Abstract
The gram-negative fastidious human oropharyngeal Aggregatibacter actinomycetemcomitans is implicated in the etiology of infective endocarditis. EmaA, an oligomeric coiled-coil adhesin homologous to YadA of Yersinia enterocolitica, was hypothesized to mediate the interaction of A. actinomycetemcomitans with collagen. Collagen, the most abundant protein in human bodies and the main component of extracellular matrix (ECM), predominates in the supporting tissue of cardiac valves. To extend our earlier studies using purified collagen to determine bacterial binding activities, we developed a tissue model using rabbit cardiac valves to investigate the interaction of A. actinomycetemcomitans with native collagen. The resected mitral valves, with or without removal of the endothelium, were incubated with equivalent numbers of the wild type and the isogenic emaA mutant defective in collagen binding. There was no difference in binding between the wild-type and the mutant strains when the endothelium remained intact. However, the emaA mutant was fivefold less effective than the wild-type strain in colonizing the exposed ECM. A 10-fold increase in the binding of the wild-type strain to ECM was observed compared with the intact endothelium. Similar observations were replicated in an in vivo endocarditis rabbit model; the emaA mutant was 10-fold less effective in the initial infection of the traumatized aortic valve. Colocalization studies indicated that A. actinomycetemcomitans bound to type I collagen. A. actinomycetemcomitans preferentially colonized the ECM and, together with the evidence that EmaA interacts with the native collagen, suggested that the adhesin is likely a potential virulence determinant of the bacterium in the initiation of infective endocarditis.
The gram-negative, nonmotile, capnophilic human oral pathogen Aggregatibacter (Actinobacillus) actinomycetemcomitans is strongly associated with localized periodontitis (13). A. actinomycetemcomitans, Haemophilus spp., Cardiobacterium hominis, Eikenella corrodens, and Kingella spp. together are categorized as HACEK organisms. The HACEK organisms represent a group of gram-negative, fastidious, slow-growing oropharyngeal bacilli, which contribute to 3% of infective endocarditis in humans. The infection mainly involves children and young adults, including both native and prosthetic valves, with a preference for the left-side valves (4, 10, 26). A. actinomycetemcomitans is the prevailing agent causing infective endocarditis within the HACEK group (4). Viable A. actinomycetemcomitans has also been isolated from atherosclerotic lesions, suggesting the periodontal pathogen is associated with the pathogenesis of this leading cause of death in the United States (20). Notably, a recent report indicated that A. actinomycetemcomitans was detected in 31% of cardiovascular specimens of endocarditis or aneurysm, using a molecular approach (24). This finding suggests that A. actinomycetemcomitans contributes to cardiovascular diseases more than was previously suggested by the blood culture data (4).
Previous studies indicated that 70% of endocarditis cases caused by A. actinomycetemcomitans have underlying heart diseases, such as valvopathy and prosthetic valves (4). The compromised condition of the host cardiac valves, together with a transient bacteremia induced through daily activities (12, 37), allows this endogenous oral bacterium to colonize, invade, and replicate at the extraoral sites. Adhesins are important in the initial recognition of receptors distributed on the surface of the host tissue. Both fimbrial and nonfimbrial adhesins have been identified on the surface of A. actinomycetemcomitans. The latter include Aae (30), Omp100 (ApiA) (39), and extracellular matrix (ECM) adhesin A (EmaA) (22). Aae and Omp100 mediate the adherence of this organism to buccal epithelial cells. Distinct from either of these two epithelial adhesins, the oligomeric EmaA forms antenna-like surface structures mediating the interaction between A. actinomycetemcomitans and acid-solubilized collagen in vitro (31, 34).
The epithelium (or endothelium), as well as the innate immune response (5), is the first defensive barrier between the environment and the host. Once the barrier is disrupted, the connective tissue becomes the target for microorganism colonization and invasion. Previous studies have shown that gram-negative periodontal pathogens, including A. actinomycetemcomitans, bind to the basement membrane-like matrix in significantly higher numbers than gram-positive oral microorganisms (38). Our earlier work also indicated that A. actinomycetemcomitans binds to acid-solublized type I, II, III, and V collagen (23), the predominant fibril-forming collagen types of the connective tissue. Collagen, the most abundant protein in the human body, forms the only supportive fiber of cardiac valves. Sixty-seven percent of the dry weight of the human mitral valve is collagen, with 74% type I, 24% type III, and 2% type V collagen (6). To date, EmaA is the only reported collagen-binding adhesin in this or any other gram-negative oral microorganism. The conservation of the emaA gene across six serotypes (a to f) of A. actinomycetemcomitans and the presence of EmaA oligomeric structures in the predominant serotypes of most populations, b, c, a, and d (34), suggest this outer membrane protein is important for the in vivo colonization of the bacterium.
Our earlier data associated with collagen-binding activities of A. actinomycetemcomitans were based on acid-solubilized collagen bound either to plastic surfaces (22, 23) or to collagen-incorporated Matrigel matrices (34). Neither of these synthetic substrates can completely imitate native collagen of animal tissue. One approach to determine the binding activities of a bacterium to native collagen is to expose the microorganism to tissue rich in the targeted collagen, such as heart valve tissue. The ECM protein composition and stratification of heart valves are conserved between human and rabbit (15), making the rabbit heart valve an ideal model system to investigate the interaction of A. actinomycetemcomitans with native collagen. In this study, a new in vitro tissue model of rabbit cardiac valves was developed. This model indicated that the wild-type strain preferentially bound to the underlying ECM of the valve compared with the endothelium. Both the wild-type strain and its isogenic emaA mutant had similar affinities for the endothelium. However, the mutant showed fivefold reduction in binding to the exposed ECM compared with the wild-type bacteria. Importantly, a comparable result was obtained in an in vivo rabbit endocarditis model.
MATERIALS AND METHODS
Bacterial strains.
