liu et al. have previously reported that MyD88−/− macrophages are able to bring spirochetes into phagosomes but show defective killing of the organism in phagosomes (28). The contribution of individual TLRs to this process for B. burgdorferi has not been reported. In this report, we detail our studies on the role of MyD88 in phagocytosis of B. burgdorferi. Surprisingly, our data show that the major defect in phagocytosis in the absence of MyD88 is not in phagolysosomal killing but in uptake of the organism. We also report our studies on the contribution of three TLRs, TLR2, TLR5, and TLR9, which were thought most likely to recognize B. burgdorferi products, to phagocytosis of B. burgdorferi, as well as their effects on inflammatory signaling.
MATERIALS AND METHODS
Mice, cells, and bacteria.
MyD88−/− mice were maintained as heterozygous breeding pairs at the sixth-generation backcross on the C57BL/6 background. MyD88−/−, MyD88+/+, and MyD88+/− littermates were genotyped as described previously (27). TLR2−/− and TLR9−/− mice on a C57BL/6 background were generated as described previously (19, 44). Age- and sex-matched C57BL/6 control mice were obtained from the Jackson Laboratory and used as controls for either TLR2−/− or TLR9−/− mice. The procedures used for our animal studies were reviewed and approved by the Tufts University Institutional Animal Care and Use Committee.
Mouse bone marrow-derived macrophages (BMDM) were recovered from mouse femurs and differentiated as described previously (41). In brief, bone marrow cells were flushed from mouse femurs with sterile RPMI medium and cultured on plastic petri dishes for 5 to 7 days in medium containing RPMI supplemented with 30% L929 cell conditioned medium, 20% fetal bovine serum (FBS), and1% penicillin-streptomycin. BMDM were harvested from 100- by 15-mm petri dishes and plated at 0.5 × 106/well in 24-well tissue culture plates. Murine macrophage cell line Raw 264.7 cells (ATCC, Manassas, VA) were grown in Dulbecco modified Eagle medium (DMEM) with l-glutamine supplemented with 10% FBS and 1% penicillin-streptomycin.
Clonal isolates of infectious low-passage B. burgdorferi sensu stricto strain N40 (clone D10E9) were used for all experiments. B. burgdorferi was cultured in Barbour-Stoenner-Kelly (BSK)-H medium (Sigma, St. Louis, MO) at 35°C as previously described (2, 22).
Phagocytosis assay.
In a 24-well plate, coverslips were coated with 1% rat collagen in 60% ethanol solution and dried overnight. Fully differentiated BMDM were plated in RPMI supplemented with 30% L-cell conditioned medium, 20% FBS, and 1% penicillin-streptomycin. The cells were maintained in this medium for 24 h and then placed into serum-free RPMI for 12 to 16 h prior to their use in assays. B. burgdorferi cells were added to the cultures at a multiplicity of infection (MOI) of 10. The plates were centrifuged at 1,200 rpm at 4°C for 5 min to bring B. burgdorferi spirochetes in contact with the cells. The plates were then moved to room temperature (time zero). For phagocytosis to occur, the plates were moved to 37°C for 5, 20, and 60 min. The coverslips were removed at various time points after the addition of B. burgdorferi and washed with cold phosphate-buffered saline (PBS) three times to remove unbound B. burgdorferi spirochetes. The cells were fixed in 3.7% paraformaldehyde with 5% sucrose in PBS for 20 min at 25°C. The coverslips were washed three times in PBS and stored at 4°C until they were used.
For inhibitor treatments, inhibitors were added to the cells 1 h prior to the addition of B. burgdorferi. SB203580 (p38 MAPK inhibitor) was purchased from Calbiochem (San Diego, CA). The activity of p38 MAPK inhibitor at the concentrations used was confirmed by measuring the effects on mRNA expression of tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) from lipopolysaccharide (LPS)-stimulated BMDM by quantitative reverse transcriptase PCR (qRT-PCR).
For experiments inhibiting phagocytosis, cytochalasin D was purchased from Tocris Bioscience (Ellisville, MO). The concentration of cytochalasin D was determined based on previous studies (24, 33). Cells were treated with 1 μM cytochalasin D 1 h before stimulation with B. burgdorferi, and the phagocytosis assay was performed as described above. Cytochalasin D treatment had no cytotoxic effect on BMDM or Raw 264.7 cells, as determined by trypan blue exclusion assay.
Immunofluorescence microscopy.
For immunofluorescence studies, coverslips were incubated three times for 5 min each time in blocking buffer (PBS containing 2% goat serum) at room temperature. All antibody incubations were continued for 1 h at 37°C in a humidified incubator. After being blocked, the coverslips were incubated for 1 h at 37°C with an anti-B. burgdorferi polyclonal rabbit antibody (a generous gift from Jenifer Coburn) diluted 1:10,000 in blocking buffer. The coverslips were then washed three times with blocking buffer and incubated with a Cascade blue-conjugated goat anti-rabbit immunoglobulin G (IgG) antibody (Molecular Probes, Eugene, OR) diluted 1:500 in blocking buffer. Samples were again washed three times in PBS for 5 min each time and then permeabilized with chilled methanol for 10 s. After being incubated three times for 5 min each time in blocking buffer, the coverslips were again incubated with the anti-B. burgdorferi rabbit antibody diluted 1:10,000 in blocking buffer. Following three 5-min washes with blocking buffer, the coverslips were incubated with an anti-lysosomal-associated membrane protein 1 (LAMP-1) rat monoclonal antibody (IB4, from the Developmental Studies Hybridoma Bank of the Department of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine, Baltimore, MD, and the Department of Biology, University of Iowa, Iowa City) diluted 1:100 in blocking buffer. After being washed three times for 5 min each time in blocking buffer, samples were incubated simultaneously with a Texas Red-conjugated goat anti-rabbit IgG antibody (Molecular Probes, Eugene, OR) and a fluorescein isothiocyanate-conjugated goat anti-rat IgG antibody (Zymed Laboratories, South San Francisco, CA). After being washed, the coverslips were mounted using Vectashield mounting medium (Vector Laboratories, Burlingame, CA) and examined by differential interference contrast and fluorescence microscopy using a Zeiss Axioplan 2 microscope (Carl Zeiss Microscopy, Jena, Germany). Images were captured with a digital charge-coupled device camera (Hamamatsu, Hamamatsu City, Japan). Analysis of the colocalization of the fluorescent labels was performed using OpenLab software (Improvision Inc., Lexington, MA).
For quantitative analyses, the percentage of cells with one or more internalized B. burgdorferi particles was determined by examining sequential fields from a minimum of three independent experiments (a minimum of three sets with 100 to 200 cells/set). Cells containing any internalized B. burgdorferi particles or cells containing internalized/intact B. burgdorferi spirochetes were counted and expressed as a percentage of the total number of cells examined. The mean percentage of a minimum of three independent experiments was plotted over time, and the statistical significance between groups was analyzed using the nonparametric Mann-Whitney U test.
Quantitative B. burgdorferi growth assay.
For quantitative analysis of B. burgdorferi survival in the BMDM from wild-type littermates and MyD88−/− mice, B. burgdorferi was incubated with BMDM as described for the phagocytosis assays. One hour after infection with B. burgdorferi, the cells were washed with cold PBS three times to remove unbound/uninternalized B. burgdorferi spirochetes. Cells (106) were resuspended in BSK liquid medium and serially diluted 1:10 in columns of 96-well plates. The plates were incubated at 37°C and monitored for color changes that would indicate the growth of bacteria. Samples from the wells were checked upon color change or every 3 days (whichever came first). The well with the highest dilution that was positive for B. burgdorferi growth was assumed to have a single bacterium at the time of initial dilution, and the number of B. burgdorferi bacteria per 106 cells was calculated by multiplying the dilution required to reach a well with no B. burgdorferi bacteria.
