Abstract
Vibrio anguillarum is the causative agent of vibriosis in fish. Hemolysins of V. anguillarum have been considered virulence factors during infection. One hemolysin gene, vah1, has been previously identified but does not account for all hemolytic activity. The mini-Tn10Km mutagenesis performed with a vah1 mutant resulted in a hemolysin-negative mutant. The region surrounding the mutation was cloned and sequenced, revealing a putative rtx operon with six genes (rtxACHBDE), where rtxA encodes an exotoxin, rtxC encodes an RtxA activator, rtxH encodes a conserved hypothetical protein, and rtxBDE encode the ABC transporters. Single mutations in rtx genes did not result in a hemolysin-negative phenotype. However, strains containing a mutation in vah1 and a mutation in an rtx gene resulted in a hemolysin-negative mutant, demonstrating that the rtx operon is a second hemolysin gene cluster in V. anguillarum M93Sm. Reverse transcription-PCR analysis revealed that the rtxC and rtxA genes are cotranscribed, as are the rtxBDE genes. Additionally, Vah1 and RtxA each have cytotoxic activity against Atlantic salmon kidney (ASK) cells. Single mutations in vah1 or rtxA attenuate the cytotoxicity of V. anguillarum M93Sm. A vah1 rtxA double mutant is no longer cytotoxic. Moreover, Vah1 and RtxA each have a distinct cytotoxic effect on ASK cells, Vah1 causes cell vacuolation, and RtxA causes cell rounding. Finally, wild-type and mutant strains were tested for virulence in juvenile Atlantic salmon. Only strains containing an rtxA mutation had reduced virulence, suggesting that RtxA is a major virulence factor for V. anguillarum.
Vibrio anguillarum is a highly motile gram-negative, curved rod bacteria. This marine member of the class Gammaproteobacteria is one of the causative agents of vibriosis, a fatal hemorrhagic septicemic disease in fish, crustaceans, and bivalves (1). Fish infected with V. anguillarum display skin discoloration and erythema around the mouth, fins, and vent. Necrotic lesions are observed in the abdominal muscle (14). Mortality rates for infected fish populations may range from 30% to as high as 100% (1). Vibriosis has resulted in severe economic losses to aquaculture worldwide (1, 45) and affects many farm-raised fish including Pacific salmon, Atlantic salmon, sea bass, cod, and eel (1, 14, 18, 45).
Several genes have been reported to be correlated with the virulence of V. anguillarum, such as the vah1 hemolysin gene cluster (40), the siderophore-mediated iron transport system (16), the empA metalloprotease gene (15, 36), and the flaA gene (37). The hemolytic activity of V. anguillarum has been considered the virulence factor responsible for hemorrhagic septicemia during infection (27). Hirono et al. (27) identified the first hemolysin gene, vah1, in V. anguillarum and suggested that the vah1 gene is broadly distributed among V. anguillarum strains. Rock and Nelson (40) described a vah1 gene cluster in V. anguillarum strain M93Sm, in which the vah1 gene was linked to two putative lipase-related genes (llpA and llpB) and a hemolysin-like gene (plp) that appeared to function as a repressor of hemolytic activity. Furthermore, mutations in the vah1 cluster of genes did not result in the loss of hemolytic activity, suggesting that V. anguillarum contained more than one hemolysin (40). Additionally, Rodkhum et al. (41) found that V. anguillarum strain H775-3 contained four hemolysin genes (vah2, vah3, vah4, and vah5) in addition to vah1. The encoded proteins showed strong similarities to hemolysins of V. vulnificus (vah2) and V. cholerae (vah3, vah4, and vah5).
The repeat-in-toxin (RTX) family is a group of related protein toxins found in gram-negative bacteria. These toxins have a broad range of distribution and activities, which includes Escherichia coli HlyA hemolytic toxin (2, 35), V. cholerae RtxA cytotoxin (32), V. vulnificus RtxA cytotoxin (31), Bordetella pertussis CyaA adenylate cyclase (26), Pseudomonas aeruginosa alkaline protease (3), and Actinobacillus pleuropneumoniae Apx toxin (28, 29). These toxin genes usually form an operon and share some common features, such as posttranslational maturation by acylation, a C-terminal calcium-binding domain with tandem glycine/aspartic acid-rich repeats, and secretion of the toxin facilitated by type I secretion systems (TISS) (6). Studies demonstrate that the rtx operons are commonly found in Vibrio species. Lin et al. (32) first identified an rtx operon in V. cholerae and showed that the Rtx toxin caused HEp-2 cells to round up. Further research demonstrated that the Rtx toxin in V. cholerae was responsible for the covalent cross-linking of cellular actin (22), and an actin cross-linking domain (ACD) was recently discovered in the RtxA protein of V. cholerae (11). The rtx operon was also found in V. vulnificus where it functioned as a cytotoxin (31). Lee et al. (31) showed that V. vulnificus virulence in mice was dependent on rtxA. Recently, Satchell (42) renamed the RtxA toxin of V. cholerae the multifunctional autoprocessing Rtx toxin (MARTX). This new family of RtxA toxins exhibits highly conserved structural domains and variable catalytic activity domains assembled as mosaics. MARTX toxins are found in at least eight gram-negative bacterial species, including members of the genera Vibrio, Aeromonas, and Yersinia (42).
