Abstract
Hookworm infection is a major cause of anemia and malnutrition in resource-poor countries. Human and animal studies suggest that infection with these intestinal nematodes is associated with impaired cellular immunity, characterized by reduced lymphocyte proliferation in response to both parasite and heterologous antigens. We report here data from studies aimed at defining mechanisms through which hookworms modulate the host cellular immune response. Splenocytes and mesenteric lymph node (MLN) cells from hamsters infected with Ancylostoma ceylanicum showed minimal proliferation in response to mitogen at days 20 and 30 postinfection (p.i.), with partial recovery noted at day 70 p.i. The proliferative capacity of enriched splenocyte T-cell preparations from infected animals following stimulation with hookworm antigens was partially restored in the presence of antigen-presenting cells from uninfected hamsters. Analysis by fluorescence-activated cell sorting revealed that hookworm infection is associated with reduced percentages of both CD4+ and surface immunoglobulin G-positive lymphocytes in the spleen and MLN cells. Splenocytes from infected hamsters also secreted more nitric oxide (NO) in culture than did those from naïve animals. Inhibition of NO secretion was associated with partial restoration of the proliferative capacity of splenocytes from infected animals in response to concanavalin A, suggesting a role for NO in mediating this effect. Together, these data demonstrate that hookworm infection is associated with impaired function of antigen-presenting cells and depletion of important lymphocyte subpopulations and also suggests a role for NO in parasite-induced immunosuppression.
It is estimated that more than 700 million people in resource-poor countries are infected with hookworms, bloodfeeding intestinal nematodes that cause anemia and malnutrition (8, 14). Together with Ascaris lumbricoides and Trichuris trichiura, the hookworms Ancylostoma duodenale and Necator americanus comprise the group of soil-transmitted nematodes that are now recognized as a major cause of global morbidity (2, 56). Significant clinical features of hookworm infection in humans include iron-deficiency anemia, hypoproteinemia, and growth delay (13, 53). Although control strategies relying on targeted delivery of benzimidazole antihelminthics are generally effective at eliminating adult worms, reinfection occurs quickly and frequent treatments may be necessary for sustained improvement in the health of at-risk populations (50, 52).
Although sterile immunity does not appear to develop following natural infection, data from human and animal studies confirm that hookworms elicit humoral and cellular immune responses in mammalian hosts (18). Although the nature of this response has yet to be elucidated fully, infection appears to be associated with a mixed Th1/Th2 host cytokine profile (22, 49). It has also been reported that hookworm infection is associated with suppression of host cellular responses to hookworm-specific and heterologous antigens (22, 45, 49). These studies suggest that hookworms, like other parasites, effectively downregulate host cell-mediated responses, blunting development of protective immunity (1, 41, 51).
We report here results of studies designed to characterize the effect of hookworm infection on cellular immune responses. These data, which were acquired using the hamster model of Ancylostoma ceylanicum infection, confirm that hookworm infection is associated with reduced lymphocyte proliferation following stimulation with parasite antigens or T-cell mitogen. These studies also demonstrate for the first time impaired antigen presentation, a reduction in CD4+ T-lymphocyte number, and a role of nitric oxide (NO) in downregulation of the hamster cellular immune response. Together, the data provide new insights into how hookworms modulate immune responses in their mammalian hosts.
MATERIALS AND METHODS
Hookworm life cycle and pathogenesis.
The A. ceylanicum life cycle was maintained by passage through Syrian hamsters as described previously (9, 30). Animals were housed in the Yale School of Medicine, and all experiments were carried out with prior approval of the Yale Animal Care and Use Committee. For studies of the cellular immune response to hookworm infection, 24 hamsters were infected with 75 third-stage larvae (L3) of A. ceylanicum by oral gavage. An equal number of uninfected animals served as naïve controls. On days 10, 20, 30, and 70 postinfection (p.i.), six animals (and an equal number of uninfected controls) were sacrificed, and blood, spleens, and mesenteric lymph nodes (MLN) were collected for analyses. Blood hemoglobin levels were measured using a total hemoglobin assay (Sigma Diagnostics, St. Louis, MO) as previously described (30), and intestinal worm burdens were evaluated at each time point.
Lymphocyte proliferation assay.
Spleens and MLN were harvested from naïve and infected hamsters (six animals/group). Splenocytes were depleted of red blood cells by lysis and washed with RPMI medium with 10% fetal bovine serum, 2-mercaptoethanol, antibiotics, and l-glutamine (RPMI-10) (45). Single-cell preparations made in RPMI-10 were plated in triplicate (105 per well) in 96-well plates (Becton Dickinson) and stimulated with concanavalin A (ConA; 1 μg/ml) (Sigma) or kept unstimulated for 24 h at 37°C with 5% CO2. The proliferation of cells was estimated by 5-bromo 2′-deoxyuridine (BrdU) incorporation using a colorimetric kit (Roche Diagnostics, Germany). The stimulation index (SI) was calculated as a ratio of the mean optical density at 450 nm of stimulated cultures to that of unstimulated cultures (60).
Purification of spleen T cells and antigen presentation assay.
T lymphocytes were isolated by passing splenocytes depleted of erythrocytes through sterile nylon wool fiber columns (Polysciences, Inc., Warrington, PA), a technique that has been shown to yield lymphocyte preparations enriched for T cells (35, 44, 59). Irradiated splenocytes from age- and sex-matched naïve or infected hamsters were used as a source of antigen-presenting cells (APCs) (29, 36, 41). Purified T cells were plated (105 per well) with APCs (5 × 104 per well) from donor hamsters in triplicate and incubated in medium containing ConA (1 μg/ml), pooled A. ceylanicum excretory/secretory proteins (ES; 5 μg/ml) (9), or adult A. ceylanicum soluble protein extracts (HEX; 25 μg/ml) for 24 h at 37°C with 5% CO2. The SIs of T lymphocytes were assessed by BrdU incorporation as described above.
Fluorescence-activated cell sorter (FACS) assay.
Spleens and MLN were harvested from naïve and infected hamsters at day 20 p.i. They were processed as previously described, and single-cell preparations were made. Approximately 106 cells were stained with fluorescein isothiocyanate-labeled goat anti-Syrian hamster immunoglobulin G (IgG) (heavy plus light chains) and phycoerythrin-labeled anti-mouse CD4 (L3T4) for 30 min on ice in the dark. The anti-mouse CD4 antibody (L3T4) has previously been shown to cross-react with the hamster CD4 molecule (15, 16, 39). Both labeled antibodies were purchased from eBiosciences (San Diego, CA). Cell surface determinant data were acquired for a total of 105 cells per sample, using a FACSCalibur flow cytometer. Data were analyzed using the FlowJo software program (Treestar, Ashland, OR), with gating on lymphocytes.