Transient transfection of MyD88 DN plasmid.
Raw 264.7 cells were transiently transfected with a dominant-negative (DN) mutant of MyD88 (15) or pCDNA3-GFP (Invitrogen, Carlsbad, CA) plasmid, using a 4:1 lipid/DNA ratio of Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol (21). The transfection mixture was added to cells in serum-free DMEM and incubated at 37°C. After 6 h, the medium was replaced with DMEM with 10% FBS added, and 24 h later, we performed a phagocytosis assay as described above. To briefly describe how we estimated the transfection efficiency of Raw 264.7 cells, we randomly chose 10 fields from each well of a 24-well plate and counted both total cells and cells expressing green fluorescent protein (GFP) after transient transfection of cells with the pCDNA3-GFP plasmid. We then divided the number of GFP-expressing cells by the total number of cells for all 10 fields. The estimated transfection efficiency for all experiments was approximately 70 to 80%.
qRT-PCR.
RNA from BMDM or Raw 264.7 cells was extracted using Trizol according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Total RNA was treated with DNase (Ambion, Austin, TX), following the manufacturer's protocol. First-strand synthesis of cDNA from total RNA was performed using Improm II RT (Promega, Madison, WI) according to the manufacturer's instructions. Quantification of cDNA was performed by quantitative PCR (iCycler; Bio-Rad) using Sybr green dye (Quantitect Sybr green PCR mix; Qiagen, Valencia, CA). The cycling parameters were 55°C for 5 min and 95°C for 15 min, followed by 40 cycles of 95°C for 30 s and 60°C for 1 min. The primers used were as previously described (5). The specificity of each reaction was checked by melting-curve analysis and electrophoresis of PCR products on agarose gels. The expression of target genes was normalized to that of β-actin. Calculations of expression were normalized using the ΔΔCT method, in which the amount of target, normalized to an endogenous reference and relative to a calibrator, is given by 2−ΔΔCT, where CT is the cycle number of the detection threshold.
siRNA transfection.
Raw 264.7 cells were transiently transfected with a 100 nM control small interfering RNA (siRNA) or custom-designed TLR5 siRNA (Sigma, St. Louis, MO) using a 2:1 lipid/siRNA ratio of Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol (21). The transfection mixture was added to the cells in serum-free DMEM. At least two different working siRNA constructs for each target were identified by their abilities to knock down targeted gene expression compared to control siRNA to ensure that the effects were due to siRNA targeting and were not nonspecific effects. Twenty-four hours later, the cells were infected with B. burgdorferi, and RNA was harvested 24 h postinfection. qRT-PCR was performed to determine the knockdown effect of TLR5, and the expression of TLR5 was normalized to that of β-actin. Transfection of siRNA had no cytotoxic effect on Raw 264.7 cells, as determined by trypan blue exclusion assay. The primers for detecting TLR5 mRNA expression were as follows: F, ATGGCATGTCAACTTGACTT, and R, GATCCTAAGATTGGGCAGGT.
Statistical analysis.
Experiments were repeated three to six times as indicated. The statistical significance between groups was analyzed using the nonparametric Mann-Whitney U test. Differences were considered statistically significant when the P values were equal to or less than 0.05.
RESULTS
MyD88 is important for uptake of B. burgdorferi by primary BMDM.
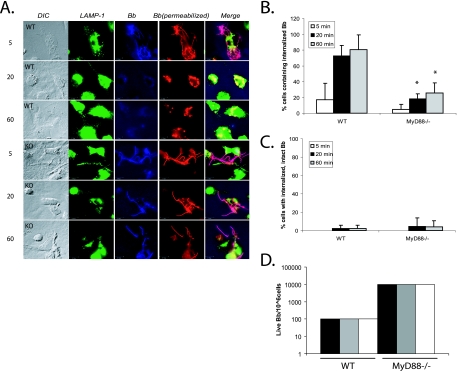
It has been shown that B. burgdorferi is efficiently phagocytosed by macrophages (28, 30-32). In order to determine whether MyD88 plays a role in the phagocytosis of B. burgdorferi, we used immunofluorescence imaging to examine the phagocytosis of B. burgdorferi by BMDM from MyD88−/− mice and their wild-type littermates. Using different fluorescent markers to label B. burgdorferi before and after cell permeabilization allowed us to distinguish between internalized and extracellular B. burgdorferi. At 5 min, most B. burgdorferi spirochetes had not been phagocytosed by either wild-type or MyD88−/− BMDM (Fig. 1A). However, by 20 min, a clear separation occurred between phagocytosis in wild-type and MyD88−/− mice. The majority of organisms incubated with the wild-type BMDM had been phagocytosed and degraded into small particles by 20 min, and almost no intact spirochetes could be seen by 60 min. In contrast, the majority of B. burgdorferi spirochetes were still intact in MyD88−/− BMDM at 20 min and even at 60 min. Almost all of these spirochetes were visualized by incubation with the B. burgdorferi antibody prior to permeabilization, suggesting a defect in the uptake into BMDM rather than a defect in degradation in the phagolysosome. At 60 min, 80.5% ± 18.7% of wild-type BMDM contained internalized spirochetes, while only 25.7% ± 12.9% of MyD88−/− BMDM contained any spirochetes (P ≤ 0.05) (Fig. 1B). Intact spirochetes were only rarely seen within either wild-type or MyD88−/− BMDM (Fig. 1C), suggesting that the difference in phagocytosis was due to uptake of the organisms and not degradation of the organisms after uptake.
FIG. 1.
MyD88−/− BMDM show deficient uptake of B. burgdorferi. (A) BMDM from wild-type littermates and MyD88−/− mice were isolated. B. burgdorferi spirochetes were added to the BMDM at an MOI of 10. The cells were incubated with B. burgdorferi for 5, 20, or 60 min. Cells were fixed at each time point and then probed with various antibodies. Lanes: DIC, differential interference contrast; LAMP-1, anti-LAMP-1 (lysosomal marker) antibody (green); Bb, rabbit anti-B. burgdorferi antibody prior to permeabilization of the cells to identify extracellular organisms (blue); Bb (permeabilized), rabbit anti-B. burgdorferi antibody after permeabilization of the cells to identify both internalized and extracellular B. burgdorferi epitopes (red). Lane Merge shows a merged image of LAMP-1, Bb, and Bb (permeabilized). Experiments were repeated five times with cells from two different mice per experiment, and representative images are shown. Scale bar, 10 μM. (B) Cells containing internalized B. burgdorferi particles were counted and expressed as a percentage of the total number of cells examined. (C) Cells containing spirochetes that appeared intact and not degraded (i.e., that maintained their spirochetal architecture) were counted and expressed as a percentage of the total cells. Each experiment was repeated five times using cells from two different mice each time. The error bars represent standard deviations. *, P ≤ 0.05 compared with wild-type (WT) cells at matched postinfection time points. (D) For quantitative analysis of B. burgdorferi survival in the BMDM from wild-type littermates and MyD88−/− mice, B. burgdorferi spirochetes were incubated with macrophages as before. After 1 h of incubation, the cells were washed three times with cold PBS to eliminate unbound B. burgdorferi spirochetes. Cells (106) were resuspended in BSK liquid medium and serially diluted in 96-well plates. The wells were monitored for growth of B. burgdorferi. Experiments were performed in duplicate and repeated three times with cells from two different mice per experiment. Each bar represents one independent experiment.
