Abstract
Two key routes of Francisella tularensis infection are through the skin and airway. We wished to understand how the route of inoculation influenced the primary acute adaptive immune response. We show that an intranasal inoculation of the F. tularensis live vaccine strain (LVS) with a 1,000-fold-smaller dose than an intradermal dose results in similar growth kinetics and peak bacterial burdens. In spite of similar bacterial burdens, we demonstrate a difference in the quality, magnitude, and kinetics of the primary acute T-cell response depending on the route of inoculation. Further, we show that prostaglandin E2 secretion in the lung is responsible for the difference in the gamma interferon (IFN-γ) response. Intradermal inoculation led to a large number of IFN-γ+ T cells 7 days after infection in both the spleen and the lung. In contrast, intranasal inoculation induced a lower number of IFN-γ+ T cells in the spleen and lung but an increased number of Th17 cells in the lung. Intranasal infection also led to a significant increase of prostaglandin E2 (PGE2) in the bronchoalveolar lavage fluid. Inhibition of PGE2 production with indomethacin treatment resulted in increased numbers of IFN-γ+ T cells and decreased bacteremia in the lungs of intranasally inoculated mice. This research illuminates critical differences in acute adaptive immune responses between inhalational and dermal infection with F. tularensis LVS mediated by the innate immune system and PGE2.
Francisella tularensis is a facultative intracellular bacterium and the causative agent of tularemia. F. tularensis has a low infective dose, high morbidity, and persistence in the environment (8). The two key routes of transmission are skin contact and inhalation (6, 43, 44). There is a significant increase in morbidity and mortality associated with inhalation infections compared to skin contact infections in humans (43). The reason for this difference is not clear. An F. tularensis live vaccine strain (LVS) has been developed from an F. tularensis type B strain as a vaccine and causes a less-severe disease in humans (31), though infection in mice causes a fulminant disease that is similar to human tularemia, which also varies in severity by inoculation route (12). (All references to F. tularensis are to LVS unless otherwise noted.) Genomic analysis demonstrates that there is a high level of conservation on the genetic level between F. tularensis LVS and both type A and type B strains, though there are differences (34). Previous studies suggest a difference in the innate immune response to F. tularensis between the intradermal and intranasal inoculation routes (2). A careful characterization of acute adaptive immune responses, comparing intradermal and intranasal infections with similar tissue bacterial burdens, has not been performed.
T-cell-mediated responses are important in clearing primary F. tularensis infection and conferring long-term immunity (4, 5, 10). Mice that lack an adaptive immune response are able to partially control bacterial growth, but they fail to resolve infection and eventually die (10). Secretion of gamma interferon (IFN-γ) by T cells is important for clearance of F. tularensis (11). Studies with IFN-γ receptor−/− bone marrow macrophages suggest other cytokines are also able to aid in killing intracellular F. tularensis. Interestingly, interleukin 12p35 knockout (IL-12p35−/−) mice are able to clear F. tularensis with little to no IFN-γ response (9). IL-12p35−/− mice still generate Th17 T-cell responses following other bacterial infections (3, 20). Th17 T cells are a third effector CD4+ T-cell subset that is important in mucosal immunity and autoimmunity (3, 20, 26, 35, 50). These T cells release IL-17, which through action on epithelial cells recruits neutrophils to the site of infection (24). These data suggest that IFN-γ-mediated responses are important in controlling F. tularensis infections, although IL-17 and other T-cell-mediated responses may play a part and become critical in the absence of IFN-γ.
We have demonstrated that F. tularensis infection of bone marrow-derived macrophages leads to the production of prostaglandin E2 (PGE2) and alters subsequent T-cell activation in vitro (49). A role for PGE2 during F. tularensis in vivo infection has not yet been reported. In both humans and mice, PGE2 can control dendritic-cell (DC) migration to the lymph node, block IL-12 production, and promote IL-23 production (17, 18, 25, 28, 36-38, 51). Increased IL-23 would enhance the development of Th17 cells (15). PGE2 also directly down modulates human and mouse T-cell activity by decreasing calcium flux (an early stage of T-cell activation) and blocking downstream production of IL-2 and IFN-γ (1, 14, 22, 29, 42). Thus, bacterial infections that induce PGE2 may alter T-cell function and gain an advantage in vivo.
In this study, we compared acute primary T-cell-mediated responses after intradermal and intranasal inoculations with F. tularensis. Intradermal inoculation led to an early and robust IFN-γ-mediated T-cell response, while intranasal inoculation led to a mixed Th1/Th17- mediated cell response. PGE2 is at least partially responsible for this difference, since inhibition of PGE2 synthesis increased the IFN-γ response and decreased the bacterial load in the lung. Studies reported in this article highlight critical differences in the adaptive immune response following different routes of inoculation with F. tularensis.
MATERIALS AND METHODS
Bacteria.
The F. tularensis LVS (obtained as a vaccine preparation for the Centers for Disease Control and Prevention) (4) was used in these studies. Viable bacteria were quantified by serial dilution on chocolate agar.
Mice.
C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All animals used in this study were maintained under specific-pathogen-free conditions in the American Association of Laboratory Animal Care-accredited University of North Carolina Department of Laboratory Animal Medicine Facilities. Female mice between 6 and 8 weeks of age were used in all studies.
Infection of mice.
Mice were inoculated with F. tularensis LVS either intranasally or intradermally. Intradermal inoculations were performed by injecting 105 CFU of F. tularensis LVS in 50 μl of phosphate-buffered saline (PBS) at the base of the tail. For intranasal inoculations, mice were anesthetized with an intraperitoneal injection of 0.4 ml of a 20-mg/ml tribromoethanol (Avertin) solution and inoculated with 5 × 102 CFU of F. tularensis LVS in 50 μl of PBS. Anesthetizing mice with 0.4 ml of 20 mg/ml Avertin prior to intradermal inoculation had no effect on the subsequent immune response (data not shown). These inoculation doses were chosen because immunocompetent mice survived and resulted in similar bacterial burdens in all tissues tested, allowing us to quantitatively compare immune responses in the absence of an altered peak antigen load.
Collection of airway, lung, and spleen cells.
Airway cells were collected by bronchoalveolar lavage as previously described (48). Bronchoalveolar lavage fluid (BALF) was collected and frozen at −80°C for later analysis. Cells were placed in complete RPMI 1640 (RPMI 1640 supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 U/ml of penicillin, 100 μg/ml streptomycin, and 50 μM β-mercaptoethanol). Mononuclear cells were isolated from the lung as previously described (48). Mononuclear cells were purified from cell suspension by density gradient centrifugation using Lympholyte M (Cedarlane Labs, Ontario, Canada). Spleens were aseptically harvested from mice, and a single cell suspension was generated. Red blood cells were removed by ammonium chloride-potassium carbonate lysis. Live cells from all tissues were determined using trypan blue exclusion and counting on a hemacytometer. Greater than 90% cell viability was noted in all samples.