Histological staining.
At day 20 p.i., sections of spleen and MLN embedded in paraffin (5 to 8 um thick) were prepared for histological staining. Tissue sections were stained with hematoxylin and eosin for examination by light microscopy.
Measurement of NO.
Nitric oxide (NO) production was assayed in the supernatants of splenocytes cultured for 72 h in RPMI-10 alone or supplemented with ConA (1 μg/ml), A. ceylanicum ES (5 μg/ml), or HEX (25 μg/ml). For this assay, 106 spleen cells/ml were plated in 24-well plates (Becton Dickinson). NO synthesis was quantified by measuring the NO oxidation products (nitrate/nitrite) in the medium as previously described (48), using a nitrate/nitrite colorimetric assay kit (Cayman Chemical, Ann Arbor, MI).
The proliferation of splenocytes from infected and uninfected hamsters in response to ConA was also measured in the presence of increasing concentrations of N-monomethyl-l-arginine (l-NMMA) (Calbiochem, La Jolla, CA), a competitive inhibitor of arginine-dependent nitric oxide synthase (31, 57). Five hamsters were orally infected with 75 A. ceylanicum L3, and four uninfected animals served as controls. At 20 days p.i., animals were sacrificed and splenocytes stimulated in vitro in the presence of ConA (1 μg/ml) mixed with splenocyte culture supernatant from either control (naïve) or previously infected (day 20 p.i.) hamsters. The effect of l-NMMA was assessed by measuring proliferation as well as the concentration of NO in splenocyte culture supernatants as described above.
Statistical analysis of data.
Data in the figures and text are presented as means ± standard errors. For comparison of data from infected and uninfected groups, F testing was employed to determine if Student's t test was appropriate; if the F test indicated a significant difference between standard deviations (P < 0.05), then the alternative Welch t test was employed. For multiple group comparisons, one-way analysis of variance was performed, followed by the Tukey-Kramer multiple comparison test. In all cases, P values of ≤0.05 were considered statistically significant.
RESULTS
Effects of A. ceylanicum infection on hamster blood hemoglobin levels and spleen weight.
Hamsters (n = 24) were infected with 75 A. ceylanicum L3 and followed for evidence of hookworm anemia (9, 30). As previously observed (9, 10, 30), blood hemoglobin levels of infected and uninfected animals were statistically equivalent at day 10 p.i., with values of 14.8 ± 0.2 g/dl and 15.6 ± 0.3 g/dl, respectively (Table 1). In contrast, blood hemoglobin levels in the infected animals (8.6 ± 0.7 g/dl) at day 20 p.i. were 51% lower than those in uninfected controls (16.9 ± 0.1 g/dl; P < 0.0001). A significant difference in blood hemoglobin was also noted at day 30 p.i., with levels in infected animals being 37% lower than those in uninfected controls (10.8 ± 0.8 g/dl versus 17.1 ± 0.2 g/dl; P = 0.0005). At day 70 p.i., the mean blood hemoglobin levels in infected hamsters (14.5 ± 0.3 g/dl) remained depressed compared to those measured in uninfected controls (16.1 ± 0.3 g/dl; P = 0.02).
TABLE 1.
Changes in blood hemoglobin level and spleen weight following infection with A. ceylanicum
| Animal group | Day p.i. | Hemoglobin concn (g/dl) | P valuea | Spleen wt (g) | P valuea |
|---|---|---|---|---|---|
| Uninfected | 10 | 15.6 ± 0.3 | 130 ± 8 | ||
| 20 | 16.9 ± 0.1 | 118 ± 7 | |||
| 30 | 17.1 ± 0.2 | 114 ± 6 | |||
| 70 | 16.1 ± 0.3 | 131 ± 4 | |||
| Infected | 10 | 14.8 ± 0.2 | NS | 161 ± 13 | 0.008 |
| 20 | 8.6 ± 0.7 | <0.0001 | 470 ± 43 | <0.0001 | |
| 30 | 10.8 ± 0.8 | 0.0005 | 365 ± 89 | 0.001 | |
| 70 | 14.5 ± 0.3 | 0.002 | 198 ± 51 | 0.02 |
Compared to uninfected group. NS, not significant.
By day 10 p.i., the mean spleen weight of infected hamsters (161 ± 5 mg) was higher than that of uninfected animals (130 ± 8 mg), a difference that was statistically significant (P = 0.008) (Table 1). By day 20 p.i., the mean weight of spleens harvested from infected hamsters (470 ± 18 mg) was fourfold higher than that for uninfected controls (118 ± 7 mg; P < 0.0001), and on day 30 p.i., infected hamster spleens had a threefold higher mean weight (365 ± 36 mg) than did those from uninfected animals (114 ± 6 mg; P = 0.001). At day 70 p.i., the difference in spleen weights between infected (198 + 21 mg) and uninfected (131 ± 4 mg) animals remained statistically significant (P = 0.02).
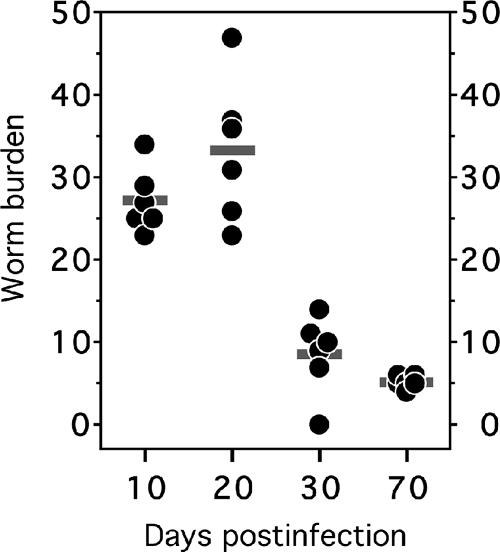
We also evaluated the intestinal worm burdens of infected animals at days 10, 20, 30, and 70 p.i. (Fig. 1). The worm burdens were highest at days 10 (27 ± 4 worms) and 20 (33 ± 9 worms) p.i., with the lowest recorded worm yield at day 70 p.i. (5 ± 1 worms). The lower mean intestinal worm burdens noted on days 30 and 70 p.i. were associated with an increase in animal weight (not shown) and blood hemoglobin level, as well as with the reduced spleen size (Table 1).