To ensure that the defect in uptake of B. burgdorferi by MyD88−/− BMDM was not due to a global defect in uptake, we compared the uptake of E. coli in MyD88−/− and wild-type BMDM. Anti-OmpA antibody was used to detect E. coli (25). E. coli bacteria were very rapidly ingested by BMDM, and no defects or delays were seen in the uptake of E. coli at 5, 20, and 60 min by MyD88−/− BMDM (data not shown). The rapid uptake of E. coli by the MyD88−/− cells suggested that the defect in uptake of B. burgdorferi was not due to an intrinsic inability of the cells to take up other organisms.
In order to further confirm the immunofluorescence data, we performed quantitative cultures for B. burgdorferi after 60-min incubations with wild-type and MyD88−/− BMDM. Unbound B. burgdorferi spirochetes were washed away prior to culture, so this assay measures the numbers of live spirochetes that are bound to the surfaces of the cells or are internalized. We recovered 2-log-units-higher numbers of spirochetes from cultures where B. burgdorferi were incubated with MyD88−/− BMDM than from wild-type BMDM (10,000 organisms/106 cells versus 100 organisms/106 cells) (Fig. 1D).
Transient transfection of MyD88 DN plasmid in Raw 264.7 cells reduces the uptake of B. burgdorferi.
To confirm the effects of MyD88 deficiency on the phagocytosis of B. burgdorferi and to eliminate the possibility of unrecognized or unintended effects related to the knockout, we wished to test the effects of blocking MyD88 signaling through a different mechanism. As one issue that has been raised in studies of phagocytosis of other organisms with MyD88-deficient cells has been the effect of the absence of TLR signaling during cellular maturation (52), we chose to transfect mature mouse macrophage (Raw 264.7) cells with a MyD88 DN plasmid. This had the advantage that the cells had matured normally in the presence of TLR signaling and that MyD88 was not eliminated by the presence of the DN protein. Only the effects of MyD88 engagement through signaling of the TLR ligands were reduced due to competitive binding to the DN protein.
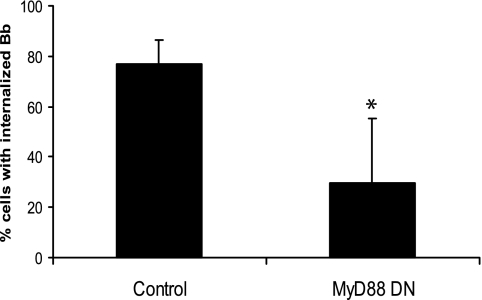
We transiently transfected a MyD88 DN plasmid or an empty-vector control into a mouse macrophage cell line, Raw 264.7. The effect of abrogating MyD88 function in these cells was confirmed by impaired TNF-α and IL-6 mRNA production in response to B. burgdorferi (data not shown). Cells transiently transfected with an empty-vector control behaved similarly to untransfected Raw 264.7 cells; B. burgdorferi spirochetes were ingested by the cells and, by 60 min, were found to be completely degraded and associated with the phagolysosomes. However, transfection with DN MyD88 resulted in a reduction in phagocytosis that was similar to that seen in the MyD88−/− BMDM (77.1% ± 9.5% control-transfected cells versus 29.6% ± 25.7% MyD88 DN-transfected cells [P ≤ 0.05]) (Fig. 2). These results confirmed the results with our MyD88−/− BMDM and strongly suggested that MyD88-mediated phagocytosis could be due to a requirement for engagement of upstream receptors that signal through MyD88.
FIG. 2.
Transient transfection of a MyD88 DN plasmid in Raw 264.7 cells reduces the uptake of B. burgdorferi. Raw 264.7 cells were transiently transfected with pCDNA3-GFP control or MyD88 DN plasmid. After 24 h, the cells were incubated with B. burgdorferi (Bb) for 60 min. Fixed cells were incubated with different fluorescently labeled antibodies as described in the legend to Fig. 1 and then visualized by fluorescence microscopy. Cells containing internalized B. burgdorferi particles at 1 h postinfection were counted and expressed as a percentage of the total number of cells examined. The data are representative of three independent experiments. The error bars represent standard deviations. *, P ≤ 0.05 compared with control-transfected cells.
TLR2, TLR5, and TLR9 are not required for the uptake of B. burgdorferi.
TLR2 has been shown to recognize borrelial lipoproteins (21), and studies of TLR2- or MyD88-deficient mice indicated that both are important in the control of B. burgdorferi infection, as bacterial burdens were greatly increased in the absence of either TLR2 or MyD88 (5, 9, 28, 49, 51). Therefore, we sought to examine whether TLR2 is also important for uptake of B. burgdorferi. We performed phagocytosis assays using BMDM from TLR2−/− mice as before. Again, wild-type control BMDM took up and degraded B. burgdorferi within phagolysosomes of macrophages by 20 min, with almost no B. burgdorferi spirochetes seen extracellularly in association with cells. In marked contrast to MyD88−/− BMDM, the absence of TLR2 did not affect the phagocytosis of B. burgdorferi, and at 20 min and 60 min, almost all the organisms were degraded, with the same percentage of cells containing degraded B. burgdorferi as control BMDM (82.2% ± 23.2% wild-type control BMDM versus 88.0% ± 13.4% TLR2−/− BMDM at 60 min postinfection) (Fig. 3A).
FIG. 3.
TLR2, TLR5, and TLR9 do not participate in the phagocytosis of B. burgdorferi. (A) Wild-type (WT) control or TLR2−/− BMDM were plated in 24-well plates and infected with B. burgdorferi spirochetes (Bb) for 5 min, 20 min, and 60 min. (B) Raw 264.7 cells were transfected with either control or TLR5 siRNAs, and after 24 h, the cells were infected with B. burgdorferi for 5 min, 20 min, and 60 min. (C) Wild-type control or TLR9−/− BMDM were plated in 24-well plates and infected with B. burgdorferi for 5 min, 20 min, and 60 min. The phagocytosis assay and staining were performed as described in the legend to Fig. 1. Cells containing internalized B. burgdorferi particles were counted and expressed as a percentage of the total number of cells examined. The data are representative of three or four independent experiments. The error bars represent standard deviations.
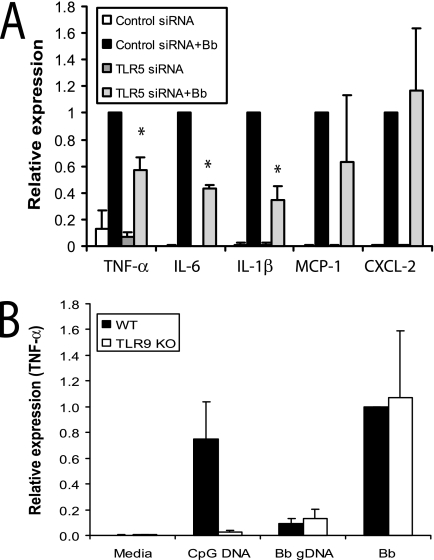
Very little is known about the interaction of TLR5 and TLR9 with B. burgdorferi products. TLR5 recognizes flagellin protein, which is required for bacterial motility (16), and TLR9 recognizes CpG motifs in bacterial DNA (3, 19). B. burgdorferi does contain flagellin, which could be recognized by TLR5, although this has not been documented. TLR5 has not been associated with the phagocytosis of any organism. To determine whether the effects of MyD88 on phagocytosis of B. burgdorferi by macrophages might be mediated by TLR5, we transfected Raw 264.7 cells with different siRNAs directed against TLR5. Unfortunately, we were unable to find an antibody suitable for determining knockdown of TLR5 at the protein level; however, we identified two separate siRNA constructs that were able to reduce mRNA expression of TLR5 by 60% 24 to 48 h posttransfection, confirmed by qRT-PCR (data not shown). In addition, these effects were specific for TLR5 ligands, as transfection with TLR5 siRNA reduced the induction of TNF-α mRNA in response to S. enterica serovar Typhimurium flagellin and did not affect cellular responses to CpG DNA (data not shown). Phagocytosis assays were performed as shown in Fig. 1. No defect in the phagocytosis of B. burgdorferi was seen with transfection of TLR5 siRNA compared with control siRNA (57.0% ± 19.5% control siRNA-transfected macrophages versus 68.8% ± 27.8% TLR5 siRNA-transfected macrophages contained B. burgdorferi particles at 60 min postinfection) (Fig. 3B). This suggests either that TLR5 is not involved in phagocytosis or that small amounts of TLR5 are sufficient to produce efficient phagocytosis.