The A. actinomycetemcomitans strains used in this study are listed in Table 1. All A. actinomycetemcomitans strains were stored at −80°C and recovered in 3% trypticase soy broth, 0.6% yeast extract (TSBYE) with or without 1.5% agar (Becton, Dickinson and Company, Franklin Lakes, NJ) in a 37°C aerobic incubator with 10% humidified carbon dioxide or under microaerophilic conditions (6% oxygen, 7% carbon dioxide, 7% hydrogen, and 80% nitrogen). The wild-type strain (VT1169) and the emaA mutant (F47) were described in our previous study (22). The strain VT1729 is the wild-type strain VT1169 transformed with plasmid pNP3 by electroporation. The plasmid pNP3, derived from pMMB67, contains a gene encoding the green fluorescent protein (GFP) of Aequorea victoria (28).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype | Phenotypic descriptiona | Source or reference |
|---|---|---|---|
| A. actinomycetemcomitans strains | |||
| SUNY465 | Clinical strain | 22 | |
| VT1169 | Wild type; a derivative of SUNY465; Rifr Nalr | 22 | |
| F47 | emaA mutant | Without EmaA structures; deficient collagen-binding activity; Rifr Nalr Spr | 22 |
| VT1729 | VT1169 transformed with pNP3 | Wild type expressing GFP | This study |
| Plasmid | |||
| pNP3 | pMMB67 with GFP gene expression controlled by spc and tacb promoters | Apr | 28 |
Ap, ampicillin; Rif, rifampin; Sp, spectinomycin; Nal, nalidixic acid.
tac is a hybrid promoter derived from the trp (tryptophan) and lac (lactose) promoters (7).
Preparation of rabbit mitral valves for the in vitro assay.
New Zealand White rabbits (3.0 to 3.5 kg) were chosen to provide atrioventricular valves for all in vitro assays. The rabbits were euthanized by exsanguination under deep pentobarbital anesthesia by intravenous administration (60 mg per kg of body weight), according to the protocols approved by the Institutional Animal Care and Use Committee of the University of Vermont. Intact mitral valves were dissected immediately after euthanasia of the rabbits and kept in sterile phosphate-buffered saline (PBS) (10 mM NaH2PO4, 150 mM NaCl, pH 7.4) on ice. One leaflet of the valve was treated with 625 U/ml trypsin from bovine pancreas (Sigma, St. Louis, MO) in 0.2 M Tris with 1 mM CaCl2, pH 9.2, for 75 min at 37°C to remove the endothelium, as well as the basement membrane (33), and to expose the ECM from the supportive tissue. The trypsin-treated valve leaflet was washed thoroughly in PBS and incubated with different strains of A. actinomycetemcomitans. The other leaflet, without trypsin treatment, was used as a control valve tissue with intact endothelium. The tissue was kept in PBS on ice before use.
Trichrome and HE stains.
Mid-logarithmic cells of the wild-type strain were collected by centrifugation at 5,000 × g for 5 min at 4°C and resuspended in sterile PBS. One milliliter of bacterial suspension, comparable to 5.0 × 108 CFU, was incubated with the trypsin-treated mitral valve at 37°C with agitation for 1 h. The infected tissue was washed with PBS twice, fixed in 10% formalin overnight at room temperature, and embedded in paraffin. The 8-μm paraffin sections on slides were prepared for trichrome and hematoxylin and eosin (HE) stains. Both HE and trichrome stains were used to show the changes in the tissue before and after trypsin treatment, as well as A. actinomycetemcomitans binding. The stained slides were observed using an Olympus BX50 light microscope, and images were collected under the bright field using an Optronics MagnaFire digital camera at magnifications of ×100, ×200, and ×400.
Colocalization of type I collagen and A. actinomycetemcomitans by immunohistochemistry.
Two leaflets of the same mitral valve, with and without trypsin treatment, were incubated with the strain expressing GFP (VT1729) at 37°C for 1 h. The bacteria, comparable to 5.0 × 108 CFU, were prepared as described above. The infected valve was fixed in 3% paraformaldehyde in PBS overnight at 4°C and embedded in paraffin. Four-micron paraffin sections were used for immunohistochemistry. The paraffin processing quenched the fluorescence of the GFP contained in VT1729. Therefore, a mouse monoclonal antibody {immunoglobulin G1(κ) [IgG1(κ)]} against the GFP (Roche, Indianapolis, IN) was used for labeling the bacterium. The slide was deparaffinized and incubated in Dako Cytomation target retrieval solution (Dako, Denmark) between 95 and 99°C for 40 min for antigen retrieval. The slides were pretreated with 1.0% bovine serum albumin in PBS with 0.1% Triton X-100 for 15 min, washed with PBS, and blocked with 10% normal donkey serum for 30 min. Afterward, the slides were incubated with primary antibody, goat polyclonal affinity-purified antibody against type I collagen (Santa Cruz, Santa Cruz, CA) and mouse monoclonal antibody against GFP, overnight at 4°C. The slides were then washed with PBS and incubated with secondary antibodies, Alexa Fluor 568 (orange-fluorescent) donkey anti-goat IgG (Invitrogen, Carlsbad, CA) and Alexa Fluor 488 (green-fluorescent) donkey anti-mouse IgG, for 1 h. The slides were washed again with PBS and incubated with DAPI (4′,6-diamidine-2-phenylindole dihydrochloride crystallized) for 15 min to label eukaryotic nuclei. The slides were visualized using an Olympus BX50 light microscope at magnifications of ×200, ×400, and ×1,000. The images were captured by using an Optronics MagnaFire digital camera with four different filters: 420 nm (blue DAPI-stained nuclei), 510 nm (green-fluorescent bacteria), 610 nm (orange-fluorescent type I collagen), and a multifluorescent 500- to 530-nm and 580- to 620-nm filter (green and orange fluorescence). Images captured by using single fluorescent filters were merged by using MagnaFire 2.0 software.
Scanning electron microscopy (SEM).
The isolated mitral valves were treated with trypsin and incubated with the wild-type strain as described above. The infected valve was fixed in 2.5% glutaraldehyde with 1.0% paraformaldehyde in 0.1 M Millonig's phosphate buffer (0.18% NaH2PO4·H2O, 2.33% Na2HPO4·7H2O, 0.50% NaCl, pH 7.2), at 4°C for 2 h (27). After fixation, the tissue was rinsed in 0.1 M Millonig's buffer and postfixed in 1% osmium tetroxide (OsO4) in Millonig's buffer at 4°C for 1 h. Afterward, the tissue was rinsed in Millonig's buffer and dehydrated using ethanol with graded concentrations (35%, 50%, 70%, 85%, 95%, and 100%). The dehydrated specimens were dried with liquid carbon dioxide in a Samdri PVT-3B critical-point dryer, mounted on a holder, and coated with gold/palladium. Data were collected at 25 kV, with magnifications of ×500, ×2,500, ×5,000, and ×10,000 using a JEOL 6060 scanning electron microscope (JEOL, Peabody, MA).