In this study, we sought to identify and characterize genes in addition to vah1 that contribute to the hemolytic activity of V. anguillarum M93Sm. Minitransposon mutagenesis was used to create and screen for hemolysin-negative mutants in a vah1 mutant background. One mutant that exhibited negative hemolytic activity was obtained, and the region surrounding this mutation was cloned and sequenced. A putative rtx operon was identified and characterized. Additionally, the contributions of the Vah1 and the Rtx hemolysins to cytotoxicity in Atlantic salmon kidney (ASK) cells and to virulence in juvenile Atlantic salmon were determined.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
All bacterial strains and plasmids used in this report are listed in Table 1. V. anguillarum strains were routinely grown in Luria-Bertani broth plus 2% NaCl (LB20) (23), supplemented with the appropriate antibiotic, in a shaking water bath at 27°C. Overnight cultures of V. anguillarum were grown in LB20 and centrifuged (8,000 × g, 10 min), and pelleted cells were washed twice with nine-salt solution (NSS) (23). Washed cells were resuspended to appropriate cell densities in experimental medium. Specific conditions are described in the text for each experiment. Antibiotics were used at the following concentrations: streptomycin, 200 μg/ml; chloramphenicol, 20 μg/ml for E. coli and 5 μg/ml for V. anguillarum; kanamycin, 80 μg/ml; ampicillin, 100 μg/ml; tetracycline, 2 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype and feature(s) | Reference |
|---|---|---|
| V. anguillarum strains | ||
| M93Sm | Spontaneous Smr mutant of M93 (serotype J-O-1) | 13 |
| S123 | Smr Cmr; M93Sm rtxA mutant | This study |
| S189 | Smr Cmr; M93Sm rtxC mutant | This study |
| S103 | Smr Cmr; M93Sm rtxB mutant | This study |
| S191 | Smr Cmr; M93Sm rtxD mutant | This study |
| S206 | Smr Cmr; M93Sm rtxE mutant | This study |
| S171 | Smr Kanr; M93Sm vah1 mutant | This study |
| S183 | Smr Cmr Kanr; M93Sm rtxA vah1 double mutant | This study |
| S193 | Smr Cmr Kanr; M93Sm rtxC vah1 double mutant | This study |
| JR7 | Smr Cmr Kanr; M93Sm rtxB vah1 double mutant | This study |
| S195 | Smr Cmr Kanr; M93Sm rtxD vah1 double mutant | This study |
| S208 | Smr Cmr Kanr; M93Sm rtxE vah1 double mutant | This study |
| E. coli strains | ||
| Sm10 | thi thr leu tonA lacY supE recA RP4-2-Tc::Mu::Km (λ pir) | 36 |
| S156 | Sm10 containing plasmid pLL1106 | This study |
| Plasmids | ||
| pNQ705-1 | Cmr; suicide vector with R6K origin | 37 |
| pDM4 | Cmr Kanr SacBCr; suicide vector | 37 |
| pJR7 | pBlueScript with partial rtx operon (for sequence) | This study |
| pBLUEscript | Cloning vector | Stratagene |
| pLL1106 | pDM4 containing the 5′ and 3′ parts of vah1 (for allelic exchange mutagenesis) | This study |
Bacterial mating.
Plasmids were introduced into V. anguillarum M93Sm from E. coli Sm10 (λ pir) by conjugation procedures described previously (37). Briefly, aliquots (100 μl) from overnight cultures of V. anguillarum and E. coli Sm10 were mixed at ratios of 1:1 in 2.5 ml of NSS plus 2.5 ml 10 mM MgSO4. The cell mixture was vacuum filtered onto a 0.45-μm-pore-size filter, which was placed on an LB15 agar plate (Luria-Bertani agar plus 1.5% NaCl) and allowed to incubate overnight at 27°C. Following incubation, the cells were removed from the filter by vigorous vortexing in 5 ml NSS. The cell suspension was spread on appropriate selection plates and allowed to incubate at 27°C until V. anguillarum colonies were observed.
Mini-Tn10Km mutagenesis.
Mini-Tn10Km mutagenesis was carried out by using a method developed by Herrero et al. (25), with a modification (40). Briefly, V. anguillarum M93Sm was mated with E. coli CC118 (λ pir)(pLOFKm) containing the mini-Tn10Km according the procedures described above. The transconjugants were selected onto LB20 plates supplemented with 200 μg/ml streptomycin (Sm200) and 80 μg/ml kanamycin (Kan80) for V. anguillarum mutants containing a mini-Tn10Km insertion. V. anguillarum colonies able to grow on LB20-Sm200-Kan80 were transferred onto trypticase soy agar (TSA)-sheep blood agar plates, and hemolytic activity was determined by measuring β-hemolysis after 24 h at 27°C.
Cloning of the mini-Tn10Km insertion mutation.
The region surrounding the gene, interrupted by the mini-Tn10Km mutagenesis, was cloned into pBluescript SKII+ (Stratagene). Briefly, genomic DNA from V. anguillarum strain JR7 was extracted and digested with SacI, and digestion fragments were ligated into the SacI-digested site of pBluescript SKII+. Then, the ligated DNA was transformed into E. coli XL1 MRF′ by electroporation, using a Bio-Rad gene pulser (at 1.5 kV, 25 μF, 200 Ω). Transformants were selected on LB agar plates supplemented with 100 μg/ml ampicillin. Plasmid DNA was purified from the clone using a Qiagen Mini-Prep kit (Qiagen). The plasmids were checked for the presence of inserted V. anguillarum DNA containing mini-Tn10Km by restriction digestion, followed by agarose gel electrophoresis. Clones of interest were saved for future study and sequencing.
Construction of the vah1::Km allelic exchange mutation.
The plasmid pDM4 (generously provided by Debra Milton) was used to construct the vah1::Km allelic exchange mutant as described previously by Milton et al. (37). The kanamycin resistance gene was amplified from the TOPO2.1 vector (Invitrogen) with the primer pair Pm152 and Pm153 (Table 2) and inserted into the XbaI and SphI sites of pDM4. The 5′ and 3′ regions of vah1 were amplified by PCR and inserted into either side of the kanamycin gene. The 5′ flanking region of vah1 was amplified from M93Sm genomic DNA with the primer pair Pm156 and Pm157 (Table 2), which amplified a 489-bp product and introduced XhoI and XbaI sites at the ends of the PCR product. The 3′ flanking region was amplified from M93Sm genomic DNA with the primer pair Pm154 and Pm155 (Table 2), which amplified a 469-bp product and introduced SphI and SacI sites at the ends of the PCR product. Both of these PCR products were cloned, sequenced, and subcloned on either side of the kanamycin resistance gene to produce the pDM4 derivative plasmid pLL1106, which was transformed into E. coli Sm10 (λ pir) to produce the transformant strain S156. S156 was mated with V. anguillarum M93Sm, and the double-crossover transconjugants were selected with LB20-Kan80-Sm200-5% sucrose plates. The resulting V. anguillarum mutants were checked for the desired allelic exchange using PCR amplification and restriction enzyme digestion. The mutant was designated S171 and used in further studies.