TLR9 is an intracellularly located receptor that would not be predicted to have an effect on phagocytosis. However, we confirmed this by utilizing TLR9−/− BMDM. As expected, no effects on phagocytosis were seen in the TLR9−/− BMDM (81.4% ± 7.1% of wild-type control BMDM versus 67.1% ± 20.3% of TLR9−/− BMDM contained degraded B. burgdorferi particles at 60 min postinfection) (Fig. 3C).
p38 MAPK does not play a role in phagocytosis of B. burgdorferi.
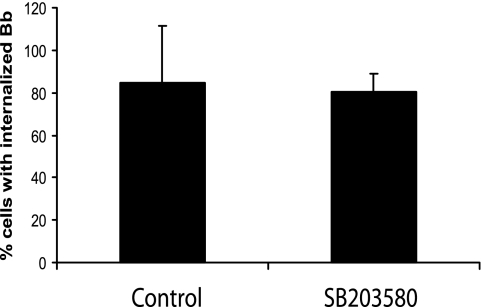
Signaling through TLRs can activate many different signaling pathways. The majority of studies of TLR involvement in phagocytosis have focused on the role of p38 MAPK (8, 12, 26). In order to determine the effect of p38 MAPK in the phagocytosis of B. burgdorferi, we performed phagocytosis assays in the presence of a p38 MAPK inhibitor. BMDM from wild-type mice were preincubated with 10 μM p38 MAPK inhibitor SB203580 for 1 h prior to the addition of B. burgdorferi. Doses of the inhibitor were chosen based on previously published studies (8, 17), and the activity of the inhibitor at these doses for suppression of downstream gene activation was confirmed by qRT-PCR in separate experiments (data not shown).
In the vehicle (dimethyl sulfoxide)-treated controls, B. burgdorferi spirochetes were found to be degraded and associated with phagolysosomes of wild-type BMDM by 60 min, with almost no B. burgdorferi spirochetes seen extracellularly in association with cells. Although it affected cytokine induction, the p38 MAPK inhibitor, SB203580, did not affect phagocytosis of B. burgdorferi, and by 60 min, almost all the organisms were degraded and the same percentage of cells as vehicle-treated cells contained degraded B. burgdorferi (84.54% ± 26.8% of vehicle-treated cells versus 84.19% ± 8.0% of SB203580-treated cells at 60 min postinfection) (Fig. 4).
FIG. 4.
p38 MAPK is not involved in the phagocytosis of B. burgdorferi. Wild-type BMDM were preincubated with either a vehicle control or 10 μM p38 MAPK inhibitor SB203580 for 1 h prior to the addition of B. burgdorferi (Bb) (MOI = 10). The phagocytosis assay and immunofluorescence staining were performed as described in the legend to Fig. 1. Cells containing internalized B. burgdorferi particles at 1 h after B. burgdorferi infection were counted and expressed as a percentage of the total number of cells examined. The data are representative of three independent experiments. The error bars represent standard deviations.
Roles of MyD88, TLR2, TLR5, and TLR9 in inflammatory signaling.
Given the important roles of TLRs and MyD88 in inflammatory signaling, we next wished to examine the impact of loss of these receptors/adaptors on the release of cytokines and chemokines in B. burgdorferi-infected BMDM. BMDM from either MyD88−/− or TLR2−/− mice were stimulated with whole B. burgdorferi spirochetes to analyze the contributions of these molecules to the cellular response in terms of cytokine and chemokine production. The cells were stimulated with B. burgdorferi, and 24 h later, RNA was harvested. The transcriptional levels of cytokines and chemokines were examined by qRT-PCR.
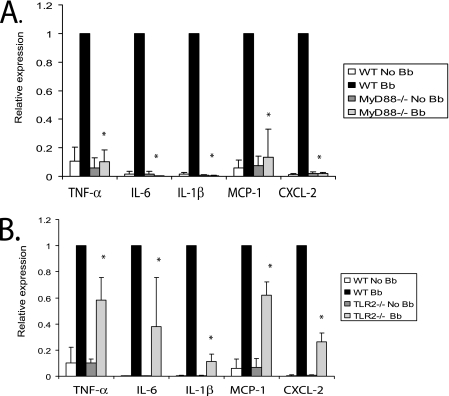
As has been well established in previous studies, the addition of B. burgdorferi to BMDM results in induction of cytokines and chemokines, such as TNF-α, IL-6, IL-1β, monocyte chemotactic protein 1 (MCP-1), and chemokine (C-X-C motif) ligand 2 (CXCL-2) (4-6, 9, 21, 53). However, MyD88−/− BMDM showed little or no induction of these cytokines and chemokines following B. burgdorferi infection (Fig. 5A).
FIG. 5.
Both MyD88 and TLR2 regulate B. burgdorferi-induced expression of inflammatory molecules from BMDM. Wild-type (WT), MyD88−/−, and TLR2−/− BMDM were infected with B. burgdorferi (Bb) at an MOI of 10 for 24 h, and transcriptional expression of TNF-α, IL-6, IL-1β, MCP-1, and CXCL-2 was measured by qRT-PCR as described in Materials and Methods. MyD88−/− and littermate wild-type control macrophages are shown in panel A, and TLR2−/− and wild-type control BMDM are shown in panel B. The expression of target genes was normalized to that of β-actin. The results shown are from three independent experiments performed in duplicate. The expression from wild-type cells infected with B. burgdorferi was arbitrarily set to 1 for all experiments, and the other values are shown relative to that expression level. The error bars represent standard deviations. *, P ≤ 0.05 compared to wild-type cells infected with B. burgdorferi.
In contrast, the absence of TLR2 only partially affected the induction of cytokines and chemokines by B. burgdorferi. The effects did show some variation, depending upon the specific cytokine or chemokine. Compared to the wild-type control, mRNA transcript expression of TNF-α, IL-6, IL-1β, MCP-1, and CXCL-2 from TLR2−/− BMDM was decreased by 41.7%, 61.9%, 88.9%, 37.9%, and 73.5% (P ≤ 0.05 for all) (Fig. 5B).
Although TLR5 did not appear to have a role in the phagocytosis of B. burgdorferi, that does not preclude a role in the recognition of B. burgdorferi products and the induction of inflammatory signaling. Recognition of B. burgdorferi products by TLR5 has not been reported. We performed siRNA knockdown of TLR5 and stimulated cells with live B. burgdorferi. Raw 264.7 cells were transfected with either control or TLR5 siRNAs and stimulated with B. burgdorferi. Cells were harvested 24 h postinfection, and total RNA was analyzed by qRT-PCR. While a nontargeting siRNA (siRNA control) had no effect on TLR5 mRNA levels, transfection with siRNA targeted at TLR5 decreased TLR5 mRNA levels by 60% after 24 to 48 h of transfection (data not shown). We found that 60% reduction of TLR5 mRNA by siRNA resulted in 43%, 57%, and 65% reductions (P ≤ 0.05 for all) in the expression of TNF-α, IL-6, and IL-1β in response to B. burgdorferi stimulation (Fig. 6A). However, knockdown of TLR5 expression did not have significant effects on the transcription of chemokines such as MCP-1 and CXCL-2.