Intracellular cytokine staining.
For stimulator cells, naive C57BL/6J splenocytes were plated in 24-well flat-bottom plates, 106 per ml, 2 ml per well, in antibiotic-free medium and infected with F. tularensis LVS at an MOI of 200:1 or mock infected. After 2 h, supernatants were removed and replaced with medium containing 5 μg/ml gentamicin to kill extracellular F. tularensis while leaving the intracellular bacteria untouched. Splenocytes were then incubated at 37°C overnight. The next day, adherent cells were extensively washed to remove T cells, secreted cytokines, and PGE2. T cells from infected spleens were undetectable after washes (data not shown). Tissue mononuclear cells from infected mice at 106 cells/ml were added to infected antigen-presenting cells (APCs) and cultured for 24 h. Tissue APCs were added along with T cells to allow cross-presentation of antigens in order to overcome any inhibitory effects on infected APCs. Streptomycin was incubated at this step to kill all bacteria and ablate their inhibitory effects. These parameters were chosen to maximize T-cell activation and minimize the inhibitory effects of F. tularensis in in vitro assays, as demonstrated in our lab (data not shown). For the last 4 h, cells were treated with 10 μg/ml brefeldin A. Cells were then stained for surface markers with fluorochrome-conjugated antibodies. Cells were then fixed and stained for intracellular cytokines, following the eBioscience protocol. Cells were analyzed on a CyAN ADP flow cytometer (Dako, Fort Collins, CO). CyAN flow cytometry data were analyzed using the Summit software program, version 4.3 (Dako). All flow data included appropriate −1 controls to allow correct compensation of multicolor analysis.
Antibodies.
The following directly conjugated antibodies were used in these studies: fluorescein isothiocyanate-labeled anti-T-cell receptor beta (TCRβ) clone H57 (eBioscience, San Diego, CA) used at 1.25 μg/106 cells, phycoerythrin (PE)-labeled anti-IFN-γ clone XMG1.2 (BD, Franklin Lakes, NJ) used at 1 μg/106 cells, PE-Texas Red-labeled anti-CD11c clone N418 (Invitrogen, Carlsbad, CA) used at 0.25 μg/106 cells, peridinin chlorophyll protein-labeled anti-CD8 clone Ly-2 (BD) used at 1 μg/106 cells, peridinin chlorophyll protein-labeled anti-GR-1 clone RB6-8C5 (BD) used at 0.33 μg/106 cells, PE-Cy7-labeled anti-CD19 clone 1D3 (BD) used at 0.2 μg/106 cells, PacBlue-labeled anti-NK1.1 clone PK136 (Biolegend, San Diego, CA) used at 1.25 μg/106 cells, PacOrange-labeled anti-CD4 clone RM4-5 (Invitrogen) used at 0.25 μg/106 cells, allophycocyanin-labeled anti-IL-17a clone TC11-18H10.1 (eBioscience) used at 2.5 μg/106 cells, allophycocyanin-labeled anti-CD8 clone Ly-2 (BD) used at 0.5 μg/106 cells, and APC-Alexa 750-labeled anti-CD11b clone M1/70 (eBioscience) used at 0.5 μg/106 cells. All antibodies were titrated and analyzed by multicolor flow cytometry with a variety of cells before use.
T-cell analysis.
After stimulation, cells were stained simultaneously with anti-TCRβ, IFN-γ, CD11c, CD8, CD19, CD4, IL-17, and CD11b. A typical gating scheme used for T-cell cytokine analysis is shown in Fig. 1A. Cells displayed in Fig. 1A are from spleens harvested 7 days after intradermal inoculation. Cells were selected by forward and side scatter; doublets were excluded by forward linear scatter versus forward area scatter. Cells were then selected as CD11c, CD11b, and CD19 negative, to exclude DCs, macrophages, and B cells. αβ T cells were then identified by TCRβ expression, followed by CD4 or CD8 expression. These TCRβ+, CD4+ or CD8+, CD11c−, CD11b−, and CD19− cells were analyzed for IFN-γ and IL-17 expression. All gating is based on isotype controls.
FIG. 1.
Gating scheme for cell identification and intracellular cytokine staining. (A) Gating scheme used for identification of T cells and characterization of intracellular cytokine expression. Staining of spleen cells from mice 7 days after intradermal inoculation is shown. (B) Cellular characterization of neutrophils, alveolar macrophages, dendritic cells, and macrophages in the bronchoalveolar spaces. Staining of BAL cells from mice 10 days post-intranasal inoculation is shown.
Bronchoalveolar lavage flow analysis.
Cells isolated from bronchoalveolar lavage were stained with anti-TCRβ, -CD4, -CD8, -CD19, -CD11c, -CD11b, -GR-1, and NK1.1. Cells were selected by forward and side scatter; doublets were excluded by forward linear scatter versus forward area scatter. The bronchoalveolar lavage cell gating scheme is shown in Fig. 1B. T cells were defined as TCRβ+, CD4+, or CD8+ and negative for all other stains. B cells were defined as CD19+, NK cells as NK1.1+, neutrophils as CD11bhi GR1hi, DCs as CD11c+, alveolar macrophages as CD11c+ CD11blow GR1mid, and macrophages as CD11b+ CD11c−. We are aware that this marker set fails to identify either B-cell or DC subsets, but since neither of these populations contributes significantly to either the IFN-γ or IL-17 responses, these markers are sufficient for our purposes.
Enumeration of bacteria in organs.
Lungs and spleens were collected and homogenized in sterile PBS. Tenfold serial dilutions were plated on chocolate agar and incubated at 37°C. Colonies were counted after 72 h.
Determination of secreted cytokines and prostaglandins.
PGE2 in BALFs was measured using a standard PGE2 enzyme immunoassay kit (Assay Design, Ann Arbor, MI) with slight modifications to the standard protocol. BALF samples were first treated with protein G-coupled Sepharose beads to remove murine immunoglobulin G (IgG) in the samples. Capture plates were than pretreated with PGE2 capture antibody for 15 min prior to addition of samples. Samples were mixed with PGE2 conjugate and then placed on plates. The manufacturer's directions were then followed. Total IgG levels in BALF were also determined at this time. There was no correlation between IgG levels and PGE2 levels (r2 = 0.3232). IL-17 was measured using an enzyme-linked immunosorbent assay (ELISA) kit (eBioscience) as per the manufacturer's instructions. Analysis of sample optical density was measured with a SpectraMax M2e microplate reader (Molecular Devices, Sunnyvale, CA).
