Abstract
Escherichia coli is an important bacterial species isolated from bovine mastitis. The rate of neutrophil recruitment into the mammary gland and their bactericidal activity largely affect the severity and outcome of the disease. Ketosis is a common metabolic disease, and affected dairy cows are known to have increased risk for mastitis and other infectious conditions. The disease is associated with high blood and milk levels of β-hydroxybutyrate (BHBA), previously shown to negatively affect neutrophil function by unknown mechanisms. We show here that the mammary pathogenic E. coli strain P4 activates normal bovine neutrophils to form neutrophil extracellular traps (NETs), which are highly bactericidal against this organism. Preincubation of these neutrophils with increasing concentrations (0.1 to 8 mmol/liter) of BHBA caused a fivefold decrease of E. coli P4 phagocytosis, though intracellular killing was unaffected. Furthermore, BHBA caused a 10-fold decrease in the NETs formed by E. coli P4-activated neutrophils and a similar decrease in NET bactericidal activity against this organism. These negative effects of BHBA on bovine neutrophils might explain the increased susceptibility of ketotic cows to mastitis and other infectious conditions.
Ketosis or hyperketonemia, a condition in which blood levels of β-hydroxybutyrate (BHBA) and its metabolite acetoacetate are elevated, is common in many animal species, resulting from impaired glucose homeostasis. Important examples are diabetes in humans and other animal species, ketosis in dairy cows, pregnancy toxemia in sheep and goats, and decreased energy intake in all animal species. In both humans and farm animals, these conditions are known to be associated with increased risk for infectious diseases (14, 16, 17, 27, 28, 33, 34). Many specific defects in innate and adaptive immune functions mediated by diverse mechanisms were identified under these diverse hyperketonemic conditions (29, 33, 35, 44, 45, 49). Abnormally high levels of BHBA and acetoacetate in blood and other body fluids is one of these deleterious mechanisms affecting immune functions in many animal species. BHBA was reported to affect human, bovine, and ovine neutrophil function and chemotaxis (7, 10, 20, 21, 36-38, 43, 48). Phagocytosis, microbial killing, and various antimicrobial mechanisms of neutrophils, like reactive oxygen species (ROS) production, were impaired by exposure to BHBA levels similar to those measured under hyperketonemic disease conditions.
Mastitis, an inflammatory response of the mammary tissue to invading bacteria, is a worldwide problem leading to multibillion dollar economic losses, and Escherichia coli is a leading cause of acute mastitis in dairy animals. Dairy cows with elevated serum, urine, and milk levels of BHBA or acetoacetate had a significantly higher risk for mastitis (14, 31, 44). Furthermore, hyperketonemia in experimentally induced E. coli bovine mastitis was associated with an increased severity of disease that was attributed to neutrophil dysfunction (22). The efficacy and speed of neutrophil recruitment are the main predictors of the outcome of mammary infection. A swift response results in the rapid clearance of infection and relatively mild clinical signs (25, 32).
An important recent advancement in our understanding of neutrophil function is the discovery of extracellular neutrophil traps (NETs), which provide an additional microbial killing mechanism affecting the pathogenesis of various infectious diseases (5). NETs are extracellular structures composed of granule and nuclear neutrophil constituents that capture and kill bacteria extracellularly. Activated neutrophils release, in a process of nonapoptotic cell death, nuclear materials that mix with granular contents and are released extracellularly to form a net of DNA, nuclear proteins, and granular enzymes.
Here we show for the first time that BHBA, in concentrations corresponding to those of bovine subclinical and clinical ketosis, negatively affect the formation and function of bovine NETs against mammary pathogenic E. coli (MPEC) and possibly other extraintestinal pathogenic E. coli strains.
MATERIALS AND METHODS
Isolation of blood neutrophils.
About 60 ml of heparinized blood was collected from clinically normal dairy cows by jugular venipuncture into sterile syringes and kept on ice until processing. For the isolation of neutrophils, 17.5 ml of whole blood was diluted with 22.5 ml phosphate-buffered saline (PBS) and centrifuged for 5 min at 1,500 × g at 4°C. The serum and upper third of the packed red blood cells were removed, and the remaining red blood cells were lysed for 1 min with double volumes of distilled water, followed by a fast recovery of isotonicity with 10× PBS. Neutrophils were pelleted by centrifugation at 500 × g for 3 min, resuspended in RPMI 1640 medium, and counted for viability (>90%) by trypan blue exclusion. Based on a Diff-Quik-stained cytospin preparation, cell suspensions contained >98% polymorphonuclear leukocytes.