FIG. 1.
Intestinal worm burdens of infected hamsters. At each time point postinfection, six infected hamsters were sacrificed and adult hookworms removed from the intestines. The horizontal bars represent the mean number of adult worms for each group.
Effect of A. ceylanicum infection on lymphocyte proliferation in response to mitogen.
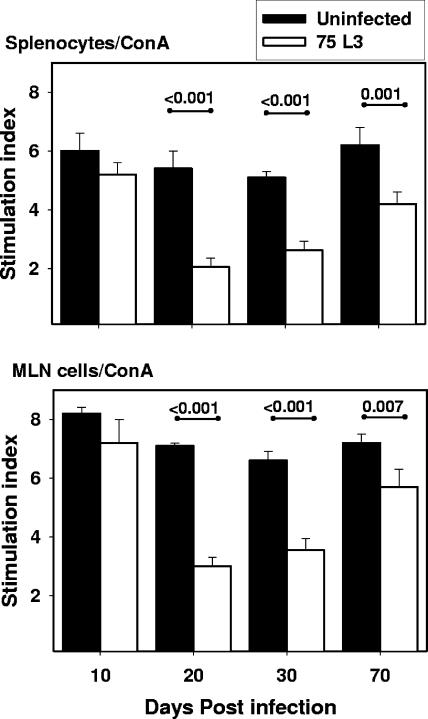
In order to define the effect of hookworm infection on lymphocyte proliferation, MLN cells and splenocytes were harvested from infected and control animals and stimulated in vitro with ConA. At day 10 p.i., the SIs of both spleen and MLN cells (Fig. 2) from infected hamsters were equivalent to those of naïve controls (6 ± 0.6 versus 5.2 ± 0.4 for splenocytes and 8.3 ± 0.2 versus 7.3 ± 0.8 for MLN cells). However, at days 20 and 30 p.i., lymphocytes from infected animals demonstrated an impaired capacity to proliferate in response to the mitogen. On day 20 p.i., the SI of splenocytes from infected hamsters (2.0 ± 0.3) was 63% lower than that for uninfected controls (5.4 ± 0.6; P < 0.001). The SI of MLN cells from infected hamsters (3.0 ± 0.3) was similarly reduced compared to that for uninfected animals (7.1 ± 0.1; P < 0.001). At day 30 p.i., the proliferative capacity of infected splenocytes remained impaired relative to that of controls (SI of 2.6 ± 0.3 versus 5.1 ± 0.2; P < 0.001), as did that of the MLN cells (SI of 3.6 + 0.4 versus 6.6 + 0.3; P < 0.001). At day 70 p.i., the reduction in SI of splenocytes from infected hamsters compared to that for uninfected controls was less dramatic than that at the earlier time points (4.2 ± 0.4 versus 6.2 ± 0.6) but was still statistically significant (P = 0.001). Similar values were observed for MLN cells (5.7 ± 0.6 versus 7.2 ± 0.3; P = 0.007) at day 70 p.i. For both organs, it was noted that SIs of cells from infected hamsters at day 10 were higher than those recorded at day 20 or 30 p.i. (P = 0.001 for each comparison) and day 70 p.i. (P = 0.01).
FIG. 2.
Proliferation of hamster splenocytes and MLN cells in response to mitogen. SIs of splenocytes (top) and MLN cells (bottom) of uninfected (solid bars; n = 5) and infected (white bars; n = 5) hamsters stimulated with ConA are shown. All values are means ± standard errors (SE). P values for differences between infected and uninfected groups that reached statistical significance (<0.05) are shown above the horizontal lines.
Infection with A. ceylanicum impairs the activity of APCs.
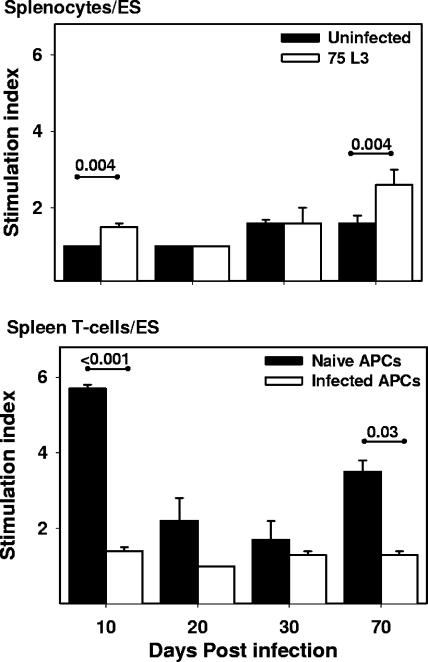
When whole splenocytes were stimulated with A. ceylanicum ES (Fig. 3), modest but statistically significant increases in SI were noted for cells from infected animals at days 10 (SI of 1.0 ± 0.0 [uninfected] versus 1.5 ± 0.1 [infected]; P = 0.004) and 70 (SI of 1.6 ± 0.2 [uninfected] versus 2.6 ± 0.4 [infected]; P = 0.004) p.i. In contrast, a complex pattern of proliferative responses was identified when splenocytes enriched for T cells were stimulated with hookworm ES antigens in the presence of APCs from infected (IAPCs) or naïve (NAPCs) donor hamsters. The presence of NAPCs significantly enhanced the proliferative response of T cells from infected animals to ES at days 10 p.i. (SI of 1.4 ± 0.1 for IAPCs versus 5.7 ± 0.1 for NAPCs; P = 0.0007) and 70 p.i. (SI of 1.3 ± 0.1 for IAPCs versus 3.5 ± 0.3 for NAPCs; P = 0.03). A similar ability of NAPCs to rescue the proliferative capacity of T cells from infected animals at days 10 and 70 p.i. was also observed following stimulation with HEX (not shown). As expected, splenocytes enriched for T cells isolated from uninfected hamsters proliferated well (SI of >6) when stimulated with ConA, independent of the infection status of the APCs (data not shown).
FIG. 3.
Proliferation of hamster splenocytes and enriched T lymphocytes in response to hookworm ES antigens. Whole splenocyte (top) or enriched splenocyte (bottom) T-cell preparations from infected (n = 5) hamsters were stimulated with hookworm ES in the presence of APCs from age-matched naïve or infected hamsters. All values are means ± SE. P values for differences that reached statistical significance (<0.05) are shown above the horizontal lines.
Hookworm infection is associated with reduced proportions of CD4+ T cells and surface IgG+ B lymphocytes.