FIG. 6.
TLR5, but not TLR9, is involved in inflammatory signaling in response to B. burgdorferi. (A) Raw 264.7 cells were transfected with either control or TLR5-specific siRNAs; 24 h later, the cells were stimulated with either medium or B. burgdorferi for 24 h. Transcriptional expression of TNF-α, IL-6, IL-1β, MCP-1, and CXCL-2 was measured by qRT-PCR, as described in Materials and Methods. Expression with cells transfected with control siRNA and infected with B. burgdorferi was arbitrarily set to 1 for all experiments, and the other values are shown relative to that expression level. (B) Wild-type (WT) and TLR9−/− BMDM were collected and plated in six-well plates. The BMDM were stimulated with either 200 nM CpG DNA, 1 μg purified B. burgdorferi genomic DNA (Bb gDNA), or whole B. burgdorferi spirochetes (Bb) for 4 h, and cells were harvested for RNA isolation. Transcriptional expression of TNF-α is shown. The expression of cytokines in wild-type BMDM infected with B. burgdorferi was arbitrarily set to 1. The real-time PCR experiments were performed in duplicate and repeated three times, and the average of all experiments is shown. The error bars represent standard deviations. *, P ≤ 0.05 compared to control siRNA stimulated with B. burgdorferi. KO, knockout.
The role of TLR9 signaling in the recognition of B. burgdorferi has also not been reported. B. burgdorferi is known to release DNA in culture and could provide a ligand for TLR9. Phagocytosis of B. burgdorferi may result in the release of CpG DNA that is sampled by TLR9 inside the cell. We stimulated wild-type and TLR9−/− BMDM with B. burgdorferi and examined the induction of mRNAs for different cytokines by qRT-PCR. However, TLR9 deficiency had no effect on cytokine secretion induced by B. burgdorferi. Stimulation with either purified genomic DNA or whole B. burgdorferi spirochetes resulted in the production of similar levels of TNF-α by BMDM from wild-type and TLR9−/− mice (Fig. 6B). Similar results were shown in the production of IL-6 and IL-1β (data not shown). Thus, TLR5, but not TLR9, is important for the induction of inflammatory responses to B. burgdorferi that we observed.
TLR2 samples B. burgdorferi lipoproteins both extracellular and intracellularly, whereas TLR5 recognizes predominantly extracellular B. burgdorferi products.
Phagocytosis of B. burgdorferi has been reported to be required for the induction of inflammatory signaling and production of cytokines (33). To determine whether the different effects of B. burgdorferi on TLR2−/− and MyD88−/− BMDM is due to the difference in phagocytosis and internalization between the cells, we examined the effects of a phagocytosis inhibitor, cytochalasin D, on signaling in wild-type and TLR2−/− BMDM. The cytochalasins have been used extensively to inhibit the phagocytosis of many different types of bacteria (10, 33, 52). First, we confirmed by immunofluorescence imaging that cytochalasin D blocked the majority (>95%) of phagocytosis of B. burgdorferi (data not shown).
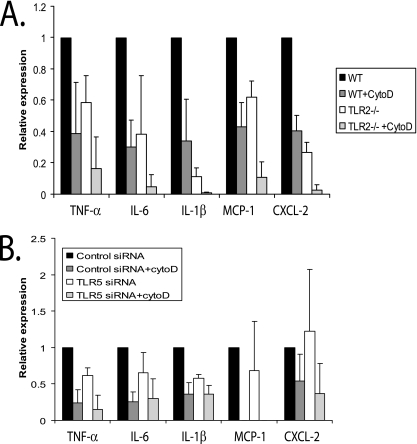
To confirm that blocking phagocytosis of B. burgdorferi by cytochalasin D treatment leads to decreased levels of cytokines and chemokines, we examined the expression of TNF-α, IL-6, IL-1β, MCP-1, and CXCL-2 in cytochalasin D-treated wild-type BMDM. Cells were harvested 24 h postinfection, and cytokine and chemokine expression was examined by qRT-PCR. The presence of cytochalasin D reduced the expression of TNF-α, IL-6, IL-1β, MCP-1, and CXCL-2 mRNA by 74%, 68%, 77%, 81%, and 72%, respectively (P ≤ 0.05 for all compared with mock-treated controls) (Fig. 7A). Of note, this level of reduction is less than was seen with the MyD88−/− BMDM.
FIG. 7.
Effects of inhibition of phagocytosis by cytochalasin D on inflammatory signaling via TLR2 and TLR5. (A) BMDM from either wild-type (WT) control or TLR2−/− mice were plated in six-well plates. Cytochalasin D (1 μM) was added to the macrophages 1 h before incubation with B. burgdorferi to block uptake of the bacteria into BMDM. The BMDM were stimulated with B. burgdorferi for 24 h, and cells were harvested for RNA isolation. Transcriptional expression of TNF-α, IL-6, IL-1β, MCP-1, and CXCL-2 was measured by qRT-PCR, as described in Materials and Methods. The expression of target genes was normalized to that of β-actin. Expression with wild-type cells infected with B. burgdorferi was arbitrarily set to 1 for all the experiments, and the other values are shown relative to that expression level. The experiments were performed three times in duplicate, and the averages of the experiments are shown. The error bars represent standard deviations. The P values for all tested cytokines and chemokines, comparing B. burgdorferi-infected wild-type and TLR2−/− BMDM, were ≤0.05. Comparisons between B. burgdorferi-infected wild-type cells treated or not with cytochalasin D and between B. burgdorferi-infected wild-type cells and TLR2−/− cells treated with cytochalasin D were also all statistically significant (P ≤ 0.05). (B) Raw 264.7 cells were transfected with either control or TLR5 siRNAs. After 24 h, cytochalasin D (1 μΜ) was added to the macrophages 1 h before incubation with B. burgdorferi to block uptake of the bacteria into the Raw 264.7 cells. The cells were stimulated with B. burgdorferi for 24 h and harvested for RNA isolation. Transcriptional expression of TNF-α, IL-6, IL-1β, MCP-1, and CXCL-2 was measured by qRT-PCR, as described in Materials and Methods. The expression of target genes was normalized to that of β-actin. Expression with control siRNA-transfected cells infected with B. burgdorferi was arbitrarily set to 1 for all the experiments, and the other values are shown relative to that expression level. The experiments were performed three times in duplicate, and the averages of the experiments are shown. The error bars represent standard deviations.