TABLE 2.
Primers used in this study
| Primera | Sequence (5′-3′)b | Targetc |
|---|---|---|
| Pm105 | ATCGAGAGCTCGCAAAATTCATGCTTATG | rtxA insertion mutation |
| Pm108 | ATCGATCTAGAGTTGTAAGCCGCAGCAC | rtxA insertion mutation |
| Pm180 | ATCGAGAGCTCGATCGTGCAATGATGCAG | rtxC insertion mutation F |
| Pm181 | ATCGATCTAGAGCGGCTTCGATTTCTCGT | rtxC insertion mutation R |
| SD rtxB F2 | GCTAGGAGCTCGTTGCGATAATTCAGGT | rtxB insertion mutation |
| SD rtxB R2 | GCTAGTCTAGATACCGCTGATCGGAATCGT | rtxB insertion mutation |
| Pm182 | ATCGAGAGCTCGCGTATTTGATGACGCAAAC | rtxD insertion mutation F |
| Pm183 | ATCGATCTAGAGCTCACCTTACTTTGGACCT | rtxD insertion mutation R |
| Pm190 | ATCGAGAGCTCGGATTTTGACCAATGCAGGT | rtxE insertion mutation |
| Pm191 | ATCGATCTAGACATTAGCGGCCCTCTCGTT | rtxE insertion mutation |
| Pm152 | ATCGATCTAGAGAACACGTAGAAAGCCAGT | kan cassette amplification |
| Pm153 | ACTGAGCATGCTCAGAAGAACTCGTCAAGAA | kan cassette amplification |
| Pm156 | ATCGACTCGAGATGTCAATAAACAGAAGAAA | vah1 allelic exchange 5′ flanking |
| Pm157 | ATCGATCTAGAGTTCGTTTCCGAACCACTAT | vah1 allelic exchange 5′ flanking |
| Pm154 | ATCGAGCATGCGGTTCATTGGCCTTACAA | vah1 allelic exchange 3′ flanking |
| Pm155 | ATCAGGAGCTCGATAAAATTAACATCGAATTAAC | vah1 allelic exchange 3′ flanking |
| Pm111 | GGAAATTATTCCGCCGACGATGGA | rtxA RT-PCR F |
| Pm112 | GCCGATACCGTATCGTTACCTGAA | rtxA RT-PCR R |
| Pm180 (RT) | GATCGTGCAATGATGCAG | rtxC RT-PCR F |
| Pm181 (RT) | GCGGCTTCGATTTCTCGT | rtxC RT-PCR R |
| Pm184 | GTTGTAGATGCGTGCCTTGCTCTG | rtxB RT-PCR |
| Pm185 | CCAATATGGAGCAAATTGCCGCCG | rtxB RT-PCR |
| Pm182 (RT) | GCGTATTTGATGACGCAAAC | rtxD RT-PCR F1 |
| Pm183 (RT) | GCTCACCTTACTTTGGACCT | rtxD RT-PCR R2 |
| Pm187 | GAGCGGGAAAAACCAACCCAAGT | rtxD RT-PCR F3 |
| Pm189 | GTGTTCACACCCTTGGGGCAGTC | rtxD RT-PCR R4 |
| Pm190 (RT) | GGATTTTGACCAATGCAGGT | rtxE RT-PCR |
| Pm191 (RT) | CATTAGCGGCCCTCTCGTT | rtxE RT-PCR |
RT, reverse-transcription primer.
Restriction sites for SacI (GAGCTC), XbaI (TCTAGA), SphI (GCATGC), and XhoI (CTCGAG) are underlined.
F, forward; R, reverse.
Insertional mutagenesis by homologous recombination of the rtx genes.
Insertional mutagenesis by homologous recombination was used to create gene interruptions within the structural genes of the rtx operon by integrating a plasmid into each rtx gene. Primers (Table 2) were designed based on the rtx gene sequence of M93Sm (GenBank accession no. EU155486). For the construction of the rtxA mutant, a 281-bp DNA fragment was PCR amplified by using primers Pm105 and Pm108 (Table 2) and cloned into the suicide vector pNQ705 by using SacI and XbaI restriction endonucleases to yield the pNQ705 derivative plasmid pLL1037, which was confirmed by both PCR amplification and restriction analysis. The mobilizable suicide vector was transferred from E. coli Sm10 containing plasmid pLL1037 into V. anguillarum M93Sm by conjugation. Transconjugants were selected by utilizing the chloramphenicol resistance gene located on the suicide plasmid. The incorporation of the suicide vector into the rtxA gene was confirmed by PCR analysis, as described previously by Milton et al. (37). The resulting V. anguillarum rtxA mutant was designated S123 (Table 1). For creating the rtxC, rtxB, rtxD, and rtxE mutants, specific DNA fragments were amplified separately and used in the same protocol as that used for the rtxA gene interruption.
RT-PCR.
The V. anguillarum wild-type strain M93Sm was grown to exponential phase (∼2 × 108 CFU/ml), and 1 ml of cells was harvested by centrifugation at 8,000 × g for 10 min. Total RNA was isolated using an RNeasy kit (Qiagen) according to the manufacturer's instructions. Isolated RNA was treated with DNase and used as the template (a 1-μg-per-50-μl reaction mixture) for reverse transcription (RT)-PCR. RT-PCR was performed using Brilliant SYBR Green single-step quantitative RT-PCR (qRT-PCR) Master Mix (Stratagene). Briefly, gene-specific primers (Table 2) were used to reverse transcribe the specific cDNA from RNA templates, and the resulting cDNA was used as the template with which to amplify the specific DNA product, using the regular PCR method. Genomic DNA (1 μg) extracted from wild-type strain M93Sm was used as the positive control. The reaction mixture without the addition of reverse transcriptase was used as a negative control. The thermal profile was 50°C for 30 min and 95°C for 15 min and then 35 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s. PCR product was visualized in a 1% agarose gel with a 100-bp DNA molecular weight ladder (Promega).