At day 20 p.i., the data showed a peak in the clinical signs of infection (lowest hemoglobin level and highest spleen weight) (Table 1) and the largest number of adult worms recovered from the intestine (Fig. 1). At this time point, the host cellular immune response to infection was highly impaired (lowest SIs of spleen and MLN cells [Fig. 2] and impairment of antigen processing/presentation [Fig. 3]). We therefore carried out a follow-up experiment in which spleen and MLN cells were harvested from infected animals at day 20 p.i. and analyzed by FACS analysis using T- and B-cell surface markers.
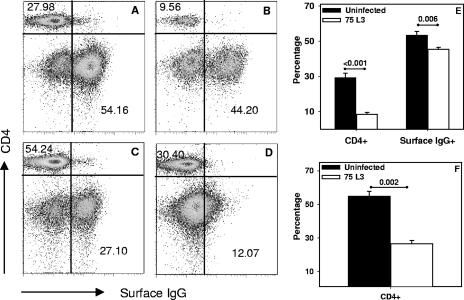
As shown in Fig. 4A and B, hookworm infection was associated with diminished percentages of both CD4+ and IgG+ lymphocyte subpopulations in the spleen. The reduction was most striking for CD4+ T cells, which accounted for 8.8% ± 1.1% of lymphocytes in the infected hamsters versus 29.1% ± 2.7% in control animals (Fig. 4E), a >3-fold reduction that was statistically significant (P < 0.001). In addition, the percentage of B cells was also reduced in the spleens of animals at day 20 p.i. This IgG+ lymphocyte population comprised 45.4% ± 1.1% of the splenocytes in infected hamsters, compared to 53.3% ± 2.3% in naïve animals, a difference that was also statistically significant (P = 0.006).
FIG. 4.
FACS analysis of splenocytes and MLN cells of naïve and infected hamsters. The data presented are representative profiles of CD4+ T-cell and surface IgG+ B-cell proportions, as shown by FACS analysis, in the spleens of an uninfected (A) and an infected (B) hamster and the MLN of an uninfected (C) and an infected (D) animal at day 20 p.i. Bar graphs represent mean percentages of CD4+ cells and surface IgG+ B cells in the spleens (E) or MLN (F) of infected and uninfected hamsters (n = 5). Values are means ± SE, and P values are shown above the horizontal lines.
Similar to the case for splenocytes, analysis of MLN cells by FACS also showed a reduction in the CD4+ T-cell population in infected hamsters (26.5% ± 2.1%) compared to that in naïve controls (54.8% ± 3%; P = 0.002) (Fig. 4C, D, and F). While sorting of MLN cells using fluorescein isothiocyanate-conjugated anti-Syrian hamster IgG showed a well-defined population bearing this surface marker in the MLN cells of naïve hamsters (Fig. 4C), this marker failed to unequivocally distinguish the stained cells from the unstained cells in infected animals at day 20 p.i. (Fig. 4D).
Histological examination of sections from enlarged MLNs taken at day 20 p.i. demonstrated the presence of activated germinal centers in samples from hookworm-infected animals (not shown). In contrast, MLNs from control (uninfected) animals were smaller, and microscopy showed no evidence of activated germinal centers. The presence of activated germinal centers is consistent with the observed loss of surface IgG found at day 20 p.i. in MLN cells and can potentially be attributed to the differentiation of B lymphocytes into mature, antibody-producing plasma cells (28).
Hookworm infection is associated with increased secretion of NO by splenocytes.
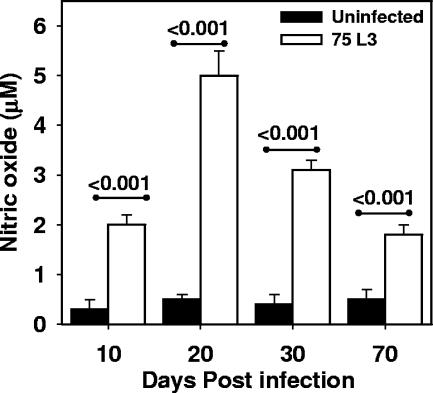
As shown in Fig. 5, cultured splenocytes from infected hamsters produced significantly greater amounts of NO than did cells from uninfected controls. Approximately 4- to 10-fold increases were noted in cells from infected compared to uninfected hamsters at every time point measured, i.e., at day 10 p.i. (2 ± 0.2 μM versus 0.3 ± 0.2 μM; P < 0.001), day 20 p.i. (5 ± 0.5 μM versus 0.5 ± 0.1 μM; P < 0.001), day 30 p.i. (3.1 ± 0.2 μM versus 0.4 ± 0.2 μM; P < 0.001), and day 70 p.i. (1.9 ± 0.4 μM versus 0.5 ± 0.2 μM; P < 0.001). The peak of NO secretion by infected splenocytes, which was measured at day 20 p.i., corresponded to the time point at which the greatest intestinal worm burden was observed (Fig. 1). Of note, the levels were unchanged by incubation with ConA or hookworm antigens (data not shown), suggesting that cells from infected animals constitutively secrete NO.
FIG. 5.
Hookworm infection is associated with secretion of nitric oxide by splenocytes. Nitric oxide levels were measured in unstimulated splenocyte culture supernatants from naïve (solid bars; n = 5) and infected (white bars; n = 5) hamsters. All values are means ± SE. P values are shown above the horizontal lines.
Impairment of lymphocyte proliferation is mediated by soluble factors.
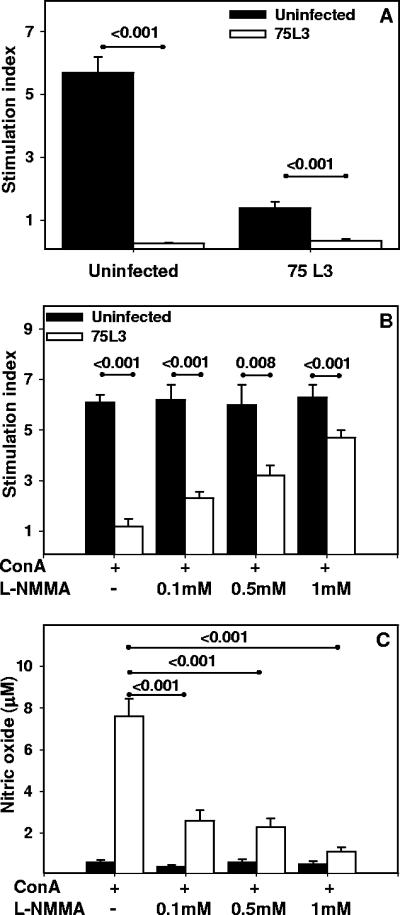
Splenocytes harvested at day 20 p.i. were stimulated with ConA in the presence of supernatant from cultured splenocytes from naïve or previously infected (day 20 p.i.) hamsters (Fig. 6A). Splenocytes from naïve animals incubated with supernatant from infected hamsters demonstrated greatly impaired proliferation in response to ConA (SI of 0.3 ± 0.03 for infected supernatant versus 5.7 ± 0.5 for naïve supernatant; P < 0.001). Concordant with previous observations, splenocytes from infected hamsters exhibited severely impaired proliferation in response to ConA in the presence of supernatant from infected hamsters (SI, 0.4 ± 0.07) or naïve animals (SI, 1.4 ± 0.2) (P = 0.001).