TLR2 is usually located on the cell surface, but upon microbial recognition, it can be recruited to phagosomes (48). In order to determine whether activation of TLR2 following B. burgdorferi infection occurs in phagosomes or through recognition of borrelial products in the extracellular space, we incubated TLR2−/− BMDM with cytochalasin D for 1 h before stimulating the cells with B. burgdorferi. The combination of cytochalasin D with TLR2−/− cells would be expected to abrogate all signaling requiring TLR2, as well as any non-TLR2-mediated signaling requiring phagocytosis. The addition of cytochalasin D to TLR2−/− BMDM resulted in additional reduction of induction of inflammatory cytokines beyond that seen with either cytochalasin D or TLR2−/− BMDM alone (Fig. 7A). The decrease in induction of inflammatory cytokines approached that seen with MyD88−/− BMDM (which would lack both phagocytosis and TLR signaling) for many molecules. This suggests that (i) based on the greater reduction of induction of inflammatory mediators with the addition of cytochalasin D to TLR2−/− BMDM, there are TLR2-independent pathways that are activated after phagocytosis of the organism, and (ii) based on greater reduction of cytokines and chemokines in TLR2−/− cells than in wild-type BMDM treated with cytochalasin D, TLR2 signaling can be activated both through recognition of external B. burgdorferi and through recognition of products after phagocytosis. The relative contributions of the recognition of external products versus phagosome sampling by TLR2 varied depending upon the cytokine involved. A rough estimation of the contribution of recognition of extracellular B. burgdorferi products by TLR2 to total induction for a specific cytokine ranged from 22 to 37% (percent wild-type BMDM treated with cytochalasin D − percent TLR2−/− BMDM treated with cytochalasin D). The contribution of recognition of intracellular B. burgdorferi products by TLR2 was estimated to range from 6 to 56% of the total induction of a specific cytokine ([100 − percent TLR2−/− BMDM] − percent TLR2 external signaling).
We also studied the effects of the addition of cytochalasin D to cells treated with TLR5 siRNA. In contrast to our findings for TLR2, the addition of cytochalasin D showed no significant effects on TLR5 signaling, suggesting that the majority of TLR5 signaling occurs through recognition of extracellular B. burgdorferi products by surface-bound receptor (Fig. 7B).
DISCUSSION
Phagocytosis involves a series of coordinated events beginning with recognition of a foreign pathogen and proceeding through activation of receptors and complex signaling networks, leading to internalization, killing, and processing of the pathogen (47). In this study, we provide evidence that MyD88 plays an important role in the internalization of B. burgdorferi into mouse macrophages. BMDM from MyD88−/− mice were unable to efficiently ingest B. burgdorferi. In addition, reduced phagocytosis in MyD88 DN plasmid-transfected macrophages indicates that even short-term inhibition of MyD88 signaling decreases phagocytosis.
Previous studies have suggested that although there may be some deficiency in the ability of MyD88−/− BMDM to ingest certain bacteria, the primary defect in their ability to kill bacteria is secondary to deficiencies in phagosome maturation or oxidative killing (8, 26). Of note, we did not observe any defect in the uptake of E. coli by BMDM from MyD88−/− mice in our hands. It is possible that E. coli may be recognized by other receptors that are important in activating phagocytosis and replacing the signals that are lost with the deletion of MyD88. For example, E. coli LPS is recognized by TLR4 (35). TLR4 can signal through both MyD88-dependent and -independent pathways. There is an overlap in the downstream signaling pathway activated by both MyD88-dependent and -independent pathways, so recognition of E. coli LPS could bypass MyD88 and still provide the required downstream signal for phagocytosis.
For B. burgdorferi, the defect in phagocytosis appears to be primarily in internalization of the organism. We did not see elongated, intact B. burgdorferi spirochetes within BMDM from MyD88−/− mice at 60 min, suggesting that once spirochetes were taken up by the macrophages, degradation occurred normally. Interestingly, loss of p38 signaling, which is important for phagosome maturation and is thought to be the defect in the lack of killing of other organisms in MyD88−/− macrophages, did not affect phagocytosis of B. burgdorferi, although it did reduce inflammatory signaling. Previous reports have suggested that phagocytosis of B. burgdorferi may occur in part through coiling phagocytosis and extraphagosomal killing (37), which would not require p38 signaling; however, our experiments did not allow us to distinguish this.
Our results differ significantly from previous reports by Liu et al. that suggested normal uptake of B. burgdorferi but impaired killing by macrophages (28). There are a number of differences in the methodologies that may account for the different results, including differences in the source and background of the mice, the use of peritoneal macrophages versus BMDM, and differences in the strain of B. burgdorferi. Most significantly, their experiments relied on H2O washes of cells incubated with B. burgdorferi to osmotically lyse noninternalized organisms without further confirmation of whether all externally bound bacteria were lysed. In our hands, while H2O washing did lyse the majority of extracellular bacteria, our staining revealed the continued presence of intact bacteria bound externally to the cells. We hardly saw intact B. burgdorferi internalized within cells. It is also possible that osmotic shock causes changes to cellular membranes or function that could affect phagocytosis.
Although MyD88 seems to be required for efficient internalization of B. burgdorferi, TLR2, TLR5, and TLR9 do not appear to play any role in the phagocytosis of B. burgdorferi. This is particularly surprising for TLR2, which is the primary host receptor for B. burgdorferi lipoproteins. It is certainly possible that the phagocytic effects involving MyD88 are mediated through an as-yet-unrecognized TLR, although none of the other TLRs have been confirmed to recognize a B. burgdorferi product. Another possibility is that the effects of MyD88 on phagocytosis are due to activation through non-TLR pathways, such as IL-1 or IL-18, which can also utilize MyD88 as an adaptor molecule (1, 29).
Several potential mechanisms have been proposed by which MyD88 may affect the internalization of organisms. One possible mechanism is that it may affect the expression of scavenger receptors on the surfaces of macrophages that are responsible for binding and internalizing bacteria (46). Some scavenger receptors have been shown to be up-regulated by TLR signaling and participate in TLR-mediated phagocytosis of other organisms (12). Another possible mechanism is that MyD88 signaling may be important in the regulation of actin polymerization and membrane extension for internalization of B. burgdorferi.
Phosphatidylinositol 3-kinase signaling is involved in membrane extension and fusion behind bound particles and may play a role in the insertion of new membrane at the site of particle internalization (47). We have found that B. burgdorferi-induced activation of phosphatidylinositol 3-kinase is dependent upon MyD88 signaling (unpublished data), but it is unclear whether this is directly responsible for the defects in phagocytosis. Third, it is possible that MyD88-mediated phagocytosis may occur through regulation of lysosomal recruitment, which is necessary to open the membrane for the organisms to enter. This mechanism has been proposed as the entry mechanism for other organisms, such as Trypanosoma cruzi (45).
The processing of microbial invaders by phagocytes plays an important role in inflammation and host defense for many different organisms (14, 24, 33). However, for B. burgdorferi, it has long been assumed that activation of TLR2 occurs through the recognition of B. burgdorferi lipoproteins by membrane-bound TLR2 prior to phagocytic processing. Recent studies have cast doubt upon the importance of recognition of B. burgdorferi lipoproteins in the extracellular space. Behera et al. have shown that antibodies to TLR2 are unable to block induction of inflammatory signaling by live B. burgdorferi, although they efficiently block cytokine induction by purified B. burgdorferi lipoproteins (6). Moore et al. have shown that treatment of human monocytes with cytochalasin D results in a reduction of TNF-α and gamma interferon induction in response to live B. burgdorferi (33). Our results confirmed the importance of phagocytosis and bacterial processing for induction of inflammatory cytokines but also suggested that there is still a more minor component of recognition of B. burgdorferi products by membrane-associated TLR2. The lack of an effect on phagocytosis by the loss of TLR2 compared with MyD88 has allowed us to distinguish the contributions of phagocytosis and external TLR2 signaling to the overall release of inflammatory products. It appears that activation of TLR2 occurs through both binding of borrelial lipoproteins to TLR on the surfaces of cells and sampling of lysosomes containing degraded bacterial products.