Statistical analysis.
Data were analyzed using Student's unpaired t test. The GraphPad Prism 4.03 software program (Graphpad Software, San Diego, CA) was used for analysis. Statistical significance was defined a P value of ≤0.05, ≤0.01, or ≤0.001. All data were pooled from a minimum of two experiments with four mice per time point per group in each experiment.
RESULTS
Route of infection did not determine peak bacterial burden in organs.
We inoculated 6- to 8-week-old C57BL/6J female mice either intradermally with 105 CFU or intranasally with 5 × 102 CFU. Inoculation doses were confirmed by immediately plating the inoculums on chocolate agar. At multiple times after inoculation, the numbers of organisms in lung and spleen tissue were determined. Early and late after inoculation, a modestly increased bacterial burden was seen in the lung of intranasally inoculated mice compared to results for intradermally inoculated mice. At day 3 through day 7 postinoculation, similar numbers of organisms were present in the lung regardless of the inoculation route (Fig. 2). Similar peak bacterial loads were seen in the lungs of intranasally and intradermally inoculated mice. A delay in clearance was seen in the lungs of intranasally inoculated mice. A trend in kinetics of accumulation and clearance of bacteria similar to that in the lung was seen in the spleen. The spleen was cleared of bacteria by day 14 postinoculation regardless of the route. The bacterial burden was also similar in the liver between the two routes of inoculation and was cleared in both groups by day 14 (data not shown). These inoculation doses were used for further studies since they resulted in similar bacterial burdens that were below the lethal dose. This allowed to us to analyze differences in acute primary adaptive immune responses. We have chosen to focus on the mechanism of effective immune responses rather than examining the failing immune response resulting in death.
FIG. 2.
Intranasal and intradermal infections have similar bacterial burdens in tissue. C57BL/6J mice were inoculated with F. tularensis LVS either intradermally (105 CFU) or intranasally (5 × 102 CFU). Spleen and lungs from each mouse were assessed for bacterial loads at indicated time points after infection. Asterisks denote statistical difference (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). Error bars represent standard errors of the means (n = 8). All comparisons are between routes of inoculation at each time point. Statistical analysis was done using Student's t test with log-transformed data. Data represented are a combination of two experiments with four mice per group for a total of eight mice per group per time point.
Route of inoculation impacted composition of cells in lung and spleen.
Since the rate of clearance but not the peak bacterial burden varied with the route of inoculation, we hypothesized that subsequent acute primary adaptive immune responses were different and were responsible for the difference in clearance rates. Furthermore, in other bacterial infections, the peak bacterial load, not the duration of infection, impacts the magnitude of the acute primary adaptive immune response (32, 46, 47). Initially we measured the total numbers of cells, CD4+ T cells, and CD8+ T cells in the lungs and spleens after both routes of infection. We intranasally or intradermally inoculated mice with F. tularensis. After inoculation, cells were collected from the airways, lungs, and spleens. Cells were counted and then stained for T-cell, B-cell, and APC markers. B-cell and APC cell markers were used to excluded these cells from T-cell analysis. Both routes of inoculation caused increased cellularity in the lung and spleen, while only the intranasal inoculation led to an increase in the number of cells in the BALF (Fig. 3). As expected with a site of inflammation, a variety of cell types increased in number. Both inoculation routes led to an increased number of B cells (CD19+), macrophages (CD11b+), DCs (CD11c+), and NK cells (NK1.1 positive) in spleen and lung tissue (data not shown). Intranasally inoculated mice showed significant cellular infiltration into the bronchoalveolar space, including neutrophils, DCs, and macrophages, with the largest percentage being neutrophils (Table 1) . Both routes of inoculation led to increased cellular inflammation in both the lung and spleen.
FIG. 3.
Inoculation route influences T-cell numbers in tissue. C57BL/6J mice were inoculated with F. tularensis LVS either intradermally (105 CFU) (○) or intranasally (5 × 102 CFU) (•). Cells were isolated from the airway, lungs, and spleen of each mouse at indicated time points after inoculation. Day zero represents mock-inoculated mice, used to determine basal numbers of cells in specific tissues. All comparisons are between routes of inoculation at each time point. Asterisks denote statistical difference (*, P ≤ 0.05; **, P ≤ 0.01; ***, P * 0.001). Error bars represent standard errors of the means (n = 8). Data represented are a combined from two experiments with four mice per group for a total of eight mice per group per time point.
TABLE 1.
Cellular infiltration into the bronchoalveolar space after intradermal or intranasal inoculation
| Daye | Groupc | Cell countab
|
|||||
|---|---|---|---|---|---|---|---|
| CD19+ | CD11c+ | CD11c+ CD11blow GR1mid | CD11b+ | CD11bhi GR1hi | NK1.1+ | ||
| 0 | Control | 18 ± 5 | 764 ± 213 | 11,000 ± 1,800 | 13 ± 3 | 152 ± 35 | 340 ± 262 |
| 7 | ID | 7 ± 7 | 875 ± 55 | 13,000 ± 2,300 | 19 ± 7 | 111 ± 26 | 1725 ± 620d |
| IN | 40 ± 10 | 2,291 ± 270d | 7,600 ± 1,200 | 1,083 ± 147d | 5,772 ± 808d | 698 ± 69 | |
| 10 | ID | 13 ± 1 | 996 ± 211 | 4,600 ± 770 | 16 ± 4 | 193 ± 27 | 376 ± 126 |
| IN | 316 ± 107d | 5,593 ± 665d | 7,300 ± 130 | 1,870 ± 361d | 8,937 ± 1375d | 2,647 ± 253d | |
| 14 | ID | 17 ± 4 | 836 ± 103 | 4,300 ± 660 | 17 ± 2 | 240 ± 36 | 84 ± 18 |
| IN | 9,644 ± 4,677d | 3,334 ± 1,670 | 10,000 ± 4,400 | 2,351 ± 1,362d | 3,452 ± 1,362d | 950 ± 586 | |
Values are means ± standard errors (eight samples per group).
Cells were defined as B cells (CD19+), DCs (CD11c+), alveolar macrophages (CD11c+ CD11blow GR1mid), macrophages (CD11b+ CD11c−), neutrophils (CD11bhi GR1hi), or NK cells (NK1.1+).
ID, intradermally inoculated mice; IN, intranasally inoculated mice.
Significant difference (P ≤ 0.05) between results for routes of inoculation at each time point as calculated by Student's t test. Data represented are combined from two experiments with four mice per group for a total of eight mice per group per time point.
Day postinoculation. “0” indicates mock inoculation.