Bacterial strains and culture conditions.
The E. coli strain P4 isolated from a case of acute bovine mastitis (3) was used in this study. The E. coli P4 strain is extensively used in experimental mastitis studies with dairy cows (18, 41) and mice (13, 23). E. coli P4 serotype O32:H37 is noncapsulated, serum resistant, motile, and highly virulent upon intramammary inoculation of dairy cows (1, 19). The plasmid pSA11 carrying lacIq and the green fluorescent protein (GFP) gene (gfp) under the regulation of the tac promoter was introduced into E. coli P4, and gfp expression was achieved by isopropyl-β-d-thiogalactopyranoside or lactose (39). Bacteria were grown to log phase in Luria-Bertani (LB) broth at 37°C. Next, bacterial suspensions were diluted in sterile, nonpyrogenic PBS to the indicated concentrations and plated on LB agar plates to determine the CFU inoculated in every experiment.
Neutrophil phagocytosis and bactericidal activity assays.
A suspension of 106 bovine neutrophils and 107 E. coli P4 CFU were seeded in 24-well plates in RPMI medium. Experimental conditions included medium alone, medium containing 20% heat-inactivated (56°C for 30 min) pooled normal bovine serum with or without cytochalasin D (10 μg/ml), and medium containing 100 U/ml DNase (Sigma). We have found in preliminary experiments that medium containing 20% serum or DNase completely abrogated NET formation by bovine neutrophils (data not shown). Control wells contained bacteria without neutrophils. Experiments were carried out in triplicate and repeated at least twice. Plates were incubated for 5, 10, 20, and 30 min at 37°C in a humidified CO2 incubator and thereafter kept on ice for further processing. Plates were centrifuged (400 × g) for 3 min at 4°C to separate neutrophils from bacteria in the suspension. At each time point, a sample of control wells (CFUcontrol) and extracellular bacteria (CFUextracellular) were separated and placed on ice before culture. Neutrophil pellets were suspended with PBS containing 50 μg/ml gentamicin to kill any adherent extracellular bacteria and washed twice to remove gentamicin. Neutrophils were lysed with 0.1% Triton on ice for 20 min to release intracellular bacteria (CFUintracellular). The lysis of neutrophils by Triton was confirmed by microscopic examination, and no deleterious influence of the detergent on the bacterial viability was observed (data not shown). All bacterial samples were serially diluted and plated onto LB agar to determine the CFU count. The percentage of phagocytosis by neutrophils in wells containing opsonizing serum or DNase to inhibit NET formation was determined by using the equation 1 − (CFUextracellular/CFUcontrol) × 100. The percentage of intracellular killing by neutrophils in replicated wells containing serum or DNase was determined by using the equation 1 − (CFUintracellular/CFUcontrol − CFUextracellular) × 100. The percentage of killing by NETs in replicated wells containing cytochalasin D to inhibit phagocytosis was determined by using the equation 1 − (CFUextracellular/CFUcontrol) × 100. In all assays, Diff-Quik-stained cytospin preparations were made with cell samples taken for direct microscopic examination.
NET formation by stimulation with bacteria.
Neutrophils (106) in RPMI medium were seeded on glass coverslips treated with 0.001% poly-l-lysine (Sigma) and placed in 24-well plates. Plates were centrifuged (400 × g at 37°C), and cells were allowed to settle and adhere by incubation for 1 h at 37°C in a humidified CO2 incubator.
Neutrophils were infected with 107 CFU of E. coli P4 and incubated for 5, 10, 20, and 30 min at 37°C in a humidified CO2 incubator. In replicated control wells, infected neutrophils were treated with 100 U/ml DNase or left uninfected. Cells were washed with sterile PBS and stained with 5 μM Sytox Orange (Invitrogen, Carlsbad, CA) in the dark for 15 min at room temperature. Coverslips were carefully washed with PBS and mounted with Gel Mount (Sigma) and viewed with a Nikon Eclipse E400 epifluorescence microscope. The percentage of NETs formed was calculated by quantifying the number of neutrophils forming NETs out of the total number of neutrophils observed under 10 high-power magnification fields (×100).
Effect of β-hydroxybutyrate on neutrophil phagocytosis and bactericidal activity.
