Abstract
Borrelia burgdorferi, the causative agent of Lyme disease in the United States, regulates numerous genes encoding lipoproteins on linear plasmid 54 in response to environmental cues. We analyzed a subset of these genes/proteins that were historically categorized as paralogous gene family 54 (BBA64, BBA65, BBA66, BBA68, BBA69, BBA70, BBA71, and BBA73) and found that the expression of several genes was influenced by the σN-σS regulatory cascade at the level of transcription and protein synthesis. Moreover, we established in this and a previous study that BBA65, BBA66, BBA69, BBA71, and BBA73 are temporally expressed during persistent infection of immunocompetent mice, as determined by quantitative real time-PCR of ear tissue, by enzyme-linked immunosorbent assay, and by immunoblotting. Correspondingly, BBA65, BBA66, BBA71, and BBA73 proteins were detectable in infectious B. burgdorferi B31 isolates but undetectable in noninfectious isolates. BBA65, BBA66, BBA71, and BBA73 proteins were also found to partition into the Triton X-114 detergent phase and were sensitive to protease treatment of intact cells, indicating that they are membrane associated and surface localized. Lastly, Southern blotting and PCR with specific gene primer/probes for BBA64, BBA65, BBA66, BBA71, and BBA73 suggest that many of these genes are conserved among the B. burgdorferi sensu lato isolates and the relapsing-fever Borrelia species. Together, the data presented suggest that these genes may play a part in Borrelia infection and/or pathogenicity that could extend beyond the sensu lato group.
Lyme disease, the most commonly reported vector-borne disease in the United States (24), is caused by the spirochete Borrelia burgdorferi. Early symptoms include fever, an erythema migrans rash, arthralgia, and myalgia; if untreated, the disease may progress to arthritic, neurologic, or cardiac manifestations (57, 74, 83, 84). The B. burgdorferi type strain, B31 MI, was sequenced in 1997 by The Institute for Genomic Research (TIGR), and many genes were categorized into paralogous gene families (pgf) (23, 34). TIGR recently reclassified the pgf, and many genes were either redistributed to new families or removed from the pgf.
Prior to this reorganization, several genes clustering to the right end of linear plasmid 54 (lp54; plasmid A) belonging to the family formerly known as pgf 54 (comprising BBA64, BBA65, BBA66, BBA68 [cspA], BBA69, BBA70, BBA71, and BBA73) were consistently shown in multiple studies and DNA microarray analyses to be the most highly regulated group of genes in response to environmental cues mimicking the mammalian host environment (20, 21, 25, 45, 46, 72, 77, 88). Importantly, many of these same genes localizing to lp54 are also up-regulated in vivo (35, 54). Recently, quantitative real time-PCR (qRT-PCR) demonstrated that BBA64, BBA65, and BBA66 are transcriptionally regulated in B. burgdorferi B31 in ear tissue during persistent infection in mice (35), and microarray analysis of B. burgdorferi 297 indicated that BBA64, BBA65, BBA66, BBA71, and BBA73 are highly expressed when bacteria are grown in dialysis membrane chamber implants (18). Furthermore, qRT-PCR has also revealed similar expression profiles for BBA65, BBA66, BBA71, and BBA73 when B. burgdorferi was grown under the combined in vitro mammal-like culture conditions of pH 7.0 and 35°C versus tick-like conditions of pH 8.0 and 23°C (25).
In addition to evidence of gene expression in vivo, antibodies specific for BBA64 (P35), BBA65, and BBA66 proteins are also detectable over the course of persistent infection in mice (35); moreover these proteins are immunogenic in humans during early- and late-disseminated disease, in rabbits, and in mice (21, 25, 35, 36, 69, 70, 91). BBA64 (P35), BBA66, and BBA69 proteins have also been shown to localize to the borrelial outer surface (12). Taken together, these data suggest that a subset of these former gene family members encode proteins that are exposed to direct interaction with the mammalian host environment and may, therefore, play an important role during mammalian infection and/or pathogenesis. This is supported by evidence that the BBA68 protein (BbCRASP-1; encoded by cspA) binds host factor H (50, 64) and that a fragment of the BBA66 protein isolated from a phage display library was capable of interacting with mouse heart tissue (3).
The alternative sigma factor cascade comprising RpoN (σN, σ54) and RpoS (σS, σ38) in B. burgdorferi has been implicated in the regulation of genes involved with infection and/or pathogenicity (17, 33, 43, 95). Both σN and σS are required for murine infection (33), and σS directly controls the expression of ospC (29, 95), which is also required for murine infection (39, 75, 86, 87). Furthermore, microarray analysis of B. burgdorferi strain B31 ΔntrA and ΔrpoS mutants established that the transcription of numerous genes, including BBA64, BBA65, BBA66, and BBA71, is influenced by the sigma factor cascade in vitro (33). Albeit in the infectious isolate 297 background, it was demonstrated recently by microarray that BBA64, BBA65, BBA66, BBA71, and BBA73 transcripts were significantly increased in the parental isolate relative to an isogenic ΔrpoS mutant when bacteria were grown in dialysis membrane chambers implanted either in rats or rabbits (18). Furthermore, an in-depth analysis of BBA66 suggests that the expression of this gene may be controlled indirectly by σS in conjunction with an as yet unidentified regulatory protein that binds to a 29-base-pair inverted repeat upstream of the −10/−35 region of the mapped promoter (25).
To further develop the evidence suggesting that these genes on lp54 may play important roles during mammalian infection, we utilized both in vivo and in vitro techniques to assess protein synthesis, gene transcription, and gene conservation. Our investigations confirmed the influence of the σN-σS regulatory cascade on transcription of target genes, correlated changes in transcription to changes in protein amount, and demonstrated that expression of these proteins was associated with infectious spirochetes. Results suggested that target genes were transcribed in ear tissue throughout persistent infection of immunocompetent mice, and orthologs of these genes of interest were detected in a broad range of Borrelia spp.
MATERIALS AND METHODS
Strains and growth conditions.
All Borrelia strains (Table 1) were grown in either Barbour-Stoenner-Kelly H (BSK-H) medium lot 045K8412 (Sigma, St. Louis, MO) or 1× liquid plating medium at 35°C under an atmosphere of 5% CO2 to 5 × 107 cells/ml, unless otherwise stated. Cells were enumerated under dark-field microscopy using a Petroff-Hausser counting chamber. pH shifts were performed as previously described (21). Escherichia coli was maintained in Luria-Bertani media (Fisher, Pittsburgh, PA) supplemented when needed with either 100 μg/ml ampicillin (Fisher) or 40 μg/ml kanamycin (Invitrogen, Carlsbad, CA).
TABLE 1.
Borrelia isolates used in this study
| Isolate or speciesa | Description | Reference(s) |
|---|---|---|
| B31 p5 | Nonclonal, infectious isolate; all plasmids present | 14 |
| clone A | Clonal, noninfectious isolate derived from B31; missing cp9, lp25, lp28-1, lp28-4, lp36, cp32-6, lp5, lp21 | 7,b76 |
| A-34 | Clonal, noninfectious isolate derived from clone A; also missing lp56 | 25c |
| A3 | Clonal, infectious isolate derived from B31 MI; missing cp9 | 31 |
| A3-Gm | A3 containing lp25::PflaB-aacC1 | 33d |
| A3ntrA-Gm | A3 with ntrA deletion | 33d |
| A3ntrA-comp | A3ntrA-Gm complemented with ntrA in trans | 33d |
| A3rpoS | A3 with rpoS deletion | 33d |
| B. californiensis CA8 | Isolated from Ixodes pacificus | 51b |
| B. afzelii PGau | Isolated from skin | 44b |
| B. garinii G1 | Isolated from human CSF | 10b |
| B. japonica HO14 | Isolated from Ixodes persulcatus | 63b |
| B. japonica IKA2 | Isolated from Ixodes ovatus | 63b |
| B. parkeri | From the Rocky Mountain Laboratories collection | 79b |
| B. crocidurae CR2A | From the Rocky Mountain Laboratories collection | 79b |
| B. andersonii 21038 | Isolated from Ixodes dentatus | 1b |
| B. valaisiana VS116 | Isolated from Ixodes ricinus | 52b |
| B. anserina | From the Rocky Mountain Laboratories collection | 79b |
| B. turicatae | From the Rocky Mountain Laboratories collection | 79b |
| B. hermsii DAH | Isolated from human blood | 41e |
The species is B. burgdorferi unless indicated otherwise.
Provided by Patricia Rosa, Rocky Mountain Laboratories, NIH.
Provided by Kit Tilly, Rocky Mountain Laboratories, NIH.
Provided by Mark Fisher and Frank Gherardini, Rocky Mountain Laboratories, NIH.
Provided by Tom Schwan, Rocky Mountain Laboratories, NIH.
Phylogenetic analysis.
Clustal V analysis was performed with a PAM250 table for the mutation probability matrix for the evolutionary distance (see Table S1 in the supplemental material) on 34 DNA sequences using the MegAlign module of the Lasergene version 4.06 software package (DNASTAR, Inc., Madison, WI). Divergence values for each gene pair are calculated with the formula Σ[residue distances + (gaps × gap penalty) + (gap residues × gap length penalty)]. A dendrogram of relatedness was prepared using the calculated divergence values as branch lengths (Fig. 1). Sequences (with accession numbers in parentheses) of B. burgdorferi B31 BBA64, BBA65, BBA66, BBA68, BBA69, BBA70, BBA71, BBA73, BBI36, BBI38, BBI39, and BBJ41 (NC_001857) and their orthologs in Borrelia garinii PBi (CP000015) were downloaded from the Comprehensive Microbial Resource website provided by TIGR (http://cmr.tigr.org/tigr-scripts/CMR/CmrHomePage.cgi). Sequence data for p46 from B. garinii ZQ1 (GenBank accession no. AJ430851), p46 from B. burgdorferi ZS7 (GenBank accession no. AJ430850), p46 from Borrelia afzelii PKo (GenBank accession no. NC_008564), p46 from B. afzelii MMS (GenBank accession no. AJ430849), and BAPKO_2061 from B. afzelii PKo (GenBank accession no. CP000395) were downloaded from the NCBI website (http://www.ncbi.nlm.nih.gov/). Sequences for BBA66, BBI36, BBI38, and BBI39 orthologs from B. burgdorferi isolates 297, JD1, and N40 were kindly provided by the Borrelia sequencing team composed of Sherwood R. Casjens, John J. Dunn, Benjamin J. Luft, Claire M. Fraser, Weigang Qiu, and Steven E. Schutzer.
FIG. 1.