Transmission electron microscopy (TEM).
The resected valve was treated with trypsin, minced into 1.0-mm3 pieces, and incubated with the wild-type strain described above. The tissue fixation process was similar to that for the SEM experiments described above. The infected tissue was fixed in 2.5% glutaraldehyde with 1.0% paraformaldehyde in 0.1 M Millonig's buffer at 4°C for 1 h, washed, and postfixed in 1.0% OsO4 at 4°C for 1 h. After graded ethanol dehydration, the specimen was cleared in propylene oxide and embedded in Spurr's epoxy resin. Semithin sections were cut in 1-μm increments using glass knives on a Reichert microtome and stained with methylene blue-azure II to briefly locate the examined area. Ultrathin sections (60 to 80 nm) were cut with a diamond knife, retrieved on 150-mesh copper grids, and stained first with 2% uranyl acetate in 50% ethanol, followed by lead citrate solution (0.13 M lead nitrate in 0.20 M sodium citrate). Data were collected at 60 kV with magnifications of ×25,000 and ×50,000 on negative films by using a JEOL 1210 transmission electron microscope (JEOL, Peabody, MA) at the Microcopy Image Center of the University of Vermont.
In vitro competition assay using a newly developed tissue model.
Mid-logarithmic-phase cells of the wild type and the emaA mutant were collected by centrifugation at 5,000 × g for 5 min at 4°C and resuspended in sterile PBS. An inoculum equivalent to 5.0 × 108 CFU, including 2.5 × 108 (each) of the wild type and the mutant in 1 ml of sterile PBS, was used for each in vitro competition assay. Two leaflets of one mitral valve, with and without trypsin treatment, were incubated with the prepared inoculum at 37°C for 1 h with agitation. The infected valve was washed with sterile PBS, homogenized, and resuspended in PBS. Serial dilutions of the original inoculum and the homogenized infected-valve samples were inoculated on both TSBYE agar (nonselective medium for both the wild type and the emaA mutant) and TSBYE with 50 μg/ml of spectinomycin (selective medium for the emaA mutant) and incubated at 37°C with 10% humidified carbon dioxide for 72 h for bacterial recovery. Plates with spectinomycin were used to determine the concentrations of the emaA mutant in the inoculum and the cardiac samples. The concentration of wild-type cells was obtained by subtracting this value from the value obtained from plates without antibiotics. A pilot study showed no antagonism between the wild type and the emaA mutant when grown in a mixed culture for 180 min (the doubling time of A. actinomycetemcomitans is 150 min). The in vitro competition assay was performed in triplicate, using mitral valves from three rabbits. The competitive index (CI) was calculated as the ratio of mutant to wild-type CFU in the cardiac-valve samples divided by the ratio of mutant to wild-type CFU in the inoculum. The CI value from each valve leaflet was compared to a value of 1, using a paired t test, through the GraphPad InStat software (version 3.0). A P value of <0.05 was set as statistically significant.
In vivo competition study using a rabbit endocarditis model.
The rabbit endocarditis model was based on a previously described protocol (25). The protocol received Institutional Animal Care and Use Committee approval and complied with all applicable federal guidelines and institutional policies. New Zealand White rabbits (3.0 to 3.5 kg) were chosen for the study. Briefly, a catheter was inserted through the internal carotid artery past the aortic valve to traumatize the valve. The catheter was sutured in place and remained throughout the period of the experiment. Two days after catheterization, equal volumes of the wild-type and the emaA mutant cultures in the stationary phase (optical density at 495 nm, 0.91 to 0.94) were combined, diluted 20-fold with fresh TSBYE medium, and incubated for 3 h at 37°C under microaerophilic conditions. The mid-logarithmic-phase bacteria, comparable to 6.4 × 107 CFU, were collected by centrifugation, resuspended in fresh medium, and inoculated into the bloodstream through the auricle vein. Serial dilutions of the inoculum were cultured on TSBYE plates with and without 50 μg/ml of spectinomycin, and the plates were incubated for 48 h for enumeration. The rabbits were euthanized approximately 3.5 h after inoculation. The heart was dissected, and the catheter was localized. The aortic-valve leaflets and any visible vegetations were removed, homogenized in PBS, and plated for enumeration as described above. The plates were then incubated for an additional 72 h to confirm that increased colony numbers were not observed. The CI value was calculated as described above and analyzed using GraphPad InStat software (version 3.0). A P value of <0.05 was set as statistically significant.
RESULTS
To extend our earlier studies using purified collagen to determine the binding activities of A. actinomycetemcomitans, a tissue model was developed in which rabbit mitral valves were resected and incubated with bacteria in vitro. By using this model, a condition of traumatized cardiac valves was imitated by removal of the endothelium using trypsin and exposure of the underlying ECM.
Colonization of A. actinomycetemcomitans on trypsin-treated cardiac valve: visualization using bright-field light microscopy.
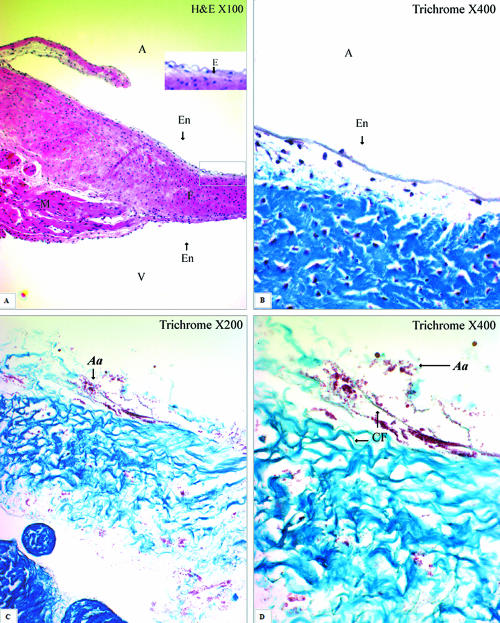
HE and trichrome stains are basic histological stains that were used in this study to visualize the valvular-structure changes before and after trypsin treatment under light microscopy. A slide with HE stain showed the cross section of the cardiac valve arising from the ventricular myocardium (Fig. 1A). The acidophilic collagen was stained pale pink, while the myocardium was stained bright pink (Fig. 1A). The rabbit mitral valve is a sheet of collagenous tissue, covered on both sides by the endocardium, without muscles and vessels. Collagen forms the dense lamina fibrosa, occupying the whole supportive tissue of the valve. The fibroblasts distributed within the collagenous tissue are recognized by the blue-stained nuclei in HE stain (Fig. 1A). Trichrome stain differentiates collagen from other tissues: collagen stains blue, nuclei stain black, and muscle and cytoplasm stain red. The collagen and fibroblasts are covered by a layer of endothelium (Fig. 1B). After 75-min trypsin treatment, the endothelium was removed and the collagen was completely exposed (Fig. 1C and D). The wild-type strain of A. actinomycetemcomitans stained red, bound to the collagen fibers. The attached bacteria were commonly found in aggregates (Fig. 1C and D).