Bovine neutrophils were preincubated for 40 min at 37°C in a humidified CO2 incubator in RPMI medium containing 0, 0.1, 1, 4, or 8 mmol/liter BHBA (Sigma). Neutrophils were tested for phagocytosis and bactericidal activity and NET formation as described above.
BHBA was tested free of DNase activity (by visualization of DNA degradation in agarose gel electrophoresis) and did not affect the pH of the RPMI medium (data not shown).
Statistical analysis.
The percentage of phagocytosis and killing and the percentage of NET formation data are reported as the means ± standard errors of the means (SEM) of values obtained from two different experiments. Comparisons of the means within and between groups were tested with Student's t tests to determine statistical significance. A P value of 0.05 or less was considered significant.
RESULTS
Phagocytosis and killing of E. coli P4 by bovine neutrophils.
The mean (± SEM) phagocytosis activity of E. coli P4 organisms by normal bovine neutrophils was 66% (±3.3%) at 5 min and increased to 88% (±3.4%) after 30 min. Preincubation of neutrophils with 10 μg/ml cytochalasin D completely abrogated phagocytosis (Fig. 1A). Similarly, nonopsonized E. coli P4 organisms were not phagocytosed by normal bovine neutrophils. The mean intracellular killing of phagocytosed E. coli P4 organisms by normal bovine neutrophils was 100% after 5 min (Fig. 1B). The mean extracellular killing of either nonopsonized E. coli P4 organisms or by cytochalasin D-treated neutrophils increased from 37% (±3.4%) at 5 min to 50% (±8.5%) at 10 min and reached a plateau at 80% (±3.4%) killing after 20 and 30 min (Fig. 1C).
FIG. 1.
Phagocytosis (A) and intracellular (B) and extracellular (C) killing of E. coli P4 by normal bovine blood neutrophils. Diff-Quik cytospin preparations of neutrophils at time zero (A, a, c, and e) and at 30 min thereafter (A, b, d, and f) are shown. Neutrophils were either noninfected controls (A, a and b), or infected (A, c to f) with serum-opsonized E. coli P4 organisms with (A, e and f) and without (A, c and d, and B) preincubation with cytochalasin D. Opsonized bacteria were phagocytosed, while cytochalasin D treatment inhibited phagocytosis. Intracellular bacteria (arrows) are killed by neutrophils (B). Mean extracellular killing of E. coli P4 organisms by cytochalasin-treated bovine neutrophils 5 to 30 min after infection is shown (C). Error bars indicate means ± SEM with different superscripts (a and b) differ significantly (P < 0.05 by t test). Scale bars are 20 μm (A) and 10 μm (B).
E. coli P4-induced NET formation by bovine neutrophils.
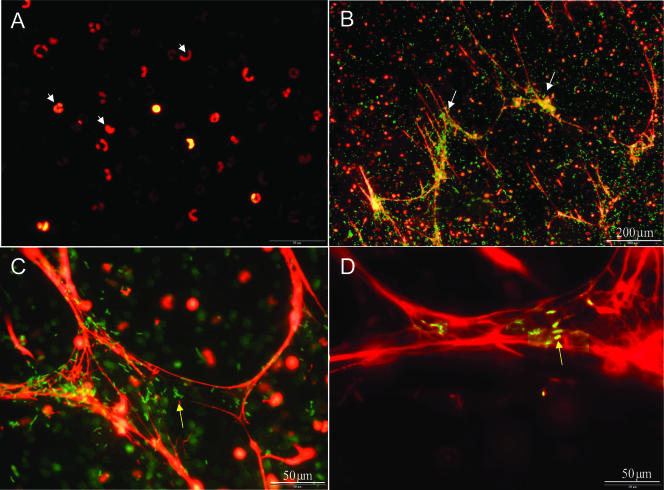
Normal bovine neutrophils adherent to poly-l-lysine-coated glass were infected with E. coli P4. NET formation was demonstrated by Sytox Orange staining of infected neutrophils, while normal, noninfected neutrophils did not form NETs at all (Fig. 2A to D). GFP-expressing E. coli P4 cells could be seen adhering to these structures (Fig. 2C to D). The addition of 10 or 20% heat-inactivated normal bovine serum (data not shown) or of DNase to the culture medium completely eliminated NET formation (Fig. 3A) and extracellular killing of E. coli P4 by bovine neutrophils (Fig. 3B).