FIG. 6.
Role of NO in hookworm-associated impairment of lymphocyte proliferation. (A) Splenocyte culture supernatants from infected and uninfected animals (see Fig. 5, day 20 p.i.) were assayed for the ability to impair proliferation of cells harvested from naïve or infected hamsters (day 20 p.i.). Spleen cells from infected and control animals were stimulated with ConA in the presence/absence of l-NMMA, and proliferation (B) and nitric oxide production (C) were measured. All values are means ± SE. P values for differences that reached statistical significance (<0.05) are shown above the horizontal lines.
Nitric oxide mediates lymphocyte responses to hookworm infection.
In order to determine whether NO might play a role in the mechanism of impaired lymphocyte proliferative responses in the setting of hookworm infection, splenocytes harvested at day 20 p.i. were stimulated with ConA in the presence of a competitive and broad-spectrum inhibitor of NO synthase, i.e., l-NMMA. As shown in Fig. 6B, the addition of l-NMMA to cultures of infected splenocytes resulted in a concentration-dependent increase in ConA-stimulated proliferation, from a baseline SI of 1.1 ± 0.4 in the absence of inhibitor to an SI of 4.7 ± 0.3 in the presence of 1 mM l-NMMA. The increase in proliferative response was statistically significant (versus that for no inhibitor) for all three concentrations of l-NMMA tested (0.1 mM, 0.5 mM, and 1 mM). As expected, the incubation with l-NMMA did not influence the proliferation of splenocytes from uninfected hamsters.
l-NMMA inhibits the release of NO by hamster spleen cells.
Supernatants collected from splenocytes cultured in the presence of l-NMMA (see above) were assayed for the presence of NO. The supernatants from infected splenocytes cultured in the presence of ConA without inhibitor contained a mean NO concentration of 7.6 ± 0.74 μM (Fig. 6C). However, consistent with the increased in lymphocyte proliferation (Fig. 6B), the addition of l-NMMA resulted in a concentration-dependent reduction in NO secretion (range, 2.6 ± 0.5 μM to 1.1 ± 0.2 μM; P < 0.001). Of note, the addition of l-NMMA did not impact the low baseline NO production of splenocytes from uninfected hamsters (range, 0.6 ± 0.1 μM to 0.5 ± 0.2 μM; P > 0.05).
DISCUSSION
Infection with hookworms, as with other helminths, is characterized by various degrees of suppression of host cellular immune responses (22, 40, 42, 47). However, few studies have attempted to probe the mechanisms underlying this phenomenon in human and/or laboratory animal hosts (23, 45, 46). In the present study, we utilized the hamster model of A. ceylanicum to characterize the modulation of host cellular immune responses and potential mechanisms underlying such immunosuppression. This model mimics the major clinical features of natural and experimental human hookworm infection and has been exploited for studies of pathogenesis as well as for drug and vaccine development (9, 10, 18, 19).
The results presented here confirm previous observations that A. ceylanicum infection is associated with impaired lymphocyte proliferation (23, 45), a phenomenon that has also been observed in filarial nematode infections (25, 26, 54). By day 70 p.i., there were very few adult worms remaining in the intestine (Fig. 1), although proliferative responses were still reduced compared to those of uninfected controls. These data provide further evidence that residual immunosuppressive effects of hookworm infection persist well beyond the peak of infection intensity and are consistent with the findings of Geiger et al. (22). Furthermore, this observation supports the concept that even light infections with hookworm have the potential to impact host immunity and to modulate susceptibility to other pathogens, as well as responses to vaccines (7, 43).
Evidence from these studies suggests that hookworm infection impairs host cellular immunity via multiple mechanisms, essentially triggering both “qualitative” and “quantitative” impairment in host immune responses. For example, the observation that lymphocyte proliferative capacity in response to hookworm proteins was effectively “rescued” in the presence of APCs from naïve (uninfected) hamsters (Fig. 3) demonstrates that impaired antigen processing and/or presentation constitutes at least one potential (qualitative) mechanism through which Ancylostoma hookworms impair the host immune response, as has been observed with other nematodes (41, 42). Importantly, the fact that APC activity in splenocytes from infected hamsters was functionally deficient also confirms that the immunosuppressive effect of hookworm infection is systemic in nature, even though adult worms reside exclusively in the intestinal tract.
These investigations also demonstrate a quantitative mechanism underlying hookworm immunosuppression, namely, a reduction in the percentages of specific lymphocyte subpopulations that are likely important in mediating acquired host defenses against the parasite. These studies, which are the first to characterize alterations in lymphocyte profile in the hamster model of hookworm infection, demonstrate moderate to severe reductions in the percentages of CD4+ T lymphocytes during the acute phase (day 20 p.i.) of infection. Our data corroborate reports from Nigeria and Brazil that describe reduced lymphocyte proportions of CD4+ T cells in human hookworm infection (21, 47). A decline in the proportion of CD4+ T cells has also been reported for other infectious diseases associated with depressed cellular immunity, most notably human immunodeficiency virus (HIV) infection (12, 27, 37, 38) but also those caused by other viral (58), bacterial (34), protozoan (11, 24), and helminth (17, 33, 55) pathogens.
The capacity for hookworms to downregulate host cellular immunity, potentially in additive or synergistic fashion with other pathogens, further supports the use of integrated control strategies in resource-limited countries where multiple infectious diseases, especially HIV infection, are endemic (5, 7, 43). Although the effect of antihelminthic treatment on CD4+ lymphocyte populations in this model has not yet been characterized, the data presented here support the concept that deworming HIV-infected individuals might confer benefit, at least in part, by mediating an increase in the percentage and/or number of CD4+ lymphocytes (5).