DNA sequencing.
All DNA sequencing was done at the URI Genomics and Sequencing Center (University of Rhode Island, Kingston, RI), using an ABI3170xl Genetic Analyzer unit (Applied Biosystems).
Complementation of the rtx mutants.
The rtx gene fragments were cloned to test the ability to complement the various rtx gene mutants, as described previously by Rock and Nelson (40). The mutants were complemented by cloning the appropriate genes into the shuttle vector pSUP202 (GenBank accession no. AY428809). Briefly, total genomic DNA from V. anguillarum M93Sm was isolated. A restriction site (PstI or EcoRI) was chosen according to the rtx operon sequence data (GenBank accession no. EU155486) and added to the PCR primer set used to amplify the gene of interest plus the putative native promoter region (Pnative), which is the 500-bp upstream region of the rtxD and rtxE genes and the whole intergenic region of the rtxC and rtxB genes (as shown in Fig. 4A). For making constitutive expressed rtx genes, a constitutive promoter for flaB (PflaB) (17), which was confirmed to constitutively express in both E. coli and V. anguillarum (L. Li and D. R. Nelson, unpublished data), was used to create PflaB-driven rtx genes (PflaB mutant) (Fig. 4A). PflaB was PCR amplified using plasmid pCE320(gfp)-PflaB (kindly supplied by Christian Eggers) (17) as the template. The restriction site SpeI was added to the primer set, which was used to insert the flaB promoter in front of the rtx gene (an SpeI site was also added to the PCR primer sets). All PCR fragments were subcloned into the PCR2.1 vector and digested with the appropriate restriction enzyme. The gel-purified gene fragment was ligated into pSUP202 and transformed into E. coli Sm10. E. coli Sm10 containing various pSUP202 plasmid derivatives (Fig. 4A) was conjugated into various rtx gene mutants by using the procedures described above. The conjugants were confirmed by using PCR amplification and restriction digestion.
FIG. 4.
Complementation of the rtx mutants and their hemolytic activities. Various gene(s) fragments driven by either a native promoter (Pnative) or a constitutive promoter (PflaB) (A) were cloned into shuttle vector pSUP202 and introduced into various V. anguillarum mutant strains by bacterial mating, as described previously (40). The resulting transconjugants were used to test the hemolytic activities on sheep blood agar plates incubated at 27°C for 48 h, and the relative hemolytic activities were compared with those of the wild-type strain M93Sm (B).
Cytotoxicity assay.
ASK cells (ATCC CRL-2747) were seeded into a six-well microtiter plate (Costar) in Leibovitz's L-15 medium (ATCC) supplemented with 10% fetal bovine serum and grown at 20°C to a cell density of ∼2 × 105 cells ml−1. V. anguillarum cultures grown overnight were harvested, washed twice in NSS, and resuspended in NSS (at a cell density of ∼2 × 109 cells ml−1). The supernatant from the overnight culture was filtered though a 0.22-μm-pore-size filter (Millipore). Washed bacterial cells were added to ASK cells at various multiplicities of infection (MOI) and incubated at 20°C for up to 4 h. Filtered supernatant (1 ml) was added directly to ASK cells containing 1 ml fresh medium and incubated at 20°C for 4 h or overnight (20 h). Changes in cell morphology were assessed and photographed by viewing live cells with an inverted microscope (Nikon TE2000 model). The concentration and viability of ASK cells were determined by the trypan blue dye exclusion method using a Vi-Cell cell viability analyzer (Beckman Coulter).
Fish infections.
Hemolysin mutants were tested for virulence with juvenile Atlantic salmon (Salmo salar L.) by intraperitoneal (i.p.) injection, as described by Denkin and Nelson (14). Briefly, V. anguillarum cells grown in LB20 supplemented with appropriate antibiotics for 18 h at 27°C were harvested by centrifugation (8,000 × g, 10 min, 4°C), washed twice in NSS, and suspended in NSS. The cell density of NSS suspensions was determined by serial dilution and spot plating. Fifteen fish (10 to 15 cm long) were used to test the virulence of each bacterial strain. Seven fish were sham inoculated with NSS as a negative control. Fish inoculated with different bacterial strains were maintained in separate tanks to prevent possible cross-contamination. Five fish were inoculated per dose, and three different doses per strain were used. Fish were inoculated i.p. with 100 μl of cells (ranging from ∼105 to 107 CFU ml−1) in NSS or with NSS alone (control fish). The fish were anesthetized in water supplemented with tricaine methane sulfonate (100 mg ml−1) prior to inoculation and allowed to recover before they were returned to their tanks. Death due to vibriosis was determined by the observation of gross clinical signs and confirmed by the recovery and isolation of V. anguillarum cells resistant to the appropriate antibiotics from infected organs of dead fish. Observations were made for 21 days. All fish used in this research project were obtained from the URI East Farm Aquaculture Center.
RESULTS
Mini-Tn10Km mutagenesis.