FIG. 2.
E. coli P4 induces NET formation by bovine neutrophils. Sytox Orange DNA staining of noninfected (A) or E. coli P4-infected (B to D) neutrophils. NET formation (white arrows) induced by GFP-expressing E. coli P4 cells (yellow arrows), which are seen trapped in NETs. Scale bars are 200 μm (A and B) and 50 μm (C and D).
FIG. 3.
DNase abrogates NET formation and eliminates E. coli P4 extracellular killing by bovine neutrophils. NETs are not observed by using Sytox Orange staining (original magnification, ×400) when E. coli P4 organisms expressing GFP (arrows) are incubated with neutrophils (arrowheads) in the presence of DNase (A). While 70% of the extracellular E. coli P4 organisms are killed by NETs 20 min after infection of bovine neutrophils, killing is completely eliminated by 100 U/ml DNase (B). Error bar indicates SEM.
β-Hydroxybutyrate inhibits E. coli P4 phagocytosis but not intracellular killing by bovine neutrophils.
Preincubation of normal bovine neutrophils with increasing concentrations of BHBA inhibited the phagocytosis of E. coli P4 organisms. While normal bovine neutrophils phagocytosed approximately 90% of the E. coli P4 organisms, a dose-response effect of increasing concentrations of BHBA on phagocytosis was observed, which was decreased to approximately 20% by 4 mmol/liter BHBA (Fig. 4A). Interestingly, increasing concentrations of BHBA had no effect on intracellular killing of the E. coli P4 organisms that were phagocytosed by the neutrophils (Fig. 4A).
FIG. 4.
BHBA inhibits E. coli P4 phagocytosis, NET formation, and extracellular killing by bovine neutrophils. Phagocytosis, but not intracellular killing, of serum-opsonized E. coli P4 is decreased from 90% (30 min after infection) to 20% by preincubation of neutrophils with increasing concentrations (0.1 to 8 mmol/liter) of BHBA (A). Extracellular killing was reduced from 55% (10 min after infection) to 5% by preincubation of neutrophils with increasing concentrations (0.1 to 8 mmol/liter) of BHBA (B). Similarly, NET formation observed by Sytox Orange staining of DNA (C to F) was decreased by preincubation of neutrophils with increasing concentrations (panel D, 1 mmol/liter; panels E and F, 8 mmol/liter) of BHBA. Error bars indicate means ± SEM with different letters (a, b, c, and d) indicating significant differences (P < 0.05 by t test). Original magnification, ×100 (C to E) and ×400 (F).
β-Hydroxybutyrate inhibits NET formation and bactericidal effect.
Preincubation of normal bovine neutrophils with increasing concentrations of BHBA inhibited NET formation and the bactericidal effect against E. coli P4 (Fig. 4). Using direct microscopic analysis, NET formation was associated with 29% of the neutrophils infected with E. coli P4. Preincubation of neutrophils with 1, 4, and 8 mmol/liter BHBA decreased NET formation to 8%, 4%, and 3.5% of neutrophils, respectively (Fig. 4).
Increasing concentrations of BHBA also decreased the NET bactericidal effect from approximately 50% to 5% of extracellular E. coli P4 organisms (Fig. 4).
DISCUSSION
Normal bovine neutrophils activated by various mastitis pathogens, including MPEC, were reported to produce NETs in milk (24). Also, lipopolysaccharide and interleukin 8 (IL-8), which are known neutrophil activators, were reported to induce NET formation (4, 15). Although the mechanism of NET formation by activated neutrophils is not fully elucidated, a recent report by Fuchs et al. (11) provided important insights into this process. These authors show that NET-forming neutrophils are undergoing a novel cell death process (neoapoptosis or netosis) that is distinct from apoptosis and necrosis. It should be noted that neoapoptosis is strongly dependent on ROS generation (11).
NET formation by recruited mammary neutrophils is likely to play an important role in the innate immune response against invading mammary pathogens (6, 24), and impaired NET formation or function is probably associated with increased sensitivity to mastitis pathogens. We investigated the capacity of MPEC to induce NET formation, and as expected, we found that MPEC strain P4 organisms activate bovine neutrophils to produce NETs with highly effective bactericidal activity. Importantly, we demonstrated a strong negative effect of BHBA on NET formation and bactericidal activity. Given that in hyperketonemic animals, the circulating blood neutrophils and recruited milk neutrophils are exposed to BHBA levels similar to those that repressed NET formation in vitro (40), our findings suggested that NET formation is impaired in hyperketonemic animals and that might render them more sensitive to infections.