Intradermal inoculation led to an increase in the number of CD4+ T cells in the spleen at days 7 and 10 postinoculation compared to results with intranasal inoculation, which led to an increase in the number of CD4+ T cells late in infection (day 14) (Fig. 3). In the lung, intradermal inoculation led to a significant increase in the numbers of both CD4+ and CD8+ T cells at day 7 postinoculation, which returned to uninfected levels by day 14. In contrast, the intranasally inoculated mice had a delay in the increase of CD4+ T cells (not increasing until day 10), while there was no significant increase in the number of CD8+ T cells. Furthermore, only intranasal inoculation led to an increase of T cells in the bronchoalveolar space. Thus, the route of inoculation influenced the type, magnitude, and kinetics of T-cell accumulation in the spleen and lung.
Intradermal but not intranasal inoculation induced a robust IFN-γ-mediated T-cell response.
IFN-γ-mediated adaptive T-cell responses are important for resolving F. tularensis infection (4, 23, 41). The lung normally has an anti-inflammatory environment characterized by high levels of PGE2 (45) which could interfere with the generation of IFN-γ-positive (IFN-γ+) T cells. We hypothesized that intranasal inoculation fails to generate a robust IFN-γ+ T-cell immune response. At various time points after inoculation, cells were collected from lungs and spleens. Leukocytes from these samples were stimulated with F. tularensis-infected splenocytes for 24 h. Cells were then stained for T-cell surface markers and intracellular IFN-γ. Bronchoalveolar lavage cells were not collected and stained for intracellular cytokines due to the low number of T cells recovered. Preliminary studies also examined NK cells; these cells did not contribute significantly to the IFN-γ response (data not shown). In fact, at a day-7 postinoculation, T cells made up more than 90% of the IFN-γ+ cells, and by days 10 and 14 postinoculation, more than 95% of the IFN-γ+ cells were T cells. We chose to do intracellular cytokine staining, versus accumulation of IFN-γ, since it allowed us to determine the total number of responding T cells in a given tissue, not just the general response. Figure 4A demonstrates representative staining profiles of CD4+ T cells in spleen tissue for day 7 postinoculation mice. Percentages shown are percentages of CD4+ T cells that were expressing either IFN-γ or IL-17. Similar responses were seen by CD8+ T cells in the spleen and by CD4+ and CD8+ T cells in the lung. As can be seen with the dot plots, intradermal inoculation led to a more-vigorous IFN-γ+ T-cell response than intranasal inoculation. By day 7 post-intradermal inoculation, there was a significant increase in the numbers of both CD4+ and CD8+ IFN-γ+ T cells in the lung and spleen (Fig. 4B). In contrast, intranasal inoculation produced only a modest number of IFN-γ+ T cells in either organ. There was no significant increase in IFN-γ+ CD8+ T cells in the lungs of intranasally inoculated mice. Thus, intradermal inoculation generated a robust IFN-γ+ T-cell-mediated response not seen in intranasal inoculation, even in the lungs.
FIG. 4.
Intradermal inoculation promotes an increased number of IFN-γ+ T cells. C57BL/6J mice were inoculated with F. tularensis LVS either intradermally (105 CFU) (○) or intranasally (5 × 102 CFU) (•). Cells were isolated from the lungs and spleens of each mouse at indicated time points after inoculation. (A) T cells were then analyzed for total numbers of CD4+ IFN-γ+ or CD8+ IFN-γ+ cells. Day zero represents mock-inoculated mice, used to determine basal numbers of cells in specific tissues. All comparisons are between routes of inoculation at each time point. Asterisks denote statistical difference (*, P ≤ 0.05; **, P * 0.01; ***, P * 0.001). Error bars represent standard errors of the means (n = 8). Data represented are combined from two experiments with four mice per group for a total of eight mice per group per time point. (B) Representative dot plot histograms of CD4+ T cells from the spleen on day 7 postinoculation.
Intranasal infection led to a Th17-cell response.
Intranasally inoculated mice still cleared a low dose of F. tularensis, even without the generation of a robust IFN-γ-mediated T-cell response. Studies have demonstrated the importance of Th17-cell-mediated responses in clearance of Klebsiella pneumoniae and Mycobacterium tuberculosis from the lung (13, 19, 52). We hypothesized that Th17 T cells were being generated after intranasal but not intradermal inoculation with F. tularensis. At various time points after inoculation, cells were collected from lungs and spleens. BALF was collected at the same time. Leukocytes from the spleen and lungs were stimulated with F. tularensis-infected splenocytes for 24 h. Cells were then stained for T-cell surface markers, intracellular IFN-γ and IL-17. The concentration of IL-17 was determined in BALF by ELISA.
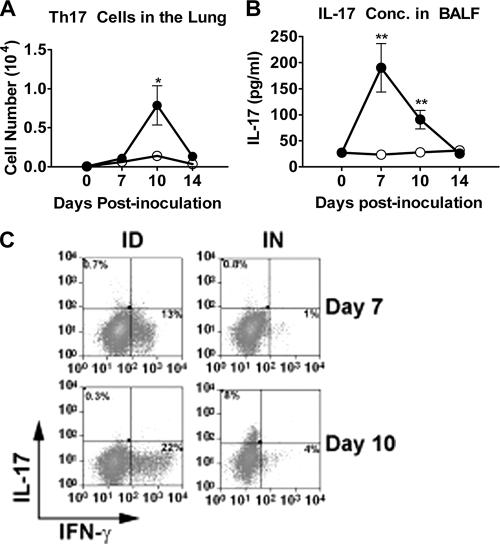
There was a significant CD4+ IL-17+ IFN-γ− T-cell (Th17) response in the lung after intranasal inoculation. By day 10, there was a significant increase in the number of Th17 cells in the lungs of intranasally inoculated mice (Fig. 5A) but not in those of intradermally inoculated mice. At day 10, 50% of the cytokine-positive CD4+ T cells against F. tularensis were making IL-17, and these were the only cells making IL-17. As expected, these Th17 cells did not make IFN-γ, as seen in Fig. 5C. A small increase in Th17 cells was seen in spleen samples from both intradermally and intranasally inoculated mice, but there was no significant difference between intranasal and intradermal inoculation, and it is only a minor component overall (6% of F. tularensis-specific CD4+ T cells) (data not shown). There was also a significant increase in the concentration of IL-17 within the BALF from intranasally inoculated mice that was absent for intradermally inoculated mice (Fig. 5B). Thus, T-cell responses in the lung after intranasal inoculation were a mix of both Th1- and Th17-mediated responses.
FIG. 5.