FIG. 1.
Cross-sectioned rabbit mitral valves in HE and trichrome stains. (A) The mitral valve arises from the ventricular myocardium (M), which stained bright pink, and mainly consists of lamina fibrosa (F), or collagen, which stained pale pink in the HE stain. The fibrosa is covered by a layer of endothelium at both atrial (A) and ventricular (V) surfaces. Elastin (E) is present mainly on the atrial side beneath the endothelium (En) (HE; magnification, ×100). (B) The fibrosa (F), dense collagen, stained blue in trichrome stain (magnification, ×400). (C) A. actinomycetemcomitans (Aa), stained red, bound to the collagen fibers of the valve, which was fully exposed after trypsin treatment (trichrome stain; magnification, ×200). (D) The same image at a higher magnification shows aggregated bacteria binding to the collagen fibers (CF) (trichrome stain; magnification, ×400).
Colocalization of A. actinomycetemcomitans and type I collagen in the infected cardiac valve by immunohistochemistry.
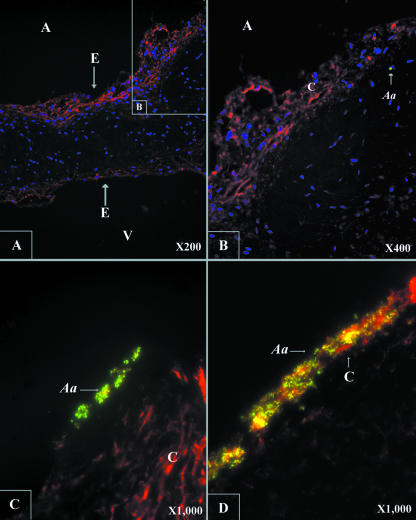
Type I collagen predominates in the supportive tissue of human cardiac valves. Type I collagen and the bacteria were immunolabeled in order to examine whether A. actinomycetemcomitans binds to native type I collagen. The GFP-labeled strain VT1729 expressed GFP in the cytoplasm and emitted green fluorescence. However, the paraffin-embedding process quenched the fluorescence of the GFP. Therefore, a monoclonal antibody with high affinity for GFP was used as the primary antibody to detect the bacterium in infected valve tissue labeled with the Alexa Fluor 488-labeled green-fluorescent secondary antibody.
The slides showed cross sections of the infected valve with and without trypsin treatment. Fifteen slides of each specimen were examined by immunolabeling the bacterium and type I collagen. Type I collagen was distributed across the connective tissue of the rabbit mitral valve but had a more dense distribution close to the endothelial surface than in the center of the valve (Fig. 2A and B). The available substrate for A. actinomycetemcomitans binding was the endothelium without trypsin treatment. In the presence of intact endothelium, only limited numbers of bacteria were seen bound to the valve (Fig. 2B). The number of attached wild-type bacteria shown on the slide was ∼200 times more than those bound to the endothelium when the underlying collagen was exposed (Fig. 2C and D). Unlike the other three colocalized images, which were merged images of three different channels (420 nm, 510 nm, and 610 nm) (Fig. 2A to C), the image in Fig. 2D was captured by using a multifluorescent filter, which showed a large number of bacteria bound to the fully exposed type I collagen. These data suggest that A. actinomycetemcomitans has higher affinity for collagen than the endothelial cells.
FIG. 2.
Colocalization of type I collagen and A. actinomycetemcomitans by immunohistochemistry. (A) Type I collagen (orange), the major collagen type of the rabbit mitral valve, was fully exposed after trypsin decellularization. Type I collagen is distributed extensively across the valve, with a denser distribution in the area close to the endothelium (E) of both the atrial (A) and ventricular (V) surfaces, especially the former. (B) Few A. actinomycetemcomitans (Aa) cells (green) bound to the intact valve covered by endothelium. (C and D) Increased numbers of bacteria attached to the exposed type I collagen fiber after removal of the endothelium. Panels A, B, and C are colocalized images based on the data collected at three different channels (blue, 420 nm; green, 510 nm; orange, 610 nm). Panel D represents a single image captured by using a multifluorescent filter.
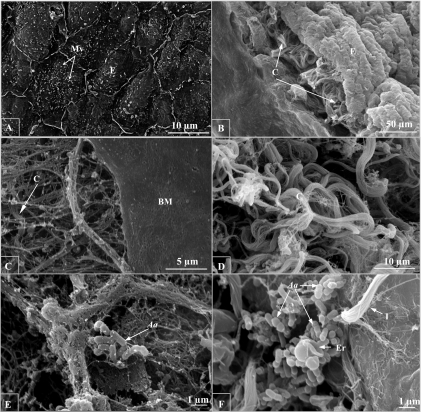
Electron microscopy observation of A. actinomycetemcomitans binding to cardiac valve tissue.
Both the trichrome stain and immunohistochemistry showed cross sections of the valve. In order to have an overview of the effects of trypsin on heart valves, the surfaces of the valve leaflets before and after treatment were examined under SEM. Before treatment, the valve was covered on the surface by a layer of endocardium, and endothelial cells were clearly differentiated from each other (Fig. 3A). From the cross section angle, dense collagen fibers were located beneath the ruffle-like endothelial layer (Fig. 3B), which was consistent with the distribution of type I collagen shown by immunohistochemistry (Fig. 2A and B). After 75 min of decellularization, more than 95% of the endothelium, as well as 50% of the basement membrane, was removed (Fig. 3C).
FIG. 3.