How BHBA inhibits NET formation is not fully clear yet, and we would like to suggest three possible mechanisms to explain the negative effect of BHBA on NET formation. The first possibility is that the BHBA primary effect is inhibition of ROS formation and that it indirectly inhibits NET formation, which is dependent upon ROS formation. In agreement with this scenario, several in vitro studies reported that exposure of neutrophils to elevated levels of BHBA resulted in decreased production of ROS (20, 26).
A second explanation is that BHBA directly inhibits the process of neoapoptosis by a yet unknown mechanism. This suggestion is somewhat speculative, but it is interesting that BHBA, in concentrations similar to those inhibiting NETs, was reported to have a strong antiapoptotic and antinecrotic effect in many cell types including fibroblasts, neurons, myocytes, and glial and epithelial cells (8, 9, 30, 50). Although this effect has yet to be tested in neutrophils, it might inhibit the neoapoptotic process inducing NET formation by lipopolysaccharide-activated neutrophils.
The third possible mechanism of BHBA-mediated inhibition of NET formation is related to the BHBA tautomerase-inhibitory activity. BHBA, in concentrations similar to those inhibiting NET formation, inhibits the tautomerase enzymatic activity of the macrophage migration inhibitory factor (MIF). MIF is a proinflammatory cytokine produced by neutrophils and other leukocytes (12), which play a critical role in inflammatory diseases, and the chemokine receptors CXCR2 and CXCR4 were identified as functional, noncognate, MIF receptors (2). MIF proinflammatory effects require IL8rb (which encodes CXCR2) and are dependent on its tautomerase enzymatic activity. Given the MIF CXCR2 agonistic activity and the IL-8 stimulatory effect on NET formation, it is likely that MIF also induce NET formation. Thus, we hypothesize that by inhibiting the MIF activity, BHBA represses the capacity of the neutrophils to form NETs.
At this point, the possibility that BHBA inhibits NET formation by blocking several pathways in parallel cannot be excluded. It is possible that BHBA-mediated inhibition of ROS formation, MIF activity, and neoapoptosis all contribute to the reduction in the neutrophils’ NET-forming capacity.
In line with previous studies, we showed here that bovine NETs are extremely sensitive to the destructive effect of DNase. Interestingly, various microbial pathogens adopted this strategy as a virulence mechanism, and they secrete DNase that enables them to evade killing by NETs (47). It is currently unknown if mammary pathogens also adopted this strategy. Nevertheless, given our results, an alternative microbial strategy to combat NET formation would be BHBA production and secretion. Of note, the synthesis of poly(3-hydroxybutyrate) and its degradation to BHBA are very common metabolic pathways in many bacteria including E. coli (42, 46). Therefore, the possible role of BHBA as a microbial virulence factor in mastitis and other extraintestinal infectious conditions warrants further research.
Acknowledgments
This research was supported in part by a grant from the Israel Dairy Board.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 14 April 2008.