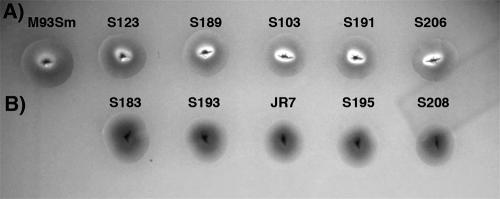
Previously, Rock and Nelson (40) found that when the vah1 hemolysin gene was mutated in V. anguillarum M93Sm, the resulting mutant exhibited little or no reduction in hemolytic activity. This result, coupled with the observation that a mutation in the adjacent and divergently transcribed plp gene increased both the vah1 transcription and the hemolytic activity, implied that there was a second hemolysin. To identify additional hemolysin genes in V. anguillarum M93Sm, mini-Tn10Km mutagenesis (25) was carried out with V. anguillarum JR1 (Table 1), a vah1 insertion mutant of M93Sm (40). Over 3,000 mini-Tn10Km-containing colonies were screened on sheep blood agar plates for altered hemolytic activity. One clone, designated JR7, exhibited negative hemolytic activity (Fig. 1). This indicated that an unknown gene, other than vah1, was disrupted by a mini-Tn10Km insertion and that the mutations in this gene and in vah1 together resulted in the complete loss of hemolytic activity in V. anguillarum M93Sm.
FIG. 1.
Hemolytic activity of the wild-type V. anguillarum M93Sm and its hemolysin mutant strains with TSA-5% sheep blood agar. Mutations in single rtx genes did not eliminate the hemolytic activity of M93Sm (A); however, mutations in both the vah1 and rtx genes resulted in the loss of hemolysin activity (B). All bacterial strains were transferred onto a sheep blood agar plate and incubated at 27°C for 48 h. S123, the rtxA mutant strain; S189, the rtxC mutant strain; S103, the rtxB mutant strain; S191, the rtxD mutant strain; S206, the rtxE mutant strain. S183, the vah1 rtxA double mutant strain; S193, the vah1 rtxC double mutant strain; JR7, the vah1 rtxB double mutant strain; S195, the vah1 rtxD double mutant strain; S208, the vah1 rtxE double mutant strain.
Cloning and identification of the putative V. anguillarum rtx hemolysin genes.
To identify the gene interrupted by the mini-Tn10Km insertion, genomic DNA from strain JR7 was isolated and digested with SacI (Fig. 2A). Digested DNA fragments were inserted into the SacI site of plasmid pBluescript IISK+ and then transformed into E. coli XL1MRF′. A fragment of ∼13 kbp containing the kanamycin resistance gene was obtained, and the plasmid designated pJR7 was isolated for DNA sequencing. Sequencing of the pJR7 plasmid revealed four complete open reading frames (ORFs) and one incomplete ORF (Fig. 2A). BLASTx analysis of the ORFs within this region (24) revealed a cluster of genes that displayed a high level of similarity to genes involved in the biogenesis of RTX toxins in several gram-negative organisms, including V. cholerae N16961 (GenBank accession number NP231094) (32) and V. vulnificus CMCP6 (GenBank accession number NP762440) and YJ016 (GenBank accession number NP937086) (9). A 13,200-bp ORF homologue of rtxA encodes a putative 440-kDa exotoxin designated rtxA of V. anguillarum (rtxVa). A 462-bp rtxC-like gene, designated rtxCVa, is upstream of rtxAVa and encodes a putative acylase for RtxAVa activation. Additionally, a 336-bp rtxH-like V. anguillarum gene, rtxHVa, is upstream of rtxCVa. Two ORFs located downstream of rtxAVa are homologous to nicotinate-nucleotide-dimethylbenzimidazole phosphoribosyltransferase (ORF1) and cobalamin synthase (ORF2). The sequence of the incomplete ORF was found to be homologous to the rtxB gene, an ABC transporter containing an ATP binding cassette that is thought to facilitate the transport of RTX toxins. Sequencing data also revealed that the mini-Tn10Km was inserted into this rtxB homologue gene (designated rtxBVa), which has an orientation opposite to that of rtxHVa, rtxCVa, and rtxAVa (Fig. 2A).
FIG. 2.
Comparison of the rtx operon organization in V. anguillarum M93Sm (A) with that of V. cholerae and V. vulnificus (B). (A) The rtx operon and its flanking DNA in V. anguillarum M93Sm, as follows: rtxAVa encodes RtxA, a secreted toxin and virulence factor; rtxCVa is a putative acylase that acts as an RtxAVa activator; rtxHVa is a conserved hypothetical gene; rtxBDEVa are putative ABC transporters of RtxAVa. The open arrow indicates the insertion of the kanamycin cassette by mini-Tn10Km mutagenesis in the rtxBVa gene in JR7 (the vah1 rtxB double mutant). The DNA fragment between the SacI sites was cloned into plasmid pJR7 and sequenced by primer walking. The rest of the DNA is cloned and sequenced using inverse PCR. Identifications based on BLASTx similarities, as follows: ORF1, nicotinate-nucleotide-dimethylbenzimidazole phosphoribosyltransferase; ORF2, cobalamin synthase; ORF3, chemotaxis gene; ORF4, Na-carboxylase symporter. (B) The rtx operon and its flanking genes in V. cholerae El Tor strain N16961 and V. vulnificus strain YJ016 (9). The diagram shows the arrangements are identical for the rtx operon and the distinct flanking genes of three Vibrio species.
Since previous studies of other rtx operons showed that at least two transport genes exist in rtx operons (32), inverse PCR was used to complete the 3′-end sequence of rtxBVa and to identify other possible downstream transporters. Several inverse PCR fragments were obtained (data not shown) and sequenced. The completely sequenced region is available as GenBank accession number EU155486. Analysis of the DNA sequences revealed two additional ORFs downstream of rtxBVa that exhibited strong homology to the ABC transporter proteins (Table 3) and were designated rtxDVa and rtxEVa. Both ORFs were located immediately downstream of rtxBVa (Fig. 2). The three putative transport genes (rtxBVa, rtxDVa, and rtxEVa) are transcribed in the same direction and exhibit overlapping regions between the start and stop codons. Like rtxBVa, rtxEVa contains an ATP binding cassette region and may act as an ABC transporter. rtxDVa encodes a putative transmembrane fusion protein and is likely part of the ABC transport system for the export of RtxAVa (6, 32). Inverse PCR and DNA sequencing also identified two other ORFs downstream of rtxEVa, which are homologous to a chemotaxis gene (ORF3) and a Na-carboxylase symporter gene (ORF4).