Dendrogram of DNA sequences demonstrating the relatedness of representatives from the original pgf 54 group from B. burgdorferi B31 sensu stricto (Bb), B. garinii (Bg), and B. afzelii (Ba). Clustal V analysis was performed as described in Materials and Methods. Divergence values used to produce the dendrogram were calculated using Lasergene software and are presented in Table S1 in the supplemental material. The new paralogous family (gbb fam_b_burgdorferi_b31.pep_35) is indicated to the right. The dashed box highlights the clade composed of closely related BBA65 and BBA66 orthologs. The scale represents the percentage of nucleotide substitutions observed out of the total number of nucleotides compared.
Preparation of membrane fractions.
Membrane fractions were prepared from spirochetes shifted to pH 7.0 as described previously (22). Soluble and membrane fractions were stored in HN buffer (10 mM HEPES, pH 8.0, 50 mM NaCl) supplemented with complete protease inhibitor, EDTA free (Roche Diagnostics, Indianapolis, IN). Protein concentrations were determined by the modified Lowry assay using bovine serum albumin (BSA) as a standard (60).
TX-114 phase partitioning.
Detergent extraction of lipidated and integral membrane proteins was performed as previously described for intact cells (9) with slight modification for cell lysate. For cell lysates, 500 μg of lysate was treated with 2% (vol/vol) Triton X-114 (TX-114; Sigma) with agitation on ice for 30 min. Centrifugation for 30 min at 10,000 × g and 10°C yielded the insoluble cold pellet. Supernatant containing TX-114-soluble material was heated, and the aqueous and detergent phases were subsequently resolved by centrifugation at 14,000 × g and 37°C for 10 min. After a washing, proteins in the aqueous and detergent phases were precipitated with 10 volumes of cold 100% acetone. All pellets were resuspended in 3× Laemmli buffer (Bio-Rad) to a final concentration of 1 × 107 cell equivalents/μl.
Protease treatment of intact B. burgdorferi isolate B31.
Protease experiments were performed according to the methods of El-Hage et al. (30) except that all cells were treated for 1 h at room temperature with 400 μg/ml proteinase K (PK; Promega, Madison, WI), trypsin (Tr; Sigma), or pronase SC (Roche Diagnostics) or with buffer as a control. Reactions were stopped by the addition of appropriate inhibitors, and samples were centrifuged and resuspended in 3× Laemmli buffer to a final concentration of 5 × 106 cells/μl.
SDS-PAGE and immunoblotting.
All samples were stored at −20°C until use. Samples resuspended in Laemmli buffer were heated to 100°C for 5 min, and proteins were separated by 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Separated proteins were either stained with the Silver Stain Plus kit (Bio-Rad) or transferred to a 0.45-μm Transblot nitrocellulose membrane (Bio-Rad) by the method of Towbin et al. (89). Membranes were stained with 0.1% naphthol blue black (Sigma) in 1% acetic acid, and standards were marked. Membranes probed with either mouse or rabbit antibodies were blocked in 5% nonfat dry milk in Tris-buffered saline-Tween 20 (TBS-T20; 10 mM Tris-HCl [pH 8], 150 mM NaCl, 0.1% Tween 20 [Fisher]), and those probed with hen antibodies were blocked in 2% BSA fraction V (Sigma) in TBS-T20. Probing and detection of reactive bands were performed as described previously (21).
Overexpression and purification of MalE fusion proteins.
Cloning and overexpression of BBA66 were described previously (25). Genes encoding either a full-length protein (BBA65, BBA69, and BBA70) or an N-terminally truncated protein (BBA68, BBA71, and BBA73) were similarly cloned using engineered specific primers (Table 2). The resulting constructs, pMAL-bba65FL, pMAL-bba68TR, pMAL-bba69FL, pMAL-bba70FL, pMAL-bba71TR, and pMAL-bba73TR, were electroporated into the E. coli strain ER2508 (New England Biochemicals, Beverly, MA), which lacks the genes encoding the Lon protease and MalE, and clones were selected with ampicillin. Positive colonies were identified by PCR using gene-specific primers (Table 2), restriction endonuclease digestion of plasmids, and sequencing. Protein overexpression and purification were carried out as described before (25). Purified fusion proteins were stored at −80°C, and protein concentrations were determined by a modified Lowry assay using BSA as a standard.
TABLE 2.
Primers used in this study
| Primer name | Sequence | Usea |
|---|---|---|
| BBA64 FL.1 | TTGAAGGATAACATTTTGAAAAAT | SB probe, PCR |
| BBA64 FL.2 | CTGAATTGGAGCAAGAATATTGG | SB probe, PCR |
| BBA64F RT.3 | CAGAGGCTTTAGCATTCAAT | qRT-PCR (in vitro), PCR |
| BBA64R RT.4 | TGTACTCGTCAATAAGGTCATCT | qRT-PCR (in vitro), PCR |
| BBA65 FL.1 | TTGAATAAAATAAAATTATCAATA | SB probe, cloning, PCR |
| BBA65 FL.2 | ATTAAATTTAAATAATGTGTC | SB probe, cloning, PCR |
| BBA65 RT.F | AGAATGGCTTAGCACAATAGA | qRT-PCR (in vitro), PCR |
| BBA65 RT.R | TCAAGCAAAGAGAAATCATAGTA | qRT-PCR (in vitro), PCR |
| BBA66 FL.1 | TTGAAAATCAAACCATTAATAC | SB probe, PCR |
| BBA66 FL.2 | TTGAGTTTGTATCAGCACTTGTTG | SB probe, PCR |
| BBA66 RT.F | ATACCTATTCAAGCCGTTAC | qRT-PCR (in vitro), PCR |
| BBA66 RT.R | ATTTACTGCCAGAAGATGTTGTTG | qRT-PCR (in vitro), PCR |
| BBA68.3 TR FWD | ACCTCATGCGCACCTTTT | Cloning |
| BBA68.2 REV+XbaI | TTTTTCTCTAGATTAGTAAAAGGCAGGTTT | Cloning |
| BBA68 RT.F | CTAAAAGCAATTGGTAAGGAACTG | qRT-PCR (in vitro), PCR |
| BBA68 RT.R | TCAATAAGATCGTAAGGACCAACT | qRT-PCR (in vitro), PCR |
| BBA68 F.rt-ms | CATGCGCACCTTTTAGCAAA | qRT-PCR (in vivo) |
| BBA68 R.rt-ms | CCCCGGATTGGTGATTTTT | qRT-PCR (in vivo) |
| BBA68 prb-ms | TCGATCCTAAAGCAAATGCAAACACTAAGCC | qRT-PCR probe (in vivo) |
| BBA69 FL.1 FWD | ATTTTGAAAAAAGCCAAACTAAAT | Cloning |
| BBA69 FL.2 REV+XbaI | TTTTATCTAGATTAATAAAAGGCAGATTG | Cloning |
| BBA69 RT.F | AAAAAGAAAACATAGAGACATT | qRT-PCR (in vitro) |
| BBA69 RT.R | AAGTGCTTTTCCAGTTTTA | qRT-PCR (in vitro) |
| BBA69 F.rt-ms | CCTTGGCGCTTCTGATGAAA | qRT-PCR (in vivo) |
| BBA69 R.rt-ms | TCTTCTAGCTCCTTACCAATTGCTTT | qRT-PCR (in vivo) |
| BBA69 prb-ms | TTATGGGAACTACCGCTTCAGAGCT | qRT-PCR probe (in vivo) |
| BBA70.1 FWD | ATGGCTCCAGAAGTAAACA | Cloning |
| BBA70.2 REV+SalI | TGGCTTATGTCGACTTATTTTATATTAGT | Cloning |
| BBA70 RT.F | TACACCAATACAAAAGAAGACACC | qRT-PCR (in vitro) |
| BBA70 RT.R | TTTGCATTTTTACATTATTTTTAG | qRT-PCR (in vitro) |
| BBA70 F.rt-ms | TGATCTCTTAGAAACTCTTGACAGTAAAATC | qRT-PCR (in vivo) |
| BBA70 R.rt-ms | CCCCTTTATCGTAATCCAAGGA | qRT-PCR (in vivo) |
| BBA70 prb-ms | AGCCTGTGTATTTTACTAAACCAGAAACAA CCGAAG | qRT-PCR probe (in vivo) |
| BBA71 FL.1 | ATGAATAAATTAAAAGAAATTCTTG | SB probe, PCR |
| BBA71 FL.2 | GTTTTGTTTAAATTCAACACTG | SB probe, cloning, PCR |
| BBA71 TR.3 | ATTATAGGACTACTTTATCACAAAGC | Cloning |
| BBA71 RT.F | AAAGGCAGCCCCGATTAC | qRT-PCR, PCR |
| BBA71 RT.R | AACTTTTCTTTTAGCATTAGGTCA | qRT-PCR, PCR |
| BBA71 F.rt-ms | CAGCCCCGATTACGAAAATATAA | qRT-PCR (in vivo) |
| BBA71 R.rt-ms | AATTCTGCGTGTATTAGTAGATCGTTTAA | qRT-PCR (in vivo) |
| BBA71 prb-ms | TATAGGACTACTTTATCACAAAGCATTGGG CATTCA | qRT-PCR probe (in vivo) |
| BBA73 FL.1 | TTGAAAAGAAACAAAATTTGGAAAAC | SB probe, PCR |
| BBA73 FL.2 | GTAGTGTATGTGGTCACAACAGG | SB probe, cloning, PCR |
| BBA73 TR.3 | TTTTATTCTAAATCAAACAACAC | Cloning |
| BBA73 RT.F | ATGACAAAAATACGGGAGGAT | qRT-PCR (in vitro), PCR |
| BBA73 RT.R | CAGGTTTTTAGCGGTGTT | qRT-PCR (in vitro), PCR |
| BBA73 F.rt-ms | GGATGCAAAGCTGATGGAAA | qRT-PCR (in vivo) |
| BBA73 R.rt-ms | GCATACTTTTATTTGAGTCGAGTTCA | qRT-PCR (in vivo) |
| BBA73 prb-ms | ATTCTCGGAGAAGTAATAAGGGTTGG | qRT-PCR probe (in vivo) |
| BBI36/38 RT.F | GTGAAAAAGAAGCCGAAAAGT | qRT-PCR (in vitro) |
| BBI36/38 RT.R | TAGTAGATAAAGAGGATGGGAATA | qRT-PCR (in vitro) |
| BBI39/J41 RT.F | GTGAAAAAGAAGCCGAAAAGT | qRT-PCR (in vitro) |
| BBI39/J41 RT.R | TAGTAGATAAAGAGGATGGGAATA | qRT-PCR (in vitro) |
| OspC.RT For | TACGGATTCTAATGCGGTTTTAC | qRT-PCR (in vitro) |
| OspC.RT Rev | GTGATTATTTTCGGTATCCAAACCA | qRT-PCR (in vitro) |
| FlaB F.rt-ms | TCTTTTCTCTGGTGAGGGAGCT | qRT-PCR (in vivo) |
| FlaB R.rt-ms | TCCTTCCTGTTGAACACCCTCT | qRT-PCR (in vivo) |
| FlaB prb-ms | AAACTGCTCAGGCTGCACCGGTTC | qRT-PCR probe (in vivo) |
SB, Southern blotting; PCR, confirmatory PCR.