REFERENCES
- 1.Anderson, J. C., M. R. Burrows, and A. J. Bramley. 1977. Bacterial adherence in mastitis caused by Escherichia coli. Vet. Pathol. 14618-628. [DOI] [PubMed] [Google Scholar]
- 2.Bernhagen, J., R. Krohn, H. Lue, J. L. Gregory, A. Zernecke, R. R. Koenen, M. Dewor, I. Georgiev, A. Schober, L. Leng, T. Kooistra, G. Fingerle-Rowson, P. Ghezzi, R. Kleemann, S. R. McColl, R. Bucala, M. J. Hickey, and C. Weber. 2007. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat. Med. 13587-596. [DOI] [PubMed] [Google Scholar]
- 3.Bramley, A. J. 1976. Variations in the susceptibility of lactating and non-lactating bovine udders to infection when infused with Escherichia coli. J. Dairy Res. 43205-211. [DOI] [PubMed] [Google Scholar]
- 4.Brinkmann, V., U. Reichard, C. Goosmann, B. Fauler, Y. Uhlemann, D. S. Weiss, Y. Weinrauch, and A. Zychlinsky. 2004. Neutrophil extracellular traps kill bacteria. Science 3031532-1535. [DOI] [PubMed] [Google Scholar]
- 5.Brinkmann, V., and A. Zychlinsky. 2007. Beneficial suicide: why neutrophils die to make NETs. Nat. Rev. Microbiol. 5577-582. [DOI] [PubMed] [Google Scholar]
- 6.Burvenich, C., D. D. Bannerman, J. D. Lippolis, L. Peelman, B. J. Nonnecke, M. E. Kehrli, Jr., and M. J. Paape. 2007. Cumulative physiological events influence the inflammatory response of the bovine udder to Escherichia coli infections during the transition period. J. Dairy Sci. 90(Suppl. 1)E39-E54. [DOI] [PubMed] [Google Scholar]
- 7.Cerone, S. I., A. S. Sansinanea, and M. C. Garcia. 2007. Effects of beta-hydroxybutyric acid on bovine milk leukocytes function in vitro. Gen. Physiol. Biophys. 2614-19. [PubMed] [Google Scholar]
- 8.Cheng, B., X. Yang, Z. Hou, X. Lin, H. Meng, Z. Li, and S. Liu. 2007. d-[beta]-hydroxybutyrate inhibits the apoptosis of PC12 cells induced by 6-OHDA in relation to up-regulating the ratio of Bcl-2/Bax mRNA. Auton. Neurosci. 13438. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, S., G.-Q. Chen, M. Leski, B. Zou, Y. Wang, and Q. Wu. 2006. The effect of d,l-[beta]-hydroxybutyric acid on cell death and proliferation in L929 cells. Biomaterials 273758. [DOI] [PubMed] [Google Scholar]
- 10.da Costa, M., V. F. Ximenes, and L. M. da Fonseca. 2004. Hypochlorous acid inhibition by acetoacetate: implications on neutrophil functions. Biol. Pharm. Bull. 271183-1187. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs, T. A., U. Abed, C. Goosmann, R. Hurwitz, I. Schulze, V. Wahn, Y. Weinrauch, V. Brinkmann, and A. Zychlinsky. 2007. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176231-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garai, J., T. Lorand, and V. Molnar. 2005. Ketone bodies affect the enzymatic activity of macrophage migration inhibitory factor. Life Sci. 771375. [DOI] [PubMed] [Google Scholar]
- 13.Gonen, E., A. Vallon-Eberhard, S. Elazar, A. Harmelin, O. Brenner, I. Rosenshine, S. Jung, and N. Y. Shpigel. 2007. Toll-like receptor 4 is needed to restrict the invasion of Escherichia coli P4 into mammary gland epithelial cells in a murine model of acute mastitis. Cell. Microbiol. 92826-2838. [DOI] [PubMed] [Google Scholar]
- 14.Grohn, Y. T., H. N. Erb, C. E. McCulloch, and H. S. Saloniemi. 1989. Epidemiology of metabolic disorders in dairy cattle: association among host characteristics, disease, and production. J. Dairy Sci. 721876-1885. [DOI] [PubMed] [Google Scholar]
- 15.Gupta, A. K., P. Hasler, W. Holzgreve, S. Gebhardt, and S. Hahn. 2005. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum. Immunol. 661146-1154. [DOI] [PubMed] [Google Scholar]
- 16.Gupta, S., J. Koirala, R. Khardori, and N. Khardori. 2007. Infections in diabetes mellitus and hyperglycemia. Infect. Dis. Clin. N. Am. 21617. [DOI] [PubMed] [Google Scholar]
- 17.Hammon, D. S., I. M. Evjen, T. R. Dhiman, J. P. Goff, and J. L. Walters. 2006. Neutrophil function and energy status in Holstein cows with uterine health disorders. Vet. Immunol. Immunopathol. 11321-29. [DOI] [PubMed] [Google Scholar]