TABLE 3.
Homology analysis of the rtx operon of V. anguillarum and other species
| ORF | Predicted no. of aa/protein mass (kDa) | Accession no. | Homology to rtx genes of other speciesa
|
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
V. vulnificus YJ016
|
V. vulnificus CMCP6
|
V. cholerae El Tor N16961
|
Yersinia enterocolitica
|
Aeromonas hydrophila subsp. ATCC 7966
|
E. coli O157:H7
|
|||||||||||||||||||||
| % of identity/% of similarity | Homologue locus | Putative function | Predicted size (aa) | % of identity/% of similarity | Homologue locus | Putative function | Predicted size (aa) | % of identity/ % of similarity | Homologue locus | Putative function | Predicted size (aa) | % of identity/ % of similarity | Homologue locus | Putative function | Predicted size (aa) | % of identity/ % of similarity | Homologue locus | Putative function | Predicted size (aa) | % of identity/ % of similarity | Homologue locus | Putative function | Predicted size (aa) | |||
| rtxAVa | 4,399/467 | EU155486 | 90/95 | NP_937086 | Cytotoxin | 5,206 | 90/95 | NP_762440 | Adhesin | 5,206 | 85/92 | NP_231094 | Cytotoxin | 4,558 | 48/67 | CAJ90394 | Cytotoxin RtxA | 3,212 | 57/72 | YP_855898 | RtxA toxin | 4,685 | N/Aa | NP_052622 | Hemolysin | 998 |
| rtxCVa | 153/18 | EU155486 | 75/89 | NP_937088 | Rtx acylase | 159 | 75/89 | NP_762441 | Acyltransferase | 153 | 73/88 | NP_231093 | Rtx acylase | 153 | 51/73 | CAJ90393 | RtxA activator | 152 | 64/84 | YP_855897 | Cytolysin-activating lysine-acyltransferase | 149 | 31/56 | NP_052623 | HlyA acylase | 163 |
| rtxHVa | 111/12 | EU155486 | 69/78 | NP_937089 | Hypothetical protein | 119 | 69/78 | NP_762442 | Hypothetical protein | 89 | 68/76 | NP_231092 | Hypothetical protein | 119 | 59/74 | CAJ90392 | Hypothetical protein | 124 | 69/82 | YP_855896 | Hypothetical protein | 132 | N/Aa | |||
| rtxBVa | 702/79 | EU155486 | 67/80 | NP_937090 | ABC transporter | 727 | 73/86 | NP_762443 | ABC transporter | 535 | 66/81 | NP_231091 | ABC transporter | 720 | 48/65 | YP_001006250 | ABC transporter | 708 | 61/77 | YP_855895 | ABC transporter | 695 | 47/63 | NP_052625 | ABC transporter | 706 |
| rtxDVa | 450/51 | EU155486 | 63/77 | NP_937091 | ABC transporter | 467 | 62/77 | NP_762444 | ABC transporter | 453 | 63/78 | NP_231090 | ABC transporter | 467 | 39/60 | YP_001006251 | ABC transporter | 464 | 58/76 | YP_855894 | ABC transporter | 454 | 30/41 | NP_052626 | ABC transporter | 479 |
| rtxEVa | 765/85 | EU155486 | 71/84 | NP_937092 | ABC transporter | 722 | 71/84 | NP_762445 | ABC transporter | 722 | 72/84 | NP_231089 | ABC transporter | 721 | 52/70 | YP_001006252 | ABC transporter | 710 | 67/80 | YP_855893 | ABC transporter | 719 | 48/64 | NP_052625 | ABC transporter | 706 |
N/A, not applicable, no homology by BLASTx; aa, amino acid(s).
V. anguillarum rtx operon shares strong homology with other rtx operons.
Examination and analysis of the predicted amino acid sequences in the V. anguillarum rtx operon by BLASTp (24) revealed important similarities to and differences from other members of the RTX family (Table 3). For example, analysis of the RtxAVa sequence identified a 95% similarity to that of RtxA of V. vulnificus and a 90% similarity to that of RtxA of V. cholerae El Tor N16961. Besides the Vibrio species, sequence analysis of RtxAVa also revealed strong homology with RtxA toxins of other gram-negative bacteria, including the putative cytotoxin RtxA in Yersinia enterocolitica, which shares 67% homology with RtxAVa. The RtxA toxin in Aeromonas hydrophila has a 72% similarity with RtxAVa. However, BLASTp analysis did not show significant similarity to HlyA, the RTX toxin/hemolysin of E. coli O157:H7 (35). It should be noted that HlyA contains only 998 amino acids (22% as long), compared to the 4,400 amino acids of RtxAVa.
In contrast to the RtxA toxins, the activator (RtxCVa), the RtxHVa, and the secretion (RtxBVa, RtxDVa, and RtxEVa) gene products for RtxAVa share significant similarities among bacterial species. For example, RtxBVa has a homology of 63% to RtxB of E. coli O157:H7 and 86% to RtxB of V. vulnificus CMCP6. High similarities of transporter proteins indicate that RtxA toxin-related TISSs have been highly conserved in gram-negative bacteria. Interestingly, there are usually three transport genes, rtxB, rtxD, and rtxE, for the secretion of RtxA toxins larger than 350 kDa or 3,200 amino acids in length, like those in Vibrio, Yersinia, or Aeromonas species (Table 3), while only two transport genes, rtxB and rtxD, are described for the secretion of relatively smaller RtxA toxins, such as those in B. pertussis, E. coli, and Pseudomonas species, which have RtxA toxins with molecular masses of 177 kDa (26), 110 kDa (35), and 55 kDa (3), respectively. Additionally, RtxE proteins are also ATP binding proteins, which share high homology with RtxB. For example, RtxEVa was found to have 64% similarity with RtxB of E. coli O157:H7 (Table 3), as well as 84% similarity with RtxE of V. vulnificus and V. cholerae.