Antibodies. (i) Primary antibodies.
Polyclonal anti-MalE-BBA66TR and anti-MalE-BBA71TR antibodies were produced at Aves Labs (Tigard, OR) in hens and used at 1:8,000 or 1:4,000, respectively. Additional antibodies to BBA65, BBA66, and BBA73 proteins were produced through immunization of 8-week-old female C3H/HeJ mice (Jackson Laboratories, Bar Harbor, ME) as follows. Fifty micrograms of purified and concentrated MalE fusion proteins and 2.5 mg Imject alum (Pierce) were injected subcutaneously in the back at days 0 and 14. Mice immunized with the BBA65 protein received an additional 50-μg protein injection at day 28 to improve seroreactivity. Mice were bled via the dorsal tail vein, and sera were collected and stored at 4°C. Polyclonal anti-oligopeptide permease A1 (OppA1) rabbit serum was provided by Linden T. Hu (42) and anti-FlaB monoclonal antibody H9724 by Nyles Charon and Mohamed A. Motaleb (5, 67). All mouse and rabbit sera were diluted 1:1,000 for immunoblots.
(ii) Secondary antibodies.
Horseradish peroxidase-conjugated protein A (Sigma) was used in conjunction with the anti-FlaB antibody at 1:10,000. For the remaining antibodies, appropriate horseradish peroxidase-conjugated secondary antibodies were purchased from Sigma and diluted 1:5,000.
Persistent infection of mice.
All animal studies were performed at the Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention, with approval from the DVBID Institutional Animal Care and Use Committee. The experimental design and procedures have been described in detail previously (35).
RNA isolation and qRT-PCR of infected mouse ear.
RNA was extracted from mouse ear tissue as described previously (35). All reactions were run in triplicate, and the primers and probes used are listed in Table 2. Gene expression was quantified relative to expression in mid-logarithmic-phase cultured B. burgdorferi, and values were normalized to the constitutively expressed flaB. Relative quantitation of gene expression was calculated by the comparative CT (2−ΔΔCT) method (55). To eliminate plate-to-plate and day-to-day variations, each sample plate also contained qRT-PCR mixtures with cultured B. burgdorferi cDNA for calculation of relative gene expression.
Serological assays of persistently infected mice.
The procedures used for analysis of mouse sera by immunoblotting and enzyme-linked immunosorbent assay (ELISA) have been previously described (35). No immunoreactivity was detected when recombinant MalE alone was probed with mouse serum (data not shown).
RNA isolation and qRT-PCR analysis from cultured spirochetes.
Total RNA was isolated from B. burgdorferi grown at pH 7.0 and was analyzed by qRT-PCR as outlined before (25). Primers used are listed in Table 2. Values were calculated using the comparative CT method and were normalized to the constitutively expressed flaB gene. Changes in fluorescence were monitored with the MyiQ single-color real-time PCR detection system using Sybr green (Bio-Rad).
Genomic DNA isolation.
Genomic DNA was prepared as described previously (66), with slight modification. Borrelia isolates grown to high cell density (approximately 1 × 108 cells/ml) were pelleted, washed once in saline-EDTA (0.15 M NaCl, 0.1 M EDTA, pH 8.0), treated with 3 mg/ml lysozyme in saline-EDTA, and then fractured by rapid freezing in a dry ice-ethanol bath and thawing with the addition of 1 volume of Tris-SDS (0.1 M Tris-Cl [pH 9.0], 0.1 M NaCl, 1% SDS). Protein was removed by phenol-chloroform-isoamyl alcohol (25:24:1) (Fisher) phase separation, and the upper aqueous phase was treated with 50 μg/ml (final) RNase A (ABgene Inc., Rochester, NY) to remove contaminating RNA. Samples were reextracted by phenol-chloroform-isoamyl alcohol, and genomic DNA was precipitated with 0.1 volume of 5 M ammonium acetate and 1 volume of 100% isopropanol. DNA was resuspended and stored at 4°C in TE (10 mM Tris-Cl [pH 8.0], 1 mM EDTA [pH 8.0]). Concentrations were determined by measuring optical density at 260 nm. For amplification of putative orthologs from various Borrelia spp., PCR conditions were as follows: 3 min at 93°C and 40 cycles of 1 min at 93°C, 1 min at 45°C, and 2 min at 72°C, followed by a single cycle of 7 min at 72°C. Primers are listed in Table 2.
Southern blotting.
One microgram of genomic DNA was digested for 1 h with EcoRI (Promega) and separated on a 0.8% agarose gel in Tris-acetate-EDTA (40 mM Tris-acetate, 2 mM EDTA) electrophoresis buffer. Bands were visualized by staining with ethidium bromide (Bio-Rad), and the DNA was transferred to Hybond XL membranes (Amersham Biosciences) as described by Marconi et al. (58, 59), except that depurination, denaturation, and neutralization steps were increased to 20 min each. Transfers were completed with a 1-h treatment with 20× sodium chloride-sodium citrate (SSC; 3 M NaCl, 0.3 M Na citrate·2H2O [pH 7.0]). Transferred material was cross-linked to membranes using the auto-cross-link function on Stratagene's UV Stratalinker 2400, and DNA was visualized by staining membranes with 0.03% methylene blue in 1% acetic acid. Blots were dried and stored at room temperature until use. Probes were prepared using the PCR digoxigenin (DIG) probe synthesis kit (Roche Diagnostics) with B. burgdorferi isolate B31 MI gene-specific primers (Table 2). Membranes were prehybridized at 42°C overnight in hybridization buffer (8) and hybridized with DIG-labeled probes diluted 1:500 (BBA65, BBA66, BBA71, BBA73, and flaB) or 1:1,000 (BBA64) in hybridization buffer at 42°C overnight. Membranes were washed under moderate-stringency conditions for 10 min at room temperature with 2 × SSC and 0.1% (wt/vol) SDS, followed by 30 min at 65°C with 0.5× SSC and 0.1% (wt/vol) SDS. Bound probes were detected using anti-DIG-peroxidase antibody from Roche Diagnostics, and bands were visualized with enhanced chemiluminescence reagents and exposure to film. Band sizes were approximated from the linear regression equation calculated for individual gels based on the mobility of λ HindIII DNA standards (Promega).
RESULTS
Phylogenetic analysis of genes previously classified as members of pgf 54.
Subsequent to sequencing and annotation of the B. burgdorferi B31 genome, TIGR classified many genes into 1 of 161 pgf (23, 34). Recently, TIGR reevaluated their pgf classifications and reorganized one such family, pgf 54, into pgf gbb fam_b_burgdorferi_b31.pep_35, which includes BBA68, BBA69, BBA70, BBA71, BBI36, BBI38, BBI39, and BBJ41, and a subset of nonparalogous genes comprising BBA64, BBA65, BBA66, and BBA73 (http://cmr.tigr.org/tigr-scripts/CMR/ParalogDescription.cgi?sub_org=&ev_type=Paralog&org_search=Borrelia&sub_org_val=gbb). Clustal V analysis of 34 selected gene sequences representing the original grouping from B. burgdorferi, as well as groupings from B. afzelii and B. garinii isolates, agrees with TIGR's reclassification and separation of a subset of the original pgf 54 into a separate pgf (Fig. 1). Genes clustering into pgf b31.pep_35 comprise a single clade and demonstrate less divergence than BBA64, BBA65, BBA66, and BBA73. Interestingly, though TIGR describes BBA65 and BBA66 as nonparalogs, our Clustal V analysis clearly indicates that these two genes and their orthologs define a specific clade that demonstrates less divergence than that observed in the newly formed pgf b31.pep_35 (Fig. 1; see Table S1 in the supplemental material). These data suggest that BBA65 and BBA66 are closely related, display less divergence than other genes within B. burgdorferi pgf, and may constitute an additional pgf not recognized by TIGR's current classification.
Influence of the σN-σS regulatory cascade upon BBA65, BBA66, BBA71, and BBA73 transcript and protein expression.
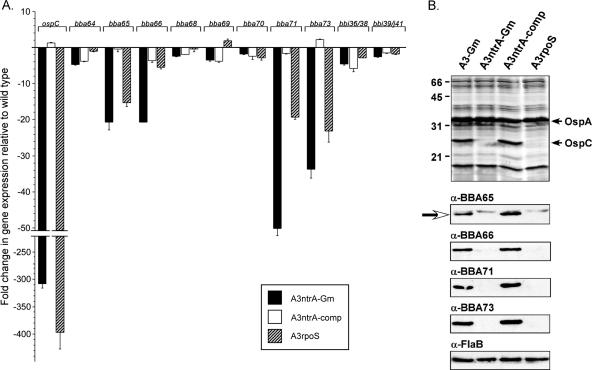
DNA microarray analyses have shown that the σN-σS regulatory cascade is involved in the transcriptional regulation of numerous outer-surface-localized lipoproteins (18, 33) and in the expression of borrelial virulence factors (17). To validate whether the σN-σS regulatory cascade influenced the expression of genes historically categorized as pgf 54, we measured transcript levels in vitro of ΔntrA and ΔrpoS mutants using qRT-PCR, which has a broader dynamic range of detection than DNA microarray analysis, as has been used in previous studies. Given that mRNA levels and protein amount do not always correlate (40), we also assessed changes at the protein level for those genes that appeared to be influenced by the σN-σS cascade in our qRT-PCR analyses. RNA and membrane-associated proteins were isolated from B31 isolates A3-Gm (wild-type control), A3ntrA-Gm (σN deletion mutant), A3ntrA-comp (complemented σN deletion mutant), and A3rpoS (σS deletion mutant) (Table 1) as described in Materials and Methods. Transcript (Fig. 2A) and protein amounts (Fig. 2B) of BBA65, BBA66, BBA71, and BBA73 were decreased in both sigma factor mutants relative to those in A3-Gm, in agreement with previous microarray studies (18, 33). Complementation of the ntrA mutation restored transcript expression of these genes (Fig. 2A) and protein expression (Fig. 2B) to levels comparable to those in A3-Gm. ospC transcript and protein amounts were substantially decreased in both sigma factor mutants and were restored with complementation of the ΔntrA mutant (Fig. 2), as expected based on previous data (29, 33, 95). Transcription of the remaining eight genes examined, including BBA64, BBA68, BBA69, BBA70, BBI36/BBI38, and BBI39/BBJ41, was minimally affected by the loss of either sigma factor under our experimental conditions (Fig. 2).