The role of TLR5 in response to B. burgdorferi has not been previously reported. Our data support the notion that TLR5 signaling may be important for induction of cytokines in response to B. burgdorferi. Interestingly, this effect was restricted to specific cytokines, and the transcription of chemokines, such as MCP-1 and CXCL-2, was not affected by the loss of TLR5 signaling. Although our data suggest that both TLR2 and TLR5 signaling contributes to the inflammatory response to B. burgdorferi, it remains possible (likely) that other receptors may contribute to the inflammatory response. Of note, our TLR5 studies utilized Raw 264.7 cells rather than primary BMDM, since primary murine BMDM express minimal or no TLR5; thus, the relative contribution of TLR5 signaling is also likely to be cell type specific. An interesting aspect of our data is that B. burgdorferi flagellin is encased within the outer surface membrane and would not be predicted to interact directly with TLR5. It is possible that some flagellin is released from the within the bacteria through bacterial death or blebbing of the membrane and is accessible to TLR5. However, we cannot rule out the possibility that a B. burgdorferi product other than flagellin is being recognized by TLR5.
In summary, we have found that MyD88, but not TLR2, TLR5, or TLR9, plays an important role in the phagocytosis of B. burgdorferi. The contribution of MyD88 to inflammatory signaling in B. burgdorferi infection is in large part due to its role in phagocytosis, but also to its transduction of TLR signals. TLR2 recognizes both extracellular and internalized products of B. burgdorferi, both of which contribute to overall inflammatory signaling. TLR5, which has not been shown to be activated by B. burgdorferi, appears to respond primarily to extracellular products. A better understanding of the complex mechanisms involved in the phagocytic responses to specific organisms and the resulting effects on the inflammasome may lead to new insights into the contributions of specific pathways to inflammation and the mechanisms by which the host response can differentiate between microbial invaders.
Acknowledgments
We thank Katherine Fitzgerald for the generous gift of MyD88 DN plasmid, Carol Kumamoto for mouse anti-OmpA antibody, Kristin Stephan for providing CpG DNA, and Honorine Ward, Carol Kumamoto, Thereza Imanish-Kari, Jin-Hwan Han, and Diem Nguyen for their assistance with TLR2−/− and TLR9−/− mice. We also thank Jenifer Coburn, Alexander Poltorak, Philip Tsichlis, Andrew Heilpern, Meghan Lavalley, Ana Kazimirova, and Deb Bhattacharya for their helpful discussions in preparing the manuscript. We thank Roberta O'Connor for help with immunofluorescence microscopy.
Work on this project was supported by grants from the National Institutes of Health (R01AI44240 [L.T.H.]) and by the American Lung Association (A.K.B.) and the Earle P. Charlton Research fund (A.K.B.).
Editor: J. B. Bliska
Footnotes
Published ahead of print on 31 March 2008.
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9143-150. [DOI] [PubMed] [Google Scholar]
- 2.Barbour, A. G. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57521-525. [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer, S., C. J. Kirschning, H. Hacker, V. Redecke, S. Hausmann, S. Akira, H. Wagner, and G. B. Lipford. 2001. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. USA 989237-9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck, G., G. S. Habicht, J. L. Benach, J. L. Coleman, R. M. Lysik, and R. F. O'Brien. 1986. A role for interleukin-1 in the pathogenesis of Lyme disease. Zentralbl. Bakteriol. Mikrobiol. Hyg. 263133-136. [DOI] [PubMed] [Google Scholar]
- 5.Behera, A. K., E. Hildebrand, R. T. Bronson, G. Perides, S. Uematsu, S. Akira, and L. T. Hu. 2006. MyD88 deficiency results in tissue-specific changes in cytokine induction and inflammation in interleukin-18-independent mice infected with Borrelia burgdorferi. Infect. Immun. 741462-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behera, A. K., E. Hildebrand, S. Uematsu, S. Akira, J. Coburn, and L. T. Hu. 2006. Identification of a TLR-independent pathway for Borrelia burgdorferi-induced expression of matrix metalloproteinases and inflammatory mediators through binding to integrin α3β1. J. Immunol. 177657-664. [DOI] [PubMed] [Google Scholar]
- 7.Bellocchio, S., C. Montagnoli, S. Bozza, R. Gaziano, G. Rossi, S. S. Mambula, A. Vecchi, A. Mantovani, S. M. Levitz, and L. Romani. 2004. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J. Immunol. 1723059-3069. [DOI] [PubMed] [Google Scholar]
- 8.Blander, J. M., and R. Medzhitov. 2004. Regulation of phagosome maturation by signals from Toll-like receptors. Science 3041014-1018. [DOI] [PubMed] [Google Scholar]
- 9.Bolz, D. D., R. S. Sundsbak, Y. Ma, S. Akira, C. J. Kirschning, J. F. Zachary, J. H. Weis, and J. J. Weis. 2004. MyD88 plays a unique role in host defense but not arthritis development in Lyme disease. J. Immunol. 1732003-2010. [DOI] [PubMed] [Google Scholar]
- 10.Chiani, P., C. Bromuro, and A. Torosantucci. 2000. Defective induction of interleukin-12 in human monocytes by germ-tube forms of Candida albicans. Infect. Immun. 685628-5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Veer, M. J., J. M. Curtis, T. M. Baldwin, J. A. DiDonato, A. Sexton, M. J. McConville, E. Handman, and L. Schofield. 2003. MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur. J. Immunol. 332822-2831. [DOI] [PubMed] [Google Scholar]
- 12.Doyle, S. E., R. M. O'Connell, G. A. Miranda, S. A. Vaidya, E. K. Chow, P. T. Liu, S. Suzuki, N. Suzuki, R. L. Modlin, W. C. Yeh, T. F. Lane, and G. Cheng. 2004. Toll-like receptors induce a phagocytic gene program through p38. J. Exp. Med. 19981-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng, C. G., C. A. Scanga, C. M. Collazo-Custodio, A. W. Cheever, S. Hieny, P. Caspar, and A. Sher. 2003. Mice lacking myeloid differentiation factor 88 display profound defects in host resistance and immune responses to Mycobacterium avium infection not exhibited by Toll-like receptor 2 (TLR2)- and TLR4-deficient animals. J. Immunol. 1714758-4764. [DOI] [PubMed] [Google Scholar]
- 14.Ferwerda, G., S. E. Girardin, B. J. Kullberg, L. Le Bourhis, D. J. de Jong, D. M. Langenberg, R. van Crevel, G. J. Adema, T. H. Ottenhoff, J. W. Van der Meer, and M. G. Netea. 2005. NOD2 and Toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog. 1279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzgerald, K. A., E. M. Palsson-McDermott, A. G. Bowie, C. A. Jefferies, A. S. Mansell, G. Brady, E. Brint, A. Dunne, P. Gray, M. T. Harte, D. McMurray, D. E. Smith, J. E. Sims, T. A. Bird, and L. A. O'Neill. 2001. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 41378-83. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 4101099-1103. [DOI] [PubMed] [Google Scholar]
- 17.Hedrick, M. N., C. M. Olson, Jr., D. B. Conze, T. C. Bates, M. Rincon, and J. Anguita. 2006. Control of Borrelia burgdorferi-specific CD4+-T-cell effector function by interleukin-12- and T-cell receptor-induced p38 mitogen-activated protein kinase activity. Infect. Immun. 745713-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helmby, H., and R. K. Grencis. 2003. Essential role for TLR4 and MyD88 in the development of chronic intestinal nematode infection. Eur. J. Immunol. 332974-2979. [DOI] [PubMed] [Google Scholar]