SEM examination of trypsin decellularization of the rabbit mitral valve. (A) Without trypsin treatment, the endothelial cells (E) remain intact, with microvilli (Mv) on the surface (magnification, ×2,500). (B) In the cross section, dense collagen fibers (C) are found underneath the endothelium (magnification, ×500). (C) After trypsin treatment, more than 95% of the endothelium was removed, with less than 50% of the area covered with basement membrane (BM) (magnification, ×5,000). (D) Bundles of collagen fibers were completely exposed (magnification, ×2,500). (E) Occasionally, a few A. actinomycetemcomitans (Aa) cells were trapped in the mesh-like, fine collagen fibers (magnification, ×10,000). Large numbers of bacteria bound to the fiber bundles, which appeared to be type I collagen. (F) Erythrocytes (Er) were also found in the aggregated bacterial cluster (magnification, ×10,000).
Unlike other fibril-forming collagen proteins (II, III, V, and XI), parallel type I collagen fibrils combine to form bundles up to 2 μm in diameter (Fig. 3D). Occasionally, a few A. actinomycetemcomitans cells were trapped by mesh-like, fine fibers (Fig. 3E); however, large numbers of bacteria were bound to type I collagen fibers (Fig. 3F). Bacterial aggregation, which was observed in the trichrome stain (Fig. 1C and D), was also found under SEM. Erythrocytes were found to be present in aggregated bacterial clusters (Fig. 3F).
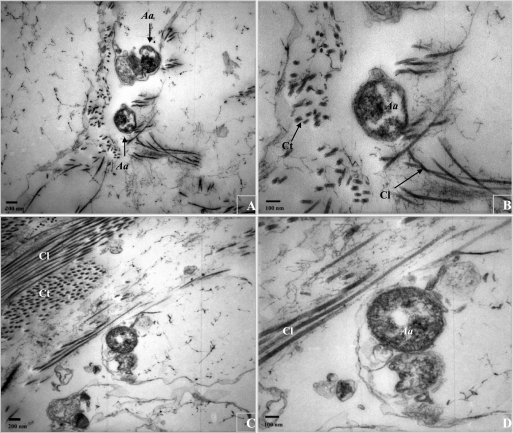
Previously, we succeeded in visualizing the EmaA structures by applying only bacteria on the grids, followed by negative staining and whole-cell mount TEM preparations (31). However, in order to preserve the tissue, as well as the bacteria, and to visualize both of them under TEM, a different technique was applied in this study: tissue fixation (2.5% glutaraldehyde with 1.0% paraformaldehyde, followed by 1.0% OsO4) and thin-section (60- to 80-nm) TEM preparation. The EmaA structures, which protrude with a minimum of ∼150 nm in length and ∼4 nm in diameter from the cell surface (31), were not visualized using this thin-sectioning technique. However, the bacteria were found in close proximity to the collagen fibrils, at a distance corresponding to ∼150 nm or less (Fig. 4).
FIG. 4.
Ultrathin-section TEM examination of A. actinomycetemcomitans (Aa) interaction with type I collagen (panels A and C, magnification, ×25,000; panels B and D, magnification, ×50,000). Type I collagen fibrils ∼17 nm in diameter (transactional [Ct] or longitudinal [Cl]) predominate in the mitral valve. A. actinomycetemcomitans was found in close proximity to the collagen fibrils, with a constant distance of ∼150 nm or less.
The emaA mutant showed defective adherence to the trypsin-treated heart valve in vitro.
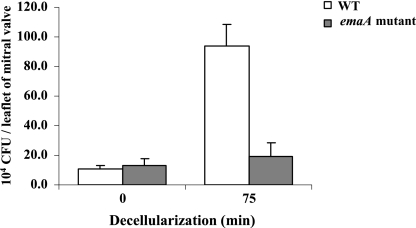
An in vitro competition assay using the resected cardiac valve was developed to determine the ability of A. actinomycetemcomitans to colonize the valves. Trypsin removal of the endothelium and the underlying basement membrane was optimized by varying the incubation time of the tissue with a constant concentration of trypsin (625 U/ml): 0, 30, 60, 75, 90, and 120 min. The binding to the endocardium of the emaA mutant was comparable to that of the wild-type strain in the absence of trypsin treatment. In contrast, the number of attached wild-type bacteria increased with the trypsin incubation time (data not shown). Maximum binding of the wild-type bacteria was reached when the valve was pretreated with trypsin for 75 min. The maximal number of the wild type binding to the valve traumatized by trypsin was 10-fold higher than the number binding to the valve with intact endothelium (Fig. 5). However, further extension of the trypsin incubation to 90 min or longer impaired bacterial binding (data not shown).
FIG. 5.
In vitro competitive binding of the wild type (WT) and the isogenic emaA mutant to rabbit mitral valves. Mid-logarithmic-phase bacteria, comparable to 5.0 × 108 CFU, with equal numbers of the wild type and the mutant were incubated with the valves, with or without trypsin treatment. The concentrations of the mutant in the inoculum or the final homogenized cardiac sample were determined by using a spectinomycin-selective medium. The wild type and the mutant showed equivalent binding to the intact valve without removal of the endocardium (0-min decellularization; P = 0.09). Removal of the endothelium only increased the wild-type bacterial binding to the valve, which was fivefold higher than that of the mutant (75-min decellularization; P = 0.02). The mutant, however, showed similar binding to the valve, with or without removal of the endothelium. The error bars indicate standard deviations.
In this in vitro tissue model, the wild type and the emaA mutant had equivalent binding to the endothelium: (10.48 ± 2.75) × 104 CFU/leaflet versus (13.33 ± 4.03) × 104 CFU/leaflet (P = 0.09), respectively. Removal of the endothelium and basement membrane increased the binding of the wild type, which was fivefold higher than that of the mutant: (93.56 ± 14.76) × 104 CFU per leaflet versus (19.37 ± 9.39) × 104 CFU per leaflet, respectively (P = 0.02) (Fig. 5). The CI values obtained from three independent experiments were 0.093, 0.207, and 0.346, with a geometric mean of 0.188 (P = 0.009). The binding efficiency of the wild type increased from 0.04% to 0.37% of the inoculated cells when the collagen of the connective tissue was exposed. The mutant, however, remained comparable with or without collagen exposure: 0.05% to 0.08% of the inoculated cells.
EmaA and initiation of endocarditis by A. actinomycetemcomitans in vivo.