FIG. 2.
Analysis of transcript levels and protein synthesis of genes formally categorized in pgf 54 in ntrA and rpoS mutants. (A) qRT-PCR was performed using RNA isolated from A3-Gm, A3ntrA-comp, A3ntrA-Gm, and A3rpoS with gene-specific primers (Table 2). Average CT values were normalized to flaB, and the differences were calculated using the comparative CT method (2−ΔΔCT). ospC served as a control. Gene pairs with greater than 99% sequence identity cannot be discerned by this method and are therefore referred to as BBI36/38 and BBI39/J41. Error bars, standard errors of the means. (B) Total membrane fractions prepared from each isolate were separated by SDS-PAGE (7.5 μg/lane), and gels were either stained with silver (top) or immunoblotted with BBA65-, BBA66-, BBA71-, BBA73-, or FlaB-specific antibodies (bottom). The arrow points to the BBA65 protein band, the lower of the two bands recognized by our polyclonal serum (see text). FlaB served as a loading control. Relative molecular masses are indicated in kilodaltons to the left. α, anti.
Temporal expression of BBA68, BBA69, BBA70, BBA71, and BBA73 in ear tissue and accompanying antibody response during persistent infection of mice.
We had previously reported the temporal expression of BBA64, BBA65, and BBA66 during persistent infection of mice with B. burgdorferi B31 (35). To complement these studies, we determined the gene expression in ear tissue by qRT-PCR and the antibody response during murine infection by ELISA and Western blotting for the genes BBA68, BBA69, BBA70, BBA71, and BBA73. A comprehensive schematic of these experiments and sample collection was recently published (35).
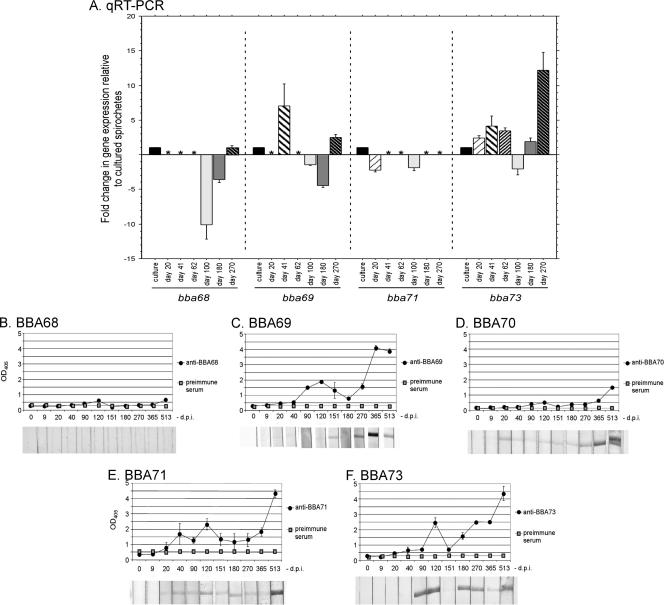
Over the 17-month infection period, BBA73 was the most dynamically expressed of the genes analyzed, demonstrating an increase in mean expression between 20 and 62 days postinfection (p.i.) and from 180 to 270 days p.i. relative to cultured organisms (Fig. 3A). Overall, the mean expression profile of BBA73 during persistent infection demonstrated a trend similar to that observed previously for BBA65 and BBA66 (35). A measurable anti-BBA73 antibody response was not observed until day 90 p.i. by immunoblotting and until day 120 p.i. by ELISA; however, this response continued from this period until the study end point (Fig. 3F). Interestingly, though this initial increase in anti-BBA73 antibodies at day 120 p.i. correlated to a 1.7-fold decrease in BBA73 transcript levels at day 100 p.i., it appeared that a subsequent increase in anti-BBA73 antibodies at later time points (days 180 and 270 p.i.) did not similarly correlate with a reduction in BBA73 transcripts (compare Fig. 3A and F). Of the remaining genes analyzed, only BBA69 displayed a trend in transcript expression similar to that displayed by BBA73. Two peaks in BBA69 expression were detected, albeit at single time points, on days corresponding to the highest levels of BBA73 expression. Moreover, an increase in BBA69 protein-specific antibody production corresponded to a decrease in BBA69 gene expression between days 40 and 151 p.i. (compare Fig. 3A and C), mirroring the trend observed with BBA73 during early stages of the infection course. Similarities between BBA69 and BBA73 gene expression and antibody production continued throughout the experiment, with low levels of anti-BBA69 antibodies detected at day 180 p.i. (day 151 p.i. for the BBA73 protein), followed by an increase in BBA69 transcripts, despite an increase in BBA69 protein-specific antibodies, at day 270 p.i. (days 180 and 270 p.i. for the BBA73 protein).
FIG. 3.
Transcript expression of BBA68, BBA70, BBA71, and BBA73 and serologic responses to their encoded proteins during persistent murine infection with B. burgdorferi. (A) RNA isolated from ear tissue of persistently infected mice was subjected to qRT-PCR using specific primers (Table 2) to measure gene transcripts. Expression of BBA70 was not detectable in any samples and therefore was omitted from the figure. Shown are the changes in gene expression relative to expression in culture following normalization to the constitutively expressed gene flaB. Note that days 120, 365, and 513 p.i. were omitted in panel A because no transcripts were detected, including flaB. The asterisks indicate that flaB transcripts were present but that the target gene was undetectable from ear tissue on that day. (B to F) ELISA data are displayed graphically above corresponding immunoblots in each panel. Preimmune serum was used to determine a baseline for ELISA data and represents the mean optical density at 405 nm (OD405) plus 3 standard deviations of the mean. Each data point represents the mean of triplicate samples from a single mouse. (B) BBA68 protein; (C) BBA69 protein; (D) BBA70 protein; (E) BBA71 protein; (F) BBA73 protein. For all panels, the days p.i. are indicated on the x axes and error bars represent the standard errors of the means.
Expression of the remaining three genes was not increased in murine ear tissue relative to cultured spirochetes. BBA70 transcripts remained undetectable throughout the course of infection (data not shown), while BBA68 and BBA71 transcript expression decreased relative to that for cultured spirochetes at various time points and failed to reach levels greater than those in spirochetes grown in vitro (Fig. 3A). For BBA68 these findings are comparable to other reports where BBA68 mRNA was undetectable in B. burgdorferi-infected ear tissues at 2 weeks p.i., in ear tissue at 4 weeks p.i., or in heart and joint tissues at any times p.i. out to 90 days (15, 65, 91). Consistent with these results, there was not a detectable antibody response against recombinant BBA68 protein (BbCRASP-1) at any time p.i. by immunoblot assay; ELISA indicated only a slight increase above background in anti-BBA68 antibodies at day 120 p.i. (Fig. 3B). Not surprisingly, the antibody response to the BBA70 protein remained weak throughout the experimental time course until 17 months (513 days) p.i. (Fig. 3D). Interestingly, however, BBA71 protein antibody responses were detected at the earliest time point of any of the proteins assessed, day 40 p.i., and antibody levels remained elevated until the end point of the experiment. It was also noted that fluctuations in anti-BBA71 antibody levels (Fig. 3E) reflected the trend observed for anti-BBA65, -BBA66, and -BBA73 antibodies (Fig. 3F) (35).
Association of BBA65, BBA66, BBA71, and BBA73 protein production with infectivity phenotypes.
We demonstrated in a previous study that synthesis of the BBA66 protein in vitro is influenced by the σN-σS regulatory cascade and is associated with infectivity phenotypes (25). Because the expression of BBA65, BBA71, and BBA73 proteins was also influenced by the σN-σS regulatory cascade (Fig. 2), we hypothesized that their expression was also linked to infectivity phenotypes. To test this, membrane-associated proteins were prepared from infectious isolates and compared to noninfectious isolates (Table 1). As demonstrated in Fig. 4A, BBA65, BBA71, and BBA73 protein expression was detected in infectious isolates but not in the noninfectious clones, similar to what was reported for the BBA66 protein (25). We noted that a second, slightly higher-molecular-weight band was commonly detected with our BBA65 protein-specific antibodies (Fig. 4A and B); however, the presence of this weakly immunoreactive band did not correlate with expected BBA65 protein expression, and the band is believed to be cross-reactive. OspC, previously linked to infectivity phenotypes (2, 22, 25, 62, 71, 87), was also produced in infectious but not noninfectious isolates. The presence of OspA indicated that lp54, which carries BBA65, BBA66, BBA71, and BBA73, is harbored by all four isolates.
FIG. 4.
In vitro expression of BBA65, BBA66, BBA71, and BBA73 proteins in association with infectious B. burgdorferi isolates and with the borrelial outer surface. All proteins were separated by SDS-PAGE and either stained with silver (upper panels) or immunoblotted with antibodies specific for BBA65, BBA66, BBA71, BBA73, FlaB, or OppAI (lower panels). OspA and OspC are indicated to the right and molecular mass markers, in kilodaltons, to the left of each silver-stained gel. Arrows point to the BBA65 protein band, the lower of two bands recognized by our anti-BBA65 polyclonal serum (see text). (A) Association of protein expression with infectious isolates. Total membrane fractions were prepared from infectious isolates (B31 and A3) and noninfectious isolates (A and A-34), and 7.5 μg of protein was loaded per lane. (B) Membrane association. Intact B31 cells were treated with TX-114, and an equivalent of 1 × 108 cells of the no-treatment (NT) control was loaded to each lane. CP, insoluble cold pellet; WP, insoluble warm pellet; AQ, aqueous phase; DT, detergent phase. FlaB and OppAI served as controls and appropriately partitioned to the expected fractions. (C) Surface localization. Intact B31 cells were treated with 400 μg/ml of the indicated protease for 1 h, and 1 × 108 cells were loaded to each lane. FlaB and OppA1 are controls for cell integrity and loading. Pn, pronase SC.
Membrane association and surface localization of BBA65, BBA66, BBA71, and BBA73 proteins.
Our immunoblot analyses of total membrane fractions in Fig. 4A also indicated that the BBA65, BBA66, BBA71, and BBA73 proteins are membrane associated. To corroborate this finding, we used the nonionic detergent TX-114, which solubilizes lipidated and integral membrane proteins (9) and has been used to fractionate borrelial lipoproteins (9, 82). Protein fractions from intact B31 cells treated with TX-114 were examined by immunoblotting, which showed that both the BBA65 and BBA71 proteins partitioned completely into the detergent phase while 19% of the total amount of the BBA73 protein partitioned into the detergent phase and the remainder into the cold pellet (Fig. 4B and data not shown). As expected, the BBA66 protein separated completely into the detergent phase (12), while FlaB remained in the cold pellet due to its association with the protoplasmic cylinder (81). OppA1, which is hypothesized to be lipidated (8, 80), was used as an additional control and partitioned into both the aqueous and detergent phases. Furthermore, the SDS-PAGE with silver staining demonstrated that known outer-surface lipoproteins OspA and OspC largely partitioned to the detergent phase. TX-114 treatment of B31 cell lysates demonstrated similar results (data not shown). Together, these data indicate that these proteins are lipidated or integral membrane proteins.