While the arrangement of rtx genes in V. anguillarum M93Sm was also found to be similar to that of other species, the flanking sequences are quite different, even for other species of Vibrio (Fig. 2). Specifically, immediately adjacent to the rtxE gene of V. anguillarum M93Sm there is a putative chemotaxis gene (ORF3). In V. cholerae, a histidine kinase/response regulator (VC1445) is adjacent to rtxE, while a ubiquitous dehydrogenase (VVA1037) is found in V. vulnificus YJ016 (9). On the other side of the rtx gene cluster (downstream of rtxA), CTX structural genes and bacteriophage elements are present in the V. cholerae genome (32), and the V. vulnificus YJ016 rtx genes are located next to an alanine racemase gene (VVA1029), whereas a cobalamin synthase gene (ORF2) is downstream of the V. anguillarum M93Sm rtxA gene (Fig. 2).
The rtx operon confers hemolytic activity in V. anguillarum M93Sm.
Mutations were constructed in each rtx gene by insertional mutagenesis, as described in Materials and Methods. Examination of each mutant for hemolytic activity revealed that a single mutation in any rtx gene gave a hemolytic activity similar to that of the wild-type strain M93Sm (Fig. 1). The results indicated that the loss of any rtx gene did not eliminate hemolytic activity, a result which was consistent with the previous report suggesting that V. anguillarum contains two hemolysin gene clusters, one of which includes the vah1 hemolysin gene (40). In order to eliminate the hemolytic effect of vah1, a mutation in vah1 was constructed by allelic exchange. The resulting mutant (S171) had a hemolytic activity similar to that of the wild type, as shown previously (40) (data not shown). The insertional mutation in each of the rtx genes was constructed in the S171 background. The vah1 rtxAVa double mutant (S183) exhibited a negative hemolytic activity (Fig. 1), which indicated that both vah1 and rtxAVa were required for hemolytic activity in V. anguillarum M93Sm. Furthermore, strains carrying the double mutations of vah1 and any other gene in the rtx operon, including vah1 rtxCVa (S193), vah1 rtxBVa (JR7), vah1 rtxDVa (S195), and vah1 rtxEVa (S208), all failed to exhibit any hemolytic activity (Fig. 1). This result indicated that all genes in the rtx operon were necessary for the hemolytic activity in V. anguillarum M93Sm.
Transcriptional analysis of the rtxVa operon.
RT-PCR was used to discover the transcriptional pattern of the rtx genes in V. anguillarum M93Sm. Primers complementary to the 3′ end of one gene and the 5′ end of the immediately adjacent downstream gene were used to determine whether transcription resulted in polycistronic mRNA (Table 2 and Fig. 3). RT-PCR data showed that a 646-bp PCR product was amplified with primers crossing the intergenic space of rtxCVa and rtxAVa (Fig. 3, lane 7), which indicated that rtxAVa and rtxCVa were transcribed together as a polycistronic mRNA, even with 22 bases between the two genes. Additionally, quantitative RT-PCR showed that rtxCVa and rtxAVa had similar numbers of transcripts, ∼106 copies per 100 ng total RNA (data not shown). Furthermore, RT-PCR products of the predicted size were generated between rtxBVa and rtxDVa (577 bp) and between rtxDVa and rtxEVa (670 bp) (Fig. 3, lanes 17 and 27, respectively), indicating that the three transporter genes were also transcribed as a polycistronic mRNA, as suggested by sequence analysis, since the three transporter genes overlap each other (Fig. 2A).
FIG. 3.
RT-PCR analysis of transcription from the rtx operon in V. anguillarum M93Sm. RT-PCR was performed with 100 ng RNA obtained from M93Sm cells grown for 12 h in LB20, using primers labeled as A (Pm112), B (Pm111), C (Pm181), D (Pm180), E (Pm185), F (Pm184), G (Pm183), H (Pm182), I (Pm189), J (Pm187), K (Pm191), and L (Pm190). Lanes 4, 14, and 24, 100-bp molecular marker; lanes 1 to 3, 11 to 13, and 21 to 23, PCRs performed with M93Sm genomic DNA as template served as positive controls; lanes 5 to 7, 15 to 17, and 25 to 27, RT-PCRs performed with 100 ng RNA from 12-h M93Sm cultures. Lanes 8 to 10, 18 to 20, and 28 to 30, PCRs performed without reverse transcriptase, as negative controls. The primers used were as follows: A and B, shown in lanes 1, 5, and 8; C and D, shown in lanes 2, 6, and 9; A and D, shown in lanes 3, 7, and 10; E and F, shown in lanes 11, 15, and 18; G and H, shown in lanes 12, 16, and 19; E and H, shown in lanes 13, 17, and 20; I and J, shown in lanes 21, 25, and 28; K and L, shown in lanes 22, 26, and 29; I and L, shown in lanes 23, 27, and 30. The map of the rtx operon shows the locations of the primers used and the lengths of the amplicons obtained.
Complementation of the rtxVa gene mutants.