- 18.Hill, A. W. 1981. Factors influencing the outcome of Escherichia coli mastitis in the dairy cow. Res. Vet. Sci. 31107-112. [PubMed] [Google Scholar]
- 19.Hill, A. W. 1991. Vaccination of cows with rough Escherichia coli mutants fails to protect against experimental intramammary bacterial challenge. Vet. Res. Commun. 157-16. [DOI] [PubMed] [Google Scholar]
- 20.Hoeben, D., R. Heyneman, and C. Burvenich. 1997. Elevated levels of beta-hydroxybutyric acid in periparturient cows and in vitro effect on respiratory burst activity of bovine neutrophils. Vet. Immunol. Immunopathol. 58165-170. [DOI] [PubMed] [Google Scholar]
- 21.Klucinski, W., A. Degorski, E. Miernik-Degorska, S. Targowski, and A. Winnicka. 1988. Effect of ketone bodies on the phagocytic activity of bovine milk macrophages and polymorphonuclear leukocytes. Zentralbl. Veterinarmed. A 35632-639. [PubMed] [Google Scholar]
- 22.Kremer, W. D. J., E. N. Noordhuizen-Stassen, F. J. Grommers, Y. H. Schukken, R. Heeringa, A. Brand, and C. Burvenich. 1993. Severity of experimental Escherichia coli mastitis in ketonemic and nonketonemic dairy cows. J. Dairy Sci. 763428-3436. [DOI] [PubMed] [Google Scholar]
- 23.Lee, J. W., M. J. Paape, and X. Zhao. 2003. Recombinant bovine soluble CD14 reduces severity of experimental Escherichia coli mastitis in mice. Vet. Res. 34307-316. [DOI] [PubMed] [Google Scholar]
- 24.Lippolis, J. D., T. A. Reinhardt, J. P. Goff, and R. L. Horst. 2006. Neutrophil extracellular trap formation by bovine neutrophils is not inhibited by milk. Vet. Immunol. Immunopathol. 113248-255. [DOI] [PubMed] [Google Scholar]
- 25.Lohuis, J. A., Y. H. Schukken, J. H. Verheijden, A. Brand, and A. S. Van Miert. 1990. Effect of severity of systemic signs during the acute phase of experimentally induced Escherichia coli mastitis on milk production losses. J. Dairy Sci. 73333-341. [DOI] [PubMed] [Google Scholar]
- 26.Marhoffer, W., M. Stein, L. Schleinkofer, and K. Federlin. 1993. Evidence of ex vivo and in vitro impaired neutrophil oxidative burst and phagocytic capacity in type 1 diabetes mellitus. Diabetes Res. Clin. Pract. 19183. [DOI] [PubMed] [Google Scholar]
- 27.Markusfeld, O. 1984. Factors responsible for post parturient metritis in dairy cattle. Vet. Rec. 114539-542. [DOI] [PubMed] [Google Scholar]
- 28.Markusfeld, O. 1985. Relationship between overfeeding, metritis and ketosis in high yielding dairy cows. Vet. Rec. 116489-491. [DOI] [PubMed] [Google Scholar]
- 29.McMurray, R. W., R. W. Bradsher, R. W. Steele, and N. S. Pilkington. 1990. Effect of prolonged modified fasting in obese persons on in vitro markers of immunity: lymphocyte function and serum effects on normal neutrophils. Am. J. Med. Sci. 299379-385. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura, S., M. Shibuya, Y. Saito, H. Nakashima, F. Saito, A. Higuchi, and K. Tsubota. 2003. Protective effect of d-beta-hydroxybutyrate on corneal epithelia in dry eye conditions through suppression of apoptosis. Investig. Ophthalmol. Vis. Sci. 444682-4688. [DOI] [PubMed] [Google Scholar]
- 31.Oltenacu, P. A., and I. Ekesbo. 1994. Epidemiological study of clinical mastitis in dairy cattle. Vet. Res. 25208-212. [PubMed] [Google Scholar]
- 32.Paape, M. J., D. D. Bannerman, X. Zhao, and J. W. Lee. 2003. The bovine neutrophil: structure and function in blood and milk. Vet. Res. 34597-627. [DOI] [PubMed] [Google Scholar]
- 33.Peleg, A. Y., T. Weerarathna, J. S. McCarthy, and T. M. Davis. 2007. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab. Res. Rev. 233-13. [DOI] [PubMed] [Google Scholar]
- 34.Reist, M., D. K. Erdin, D. von Euw, K. M. Tschumperlin, H. Leuenberger, H. M. Hammon, N. Kunzi, and J. W. Blum. 2003. Use of threshold serum and milk ketone concentrations to identify risk for ketosis and endometritis in high-yielding dairy cows. Am. J. Vet. Res. 64188-194. [DOI] [PubMed] [Google Scholar]