To address whether membrane-associated BBA65, BBA71, and BBA73 proteins were also localized to the outer surface, intact infectious B31 cells were treated with PK, Tr, or pronase SC and examined by immunoblotting (Fig. 4C). Detectable levels of BBA65 and BBA73 proteins were decreased by all treatments and to the greatest extent by PK and Tr, respectively. The BBA71 protein was undetectable following treatment with all proteases utilized. Agreeing with a previous report (12), detectable BBA66 protein was also decreased following protease treatment of intact cells. Cell integrity was maintained throughout the experiment, as demonstrated by constant levels of periplasmically localized FlaB and inner membrane-localized OppA1 (Fig. 4C).
Analysis of B. burgdorferi sensu lato and relapsing-fever Borrelia spp. for the presence of BBA64, BBA65, BBA66, BBA71, and BBA73 orthologs.
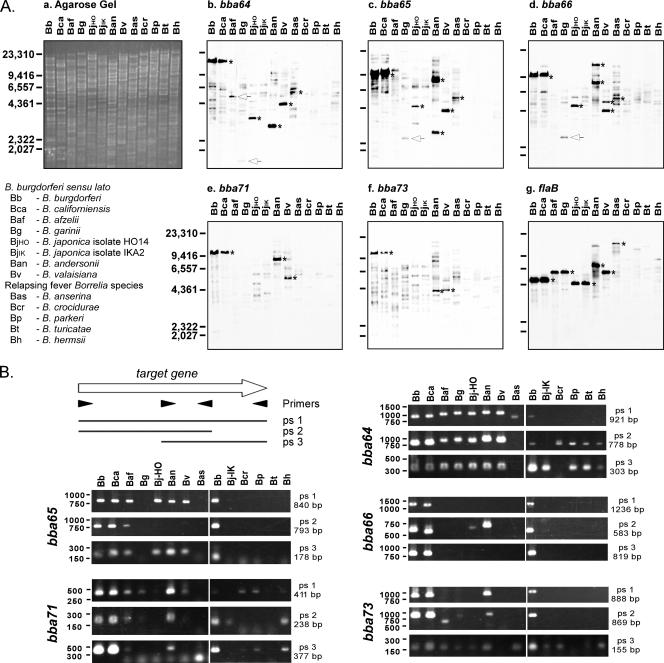
Previous studies (11, 25, 35, 53, 72, 77, 88, 91) and the data presented thus far suggested that BBA64, BBA65, BBA66, BBA71, and BBA73 may be important factors for borrelial pathogenesis that are expressed by infectious B. burgdorferi B31 throughout murine infection. Though it has been shown that orthologs to these genes are harbored by B. burgdorferi, B. afzelii, and B. garinii (36-38, 49, 91), this has not been demonstrated experimentally in other sensu lato species. Thus, to determine if these genes are conserved in other Borrelia spp. in and beyond the sensu lato group, genomic DNA from various B. burgdorferi sensu lato and relapsing-fever Borrelia spp. (Table 1) was analyzed for the presence of gene orthologs by Southern blotting with B. burgdorferi isolate B31 gene-specific probes or by PCR using B. burgdorferi gene-specific primers (Fig. 5).
FIG. 5.
Southern blot and PCR analyses of B. burgdorferi sensu lato and relapsing-fever spirochetes for BBA64, BBA65, BBA66, BBA71, and BBA73 gene orthologs. (A) Southern blotting analysis. Genomic DNA prepared from various Borrelia spp. (lower left and Table 1) was digested with EcoRI and separated on 0.8% agarose gels (1 μg/lane). A representative ethidium bromide-stained gel is shown in panel a. In panels b to g, DNA was transferred to nylon membranes and probed with gene-specific probes based on B31 type strain sequences. Wash conditions are noted in Materials and Methods. DNA standard values are given in base pairs to the left of panels a and e and are indicated by hash marks to the left of the remaining panels. The asterisks denote regions of strong hybridization. Arrows indicate weak hybridization corresponding to the predicted restriction digest DNA fragment according to the genome sequences of known gene orthologs. (B) PCR analysis. A schematic representation of the primer sets (ps) used for each target gene is depicted at the upper left. In the remaining panels, products amplified from genomic DNA by PCR were separated on agarose gels, stained with ethidium bromide, and photographed under UV light. Target genes are shown vertically to the left of corresponding agarose gels. Standards are shown in base pairs to the left of each panel. The ps used and the expected size from B. burgdorferi B31 genomic DNA are indicated to the right of each agarose gel. ps 1, both full-length (FL) primers specific for the target gene; ps 2, the forward FL primer and reverse reverse transcription (rt) primer specific for the target gene; ps 3, the forward rt primer and reverse FL primer specific for the target gene. Species abbreviations are as given in panel A.
For Southern blots, moderate-stringency wash conditions were used to increase our ability to identify putative orthologs in more distantly related species. A representative agarose gel stained with ethidium bromide shows equal loading and the expected laddering of genomic DNA following restriction endonuclease digestion (Fig. 5A, a). When membranes were hybridized with BBA64, BBA65, and BBA66 gene probes (Fig. 5A, b to d) intense bands were apparent in the B. burgdorferi B31 control, “Borrelia californiensis” (proposed name), Borrelia japonica (HO14), “Borrelia andersonii” (proposed name), Borrelia valaisiana, and Borrelia anserina. Hybridizing bands were also evident with BBA71 and BBA73 gene probes in B. burgdorferi, B. californiensis, B. andersonii, and B. valaisiana (Fig. 5A, e and f). Weakly hybridizing bands detected in B. afzelii PGau and B. garinii isolate G1 with BBA64, BBA65, and BBA66 gene probes corresponded to the expected restriction fragment sizes of the sequenced B. afzelii isolate PKo (GenBank accession no. CP000395) or B. garinii isolate PBi (GenBank accession no. CP000015); the intensities of these bands, though not increased over background (Fig. 5A, b to d), did correlate with findings for BBA64 by Gilmore et al. (36). flaB was used as a hybridization control and demonstrated definitive hybridization to genomic DNA from species containing orthologs with 84% sequence identity or higher to the B. burgdorferi isolate B31 flaB sequence (Fig. 5A, g).
To expand our findings from the Southern blotting analyses, we examined whether we could amplify products from diverse Borrelia spp. using B. burgdorferi B31-specific PCR primers and, if so, whether those PCR products were of a similar size to those produced using B. burgdorferi B31 genomic DNA. All species examined by Southern blotting were analyzed with three pairings of primers for each gene (Table 2 and Fig. 5B). Using the three BBA64 primers sets, an appropriately sized product was obtained from at least one PCR from all Borrelia spp. tested (Fig. 5B). We amplified an appropriately sized product using primers specific for BBA65 with DNA isolated from B. burgdorferi B31, B. californiensis, B. afzelii, B. japonica isolate HO14, B. andersonii, and B. valaisiana. All three BBA66 primers yielded products for B. burgdorferi B31 and B. californiensis, while PCR products were produced only from B. japonica isolate HO14 and B. andersonii using BBA66-specific primer set 2 (Fig. 5B). For BBA71, definitive PCR products were obtained from all Borrelia spp. examined using at least one primer set, except for B. anserina and B. turicatae. Lastly, PCR products were obtained with one or more of the BBA73-specific primer sets from DNA isolated from B. burgdorferi B31, B. californiensis, B. andersonii, B. afzelii, B. garinii, both B. japonica isolates, B. anserina, B. crocidurae, and B. hermsii. The Southern blot and PCR results are summarized in Table 3.
TABLE 3.
Southern blotting and PCR summary for diverse Borrelia spp.
| Species (isolate) | Resulta of indicated procedureb for gene:
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BBA64
|
BBA65
|
BBA66
|
BBA71
|
BBA73
|
||||||||||||||||
| SB | PCR
|
SB | PCR
|
SB | PCR
|
SB | PCR
|
SB | PCR
|
|||||||||||
| ps 1 | ps 2 | ps 3 | ps 1 | ps 2 | ps 3 | ps 1 | ps 2 | ps 3 | ps 1 | ps 2 | ps 3 | ps 1 | ps 2 | ps 3 | ||||||
| B. burgdorferi | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| B. californiensis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| B. afzelii | ± | + | + | + | − | + | + | + | − | − | − | − | − | + | + | + | − | − | + | + |
| B. garinii | ± | + | + | + | ± | − | − | − | ± | − | − | − | − | + | + | − | − | − | + | + |
| B. japonica (HO14) | + | + | + | + | + | + | − | + | + | − | + | − | − | + | − | − | − | − | − | + |
| B. japonica (IKA2) | − | − | − | + | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | + |
| B. andersonii | + | + | + | + | + | + | − | + | + | − | + | − | + | + | + | + | + | + | + | + |
| B. valaisiana | + | + | + | + | + | + | − | + | + | − | − | − | + | + | − | − | + | − | − | − |
| B. aserina | + | + | − | − | + | − | − | − | + | − | − | − | − | − | − | − | − | − | − | + |
| B. crocidurae | − | − | + | − | − | − | − | − | − | − | − | − | − | + | − | + | − | − | − | + |
| B. parkeri | − | − | + | + | − | − | − | − | − | − | − | − | − | + | − | + | − | − | − | − |
| B. turicatae | − | − | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + |
| B. hermsii | − | − | + | + | − | − | − | − | − | − | − | − | − | + | + | + | − | − | − | + |
Immunoblot analysis of B. burgdorferi sensu lato and relapsing-fever Borrelia spp. for the presence of proteins cross-reactive to anti-BBA64, -BBA65, -BBA66, -BBA71, and -BBA73 antibodies.
Immunoblotting was utilized to assess the cross-reactivity of anti-BBA64, -BBA65, -BBA66, -BBA71, and -BBA73 B. burgdorferi-specific antibodies with total membrane fractions prepared from seven of the Borrelia spp. demonstrating hybridization with our gene probes by Southern blotting (Fig. 5A). B. japonica isolate HO14 was not cultivable in the BSK-H lot available to us (lot 057K8413) and thus was eliminated from this analysis. The high-passage-number B. burgdorferi-derived clone A, which does not express the proteins under examination (25), was used as a negative control. Cross-reactive proteins were observed for B. californiensis with anti-BBA66 and -BBA73 antibodies, for B. afzelii with anti-BBA66 and -BBA71 antibodies, and for B. garinii with anti-BBA71 antibodies (Fig. 6). Cross-reactive proteins were not detected using anti-BBA64 or -BBA65 (data not shown). All species examined were cross-reactive with anti-OppAI antibodies, which served as a loading control.