Intranasally inoculated mice have increased number of Th17 cells and increased IL-17 in lung tissue. C57BL/6J mice were inoculated with F. tularensis LVS either intradermally (105 CFU) or intranasally (5 × 102 CFU). BALF and cells were isolated from the lungs of each mouse at indicated time points after inoculation. Day zero represents mock inoculation mice, used to determine basal numbers of cells in lung tissue and the concentration of IL-17 in BLF. (A) T cells were analyzed for total number of CD4+ IL-17+ cells. (B) BALF was analyzed for IL-17 by ELISA. All comparisons are between routes of inoculation at each time point. (C) Representative staining of CD4+ T cells from the lungs of mice days 7 and 10 post-intranasal (IN) and intradermal (ID) inoculation. Asterisks denote statistical difference (**, P ≤ 0.01). Error bars represent standard errors of the means (n = 8). Error bars on some samples are smaller than the symbols. Data represented are combined from two experiments with four mice per group for a total of eight mice per group per time point.
PGE2 release after intranasal inoculation affected generation of IFN-γ+ T-cell responses and clearance of F. tularensis.
We previously demonstrated that F. tularensis infection of macrophages in vitro leads to the production of PGE2 and dampens Th1-cell development (49). PGE2 can also block an IFN-γ response and promote a Th17-cell response (14, 22, 39). We hypothesized that PGE2 was affecting the acute adaptive immune response generated after intranasal but not intradermal inoculation. BALF collected at various time points after intranasal and intradermal inoculation was tested for the accumulation of PGE2. Both routes led to a significant increase in the PGE2 concentration in the BALF on day 7 postinoculation compared to results for mock-inoculated mice (day 0) (Fig. 6A). PGE2 levels in the BALF of intradermally inoculated mice returned to control levels by day 10, while levels in the BALF of intranasally inoculated mice continued to increase from day 7 to day 14. Since we can detect PGE2 in the BALF by day 7 postinoculation, it is likely that PGE2 is functioning earlier and shaping the adaptive immune response in the lung. Thus, intranasal inoculation led to a prolonged elevation of PGE2 in the BALF.
FIG. 6.
PGE2 delays the generation of IFN-γ+ T cells and inhibits effective clearance of F. tularensis from the lung. C57BL/6J mice were inoculated with F. tularensis LVS either intradermally (105 CFU) or intranasally (5 × 102 CFU). (A) BALF was collected from all mice (n = 8) at indicated time points. BALF was analyzed for the concentration of PGE2 using a standard ELISA. (B, C, D) C57BL/6J mice were inoculated with F. tularensis LVS intranasally (5 × 102 CFU). At days 5, 6, and 7 postinoculation, mice were treated with 1 mg/kg indomethacin in intraperitoneal injections. Untreated mice were injected with 1% DMSO. (B) On day 7, BALF was isolated from all mice and analyzed for the concentration of PGE2 using a commercial ELISA. (C) On days 7, 10, and 14 postinoculation, cells were isolated from the lungs of all mice. T cells were then analyzed for IL-17 and IFN-γ. (D) On days 7, 10, and 14 postinoculation, the bacterial load was determined in the lungs of all mice. Panel A comparisons are between routes of inoculation at each given time point; panel B, C, and D comparisons are between treated mice and untreated mice. Asterisks denote statistical difference (*, P ≤ 0.05; **, P ≤ 0.01, ***, P ≤ 0.001). Error bars represent standard errors of the means (n = 8). Data represented are combined from two experiments with four mice per group for a total of eight mice per group per time point.
We hypothesized that indomethacin treatment of intranasally inoculated mice would lead to an increased IFN-γ+ T-cell response and a decreased bacterial burden. Mice were either intranasally or intradermally inoculated with F. tularensis and then treated with indomethacin by intraperitoneal (i.p.) injection of 1 mg of indomethacin/kg of body weight in 1% dimethylsulfoxide (DMSO) on days 5, 6, and 7 postinoculation. Untreated mice were i.p. injected with 1% DMSO. To determine if indomethacin treatment was altering PGE2 levels, BALF was collected at day 7. In preliminary studies, we demonstrated no difference in PGE2 levels in the BALF of intranasally inoculated mice treated with i.p. injections of PBS or 1% DMSO (data not shown). Treatment of mice with indomethacin led to a significant decrease in the amount of PGE2 in the BALF of intranasally inoculated mice (Fig. 6B). A similar response was seen with intradermally inoculated mice (data not shown).
Mice were intranasally or intradermally inoculated and treated with indomethacin as described above. At days 7, 10, and 14 postinoculation, leukocytes from spleen and lung tissue were isolated and stimulated with F. tularensis-infected splenocytes for 24 h, after which the numbers of IFN-γ+ cells, IL-17+ cells, total cell populations, and bacteria were determined. The treatment of mice with indomethacin changed the kinetics of IFN-γ+ T-cell accumulation but not the peak level. Indomethacin treatment of mice did not affect the dynamics of the responding cell populations, since there was no change in the numbers of CD19+, CD11c+, CD11b+, TCRβ+ CD4+, or CD8+ cells in indomethacin-treated mice versus untreated mice (data not shown). Intranasally inoculated mice treated with indomethacin showed a significant increase in the numbers of both CD4+ and CD8+ IFN-γ-producing cells in the lungs by day 10 postinoculation (Fig. 6C). Importantly, at this time there was a 94% reduction in the absolute number of bacteria within the lung tissue of treated mice (Fig. 6D). By day 14 postinoculation, the number of IFN-γ+ CD4+ T cells for the untreated mice was equivalent to that for the treated mice; this also corresponds to increased clearance of organisms from the lungs of the untreated mice. By day 7 postinoculation, 90% of the IFN-γ+ cells in the lungs were also TCRβ+, with the other 10% not being any one dominant cell population, regardless of treatment. At days 10 and 14 postinoculation, more than 95% of the IFN-γ+ cells were also TCRβ+ (data not shown). There was no change in the number of Th17 cells for indomethacin-treated mice from that for untreated mice, suggesting that Th17 and Th1 development are independent. Treatment of intranasally inoculated mice with indomethacin had no effect on the number of organisms in the spleen or the number of IFN-γ+ T cells, nor did treatment of intradermally inoculated mice with indomethacin have an effect on either the organism burden or the T-cell response (data not shown). Thus, intranasal infection of mice with F. tularensis led to a prolonged elevation of PGE2 levels that altered the subsequent immune response and organism clearance.