- 19.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408740-745. [DOI] [PubMed] [Google Scholar]
- 20.Henneke, P., O. Takeuchi, R. Malley, E. Lien, R. R. Ingalls, M. W. Freeman, T. Mayadas, V. Nizet, S. Akira, D. L. Kasper, and D. T. Golenbock. 2002. Cellular activation, phagocytosis, and bactericidal activity against group B streptococcus involve parallel myeloid differentiation factor 88-dependent and independent signaling pathways. J. Immunol. 1693970-3977. [DOI] [PubMed] [Google Scholar]
- 21.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by Toll-like receptor 2. J. Immunol. 1632382-2386. [PubMed] [Google Scholar]
- 22.Hu, L. T., G. Perides, R. Noring, and M. S. Klempner. 1995. Binding of human plasminogen to Borrelia burgdorferi. Infect. Immun. 633491-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5987-995. [DOI] [PubMed] [Google Scholar]
- 24.Kapetanovic, R., M. A. Nahori, V. Balloy, C. Fitting, D. J. Philpott, J. M. Cavaillon, and M. Adib-Conquy. 2007. Contribution of phagocytosis and intracellular sensing for cytokine production by Staphylococcus aureus-activated macrophages. Infect. Immun. 75830-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumamoto, C. A. 1989. Escherichia coli SecB protein associates with exported protein precursors in vivo. Proc. Natl. Acad. Sci. USA 865320-5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laroux, F. S., X. Romero, L. Wetzler, P. Engel, and C. Terhorst. 2005. Cutting edge: MyD88 controls phagocyte NADPH oxidase function and killing of gram-negative bacteria. J. Immunol. 1755596-5600. [DOI] [PubMed] [Google Scholar]
- 27.Leadbetter, E. A., I. R. Rifkin, A. M. Hohlbaum, B. C. Beaudette, M. J. Shlomchik, and A. Marshak-Rothstein. 2002. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416603-607. [DOI] [PubMed] [Google Scholar]
- 28.Liu, N., R. R. Montgomery, S. W. Barthold, and L. K. Bockenstedt. 2004. Myeloid differentiation antigen 88 deficiency impairs pathogen clearance but does not alter inflammation in Borrelia burgdorferi-infected mice. Infect. Immun. 723195-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medzhitov, R., P. Preston-Hurlburt, E. Kopp, A. Stadlen, C. Chen, S. Ghosh, and C. A. Janeway, Jr. 1998. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 2253-258. [DOI] [PubMed] [Google Scholar]
- 30.Montgomery, R. R., D. Lusitani, A. de Boisfleury Chevance, and S. E. Malawista. 2002. Human phagocytic cells in the early innate immune response to Borrelia burgdorferi. J. Infect. Dis. 1851773-1779. [DOI] [PubMed] [Google Scholar]
- 31.Montgomery, R. R., and S. E. Malawista. 1996. Entry of Borrelia burgdorferi into macrophages is end-on and leads to degradation in lysosomes. Infect. Immun. 642867-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montgomery, R. R., M. H. Nathanson, and S. E. Malawista. 1993. The fate of Borrelia burgdorferi, the agent for Lyme disease, in mouse macrophages. Destruction, survival, recovery. J. Immunol. 150909-915. [PubMed] [Google Scholar]
- 33.Moore, M. W., A. R. Cruz, C. J. LaVake, A. L. Marzo, C. H. Eggers, J. C. Salazar, and J. D. Radolf. 2007. Phagocytosis of Borrelia burgdorferi and Treponema pallidum potentiates innate immune activation and induces gamma interferon production. Infect. Immun. 752046-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasare, C., and R. Medzhitov. 2005. Toll-like receptors: linking innate and adaptive immunity. Adv. Exp. Med. Biol. 56011-18. [DOI] [PubMed] [Google Scholar]
- 35.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 2822085-2088. [DOI] [PubMed] [Google Scholar]
- 36.Radolf, J. D., L. L. Arndt, D. R. Akins, L. L. Curetty, M. E. Levi, Y. Shen, L. S. Davis, and M. V. Norgard. 1995. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J. Immunol. 1542866-2877. [PubMed] [Google Scholar]
- 37.Rittig, M. G., A. Krause, T. Haupl, U. E. Schaible, M. Modolell, M. D. Kramer, E. Lutjen-Drecoll, M. M. Simon, and G. R. Burmester. 1992. Coiling phagocytosis is the preferential phagocytic mechanism for Borrelia burgdorferi. Infect. Immun. 604205-4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scanga, C. A., J. Aliberti, D. Jankovic, F. Tilloy, S. Bennouna, E. Y. Denkers, R. Medzhitov, and A. Sher. 2002. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 1685997-6001. [DOI] [PubMed] [Google Scholar]
- 39.Seki, E., H. Tsutsui, N. M. Tsuji, N. Hayashi, K. Adachi, H. Nakano, S. Futatsugi-Yumikura, O. Takeuchi, K. Hoshino, S. Akira, J. Fujimoto, and K. Nakanishi. 2002. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J. Immunol. 1693863-3868. [DOI] [PubMed] [Google Scholar]
- 40.Sellati, T. J., D. A. Bouis, M. J. Caimano, J. A. Feulner, C. Ayers, E. Lien, and J. D. Radolf. 1999. Activation of human monocytic cells by Borrelia burgdorferi and Treponema pallidum is facilitated by CD14 and correlates with surface exposure of spirochetal lipoproteins. J. Immunol. 1632049-2056. [PubMed] [Google Scholar]
- 41.Swanson, M. S., and R. R. Isberg. 1995. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect. Immun. 633609-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeda, K., and S. Akira. 2005. Toll-like receptors in innate immunity. Int. Immunol. 171-14. [DOI] [PubMed] [Google Scholar]
- 43.Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 1655392-5396. [DOI] [PubMed] [Google Scholar]
- 44.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11443-451. [DOI] [PubMed] [Google Scholar]
- 45.Tardieux, I., P. Webster, J. Ravesloot, W. Boron, J. A. Lunn, J. E. Heuser, and N. W. Andrews. 1992. Lysosome recruitment and fusion are early events required for trypanosome invasion of mammalian cells. Cell 711117-1130. [DOI] [PubMed] [Google Scholar]
- 46.Underhill, D. M., and B. Gantner. 2004. Integration of Toll-like receptor and phagocytic signaling for tailored immunity. Microbes Infect. 61368-1373. [DOI] [PubMed] [Google Scholar]
- 47.Underhill, D. M., and A. Ozinsky. 2002. Phagocytosis of microbes: complexity in action. Annu. Rev. Immunol. 20825-852. [DOI] [PubMed] [Google Scholar]
- 48.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401811-815. [DOI] [PubMed] [Google Scholar]
- 49.Wang, X., Y. Ma, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2005. Relative contributions of innate and acquired host responses to bacterial control and arthritis development in Lyme disease. Infect. Immun. 73657-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watanabe, I., M. Ichiki, A. Shiratsuchi, and Y. Nakanishi. 2007. TLR2-mediated survival of Staphylococcus aureus in macrophages: a novel bacterial strategy against host innate immunity. J. Immunol. 1784917-4925. [DOI] [PubMed] [Google Scholar]
- 51.Wooten, R. M., Y. Ma, R. A. Yoder, J. P. Brown, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2002. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168348-355. [DOI] [PubMed] [Google Scholar]
- 52.Yates, R. M., and D. G. Russell. 2005. Phagosome maturation proceeds independently of stimulation of toll-like receptors 2 and 4. Immunity 23409-417. [DOI] [PubMed] [Google Scholar]
- 53.Zhao, Z., B. McCloud, R. Fleming, and M. S. Klempner. 2007. Borrelia burgdorferi-induced monocyte chemoattractant protein-1 production in vivo and in vitro. Biochem. Biophys. Res. Commun. 358528-533. [DOI] [PubMed] [Google Scholar]