The data reported here strongly suggest a role for NO in suppressing lymphocyte proliferation in the context of Ancylostoma hookworm infection, as supernatants of cells harvested from infected hamsters contained up to 10-fold higher concentrations of NO than did those from naïve controls (Fig. 5), and the proliferation of spleen cells from both naïve and infected hosts was effectively inhibited by supernatant from splenocytes of infected hamsters cultured at day 20 p.i. (Fig. 6A). This observation indicates that the impairment of host cell proliferation, at least within the spleen, is mediated by soluble factors secreted in response to infection. The capacity of l-NMMA to rescue some of the proliferative capacity of splenocytes in response to ConA (Fig. 6B), in association with dramatic reductions in NO secretion (Fig. 6C), provides further evidence of a role for NO in mediating this effect, either directly or by stimulating secretion of downstream effector molecules. The fact that NO has a very short half-life in solution would suggest that the effect is due to the latter.
Depending on the cell type and model, NO has been reported to mediate both pro- and anti-inflammatory effects (4). In light of this, it is not surprising that increased secretion of NO has been associated with either a reduction in or exacerbation of pathology in various models of parasitic infection (3, 6, 20). While NO facilitates pathogen killing by host cells, it may also exacerbate inflammatory responses that worsen disease manifestations. In the case of helminth infections, NO may play a similar role in regulating the host inflammatory response directed at parasite antigens within tissues, thus defining the regulation of NO activity as potentially important for both the host and parasite. Although much has been written about the role of NO and various cytokines in the immunopathogenesis of tissue nematode infections, e.g., filarial worms, relatively little is known about the mechanisms of immunosuppression in intestinal nematodes. Based on the cumulative data presented, we hypothesize that NO plays a direct and significant role in mediating hookworm-associated immunosuppression through its negative effects on lymphocyte proliferation. Work is currently under way in order to determine whether NO-mediated impairment of lymphocyte proliferation represents an evolutionary survival strategy of the parasite or, rather, a host defense response designed to blunt a pathogenic inflammatory response.
In summary, we report that Ancylostoma hookworm infection is associated with reduced lymphocyte proliferative capacity, impaired antigen processing/presentation, depletion of CD4+ and surface IgG+ lymphocyte subpopulations, and increased production of the immunomodulatory compound NO. More importantly, we show that NO produced in the context of hookworm infection impairs host lymphocyte proliferation and that local inhibition of NO secretion restores proliferative capacity. These observations with the hamster model of A. ceylanicum infection are in general agreement with what has been observed in humans, namely, cellular hyporesponsiveness induced by hookworm infection (40), with diminished T-cell populations (47). Although impaired antigen processing/presentation and NO-dependent lymphoproliferation have not previously been reported in the context of human infection, these data from a reproducible animal model suggest that the potential impact of hookworm-induced immunosuppression should be considered in the development of strategies to reduce the global burden of this and other important parasitic diseases (7, 32).
Acknowledgments
This work was supported by National Institutes of Health grants AI58980 (M.C.) and AI63110 (R.D.B.). M.C. is a recipient of a Hellman Family Fellowship from the Office of the President of Yale University.
We acknowledge Lisa DiFedele, Isacc Howley, Christopher Wendler, and George Porter of Yale University for technical assistance. We thank Peter Melby (University of Texas at San Antonio) for thoughtful suggestions during the course of this work.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 17 March 2008.
REFERENCES
- 1.Allen, J. E., R. A. Lawrence, and R. M. Maizels. 1996. APC from mice harbouring the filarial nematode, Brugia malayi, prevent cellular proliferation but not cytokine production. Int. Immunol. 8143-151. [DOI] [PubMed] [Google Scholar]
- 2.Bethony, J., S. Brooker, M. Albonico, S. M. Geiger, A. Loukas, D. Diemert, and P. J. Hotez. 2006. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 3671521-1532. [DOI] [PubMed] [Google Scholar]
- 3.Bian, K., M. Zhong, Y. Harari, M. Lai, N. Weisbrodt, and F. Murad. 2005. Helminth regulation of host IL-4Ralpha/Stat6 signaling: mechanism underlying NOS-2 inhibition by Trichinella spiralis. Proc. Natl. Acad. Sci. USA 1023936-3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogdan, C. 2001. Nitric oxide and the immune response. Nat. Immunol. 2907-916. [DOI] [PubMed] [Google Scholar]
- 5.Borkow, G., C. Teicher, and Z. Bentwich. 2007. Helminth-HIV coinfection: should we deworm? PLoS Negl. Trop. Dis. 1e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunet, L. R. 2001. Nitric oxide in parasitic infections. Int. Immunopharmacol. 11457-1467. [DOI] [PubMed] [Google Scholar]
- 7.Bundy, D., A. Sher, and E. Michael. 2000. Good worms or bad worms: do worm infections affect the epidemiological patterns of other diseases? Parasitol. Today 16273-274. [DOI] [PubMed] [Google Scholar]
- 8.Bungiro, R., and M. Cappello. 2004. Hookworm infection: new developments and prospects for control. Curr. Opin. Infect. Dis. 17421-426. [DOI] [PubMed] [Google Scholar]
- 9.Bungiro, R. D., Jr., J. Greene, E. Kruglov, and M. Cappello. 2001. Mitigation of hookworm disease by immunization with soluble extracts of Ancylostoma ceylanicum. J. Infect. Dis. 1831380-1387. [DOI] [PubMed] [Google Scholar]