DISCUSSION
F. tularensis is able to infect through both the skin and lungs, and the route has a dramatic impact on the disease course and disease outcome. Here we demonstrate that acute primary T-cell responses depend on the route of inoculation, since regardless of the route, similar peak bacteria burdens are seen in both the spleen and lung. Intradermal inoculation led to a dominant IFN-γ+ T-cell response in both the lung and spleen. Intranasal inoculation, however, produced a lower number of IFN-γ+ T cells and a significant increase in the number of Th17 cells. Interestingly, we see differences in the number of responding T cells, even though the peak bacterial burden is similar, which would suggest similar antigen loads for T-cell stimulation. In fact, with delayed clearance of organisms from the intranasally inoculated mice, we would expect increased numbers of IFN-γ+ T cells. With other bacterial infection models, it is the peak bacterial load that determines the magnitude of the T-cell response (32, 46, 47), though this may differ in F. tularensis infections. Since there are fewer IFN-γ+ T cells in intranasally inoculated mice, we believe it is the microenvironment of the initial T-cell activation that is shaping the subsequent T-cell response, not the size of the bacterial burden.
IFN-γ-mediated T-cell responses are an important arm of the immune response against F. tularensis (10, 11, 23). We found that intradermal inoculation led to a significant increase in the numbers of IFN-γ+ CD4+ and CD8+ T cells 7 days postinoculation in both the lung and spleen. This response rapidly returned to control levels by day 14 postinoculation, and accordingly, bacteremia was resolved by day 14. In contrast, the accumulation of T cells in the spleen and lung after intranasal inoculation was delayed. Even when there was an increase in cell numbers, the numbers of IFN-γ+ T cells in the lung after intranasal inoculation was significantly lower than that after intradermal inoculation. Intranasally inoculated mice fail to generate the same robust IFN-γ+ T-cell response observed in intradermally inoculated mice.
Intranasally inoculated mice clear F. tularensis without a robust IFN-γ-mediated response. This is not surprising, since IL-12p35−/− mice clear F. tularensis and generate long-term immunity, even in the presence of little to no IFN-γ (9). IL-12p35−/− mice still produce the IL-12p40 subunit, which combines with the IL-23p19 subunit to make functional IL-23 (30), an important mediator in stabilizing IL-17 expression in T cells (16), consistent with the IL-17 responses we detected. This would suggest that IL-17 can at least partially substitute for IFN-γ in controlling F. tularensis. Th17 cells aid in the recruitment of neutrophils by stimulating epithelial cells to secrete granulocyte-macrophage colony-stimulating factor (21, 53). Along with T-cell responses, we saw a significant neutrophil infiltrate in the bronchoalveolar space of intranasally inoculated mice. Interestingly, human neutrophils are unable to kill F. tularensis (27). Other reports suggest that neutrophils are important for intradermal infections (2, 40); however, these studies are difficult to interpret, since they never determine which cells are depleted by the RB6-8C5 antibody treatment. This antibody, which depletes GR-1 cells, can deplete a variety of cells, including DCs, macrophages, and lymphocytes (7). Furthermore, a variety of macrophages express GR-1. In fact, one study demonstrates that in the liver, the major cell group infected with F. tularensis is GR-1+ macrophages (33). Thus, making unequivocal conclusions about neutrophils is impossible. Further studies need to be done to clarify the role of neutrophils and IL-17 in tularemia. Since in humans neutrophils seem unlikely to kill F. tularensis, it would suggest that the neutrophil response seen in intranasal infection, along with IL-17, would be ill equipped to deal with a large dose of F. tularensis.
Prostaglandins are known to be powerful mediators of immune responses. The immunoregulatory properties of PGE2 are the best characterized of those of the known prostaglandins. We have previously demonstrated the importance of PGE2 in in vitro F. tularensis infection of macrophages (49). We have also demonstrated in our laboratory that both Francisella novicida strain U112 and F. tularensis subsp. tularensis strain Schu S4 induce PGE2 production from bone marrow-derived macrophages (data not shown). It has also been clearly defined that PGE2 is a major contributor to the microenvironment of the lung (45). Intranasal inoculation led to a significant increase in the amounts of PGE2 in the lung. Due to the multiple receptors that PGE2 can bind, it has both inflammatory and anti-inflammatory effects on immune cells (28). It is well established that in both humans and mice, PGE2 leads to increased cAMP levels within T cells, which blocks IFN-γ and IL-2 production and subsequent T-cell proliferation and promotes the production of the Th2 cytokine IL-5 (1, 14, 22). This has also been documented in an in vivo setting (54). We demonstrate that pharmacological inhibition of PGE2 production led to increased IFN-γ+ T-cell numbers and a decreased bacterial burden in intranasally inoculated mice earlier than in untreated mice. Indomethacin treatment had no effect on intradermally inoculated mice (data not shown), suggesting that PGE2 is more important in altering T-cell responses in the lung. We specifically waited until day 5 postinoculation to treat mice with indomethacin to allow innate events to function normally in the context of PGE2-mediated signaling but decrease during activation of adaptive T-cell responses. It is possible that the effect of decreased PGE2 is not directly affecting the T-cell response but rather is affecting some unknown function. The simplest explanation, however, is that PGE2 is directly blocking the generation of IFN-γ+ T cells and the inhibition of PGE2 production alleviates this inhibition. Regardless of the mechanism, inhibition of PGE2 production in mice leads to both increased IFN-γ+ T cells and a decreased bacterial burden, demonstrating that PGE2 is detrimental to the host in clearance of F. tularensis. Further studies on the in vivo role of PGE2 during F. tularensis infection are ongoing in our laboratory.
Acknowledgments
We thank Robert Maile and Benjamin G. Vincent for helpful conversations and critical comments on the manuscript.
This work was supported by the NIH/NIAID Southeast Regional Center of Excellence for Emerging Infections and Biodefense (SERCEB) (grant no. U54 AI 057157); M.D.W. was supported by a T32 training grant (AI-007062) to the Division of Infectious Disease.
Author contributions are as follows: M.D.W., L.L.H., T.H.K., and J.A.F. designed research; M.D.W. and L.L.H. performed research; M.D.W., L.L.H., T.H.K., and J.A.F. analyzed data; and M.D.W. and J.A.F. wrote the paper.
There is no conflicting financial interest among the authors.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 7 April 2008.