- 35.Saeed, F. A., and G. E. Castle. 1998. Neutrophil chemiluminescence during phagocytosis is inhibited by abnormally elevated levels of acetoacetate: implications for diabetic susceptibility to infections. Clin. Diagn. Lab. Immunol. 5740-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sartorelli, P., S. Paltrinieri, and F. Agnes. 1999. Non-specific immunity and ketone bodies. I. In vitro studies on chemotaxis and phagocytosis in ovine neutrophils. J. Vet. Med. A 46613-619. [DOI] [PubMed] [Google Scholar]
- 37.Sartorelli, P., S. Paltrinieri, and S. Comazzi. 2000. Non-specific immunity and ketone bodies. II: In vitro studies on adherence and superoxide anion production in ovine neutrophils. J. Vet. Med. A 471-8. [DOI] [PubMed] [Google Scholar]
- 38.Sato, N., H. Shimizu, Y. Shimomura, K. Suwa, M. Mori, and I. Kobayashi. 1992. Mechanism of inhibitory action of ketone bodies on the production of reactive oxygen intermediates (ROIS) by polymorphonuclear leukocytes. Life Sci. 51113-118. [DOI] [PubMed] [Google Scholar]
- 39.Schlosser-Silverman, E., M. Elgrably-Weiss, I. Rosenshine, R. Kohen, and S. Altuvia. 2000. Characterization of Escherichia coli DNA lesions generated within J774 macrophages. J. Bacteriol. 1825225-5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shpigel, N. Y., R. Chen, Y. Avidar, and E. Bogin. 1996. Use of corticosteroids alone or combined with glucose to treat ketosis in dairy cows. J. Am. Vet. Med. Assoc. 2081702-1704. [PubMed] [Google Scholar]
- 41.Shpigel, N. Y., D. Levin, M. Winkler, A. Saran, G. Ziv, and A. Bottner. 1997. Efficacy of cefquinome for treatment of cows with mastitis experimentally induced using Escherichia coli. J. Dairy Sci. 80318-323. [DOI] [PubMed] [Google Scholar]
- 42.Spiekermann, P., B. H. Rehm, R. Kalscheuer, D. Baumeister, and A. Steinbuchel. 1999. A sensitive, viable-colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. Arch. Microbiol. 17173-80. [DOI] [PubMed] [Google Scholar]
- 43.Suriyasathaporn, W., A. J. Daemen, E. N. Noordhuizen-Stassen, S. J. Dieleman, M. Nielen, and Y. H. Schukken. 1999. Beta-hydroxybutyrate levels in peripheral blood and ketone bodies supplemented in culture media affect the in vitro chemotaxis of bovine leukocytes. Vet. Immunol. Immunopathol. 68177-186. [DOI] [PubMed] [Google Scholar]
- 44.Suriyasathaporn, W., C. Heuer, E. N. Noordhuizen-Stassen, and Y. H. Schukken. 2000. Hyperketonemia and the impairment of udder defense: a review. Vet. Res. 31397-412. [DOI] [PubMed] [Google Scholar]
- 45.Tater, D., B. Tepaut, J. P. Bercovici, and P. Youinou. 1987. Polymorphonuclear cell derangements in type I diabetes. Horm. Metab. Res. 19642-647. [DOI] [PubMed] [Google Scholar]
- 46.Uchino, K., T. Saito, B. Gebauer, and D. Jendrossek. 2007. Isolated poly(3-hydroxybutyrate) (PHB) granules are complex bacterial organelles catalyzing formation of PHB from acetyl coenzyme A (CoA) and degradation of PHB to acetyl-CoA. J. Bacteriol. 1898250-8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urban, C. F., S. Lourido, and A. Zychlinsky. 2006. How do microbes evade neutrophil killing? Cell. Microbiol. 81687-1696. [DOI] [PubMed] [Google Scholar]
- 48.Wilson, R. M., and W. G. Reeves. 1986. Neutrophil phagocytosis and killing in insulin-dependent diabetes. Clin. Exp. Immunol. 63478-484. [PMC free article] [PubMed] [Google Scholar]
- 49.Woody, R. C., R. W. Steele, W. L. Knapple, and N. S. Pilkington, Jr. 1989. Impaired neutrophil function in children with seizures treated with the ketogenic diet. J. Pediatr. 115427-430. [DOI] [PubMed] [Google Scholar]
- 50.Xiao, X. Q., Y. Zhao, and G. Q. Chen. 2007. The effect of 3-hydroxybutyrate and its derivatives on the growth of glial cells. Biomaterials 283608-3616. [DOI] [PubMed] [Google Scholar]