FIG. 6.

BBA66, BBA71, and BBA73 protein antibody cross-reactivity with total cell membranes from diverse Borrelia spp. Borrelia spp. were grown at pH 7.0, and total cell membranes were prepared as described in Materials and Methods. Five micrograms of protein was separated per lane by SDS-PAGE and either stained with silver (top) or transferred for immunoblotting with anti-BBA66, -BBA71, -BBA73, or -OppAI specific antibodies (indicated to the left of each lower panel). No cross-reactive bands were observed with either anti-BBA64 or -BBA65 polyclonal antibodies (not shown). Molecular mass standards are indicated in kilodaltons to the left of the upper panel. Species abbreviations are as listed in Fig. 5A. OppAI served as a loading control.
DISCUSSION
The alternative sigma factor σS has been implicated in the regulation of virulence factors in many diverse bacterial species (32, 48, 85). In B. burgdorferi the expression of σS is directly controlled by the alternative sigma factor σN, and several reports have highlighted the importance of this unique regulatory cascade in B. burgdorferi for gene regulation and infectivity (16-18, 33, 43, 93, 94). Comprehensive transcriptome analyses of ntrA (encoding σN) and rpoS (encoding σS) mutants in strains B31 and 297 have demonstrated the pleiotropic effect of knocking out these sigma factors upon transcription in B. burgdorferi, and numerous genes appear to be influenced by this cascade, including BBA64, BBA65, BBA66, BBA71, and BBA73 (18, 33). Using qRT-PCR, a technique with a broader dynamic range, we too demonstrated the effects upon in vitro expression of these genes when either σN or σS was absent (Fig. 2A). Our results agreed with previous microarray analyses for BBA65, BBA66, and BBA71 (18, 33), but our observed changes in levels of transcription were in better agreement with the findings of Caimano et al. (18, 33), demonstrating 21- to 50-fold decreases in gene transcripts with mutation of ntrA or rpoS versus 1.5- to 3.8-fold decreases measured by Fisher and colleagues (18, 33). The lack of an appreciable change in BBA64 transcript levels in the absence of either σN or σS (Fig. 2A) is likely due to the specific lot of BSK-H media used for these experiments, as has been well documented before (4, 19, 25, 92). Immunoblotting of total membrane fractions demonstrated that changes observed in gene transcript levels correlated well with BBA65, BBA66, BBA71, and BBA73 protein levels (Fig. 2B).
We previously reported the temporal expression of BBA64, BBA65, and BBA66 in ear tissue throughout persistent infection of immunocompetent mice (35). To complement these studies, we analyzed the same sample sets for gene expression of BBA68 (cspA), BBA69, BBA70, BBA71 and BBA73 and murine antibody responses to their products (Fig. 3). We detected the expression of several of these genes in ear tissue at various times during persistent infection. Interestingly, BBA73 demonstrated an expression pattern similar to what had been reported for BBA65 and BBA66 (35), and serological evidence indicated that the BBA73 protein is immunogenic and recognized throughout persistent infection. Continued expression of BBA73 despite increases in specific antibodies during persistent infection suggests that the protein may be involved in the long-term survival or pathogenesis of B. burgdorferi in mice.
Though BBA68 expression in ear tissue was detectable in small amounts at three independent time points, we were unable to detect a sufficient antibody response to denatured (Western blotting) or to nondenatured (ELISA) recombinant BBA68 protein (BbCRASP-1) at any time point. Our data indicate that BBA68 is expressed below the level of detection once B. burgdorferi has disseminated from the site of inoculation to ear tissues (day 20 p.i.). Moreover, our serological results support previous reports of an undetectable antibody response to the BBA68 protein in mice and Lyme disease patients, but our ELISA data are at odds with a report by Rossmann et al. describing a detectable response with nondenatured BBA68 protein (65, 78). Our results, however, are consistent with the hypothesis that BBA68 may initially protect transmitted organisms against the serum complement-mediated killing activity of the host by binding factor H (13, 90) but then is rapidly decreased.
Our results with BBA69 expression and serology, which demonstrated detectable responses up to 1 year 5 months p.i., agreed with the assessment of BBA69 protein immunoreactivity with sera from tick-infected nonhuman primates (12), which showed small but sustainable titers against recombinant BBA69 protein up to a year p.i. It was also interesting to note similar trends between BBA69 and BBA73 transcript expression and antibody detection, though in vitro analyses of these genes/proteins in sigma factor mutants would suggest that they are regulated via differing pathways (Fig. 2). Despite undetectable expression of BBA70 and low expression of BBA71 in ear tissue of persistently infected mice, serology indicated detectable specific antibodies either late in (BBA70 protein) or throughout (BBA71 protein) the study. This suggests that the associated genes may be expressed in greater amounts in tissues other than mouse ear during persistence. Differential expression of B. burgdorferi proteins in various animal tissues has been previously demonstrated for other surface-localized proteins (26, 27) and is likely important in pathogenesis and tissue tropism.
Our results also indicate that BBA65, BBA66, BBA71, and BBA73 proteins are either lipidated or integral membrane proteins that localize to the outer surface of B. burgdorferi B31, as all partitioned to the TX-114 detergent phase and were sensitive to protease treatment of intact spirochetes. Our findings for the BBA66 protein were in agreement with those of Brooks et al., who reported similar partitioning and surface localization for the BBA64, BBA66, and BBA69 proteins (12). It was surprising to find that the BBA73 protein did not demonstrate complete partitioning into the detergent phase, given that a spirochete-specific lipidation motif in this protein suggests that it is lipidated (80). However, it is possible that the BBA73 protein may possess biochemical characteristics that do not allow it to be easily solubilized in TX-114. Our findings for the BBA71 protein were also interesting, as this protein was dissimilar from others examined in this study. The BBA71 protein is not predicted to contain a recognizable signal sequence or lipidation motif by either the SignalP (6, 68) or LipoP algorithm (47), nor is it predicted to be lipidated by the SpLip algorithm (80) (data not shown). Upon further analysis, however, we identified a putative transmembrane domain (20I-I-I-G-L-L-Y-H-K28) using the DAS algorithm (28) that was recognized only using the weakest cutoff setting (data not shown).
Partial genome sequencing of B. afzelii PKo and B. garinii PBi indicates that orthologs with various identities to BBA64, BBA65, BBA66, BBA71, and BBA73 are present in these isolates. Southern blotting and PCR experiments performed to assess whether these genes were conserved by other diverse Borrelia spp. demonstrated that putative orthologs may indeed be present in all Borrelia spp. examined. Hybridization results were varied for the samples tested, and the appearance of weak hybridization bands or nondefinitive bands in genomic DNA from B. afzelii, B. garinii, or less-related spirochetes was not unexpected. The sequence identity of the gene orthologs in these species ranges from 82% to 32% in comparison to B. burgdorferi B31, decreasing the likelihood of hybridization under our experimental conditions (see Table S1 in the supplemental material). Notably, our level of detection of BBA66 orthologs was similar to that from a previous study (91), but BBA64 orthologs were more readily detected by Gilmore et al. in B. afzelii and B. garinii genomic DNA and might reflect differences in gene probes or stains used in the study (36). Interestingly, products of the appropriate size were amplified by PCR from at least one of our primer sets from the majority of DNA samples examined from our representative panel of Borrelia spp. Ultimately, the lack of an observable hybridizing band and/or PCR product from several Borrelia spp. is not indicative of the absence of an ortholog to our genes of interest, as there may be enough divergence in the sequences that our B. burgdorferi B31-derived gene-specific probes/primers were unable to hybridize/anneal. This point is highlighted by (i) the lack of hybridization of our flaB probe in all Borrelia spp. whose flaB was less than 84% similar to that of B. burgdorferi B31 and (ii) the failure to amplify PCR products from some species using B. burgdorferi B31-specific primers (Fig. 5B) that demonstrated hybridization by Southern blotting (Fig. 5A). However, we cannot rule out the possibilities that plasmids encoding putative orthologs in other species have been lost during culture or that genomic DNA from nonsequenced isolates may have been too fragmented by EcoRI digestion to detect gene orthologs.
The culmination of the information to date from this study and other reports indicates that many of the genes that cluster on lp54 (i.e., BBA64, BBA65, BBA66, BBA69, BBA71, and BBA73) are influenced by the σN-σS regulatory cascade in response to environmental signals and are expressed during persistent infection and that the encoded proteins are exposed on the spirochetal cell surface. Furthermore, putative orthologs to BBA64, BBA65, BBA66, BBA71, and BBA73 were detected in select B. burgdorferi sensu lato strains and in several relapsing-fever Borrelia spp. This indicates that this set of genes, formally categorized as pgf 54, are conserved in both Lyme disease-associated and relapsing-fever Borrelia spp. Though the function of these proteins during the infection process, pathogenesis, or tissue tropism is currently undefined, the findings presented here and in previous studies suggest that the BBA64, BBA65, BBA66, BBA71, and BBA73 proteins may play a role in Borrelia tissue-specific pathogenicity in mammalian hosts (3, 35, 56, 61). Further investigation of the role of these outer-surface proteins in host-pathogen interactions and during the tick-mouse infection cycle is clearly warranted.
Supplementary Material
Acknowledgments
We thank Mohamed A. Motaleb and Nyles Charon for the monoclonal anti-FlaB, Linden T. Hu for the polyclonal anti-OppA1 rabbit serum, Kit Tilly for B. burgdorferi B31 A-34, Patricia Rosa and Tom Schwan for various RML Borrelia isolates, and Mark A. Fisher and Frank C. Gherardini for the strains A3-Gm, A3ntrA-Gm, A3ntrA-comp, and A3rpoS. We acknowledge the Borrelia sequencing team of Sherwood R. Casjens, John J. Dunn, Benjamin J. Luft, Claire M. Fraser, Weigang Qiu, and Steven E. Schutzer, working under grants from the Lyme Disease Association and National Institutes of Health (AI37256, and AI49003), for access to unpublished sequence information.