REFERENCES
- 1.Betz, M., and B. S. Fox. 1991. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J. Immunol. 146108-113. [PubMed] [Google Scholar]
- 2.Conlan, J. W., R. KuoLee, H. Shen, and A. Webb. 2002. Different host defences are required to protect mice from primary systemic vs pulmonary infection with the facultative intracellular bacterial pathogen, Francisella tularensis LVS. Microb. Pathog. 32127-134. [DOI] [PubMed] [Google Scholar]
- 3.Cooper, A. M., A. Kipnis, J. Turner, J. Magram, J. Ferrante, and I. M. Orme. 2002. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J. Immunol. 1681322-1327. [DOI] [PubMed] [Google Scholar]
- 4.Cowley, S. C., and K. L. Elkins. 2003. Multiple T cell subsets control Francisella tularensis LVS intracellular growth without stimulation through macrophage interferon gamma receptors. J. Exp. Med. 198379-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowley, S. C., E. Hamilton, J. A. Frelinger, J. Su, J. Forman, and K. L. Elkins. 2005. CD4-CD8- T cells control intracellular bacterial infections both in vitro and in vivo. J. Exp. Med. 202309-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cronquist, S. D. 2004. Tularemia: the disease and the weapon. Dermatol. Clin. 22313-320, vi-vii. [DOI] [PubMed] [Google Scholar]
- 7.Daley, J. M., A. A. Thomay, M. D. Connolly, J. S. Reichner, and J. E. Albina. 2008. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 8364-70. [DOI] [PubMed] [Google Scholar]
- 8.Dennis, D. T., T. V. Inglesby, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, M. Layton, S. R. Lillibridge, J. E. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, and K. Tonat. 2001. Tularemia as a biological weapon: medical and public health management. JAMA 2852763-2773. [DOI] [PubMed] [Google Scholar]
- 9.Elkins, K. L., A. Cooper, S. M. Colombini, S. C. Cowley, and T. L. Kieffer. 2002. In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin-12 (IL-12) but not on IL-12 p70. Infect. Immun. 701936-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elkins, K. L., T. R. Rhinehart-Jones, S. J. Culkin, D. Yee, and R. K. Winegar. 1996. Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect. Immun. 643288-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fortier, A. H., T. Polsinelli, S. J. Green, and C. A. Nacy. 1992. Activation of macrophages for destruction of Francisella tularensis: identification of cytokines, effector cells, and effector molecules. Infect. Immun. 60817-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fortier, A. H., M. V. Slayter, R. Ziemba, M. S. Meltzer, and C. A. Nacy. 1991. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect. Immun. 592922-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Happel, K. I., E. A. Lockhart, C. M. Mason, E. Porretta, E. Keoshkerian, A. R. Odden, S. Nelson, and A. J. Ramsay. 2005. Pulmonary interleukin-23 gene delivery increases local T-cell immunity and controls growth of Mycobacterium tuberculosis in the lungs. Infect. Immun. 735782-5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris, S. G., J. Padilla, L. Koumas, D. Ray, and R. P. Phipps. 2002. Prostaglandins as modulators of immunity. Trends Immunol. 23144-150. [DOI] [PubMed] [Google Scholar]
- 15.Hunter, C. A. 2005. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 5521-531. [DOI] [PubMed] [Google Scholar]
- 16.Ivanov, I. I., B. S. McKenzie, L. Zhou, C. E. Tadokoro, A. Lepelley, J. J. Lafaille, D. J. Cua, and D. R. Littman. 2006. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 1261121-1133. [DOI] [PubMed] [Google Scholar]
- 17.Kalinski, P., C. M. Hilkens, A. Snijders, F. G. Snijdewint, and M. L. Kapsenberg. 1997. Dendritic cells, obtained from peripheral blood precursors in the presence of PGE2, promote Th2 responses. Adv. Exp. Med. Biol. 417363-367. [DOI] [PubMed] [Google Scholar]
- 18.Kalinski, P., P. L. Vieira, J. H. Schuitemaker, E. C. de Jong, and M. L. Kapsenberg. 2001. Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood 973466-3469. [DOI] [PubMed] [Google Scholar]
- 19.Khader, S. A., G. K. Bell, J. E. Pearl, J. J. Fountain, J. Rangel-Moreno, G. E. Cilley, F. Shen, S. M. Eaton, S. L. Gaffen, S. L. Swain, R. M. Locksley, L. Haynes, T. D. Randall, and A. M. Cooper. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8369-377. [DOI] [PubMed] [Google Scholar]
- 20.Khader, S. A., J. E. Pearl, K. Sakamoto, L. Gilmartin, G. K. Bell, D. M. Jelley-Gibbs, N. Ghilardi, F. deSauvage, and A. M. Cooper. 2005. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J. Immunol. 175788-795. [DOI] [PubMed] [Google Scholar]
- 21.Kolls, J. K., and A. Linden. 2004. Interleukin-17 family members and inflammation. Immunity 21467-476. [DOI] [PubMed] [Google Scholar]
- 22.Kvirkvelia, N., I. Vojnovic, T. D. Warner, V. Athie-Morales, P. Free, N. Rayment, B. M. Chain, T. W. Rademacher, T. Lund, I. M. Roitt, and P. J. Delves. 2002. Placentally derived prostaglandin E2 acts via the EP4 receptor to inhibit IL-2-dependent proliferation of CTLL-2 T cells. Clin. Exp. Immunol. 127263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leiby, D. A., A. H. Fortier, R. M. Crawford, R. D. Schreiber, and C. A. Nacy. 1992. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect. Immun. 6084-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linden, A. 2001. Role of interleukin-17 and the neutrophil in asthma. Int. Arch. Allergy Immunol. 126179-184. [DOI] [PubMed] [Google Scholar]
- 25.Luft, T., M. Jefford, P. Luetjens, T. Toy, H. Hochrein, K. A. Masterman, C. Maliszewski, K. Shortman, J. Cebon, and E. Maraskovsky. 2002. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: prostaglandin E(2) regulates the migratory capacity of specific DC subsets. Blood 1001362-1372. [DOI] [PubMed] [Google Scholar]
- 26.Matsuzaki, G., and M. Umemura. 2007. Interleukin-17 as an effector molecule of innate and acquired immunity against infections. Microbiol. Immunol. 511139-1147. [DOI] [PubMed] [Google Scholar]
- 27.McCaffrey, R. L., and L. A. Allen. 2006. Francisella tularensis LVS evades killing by human neutrophils via inhibition of the respiratory burst and phagosome escape. J. Leukoc. Biol. 801224-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nataraj, C., D. W. Thomas, S. L. Tilley, M. T. Nguyen, R. Mannon, B. H. Koller, and T. M. Coffman. 2001. Receptors for prostaglandin E(2) that regulate cellular immune responses in the mouse. J. Clin. Investig. 1081229-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okano, M., Y. Sugata, T. Fujiwara, R. Matsumoto, M. Nishibori, K. Shimizu, M. Maeda, Y. Kimura, S. Kariya, H. Hattori, M. Yokoyama, K. Kino, and K. Nishizaki. 2006. E prostanoid 2 (EP2)/EP4-mediated suppression of antigen-specific human T-cell responses by prostaglandin E2. Immunology 118343-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oppmann, B., R. Lesley, B. Blom, J. C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, F. Zonin, E. Vaisberg, T. Churakova, M. Liu, D. Gorman, J. Wagner, S. Zurawski, Y. Liu, J. S. Abrams, K. W. Moore, D. Rennick, R. de Waal-Malefyt, C. Hannum, J. F. Bazan, and R. A. Kastelein. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13715-725. [DOI] [PubMed] [Google Scholar]
- 31.Pollitzer, R. 1967. History and incidence of tularemia in the Soviet Union; a review, p. 1-103. Institute for Contemporary Russian Studies, Fordham University, New York, NY.