The rabbit model of endocarditis is well established for the study of endocarditis-associated virulence factors of streptococci (8, 14, 17, 25) and staphylococci (2, 35). In that model, a catheter is introduced from the carotid artery past the aortic valve to induce minor damage to the valve tissue (8). This results in the formation of a sterile vegetation composed mostly of platelets and fibrin, to which the majority of these bacteria attach (9). Based on previous work, we attempted to apply a similar model to study collagen adhesin (EmaA) and its contribution to A. actinomycetemcomitans initiation of endocarditis in our pilot studies. Three rabbits were inoculated with 1.5 × 107 or 15 × 107 CFU of the bacterium 48 h after catheterization. Large, visible vegetations formed in all three rabbits after 72 h. However, only ∼20 CFU were recovered from the vegetation of one rabbit and no bacteria were recovered from the other two vegetations, which was in sharp contrast to the high recovery rate typically obtained for streptococci (17) and staphylococci (2, 35). Together with the observation based on the in vitro tissue model, we hypothesized that A. actinomycetemcomitans might attach directly to the damaged valve tissue rather than the vegetations.
In the next set of experiments, the rabbits were either singly or repeatedly inoculated with the bacterium at different time points, immediately or 48 h after catheterization or at both times. The animals were euthanized ∼3.5 h after the second inoculation. Entire aortic valves, as well as any visible vegetation, were isolated for bacterial recovery. The vegetations appeared smaller than in the initial experiment. A. actinomycetemcomitans was recovered from all three rabbits, with yields ranging from 2.2 × 104 to 4.3 × 104 CFU per rabbit (data not shown). The valve tissue and vegetations from a fourth rabbit with repeated inoculations were examined by SEM, and A. actinomycetemcomitans was not observed in association with the vegetations (data not shown).
Based on these preliminary observations, a final study was performed in which three rabbits were inoculated 48 h after catheterization and euthanized ∼3.5 h after the inoculation. Complete aortic valves were collected for bacterial recovery. The recovered CFU ranged from 1.3 × 104 to 3.1 × 105 per rabbit. The CI values for these three rabbits ranged from 0.044 to 0.155, with a geometric mean value of 0.089 (P = 0.001). A CI value of 1 indicates no difference in competitiveness between the mutant and wild-type strains. The data suggest that the emaA mutant colonized the traumatized heart valve approximately 10-fold less effectively than the wild-type strain.
DISCUSSION
More than 500 species of microorganisms are found in the human oral cavity (19), which makes it a potential reservoir for endogenous infection of neighboring tissues, as well as distal essential organs. Recent studies have linked the gram-negative periodontal pathogen A. actinomycetemcomitans with cardiovascular diseases, including infective endocarditis (4, 10, 26), atherosclerosis (20), and aneurysm (24). These findings are not surprising, since periodontal pathogens can enter the bloodstream through daily activities, including tooth brushing and chewing food, especially in individuals with poor oral hygiene (12, 37).
A. actinomycetemcomitans binds to acid-solubilized type I, III, and V collagen in vitro (23), the most important collagen types present in the periodontium (3), as well as arteries (36) and cardiac valves (6). Additionally, increasing evidence shows abnormal accumulation of ECM, including type I and III collagen, in the adventitia of patients with arteriosclerosis and hypertension (18, 36). Further, transient bacteremia is easily induced by periodontal pathogens through daily activities. Therefore, understanding the mechanism by which this endogenous oral microorganism colonizes extraoral organs is an important approach to prevent the progression of the extraoral diseases induced by A. actinomycetemcomitans.
EmaA, an oligomeric coiled-coil adhesin (22, 31, 34), is an ortholog of the well-studied, multifunctional collagen adhesin YadA, found in Yersinia enterocolitica (29). EmaA is one of two identified ECM adhesins found in gram-negative oropharyngeal bacteria. The other adhesin, BspA, is found on the cell surface of the periodontal pathogen Tannerella forsythus (formerly Bacteroides forsythus) and interacts with fibronectin and fibrinogen (16, 32). Unlike BspA, EmaA has higher affinity for collagen than for fibronectin (22). EmaA forms antenna-like protrusions on the cell surface (31, 34). These protrusions make EmaA different from either the long fimbriae of the organism or other nonfimbrial adhesins that are part of the membrane, such as Omp100 (1) and Aae (30). The antenna-like protrusions have the advantage of reaching the collagenous tissue through a discontinuous epithelium.
Our previous studies employed two different techniques to compare the collagen-binding activities of A. actinomycetemcomitans strains: enzyme-linked immunosorbent assay and collagen-incorporated Matrigel assay (22, 34). In the enzyme-linked immunosorbent assay, the emaA mutant showed a 50% reduction in binding to collagen compared with the parent strain (22). In a subsequent study using the collagen-incorporated Matrigel matrix as a substrate and enumeration of colonized bacteria, strains displaying EmaA structures showed 10- to 60-fold higher binding to the collagen than the EmaA-null alleles (34). In this study, the native collagen from animal heart valves replaced the previous simulated collagen substrates. Consistent with the previous results, the competition assays showed the emaA mutant was 5- to 10-fold less effective in colonizing the traumatized heart valve than the parent strain. This held true whether the trauma was induced by trypsin treatment of valve tissue in vitro or catheterization in vivo. More importantly, we have demonstrated that EmaA mediates A. actinomycetemcomitans interaction with native collagen. With the same mechanism, EmaA may directly contribute to the microorganism binding to human cardiac valves in vivo.
The large number of bacteria bound to the exposed collagen fibers, which were visualized either by trichrome stain (Fig. 1C and D) or by colocalization in immunohistochemistry experiments (Fig. 2C and D), sharply contrasts with the few bacteria bound to the endothelium (Fig. 2A and B). The ratio of wild-type bacteria bound to the exposed collagen versus those bound to the endothelium, based on these images, was ∼200. This number is much higher than the number obtained based on CFUs, which was only a 10-fold difference (Fig. 5). The variation may be associated with different abilities of the bacterium to be disassociated from the endothelium or the collagenous tissue. Secondly, the extensive distribution of type I collagen increases the difficulty of releasing all attached bacteria during homogenization of the heart valves. Furthermore, aggregation was commonly found in bacteria attached to the collagenous tissue (Fig. 1 to 3), and bacterial aggregation remained even after bacteria were released from the valve tissue (data not shown). All of these factors may have resulted in an underestimation of the number of bound bacteria determined by plating compared with microscopy examination for bacterial binding. Regardless, the data suggest A. actinomycetemcomitans has higher affinity for collagen than the endothelial cells.