This research was supported in part by CDC cooperative agreement number CI000181. J.L.H. was supported by NIH predoctoral training grant number AI060525. A.J.N. was supported by NIH training grant number HD042987.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 7 April 2008.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Anderson, J. F., L. A. Magnarelli, R. B. LeFebvre, T. G. Andreadis, J. B. McAninch, G. C. Perng, and R. C. Johnson. 1989. Antigenically variable Borrelia burgdorferi isolated from cottontail rabbits and Ixodes dentatus in rural and urban areas. J. Clin. Microbiol. 2713-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anguita, J., S. Samanta, B. Revilla, K. Suk, S. Das, S. W. Barthold, and E. Fikrig. 2000. Borrelia burgdorferi gene expression in vivo and spirochete pathogenicity. Infect. Immun. 681222-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonara, S., R. M. Chafel, M. Lafrance, and J. Coburn. 2007. Borrelia burgdorferi adhesins identified using in vivo phage display. Mol. Microbiol. 66262-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babb, K., N. El-Hage, J. C. Miller, J. A. Carroll, and B. Stevenson. 2001. Distinct regulatory pathways control expression of Borrelia burgdorferi infection-associated OspC and Erp surface proteins. Infect. Immun. 694146-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbour, A. G., S. F. Hayes, R. A. Heiland, M. E. Schrumpf, and S. L. Tessier. 1986. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect. Immun. 52549-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340783-795. [DOI] [PubMed] [Google Scholar]
- 7.Bono, J. L., A. F. Elias, J. J. Kupko III, B. Stevenson, K. Tilly, and P. Rosa. 2000. Efficient targeted mutagenesis in Borrelia burgdorferi. J. Bacteriol. 1822445-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bono, J. L., K. Tilly, B. Stevenson, D. Hogan, and P. Rosa. 1998. Oligopeptide permease in Borrelia burgdorferi: putative peptide-binding components encoded by both chromosomal and plasmid loci. Microbiology 1441033-1044. [DOI] [PubMed] [Google Scholar]
- 9.Brandt, M. E., B. S. Riley, J. D. Radolf, and M. V. Norgard. 1990. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect. Immun. 58983-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breitner-Ruddock, S., R. Wurzner, J. Schulze, and V. Brade. 1997. Heterogeneity in the complement-dependent bacteriolysis within the species of Borrelia burgdorferi. Med. Microbiol. Immunol. 185253-260. [DOI] [PubMed] [Google Scholar]
- 11.Brooks, C. S., P. S. Hefty, S. E. Jolliff, and D. R. Akins. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 713371-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks, C. S., S. R. Vuppala, A. M. Jett, and D. R. Akins. 2006. Identification of Borrelia burgdorferi outer surface proteins. Infect. Immun. 74296-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks, C. S., S. R. Vuppala, A. M. Jett, A. Alitalo, S. Meri, and D. R. Akins. 2005. Complement regulator-acquiring surface protein 1 imparts resistance to human serum in Borrelia burgdorferi. J. Immunol. 1753299-3308. [DOI] [PubMed] [Google Scholar]
- 14.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease—a tick borne spirochetosis? Science 2161317-1319. [DOI] [PubMed] [Google Scholar]
- 15.Bykowski, T., M. E. Woodman, A. E. Cooley, C. A. Brissette, V. Brade, R. Wallich, P. Kraiczy, and B. Stevenson. 2007. Coordinated expression of Borrelia burgdorferi complement regulator-acquiring surface proteins during the Lyme disease spirochete's mammal-tick infection cycle. Infect. Immun. 754227-4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caimano, M. J., C. H. Eggers, C. A. Gonzalez, and J. D. Radolf. 2005. Alternate sigma factor RpoS is required for the in vivo-specific repression of Borrelia burgdorferi plasmid lp54-borne ospA and lp6.6 genes. J. Bacteriol. 1877845-7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caimano, M. J., C. H. Eggers, K. R. Hazlett, and J. D. Radolf. 2004. RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect. Immun. 726433-6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caimano, M. J., R. Iyer, C. H. Eggers, C. Gonzalez, E. A. Morton, M. A. Gilbert, I. Schwartz, and J. D. Radolf. 2007. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol. Microbiol. 651193-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callister, S. M., K. L. Case, W. A. Agger, R. F. Schell, R. C. Johnson, and J. L. Ellingson. 1990. Effects of bovine serum albumin on the ability of Barbour-Stoenner-Kelly medium to detect Borrelia burgdorferi. J. Clin. Microbiol. 28363-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carroll, J. A., R. M. Cordova, and C. F. Garon. 2000. Identification of 11 pH-regulated genes in Borrelia burgdorferi localizing to linear plasmids. Infect. Immun. 686677-6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carroll, J. A., C. F. Garon, and T. G. Schwan. 1999. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect. Immun. 673181-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carroll, J. A., and F. C. Gherardini. 1996. Membrane protein variations associated with in vitro passage of Borrelia burgdorferi. Infect. Immun. 64392-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35490-516. [DOI] [PubMed] [Google Scholar]
- 24.CDC. 2007. Lyme disease—United States, 2003-2005. MMWR Morb. Mortal. Wkly. Rep. 56573-576. [PubMed] [Google Scholar]
- 25.Clifton, D. R., C. L. Nolder, J. L. Hughes, A. J. Nowalk, and J. A. Carroll. 2006. Regulation and expression of bba66 encoding an immunogenic infection-associated lipoprotein in Borrelia burgdorferi. Mol. Microbiol. 61243-258. [DOI] [PubMed] [Google Scholar]
- 26.Crother, T. R., C. I. Champion, J. P. Whitelegge, R. Aguilera, X. Y. Wu, D. R. Blanco, J. N. Miller, and M. A. Lovett. 2004. Temporal analysis of the antigenic composition of Borrelia burgdorferi during infection in rabbit skin. Infect. Immun. 725063-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crother, T. R., C. I. Champion, X. Y. Wu, D. R. Blanco, J. N. Miller, and M. A. Lovett. 2003. Antigenic composition of Borrelia burgdorferi during infection of SCID mice. Infect. Immun. 713419-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cserzo, M., E. Wallin, I. Simon, G. von Heijne, and A. Elofsson. 1997. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 10673-676. [DOI] [PubMed] [Google Scholar]
- 29.Eggers, C. H., M. J. Caimano, and J. D. Radolf. 2004. Analysis of promoter elements involved in the transcriptional initiation of RpoS-dependent Borrelia burgdorferi genes. J. Bacteriol. 1867390-7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Hage, N., K. Babb, J. A. Carroll, N. Lindstrom, E. R. Fischer, J. C. Miller, R. D. Gilmore, Jr., M. L. Mbow, and B. Stevenson. 2001. Surface exposure and protease insensitivity of Borrelia burgdorferi Erp (OspEF-related) lipoproteins. Microbiology 147821-830. [DOI] [PubMed] [Google Scholar]
- 31.Elias, A. F., P. E. Stewart, D. Grimm, M. J. Caimano, C. H. Eggers, K. Tilly, J. L. Bono, D. R. Akins, J. D. Radolf, T. G. Schwan, and P. Rosa. 2002. Clonal polymorphism of Borrelia burgdorferiB31 MI: implications for mutagenesis in an infectious strain background. Infect. Immun. 702139-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang, F. C., S. J. Libby, N. A. Buchmeier, P. C. Loewen, J. Switala, J. Harwood, and D. G. Guiney. 1992. The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc. Natl. Acad. Sci. USA 8911978-11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher, M. A., D. Grimm, A. K. Henion, A. F. Elias, P. E. Stewart, P. A. Rosa, and F. C. Gherardini. 2005. Borrelia burgdorferi σ54 is required for mammalian infection and vector transmission but not for tick colonization. Proc. Natl. Acad. Sci. USA 1025162-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. C. Venter, et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390580-586. [DOI] [PubMed] [Google Scholar]
- 35.Gilmore, R. D., Jr., R. R. Howison, V. L. Schmit, A. J. Nowalk, D. R. Clifton, C. Nolder, J. L. Hughes, and J. A. Carroll. 2007. Temporal expression analysis of the Borrelia burgdorferi paralogous gene family 54 genes BBA64, BBA65, and BBA66 during persistent infection in mice. Infect. Immun. 752753-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilmore, R. D., Jr., K. J. Kappel, and B. J. Johnson. 1997. Molecular characterization of a 35-kilodalton protein of Borrelia burgdorferi, an antigen of diagnostic importance in early Lyme disease. J. Clin. Microbiol. 3586-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glockner, G., R. Lehmann, A. Romualdi, S. Pradella, U. Schulte-Spechtel, M. Schilhabel, B. Wilske, J. Suhnel, and M. Platzer. 2004. Comparative analysis of the Borrelia garinii genome. Nucleic Acids Res. 326038-6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glockner, G., U. Schulte-Spechtel, M. Schilhabel, M. Felder, J. Suhnel, B. Wilske, and M. Platzer. 2006. Comparative genome analysis: selection pressure on the Borrelia vls cassettes is essential for infectivity. BMC Genomics 7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grimm, D., K. Tilly, R. Byram, P. E. Stewart, J. G. Krum, D. M. Bueschel, T. G. Schwan, P. F. Policastro, A. F. Elias, and P. A. Rosa. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. USA 1013142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gygi, S. P., Y. Rochon, B. R. Franza, and R. Aebersold. 1999. Correlation between protein and mRNA abundance in yeast. Mol. Cell. Biol. 191720-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hinnebusch, B. J., A. G. Barbour, B. I. Restrepo, and T. G. Schwan. 1998. Population structure of the relapsing fever spirochete Borrelia hermsii as indicated by polymorphism of two multigene families that encode immunogenic outer surface lipoproteins. Infect. Immun. 66432-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu, L. T., S. D. Pratt, G. Perides, L. Katz, R. A. Rogers, and M. S. Klempner. 1997. Isolation, cloning, and expression of a 70-kilodalton plasminogen binding protein of Borrelia burgdorferi. Infect. Immun. 654989-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hubner, A., X. Yang, D. M. Nolen, T. G. Popova, F. C. Cabello, and M. V. Norgard. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. USA 9812724-12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hughes, C. A., and R. C. Johnson. 1990. Methylated DNA in Borrelia species. J. Bacteriol. 1726602-6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hyde, J. A., J. P. Trzeciakowski, and J. T. Skare. 2007. Borrelia burgdorferi alters its gene expression and antigenic profile in response to CO2 levels. J. Bacteriol. 189437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Indest, K. J., R. Ramamoorthy, M. Sole, R. D. Gilmore, B. J. Johnson, and M. T. Philipp. 1997. Cell-density-dependent expression of Borrelia burgdorferi lipoproteins in vitro. Infect. Immun. 651165-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Juncker, A. S., H. Willenbrock, G. Von Heijne, S. Brunak, H. Nielsen, and A. Krogh. 2003. Prediction of lipoprotein signal peptides in gram-negative bacteria. Protein Sci. 121652-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kowarz, L., C. Coynault, V. Robbe-Saule, and F. Norel. 1994. The Salmonella typhimurium katF (rpoS) gene: cloning, nucleotide sequence, and regulation of spvR and spvABCD virulence plasmid genes. J. Bacteriol. 1766852-6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kraiczy, P., E. Rossmann, V. Brade, M. M. Simon, C. Skerka, P. F. Zipfel, and R. Wallich. 2006. Binding of human complement regulators FHL-1 and factor H to CRASP-1 orthologs of Borrelia burgdorferi. Wien Klin. Wochenschr. 118669-676. [DOI] [PubMed] [Google Scholar]