- 32.Porter, B. B., and J. T. Harty. 2006. The onset of CD8+-T-cell contraction is influenced by the peak of Listeria monocytogenes infection and antigen display. Infect. Immun. 741528-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasmussen, J. W., J. Cello, H. Gil, C. A. Forestal, M. B. Furie, D. G. Thanassi, and J. L. Benach. 2006. Mac-1+ cells are the predominant subset in the early hepatic lesions of mice infected with Francisella tularensis. Infect. Immun. 746590-6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rohmer, L., M. Brittnacher, K. Svensson, D. Buckley, E. Haugen, Y. Zhou, J. Chang, R. Levy, H. Hayden, M. Forsman, M. Olson, A. Johansson, R. Kaul, and S. I. Miller. 2006. Potential source of Francisella tularensis live vaccine strain attenuation determined by genome comparison. Infect. Immun. 746895-6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salvatore, C. M., M. Fonseca-Aten, K. Katz-Gaynor, A. M. Gomez, A. Mejias, C. Somers, S. Chavez-Bueno, G. H. McCracken, and R. D. Hardy. 2007. Respiratory tract infection with Mycoplasma pneumoniae in interleukin-12 knockout mice results in improved bacterial clearance and reduced pulmonary inflammation. Infect. Immun. 75236-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scandella, E., Y. Men, S. Gillessen, R. Forster, and M. Groettrup. 2002. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood 1001354-1361. [DOI] [PubMed] [Google Scholar]
- 37.Schnurr, M., T. Toy, A. Shin, M. Wagner, J. Cebon, and E. Maraskovsky. 2005. Extracellular nucleotide signaling by P2 receptors inhibits IL-12 and enhances IL-23 expression in human dendritic cells: a novel role for the cAMP pathway. Blood 1051582-1589. [DOI] [PubMed] [Google Scholar]
- 38.Sheibanie, A. F., I. Tadmori, H. Jing, E. Vassiliou, and D. Ganea. 2004. Prostaglandin E2 induces IL-23 production in bone marrow-derived dendritic cells. FASEB J. 181318-1320. [DOI] [PubMed] [Google Scholar]
- 39.Sheibanie, A. F., J. H. Yen, T. Khayrullina, F. Emig, M. Zhang, R. Tuma, and D. Ganea. 2007. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23→IL-17 axis. J. Immunol. 1788138-8147. [DOI] [PubMed] [Google Scholar]
- 40.Sjostedt, A., J. W. Conlan, and R. J. North. 1994. Neutrophils are critical for host defense against primary infection with the facultative intracellular bacterium Francisella tularensis in mice and participate in defense against reinfection. Infect. Immun. 622779-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sjostedt, A., R. J. North, and J. W. Conlan. 1996. The requirement of tumour necrosis factor-alpha and interferon-gamma for the expression of protective immunity to secondary murine tularaemia depends on the size of the challenge inoculum. Microbiology 1421369-1374. [DOI] [PubMed] [Google Scholar]
- 42.Snijdewint, F. G., P. Kalinski, E. A. Wierenga, J. D. Bos, and M. L. Kapsenberg. 1993. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J. Immunol. 1505321-5329. [PubMed] [Google Scholar]
- 43.Tarnvik, A., and L. Berglund. 2003. Tularaemia. Eur. Respir. J. 21361-373. [DOI] [PubMed] [Google Scholar]
- 44.Titball, R. W., and P. C. Oyston. 2003. A vaccine for tularaemia. Expert Opin. Biol. Ther. 3645-653. [DOI] [PubMed] [Google Scholar]
- 45.Vancheri, C., C. Mastruzzo, M. A. Sortino, and N. Crimi. 2004. The lung as a privileged site for the beneficial actions of PGE2. Trends Immunol. 2540-46. [DOI] [PubMed] [Google Scholar]
- 46.Williams, M. A., and M. J. Bevan. 2004. Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J. Immunol. 1736694-6702. [DOI] [PubMed] [Google Scholar]
- 47.Wong, P., and E. G. Pamer. 2001. Cutting edge: antigen-independent CD8 T cell proliferation. J. Immunol. 1665864-5868. [DOI] [PubMed] [Google Scholar]
- 48.Woolard, M. D., L. M. Hodge, H. P. Jones, T. R. Schoeb, and J. W. Simecka. 2004. The upper and lower respiratory tracts differ in their requirement of IFN-gamma and IL-4 in controlling respiratory mycoplasma infection and disease. J. Immunol. 1726875-6883. [DOI] [PubMed] [Google Scholar]
- 49.Woolard, M. D., J. E. Wilson, L. L. Hensley, L. A. Jania, T. H. Kawula, J. R. Drake, and J. A. Frelinger. 2007. Francisella tularensis-infected macrophages release prostaglandin E2 that blocks T cell proliferation and promotes a Th2-like response. J. Immunol. 1782065-2074. [DOI] [PubMed] [Google Scholar]
- 50.Wu, Q., R. J. Martin, J. G. Rino, R. Breed, R. M. Torres, and H. W. Chu. 2007. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 978-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang, L., N. Yamagata, R. Yadav, S. Brandon, R. L. Courtney, J. D. Morrow, Y. Shyr, M. Boothby, S. Joyce, D. P. Carbone, and R. M. Breyer. 2003. Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J. Clin. Investig. 111727-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ye, P., P. B. Garvey, P. Zhang, S. Nelson, G. Bagby, W. R. Summer, P. Schwarzenberger, J. E. Shellito, and J. K. Kolls. 2001. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am. J. Respir. Cell Mol. Biol. 25335-340. [DOI] [PubMed] [Google Scholar]
- 53.Ye, P., F. H. Rodriguez, S. Kanaly, K. L. Stocking, J. Schurr, P. Schwarzenberger, P. Oliver, W. Huang, P. Zhang, J. Zhang, J. E. Shellito, G. J. Bagby, S. Nelson, K. Charrier, J. J. Peschon, and J. K. Kolls. 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194519-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yin, H., L. Cheng, R. Langenbach, and C. Ju. 2007. Prostaglandin I(2) and E(2) mediate the protective effects of cyclooxygenase-2 in a mouse model of immune-mediated liver injury. Hepatology 45159-169. [DOI] [PubMed] [Google Scholar]