Trypsin is commonly used for valvular decellularization in tissue engineering for prosthetic heart valves (11). In this study, trypsin was successfully applied for the removal of the endothelium and the basement membrane. Maximum binding of the wild-type bacteria was reached when the valve was pretreated with trypsin for 75 min. The 75-min incubation removed more than 95% of the endothelium and 50% of the basement membrane, as shown by SEM. Further extension of the incubation time may remove all of the endocardium but at the same time may also hydrolyze the exposed collagen protein, consequently affecting interaction with the adhesin (data not shown). Trypsin is a serine protease that displays collagenolytic activity (21). The wild-type strain showed 10-fold-greater binding after the removal of the endothelium than with no treatment. However, removal of the endothelium did not affect the binding of the isogenic emaA mutant (Fig. 5). The data lend strong support to our hypothesis that EmaA is specific for mediating the interaction of A. actinomycetemcomitans with collagen, but not the endothelium.
To the best of our knowledge, this is the first study in which a nonfimbrial adhesin (EmaA) contributed to the colonization of an endocarditis-implicated, gram-negative oral pathogen on heart valves in vitro, as well as in vivo. The present data suggest that EmaA is an important virulence determinant for inducing infectious endocarditis and contributes to A. actinomycetemcomitans colonization in vivo. The adhesin may be a tropism mechanism for the infections of A. actinomycetemcomitans in the oral cavity, as well as the extraoral tissue.
Acknowledgments
We are grateful to Matthew Nystoriak, Micah Beem-Miller, and Kevin P. O'Connor for their collaboration for rabbit surgery and Michele Von Turkovich, Nicole M. Bishop, and Marilyn Wadsworth for their technical support in the microscopy studies. We also thank Teresa Ruiz for her helpful discussion and Paula Fives-Taylor for her encouragement and critical review of the manuscript.
This research was supported by National Institutes of Health-National Institute of Dental and Craniofacial Research (NIH-NIDCR) grants RO1-DE13824 and RO1-DE09760 (K.P.M.) and National Institute of Allergy and Infectious Diseases grants K02AI054908 and R01AI47841 (T.K.).
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 17 March 2008.
REFERENCES
- 1.Asakawa, R., H. Komatsuzawa, T. Kawai, S. Yamada, R. Goncalves, S. Izumi, T. Fujiwara, Y. Nakano, N. Suzuki, Y. Uchida, K. Ouhara, H. Shiba, M. Taubman, H. Kurihara, and M. Sugai. 2003. Outer membrane protein 100, a versatile virulence factor of Actinobacillus actinomycetemcomitans. Mol. Microbiol. 501125-1139. [DOI] [PubMed] [Google Scholar]
- 2.Bayer, A. S., S. N. Coulter, C. K. Stover, and W. R. Schwan. 1999. Impact of the high-affinity proline permease gene (putP) on the virulence of Staphylococcus aureus in experimental endocarditis. Infect. Immun. 67740-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker, J., D. Schuppan, J. Rabanus, R. Rauch, U. Niechoy, and H. Gelderblom. 1991. Immunoelectron microscopic localization of collagens type I, V, VI and of procollagen type III in human periodontal ligament and cementum. J. Histochem. Cytochem. 39103-110. [DOI] [PubMed] [Google Scholar]
- 4.Brouqui, P., and D. Raoult. 2001. Endocarditis due to rare and fastidious bacteria. Clin. Microbiol. Rev. 14177-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung, W., and B. Dale. 2004. Innate immune response of oral and foreskin keratinocytes: utilization of different signaling pathways by various bacterial species. Infect. Immun. 72352-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole, W., D. Chan, A. Hickey, and D. Wilcken. 1984. Collagen composition of normal and myxomatous human mitral heart valves. Biochem. J. 219451-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Boer, H., L. Comstock, and M. Vasser. 1983. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc. Natl. Acad. Sci. USA 8021-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durack, D. T., P. B. Beeson, and R. G. Petersdorf. 1973. Experimental bacterial endocarditis. 3. Production and progress of the disease in rabbits. Br. J. Exp. Pathol. 54142-151. [PMC free article] [PubMed] [Google Scholar]
- 9.Durack, D. T. 1975. Experimental bacterial endocarditis. IV. Structure and evolution of very early lesions. J. Pathol. 11581-89. [DOI] [PubMed] [Google Scholar]
- 10.Grace, C., R. Levitz, H. Katz-Pollak, and L. Brettman. 1988. Actinobacillus actinomycetemcomitans prosthetic valve endocarditis. Rev. Infect. Dis. 10922-929. [DOI] [PubMed] [Google Scholar]
- 11.Grauss, R., M. Hazekamp, F. Oppenhuizen, C. van Munsteren, A. Gittenberger-de Groot, and M. DeRuiter. 2005. Histological evaluation of decellularised porcine aortic valves: matrix changes due to different decellularisation methods. Eur. J. Cardiothorac. Surg. 27566-571. [DOI] [PubMed] [Google Scholar]
- 12.Guntheroth, W. G. 1984. How important are dental procedures as a cause of infective endocarditis? Am. J. Cardiol. 54797-801. [DOI] [PubMed] [Google Scholar]
- 13.Haubek, D., O. Ennibi, K. Poulsen, N. Benzarti, and V. Baelum. 2004. The highly leukotoxic JP2 clone of Actinobacillus actinomycetemcomitans and progression of periodontal attachment loss. J. Dent. Res. 83767-770. [DOI] [PubMed] [Google Scholar]
- 14.Herzberg, M. C., G. D. MacFarlane, K. Gong, N. N. Armstrong, A. R. Witt, P. R. Erickson, and M. W. Meyer. 1992. The platelet interactivity phenotype of Streptococcus sanguis influences the course of experimental endocarditis. Infect. Immun. 604809-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinton, R. J., J. Lincoln, G. Deutsch, H. Osinska, P. Manning, D. Benson, and K. Yutzey. 2006. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ. Res. 981431-1438. [DOI] [PubMed] [Google Scholar]