- 50.Kraiczy, P., C. Skerka, V. Brade, and P. F. Zipfel. 2001. Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect. Immun. 697800-7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lane, R. S., and J. A. Pascocello. 1989. Antigenic characteristics of Borrelia burgdorferi isolates from ixodid ticks in California. J. Clin. Microbiol. 272344-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Le Fleche, A., D. Postic, K. Girardet, O. Peter, and G. Baranton. 1997. Characterization of Borrelia lusitaniae sp. nov. by 16S ribosomal DNA sequence analysis. Int. J. Syst. Bacteriol. 47921-925. [DOI] [PubMed] [Google Scholar]
- 53.Liang, F. T., F. K. Nelson, and E. Fikrig. 2002. DNA microarray assessment of putative Borrelia burgdorferi lipoprotein genes. Infect. Immun. 703300-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang, F. T., F. K. Nelson, and E. Fikrig. 2002. Molecular adaptation of Borrelia burgdorferi in the murine host. J. Exp. Med. 196275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 56.Livengood, J. A., V. L. Schmit, and R. D. Gilmore, Jr. 2008. Global transcriptome analysis of Borrelia burgdorferi during association with human neuroglial cells. Infect. Immun. 76298-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Logigian, E. L., R. F. Kaplan, and A. C. Steere. 1990. Chronic neurologic manifestations of Lyme disease. N. Engl. J. Med. 3231438-1444. [DOI] [PubMed] [Google Scholar]
- 58.Marconi, R. T., D. S. Samuels, and C. F. Garon. 1993. Transcriptional analyses and mapping of the ospC gene in Lyme disease spirochetes. J. Bacteriol. 175926-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marconi, R. T., D. S. Samuels, T. G. Schwan, and C. F. Garon. 1993. Identification of a protein in several Borrelia species which is related to OspC of the Lyme disease spirochetes. J. Clin. Microbiol. 312577-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Markwell, M. A., S. M. Haas, L. L. Bieber, and N. E. Tolbert. 1978. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 87206-210. [DOI] [PubMed] [Google Scholar]
- 61.Maruskova, M., M. D. Esteve-Gassent, V. L. Sexton, and J. Seshu. 2008. Role of the BBA64 locus of Borrelia burgdorferi in early stages of infectivity in a murine model of Lyme disease. Infect. Immun. 76391-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Masuzawa, T., T. Kurita, H. Kawabata, and Y. Yanagihara. 1994. Relationship between infectivity and OspC expression in Lyme disease Borrelia. FEMS Microbiol. Lett. 123319-324. [DOI] [PubMed] [Google Scholar]
- 63.Masuzawa, T., Y. Okada, Y. Yanagihara, and N. Sato. 1991. Antigenic properties of Borrelia burgdorferi isolated from Ixodes ovatus and Ixodes persulcatus in Hokkaido, Japan. J. Clin. Microbiol. 291568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McDowell, J. V., M. E. Harlin, E. A. Rogers, and R. T. Marconi. 2005. Putative coiled-coil structural elements of the BBA68 protein of Lyme disease spirochetes are required for formation of its factor H binding site. J. Bacteriol. 1871317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McDowell, J. V., K. M. Hovis, H. Zhang, E. Tran, J. Lankford, and R. T. Marconi. 2006. Evidence that the BBA68 protein (BbCRASP-1) of the Lyme disease spirochetes does not contribute to factor H-mediated immune evasion in humans and other animals. Infect. Immun. 743030-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meier, J. T., M. I. Simon, and A. G. Barbour. 1985. Antigenic variation is associated with DNA rearrangements in a relapsing fever Borrelia. Cell 41403-409. [DOI] [PubMed] [Google Scholar]
- 67.Motaleb, M. A., L. Corum, J. L. Bono, A. F. Elias, P. Rosa, D. S. Samuels, and N. W. Charon. 2000. Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. Proc. Natl. Acad. Sci. USA 9710899-10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 101-6. [DOI] [PubMed] [Google Scholar]
- 69.Nowalk, A. J., R. D. Gilmore, Jr., and J. A. Carroll. 2006. Serologic proteome analysis of Borrelia burgdorferi membrane-associated proteins. Infect. Immun. 743864-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nowalk, A. J., C. Nolder, D. R. Clifton, and J. A. Carroll. 2006. Comparative proteome analysis of subcellular fractions from Borrelia burgdorferi by NEPHGE and IPG. Proteomics 62121-2134. [DOI] [PubMed] [Google Scholar]
- 71.Ohnishi, J., J. Piesman, and A. M. de Silva. 2001. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. USA 98670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ojaimi, C., C. Brooks, S. Casjens, P. Rosa, A. Elias, A. Barbour, A. Jasinskas, J. Benach, L. Katona, J. Radolf, M. Caimano, J. Skare, K. Swingle, D. Akins, and I. Schwartz. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 711689-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reference deleted.
- 74.Pachner, A. R., P. Duray, and A. C. Steere. 1989. Central nervous system manifestations of Lyme disease. Arch. Neurol. 46790-795. [DOI] [PubMed] [Google Scholar]
- 75.Pal, U., X. Yang, M. Chen, L. K. Bockenstedt, J. F. Anderson, R. A. Flavell, M. V. Norgard, and E. Fikrig. 2004. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Investig. 113220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pinne, M., K. Denker, E. Nilsson, R. Benz, and S. Bergstrom. 2006. The BBA01 protein, a member of paralog family 48 from Borrelia burgdorferi, is potentially interchangeable with the channel-forming protein P13. J. Bacteriol. 1884207-4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Revel, A. T., A. M. Talaat, and M. V. Norgard. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 991562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rossmann, E., V. Kitiratschky, H. Hofmann, P. Kraiczy, M. M. Simon, and R. Wallich. 2006. Borrelia burgdorferi complement regulator-acquiring surface protein 1 of the Lyme disease spirochetes is expressed in humans and induces antibody responses restricted to nondenatured structural determinants. Infect. Immun. 747024-7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schwan, T. G., W. J. Simpson, M. E. Schrumpf, and R. H. Karstens. 1989. Identification of Borrelia burgdorferi and B. hermsii using DNA hybridization probes. J. Clin. Microbiol. 271734-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Setubal, J. C., M. Reis, J. Matsunaga, and D. A. Haake. 2006. Lipoprotein computational prediction in spirochaetal genomes. Microbiology 152113-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shin, J. J., A. V. Bryksin, H. P. Godfrey, and F. C. Cabello. 2004. Localization of BmpA on the exposed outer membrane of Borrelia burgdorferi by monospecific anti-recombinant BmpA rabbit antibodies. Infect. Immun. 722280-2287. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82.Skare, J. T., E. S. Shang, D. M. Foley, D. R. Blanco, C. I. Champion, T. Mirzabekov, Y. Sokolov, B. L. Kagan, J. N. Miller, and M. A. Lovett. 1995. Virulent strain associated outer membrane proteins of Borrelia burgdorferi. J. Clin. Investig. 962380-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Steere, A. C., J. E. Craft, G. J. Hutchinson, J. H. Newman, D. W. Rhan, L. H. Sigal, P. N. Spieler, K. S. Stenn, and S. E. Malawista. 1983. The early clinical manifestations of Lyme disease. Ann. Intern. Med. 9976-82. [DOI] [PubMed] [Google Scholar]
- 84.Steere, A. C., R. L. Grodzicki, A. N. Kornblatt, J. E. Craft, A. G. Barbour, W. Burgdorfer, G. P. Schmid, E. Johnson, and S. E. Malawista. 1983. The spirochetal etiology of Lyme disease. N. Engl. J. Med. 308733-740. [DOI] [PubMed] [Google Scholar]
- 85.Suh, S. J., L. Silo-Suh, D. E. Woods, D. J. Hassett, S. E. West, and D. E. Ohman. 1999. Effect of rpoS mutation on the stress response and expression of virulence factors in Pseudomonas aeruginosa. J. Bacteriol. 1813890-3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tilly, K., A. Bestor, M. W. Jewett, and P. Rosa. 2007. Rapid clearance of Lyme disease spirochetes lacking OspC from skin. Infect. Immun. 751517-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tilly, K., J. G. Krum, A. Bestor, M. W. Jewett, D. Grimm, D. Bueschel, R. Byram, D. Dorward, M. J. Vanraden, P. Stewart, and P. Rosa. 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect. Immun. 743554-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tokarz, R., J. M. Anderton, L. I. Katona, and J. L. Benach. 2004. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect. Immun. 725419-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 764350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.von Lackum, K., J. C. Miller, T. Bykowski, S. P. Riley, M. E. Woodman, V. Brade, P. Kraiczy, B. Stevenson, and R. Wallich. 2005. Borrelia burgdorferi regulates expression of complement regulator-acquiring surface protein 1 during the mammal-tick infection cycle. Infect. Immun. 737398-7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wallich, R., O. Jahraus, T. Stehle, T. T. Tran, C. Brenner, H. Hofmann, L. Gern, and M. M. Simon. 2003. Artificial-infection protocols allow immunodetection of novel Borrelia burgdorferi antigens suitable as vaccine candidates against Lyme disease. Eur. J. Immunol. 33708-719. [DOI] [PubMed] [Google Scholar]
- 92.Wang, G., R. Iyer, S. Bittker, D. Cooper, J. Small, G. P. Wormser, and I. Schwartz. 2004. Variations in Barbour-Stoenner-Kelly culture medium modulate infectivity and pathogenicity of Borrelia burgdorferi clinical isolates. Infect. Immun. 726702-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang, X., M. S. Goldberg, T. G. Popova, G. B. Schoeler, S. K. Wikel, K. E. Hagman, and M. V. Norgard. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol. Microbiol. 371470-1479. [DOI] [PubMed] [Google Scholar]
- 94.Yang, X. F., A. Hubner, T. G. Popova, K. E. Hagman, and M. V. Norgard. 2003. Regulation of expression of the paralogous Mlp family in Borrelia burgdorferi. Infect. Immun. 715012-5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang, X. F., M. C. Lybecker, U. Pal, S. M. Alani, J. Blevins, A. T. Revel, D. S. Samuels, and M. V. Norgard. 2005. Analysis of the ospC regulatory element controlled by the RpoN-RpoS regulatory pathway in Borrelia burgdorferi. J. Bacteriol. 1874822-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.