- 10.Cappello, M., R. D. Bungiro, L. M. Harrison, L. J. Bischof, J. S. Griffitts, B. D. Barrows, and R. V. Aroian. 2006. A purified Bacillus thuringiensis crystal protein with therapeutic activity against the hookworm parasite Ancylostoma ceylanicum. Proc. Natl. Acad. Sci. USA 10315154-15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carneiro, C. M., O. A. Martins-Filho, A. B. Reis, V. M. Veloso, F. M. Araujo, M. T. Bahia, M. de Lana, G. L. Machado-Coelho, G. Gazzinelli, R. Correa-Oliveira, and W. L. Tafuri. 2007. Differential impact of metacyclic and blood trypomastigotes on parasitological, serological and phenotypic features triggered during acute Trypanosoma cruzi infection in dogs. Acta Trop. 101120-129. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhuri, R., O. W. Lindwasser, W. J. Smith, J. H. Hurley, and J. S. Bonifacino. 2007. Downregulation of CD4 by human immunodeficiency virus type 1 Nef is dependent on clathrin and involves direct interaction of Nef with the AP2 clathrin adaptor. J. Virol. 813877-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crompton, D. W., and M. C. Nesheim. 2002. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu. Rev. Nutr. 2235-59. [DOI] [PubMed] [Google Scholar]
- 14.de Silva, N. R., S. Brooker, P. J. Hotez, A. Montresor, D. Engels, and L. Savioli. 2003. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 19547-551. [DOI] [PubMed] [Google Scholar]
- 15.DuChateau, B. K., J. R. Jensen, D. M. England, S. M. Callister, S. D. Lovrich, and R. F. Schell. 1997. Macrophages and enriched populations of T lymphocytes interact synergistically for the induction of severe, destructive Lyme arthritis. Infect. Immun. 652829-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DuChateau, B. K., E. L. Munson, D. M. England, S. D. Lovrich, S. M. Callister, J. R. Jensen, and R. F. Schell. 1999. Macrophages interact with enriched populations of distinct T lymphocyte subsets for the induction of severe destructive Lyme arthritis. J. Leukoc. Biol. 65162-170. [DOI] [PubMed] [Google Scholar]
- 17.Else, K. J., and R. K. Grencis. 1991. Cellular immune responses to the murine nematode parasite Trichuris muris. I. Differential cytokine production during acute or chronic infection. Immunology 72508-513. [PMC free article] [PubMed] [Google Scholar]
- 18.Fujiwara, R. T., S. M. Geiger, J. Bethony, and S. Mendez. 2006. Comparative immunology of human and animal models of hookworm infection. Parasite Immunol. 28285-293. [DOI] [PubMed] [Google Scholar]
- 19.Garside, P., and J. M. Behnke. 1989. Ancylostoma ceylanicum in the hamster: observations on the host-parasite relationship during primary infection. Parasitology 98283-289. [DOI] [PubMed] [Google Scholar]
- 20.Garside, P., M. W. Kennedy, D. Wakelin, and C. E. Lawrence. 2000. Immunopathology of intestinal helminth infection. Parasite Immunol. 22605-612. [DOI] [PubMed] [Google Scholar]
- 21.Geiger, S. M., I. R. Caldas, B. E. Mc Glone, A. C. Campi-Azevedo, L. M. De Oliveira, S. Brooker, D. Diemert, R. Correa-Oliveira, and J. M. Bethony. 2007. Stage-specific immune responses in human Necator americanus infection. Parasite Immunol. 29347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geiger, S. M., C. L. Massara, J. Bethony, P. T. Soboslay, and R. Correa-Oliveira. 2004. Cellular responses and cytokine production in post-treatment hookworm patients from an endemic area in Brazil. Clin. Exp. Immunol. 136334-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh, K., W. Wu, A. D. Antoine, M. E. Bottazzi, J. G. Valenzuela, P. J. Hotez, and S. Mendez. 2006. The impact of concurrent and treated Ancylostoma ceylanicum hookworm infections on the immunogenicity of a recombinant hookworm vaccine in hamsters. J. Infect. Dis. 193155-162. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh, M. K., A. K. Ghosh, M. Addy, A. Nandy, and A. C. Ghose. 1996. Subpopulations of T lymphocytes in the peripheral blood and lymph nodes of Indian kala-azar patients. Med. Microbiol. Immunol. 185183-187. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh, R. P., P. K. Murthy, K. Tyagi, P. S. Murthy, and R. K. Chatterjee. 1999. Longitudinal cellular immune responses in asymptomatic and symptomatic Brugia malayi-infected Indian leaf monkey Presbytis entellus. J. Parasitol. 85861-866. [PubMed] [Google Scholar]
- 26.Graham, S. P., A. J. Trees, R. A. Collins, D. M. Moore, F. M. Guy, M. J. Taylor, and A. E. Bianco. 2001. Down-regulated lymphoproliferation coincides with parasite maturation and with the collapse of both gamma interferon and interleukin-4 responses in a bovine model of onchocerciasis. Infect. Immun. 694313-4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graziosi, C., H. Soudeyns, G. P. Rizzardi, P. A. Bart, A. Chapuis, and G. Pantaleo. 1998. Immunopathogenesis of HIV infection. AIDS Res. Hum. Retrovir. 14(Suppl. 2)S135-S142. [PubMed] [Google Scholar]
- 28.Hansson, M. 1990. Growth and differentiation factors for B and T cells. Leukoc. Res. 14705-710. [DOI] [PubMed] [Google Scholar]
- 29.Hara, Y., Y. Kitazawa, N. Funeshima, M. Kawasaki, Y. Sato, K. Tezuka, H. Kimura, K. Hatakeyama, and X. K. Li. 2006. Anergic lymphocytes generated by blocking CD28 and ICOS pathways in vitro prolong rat cardiac graft survival. Int. Immunopharmacol. 61143-1151. [DOI] [PubMed] [Google Scholar]
- 30.Held, M. R., R. D. Bungiro, L. M. Harrison, I. Hamza, and M. Cappello. 2006. Dietary iron content mediates hookworm pathogenesis in vivo. Infect. Immun. 74289-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hibbs, J. B., Jr., Z. Vavrin, and R. R. Taintor. 1987. l-Arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J. Immunol. 138550-565. [PubMed] [Google Scholar]
- 32.Hotez, P. J., D. H. Molyneux, A. Fenwick, E. Ottesen, S. Ehrlich Sachs, and J. D. Sachs. 2006. Incorporating a rapid-impact package for neglected tropical diseases with programs for HIV/AIDS, tuberculosis, and malaria. PLoS Med. 3e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalinkovich, A., Z. Weisman, Z. Greenberg, J. Nahmias, S. Eitan, M. Stein, and Z. Bentwich. 1998. Decreased CD4 and increased CD8 counts with T cell activation is associated with chronic helminth infection. Clin. Exp. Immunol. 114414-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kittner, J. M., R. Jacobs, S. Buyny, D. Peest, M. Stoll, and R. E. Schmidt. 2007. Adult onset of T-cell deficiency with impaired CD2 expression complicated by Rhodococcus infection: a case report. Ann. Allergy Asthma Immunol. 98294-298. [DOI] [PubMed] [Google Scholar]