- 16.Honma, K., H. Kuramitsu, R. Genco, and A. Sharma. 2001. Development of a gene inactivation system for Bacteroides forsythus: construction and characterization of a BspA mutant. Infect. Immun. 694686-4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hook, E. W., III, and M. A. Sande. 1974. Role of the vegetation in experimental Streptococcus viridans endocarditis. Infect. Immun. 101433-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishikawa, J., K. Kario, Y. Matsui, S. Shibasaki, M. Morinari, R. Kaneda, S. Hoshide, K. Eguchi, Y. Hojo, and K. Shimada. 2005. Collagen metabolism in extracellular matrix may be involved in arterial stiffness in older hypertensive patients with left ventricular hypertrophy. Hypertens Res. 28995-1001. [DOI] [PubMed] [Google Scholar]
- 19.Kolenbrander, P. 2000. Oral microbial communities: biofilms, interactions, and genetic systems. Annu. Rev. Microbiol. 54413-437. [DOI] [PubMed] [Google Scholar]
- 20.Kozarov, E., B. Dorn, C. Shelburne, W. J. Dunn, and A. Progulske-Fox. 2005. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler. Thromb. Vasc. Biol. 25e17-18. [DOI] [PubMed] [Google Scholar]
- 21.Kusano, A., Y. Seyama, M. Nagai, M. Shibano, and G. Kusano. 2001. Effects of fukinolic acid and cimicifugic acids from Cimicifuga species on collagenolytic activity. Biol. Pham. Bull. 241198-1201. [DOI] [PubMed] [Google Scholar]
- 22.Mintz, K. 2004. Identification of an extracellular matrix protein adhesin, EmaA, which mediates the adhesion of Actinobacillus actinomycetemcomitans to collagen. Microbiology 1502677-2688. [DOI] [PubMed] [Google Scholar]
- 23.Mintz, K., and P. Fives-Taylor. 1999. Binding of the periodontal pathogen Actinobacillus actinomycetemcomitans to extracellular matrix proteins. Oral Microbiol. Immunol. 14109-116. [DOI] [PubMed] [Google Scholar]
- 24.Nakano, K., H. Inaba, R. Nomura, H. Nemoto, K. Tamura, E. Miyamoto, H. Yoshioka, K. Taniguchi, A. Amano, and T. Ooshima. 2007. Detection and serotype distribution of Actinobacillus actinomycetemcomitans in cardiovascular specimens from Japanese patients. Oral Microbiol. Immunol. 22136-139. [DOI] [PubMed] [Google Scholar]
- 25.Paik, S., L. Senty, S. Das, J. Noe, C. Munro, and T. Kitten. 2005. Identification of virulence determinants for endocarditis in Streptococcus sanguinis by signature-tagged mutagenesis. Infect. Immun. 736064-6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paturel, L., J. P. Casalta, G. Habib, M. Nezri, and D. Raoult. 2004. Actinobacillus actinomycetemcomitans endocarditis. Clin. Microbiol. Infect. 1098-118. [DOI] [PubMed] [Google Scholar]
- 27.Pease, D. 1964. Histological technique for electron microscopy, p.381. Academic Press, New York, NY.
- 28.Permpanich, P., M. J. Kowolik, and D. M. Galli. 2006. Resistance of fluorescent-labelled Actinobacillus actinomycetemcomitans strains to phagocytosis and killing by human neutrophils. Cell. Microbiol. 872-84. [DOI] [PubMed] [Google Scholar]
- 29.Roggenkamp, A., N. Ackermann, C. Jacobi, K. Truelzsch, H. Hoffmann, and J. Heesemann. 2003. Molecular analysis of transport and oligomerization of the Yersinia enterocolitica adhesin YadA. J. Bacteriol. 1853735-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose, J., D. Meyer, and P. Fives-Taylor. 2003. Aae, an autotransporter involved in adhesion of Actinobacillus actinomycetemcomitans to epithelial cells. Infect. Immun. 715284-5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruiz, T., C. Lenox, M. Radermacher, and K. Mintz. 2006. Novel surface structures are associated with the adhesion of Actinobacillus actinomycetemcomitans to collagen. Infect. Immun. 746163-6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma, A., H. Sojar, I. Glurich, K. Honma, H. Kuramitsu, and R. Genco. 1998. Cloning, expression, and sequencing of a cell surface antigen containing a leucine-rich repeat motif from Bacteroides forsythus ATCC 43037. Infect. Immun. 665703-5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suresh, A., M. Stemerman, and T. Spaet. 1973. Rabbit heart valve basement membrane: low platelet reactivity. Blood 41359-367. [PubMed] [Google Scholar]
- 34.Tang, G., T. Ruiz, R. Barrantes-Reynolds, and K. Mintz. 2007. Molecular heterogeneity of EmaA, an oligomeric autotransporter adhesin of Aggregatibacter (Actinobacillus) actinomycetemcomitans. Microbiology 1532447-2457. [DOI] [PubMed] [Google Scholar]
- 35.Veltrop, M. H., M. J. Bancsi, R. M. Bertina, and J. Thompson. 2000. Role of monocytes in experimental Staphylococcus aureus endocarditis. Infect. Immun. 684818-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber, K. 1998. Monitoring vascular sclerosis in hypertension: a new window of opportunity. Circulation 98498-500. [DOI] [PubMed] [Google Scholar]
- 37.Wilson, W., K. A. Taubert, M. Gewitz, P. B. Lockhart, L. M. Baddour, M. Levison, A. Bolger, C. H. Cabell, M. Takahashi, R. S. Baltimore, J. W. Newburger, B. L. Strom, L. Y. Tani, M. Gerber, R. O. Bonow, T. Pallasch, S. T. Shulman, A. H. Rowley, J. C. Burns, P. Ferrieri, T. Gardner, D. Goff, D. T. Durack; American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; Council on Cardiovascular Surgery and Anesthesia; Quality of Care and Outcomes Research Interdisciplinary Working Group; and American Dental Association. 2007. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. J. Am. Dent. Assoc. 138739-745, 747-760. [DOI] [PubMed] [Google Scholar]
- 38.Winkler, J., S. John, R. Kramer, C. Hoover, and P. Murray. 1987. Attachment of oral bacteria to a basement-membrane-like matrix and to purified matrix proteins. Infect. Immun. 552721-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yue, G., J. Kaplan, D. Furgang, K. Mansfield, and D. Fine. 2007. A second Aggregatibacter actinomycetemcomitans autotransporter adhesin exhibits specificity for buccal epithelial cells in humans and old world primates. Infect. Immun. 754440-4448. [DOI] [PMC free article] [PubMed] [Google Scholar]