- 35.Korn, A., H. P. Kroll, H. P. Berger, A. Kahler, R. Hessler, J. Brauburger, K. P. Muller, and K. Nixdorff. 1993. The 39-kilodalton outer membrane protein of Proteus mirabilis is an OmpA protein and mitogen for murine B lymphocytes. Infect. Immun. 614915-4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwun, J., S. J. Knechtle, and H. Hu. 2006. Determination of the functional status of alloreactive T cells by interferon-gamma kinetics. Transplantation 81590-598. [DOI] [PubMed] [Google Scholar]
- 37.Levy, J. A. 1993. Pathogenesis of human immunodeficiency virus infection. Microbiol. Rev. 57183-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindwasser, O. W., R. Chaudhuri, and J. S. Bonifacino. 2007. Mechanisms of CD4 downregulation by the Nef and Vpu proteins of primate immunodeficiency viruses. Curr. Mol. Med. 7171-184. [DOI] [PubMed] [Google Scholar]
- 39.Liu, H., J. D. Alder, B. M. Steiner, J. Stein-Streilein, L. Lim, and R. F. Schell. 1991. Role of L3T4+ and 38+ T-cell subsets in resistance against infection with Treponema pallidum subsp. pertenue in hamsters. Infect. Immun. 59529-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loukas, A., S. L. Constant, and J. M. Bethony. 2005. Immunobiology of hookworm infection. FEMS Immunol. Med. Microbiol. 43115-124. [DOI] [PubMed] [Google Scholar]
- 41.MacDonald, A. S., P. Loke, and J. E. Allen. 1999. Suppressive antigen-presenting cells in helminth infection. Pathobiology 67265-268. [DOI] [PubMed] [Google Scholar]
- 42.Maizels, R. M., and M. Yazdanbakhsh. 2003. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat. Rev. Immunol. 3733-744. [DOI] [PubMed] [Google Scholar]
- 43.Markus, M. B., and J. E. Fincham. 2007. Helminthiasis, bystander diseases and vaccines: analysis of interaction. Trends Parasitol. 23517-519. [DOI] [PubMed] [Google Scholar]
- 44.McDonagh, P. F., and D. S. Wilson. 1995. The initial response of blood leukocytes to incubation with perfluorocarbon blood substitute emulsions. Artif. Cells Blood Substit. Immobil. Biotechnol. 23439-447. [DOI] [PubMed] [Google Scholar]
- 45.Mendez, S., J. G. Valenzuela, W. Wu, and P. J. Hotez. 2005. Host cytokine production, lymphoproliferation, and antibody responses during the course of Ancylostoma ceylanicum infection in the Golden Syrian hamster. Infect. Immun. 733402-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olatunde, B. O., and G. C. Onyemelukwe. 1994. Immunosuppression in Nigerians with hookworm infection. Afr. J. Med. Med. Sci. 23221-225. [PubMed] [Google Scholar]
- 47.Onyemelukwe, G. C., and B. O. Musa. 2001. T-lymphocyte subsets in patients with hookworm infection in Zaria, Nigeria. Afr. J. Med. Med. Sci. 30255-259. [PubMed] [Google Scholar]
- 48.Perez, L. E., B. Chandrasekar, O. A. Saldarriaga, W. Zhao, L. T. Arteaga, B. L. Travi, and P. C. Melby. 2006. Reduced nitric oxide synthase 2 (NOS2) promoter activity in the Syrian hamster renders the animal functionally deficient in NOS2 activity and unable to control an intracellular pathogen. J. Immunol. 1765519-5528. [DOI] [PubMed] [Google Scholar]
- 49.Quinnell, R. J., D. I. Pritchard, A. Raiko, A. P. Brown, and M. A. Shaw. 2004. Immune responses in human necatoriasis: association between interleukin-5 responses and resistance to reinfection. J. Infect. Dis. 190430-438. [DOI] [PubMed] [Google Scholar]
- 50.Quinnell, R. J., A. F. Slater, P. Tighe, E. A. Walsh, A. E. Keymer, and D. I. Pritchard. 1993. Reinfection with hookworm after chemotherapy in Papua New Guinea. Parasitology 106379-385. [DOI] [PubMed] [Google Scholar]
- 51.Rodrigues, V., Jr., J. Santana da Silva, and A. Campos-Neto. 1998. Transforming growth factor beta and immunosuppression in experimental visceral leishmaniasis. Infect. Immun. 661233-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saathoff, E., A. Olsen, B. Sharp, J. D. Kvalsvig, C. C. Appleton, and I. Kleinschmidt. 2005. Ecologic covariates of hookworm infection and reinfection in rural Kwazulu-natal/South Africa: a geographic information system-based study. Am. J. Trop. Med. Hyg. 72384-391. [PubMed] [Google Scholar]
- 53.Sakti, H., C. Nokes, W. S. Hertanto, S. Hendratno, A. Hall, D. A. Bundy, and Satoto. 1999. Evidence for an association between hookworm infection and cognitive function in Indonesian school children. Trop. Med. Int. Health 4322-334. [DOI] [PubMed] [Google Scholar]
- 54.Soboslay, P. T., C. M. Dreweck, H. R. Taylor, B. Brotman, P. Wenk, and B. M. Greene. 1991. Experimental onchocerciasis in chimpanzees. Cell-mediated immune responses, and production and effects of IL-1 and IL-2 with Onchocerca volvulus infection. J. Immunol. 147346-353. [PubMed] [Google Scholar]
- 55.Soltys, J., P. K. Goyal, and D. Wakelin. 1999. Cellular immune responses in mice infected with the intestinal nematode Trichuris muris. Exp. Parasitol. 9240-47. [DOI] [PubMed] [Google Scholar]
- 56.Stephenson, L. S., M. C. Latham, and E. A. Ottesen. 2000. Malnutrition and parasitic helminth infections. Parasitology 121(Suppl. 1):S23-S38. [DOI] [PubMed] [Google Scholar]
- 57.Sternberg, J., and F. McGuigan. 1992. Nitric oxide mediates suppression of T cell responses in murine Trypanosoma brucei infection. Eur. J. Immunol. 222741-2744. [DOI] [PubMed] [Google Scholar]
- 58.Wang, Y., P. H. Dennehy, H. L. Keyserling, K. Tang, J. R. Gentsch, R. I. Glass, and B. Jiang. 2007. Rotavirus infection alters peripheral T-cell homeostasis in children with acute diarrhea. J. Virol. 813904-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong, D. W., and K. K. Mittal. 1981. HLA-DR typing: a comparison between nylon wool adherence and T cell rosetting in the isolation of B cells. J. Immunol. Methods 46177-186. [DOI] [PubMed] [Google Scholar]
- 60.Zhang, W., E. Moreau, F. Peigne, W. Huang, and A. Chauvin. 2005. Comparison of modulation of sheep, mouse and buffalo lymphocyte responses by Fasciola hepatica and Fasciola gigantica excretory-secretory products. Parasitol. Res. 95333-338. [DOI] [PubMed] [Google Scholar]