Plasmid constructions were made to complement the rtx gene mutants as described previously (40) and shown in Fig. 4A. With the exception of the plasmid constructed for rtxE, complementation plasmids for each expression unit were made in two ways: (i) those containing the structural genes plus enough of the upstream sequence to contain the putative native promoter, Pnative; and (ii) those in which the structural genes were inserted behind a strong constitutive promoter for flaB, PflaB (7, 17), to drive the genes in question. For rtxE, only the putative Pnative was used. Surprisingly, the plasmid containing the rtxCH/B intergenic region plus the rtxBDE gene fragment (Pnative-rtxBDE) (Fig. 4A) did not restore the hemolytic activities of JR7 (the vah1 rtxB double mutant) and S195 (the vah1 rtxD double mutant); instead, the plasmid containing the rtxBDE genes driven by the constitutive PflaB (PflaB-rtxBDE) restored the hemolytic activities of both strains JR7 and S195 (Fig. 4). The results suggested the possibility of a regulatory site adjacent to or in the putative native promoter of the rtxB gene that is necessary for transcription but does not function in trans. Additionally, plasmids harboring the rtxBD fragment driven by the flaB promoter failed to restore hemolytic activity in either the vah1 rtxB (JR7) or the vah1 rtxD (S195) double mutant (Fig. 4). This suggested that mutations in rtxB or rtxD had a polar effect on rtxE expression. Similarly, the plasmid harboring the rtxDE gene plus its 500-bp upstream region (Pnative-rtxDE) (Fig. 4) could not complement the hemolytic activity of vah1/rtxD mutant (S195), while the rtxDE gene, driven by the constitutive PflaB (PflaB-rtxDE), restored the hemolytic activity of strain S195 (Fig. 4). Surprisingly, the vah1 rtxE mutant (S208) complemented with pSUP202 containing rtxE plus its 500-bp upstream fragment (Pnative-rtxE) completely restored hemolytic activity. This result indicated that the rtxE gene was able to be transcribed from its own native promoter, even though rtxE is also transcribed as a polycistronic mRNA together with rtxB and rtxD (Fig. 3, lane 27).
Plasmids containing the rtxB/HC intergenic region plus the rtxC gene (Pnative-rtxC) or the PflaB-driven rtxC gene (PflaB-rtxC) (Fig. 4) did not restore the hemolytic activity of strain S193 (the vah1 rtxC mutant). Thus, the mutation in rtxC had a polar effect on the rtxA gene. Attempts to complement the vah1 rtxA double mutant were not successful due to the difficulty of cloning and conjugating the 13-kbp-long rtxA or rtxHCA gene fragment.
Cytotoxic activities of Vah1 and RtxAVa against ASK cells.
The RTX toxins produced in V. cholerae and other gram-negative bacteria typically display cytotoxic or hemolytic activities (31, 32, 35). In V. cholerae, RTX toxin causes HEp-2 cells to round up and detach from surfaces (32). In this study, an ASK cell line was used to test the cytotoxic activity of V. anguillarum M93Sm and its various hemolysin mutants. Briefly, ASK cells (∼3 × 105 cells/ml) were exposed to V. anguillarum cells (MOI, 500) for up to 4 h. As shown in Fig. 5A, more than 99% of the ASK cells were detached and killed (P < 0.01) when exposed to washed M93Sm cells. ASK cells also exhibited extensive rounding prior to detachment (Fig. 5B-1). Exposing the ASK cells to washed S171 (the vah1 deletion mutant) cells also resulted in rounding and a significant decrease in attachment (Fig. 5B-2). After 4 h of exposure to S171 cells, only 8% of the ASK cells were still attached to the surface (P < 0.01) compared to that attached to NSS-treated cells. The rtxA mutant strain (S123) exhibited weaker cytotoxicity against ASK cells, with about 60% of the cells still attached after treatment (P < 0.1). No rounding of the ASK cells was observed when they were treated with S123 cells (Fig. 5B-3). When ASK cells were exposed to the hemolysin-negative strain S183 (the vah1 rtxA double mutant), no cytotoxic activity was observed (Fig. 5A) during the 4-h exposure, and ASK cells did not exhibit rounding, detachment, or cell death (Fig. 5B-4). The data strongly suggest that while both Vah1 and RtxAVa contribute to the cytotoxicity of V. anguillarum cells, ASK cell rounding was observed only when RtxAVa was present. Additionally, the occurrence of ASK cell rounding was observed in the presence of the wild-type M93Sm after only 1 h at a lower MOI value (MOI, 100). It should be noted that at this time and MOI, most ASK cells were still attached and cell viability was similar to that of the NSS-treated cells (data not shown). This suggested that cell rounding precedes cell detachment and death.
FIG. 5.
The cytotoxicity of V. anguillarum M93Sm and hemolysin mutant strains against ASK cells. (A) ASK cells were treated with M93Sm and its derivative hemolysin mutants at an MOI of 500 for 4 h. Determination of the ASK cell density and viability is described in Materials and Methods and was carried out using a trypan blue dye exclusion assay. Bars represent the standard deviations of three independent measurements. P values above each bar of assays were calculated by t test analysis. (B) The morphological changes of treated ASK cells were observed by inverted microscopy at a magnification of ×100. ASK cells were incubated for 1 h with NSS-washed V. anguillarum cells at an MOI of 100. The V. anguillarum strains added were M93Sm (B-1), S171 (vah1 mutant) (B-2), S123 (rtxA mutant) (B-3), S183 (vah1 rtxA double mutant) (B-4), and mock (NSS).
Culture supernatants from V. anguillarum strains grown overnight in LB20 were collected by centrifugation, passed through a 0.2-μm filter, and tested for cytotoxic activity against ASK cells. ASK cells became highly vacuolated after 4 h of incubation with the M93Sm supernatant; subsequently, ASK cells became rounded and detached when the incubation was continued overnight (Fig. 6, panels M93Sm 4h and M93Sm o/n). This suggested that cell vacuolation and cell rounding are separate events for ASK cells when they are exposed to the V. anguillarum supernatant. When the supernatant from S171 (the vah1 mutant) was added to the ASK cell culture, no vacuolation was observed at any time during the 24-h incubation; however, ASK cells were observed to round up after an overnight (24-h) incubation (Fig. 6, panels vah1 mutant 4h and vah1 mutant o/n). This observation suggested that the Vah1 hemolysin was responsible for the vacuolation of ASK cells and that RtxAVa is secreted and is responsible for ASK cell rounding. To confirm these observations, culture supernatants from S123 (the rtxA mutant) were added to ASK cells and incubated for 24 h. Vacuolation, but not rounding, of ASK cells was observed in the presence of the S123 supernatant. Furthermore, more vacuoles formed as the incubation time increased (Fig. 6, panels rtxA mutant 4h and rtxA o/n), which indicated that the Vah1 hemolysin is also a secretory protein and responsible for the vacuolation of ASK cells. When the culture supernatant from S183 (the vah1 rtxA double mutant) was added to ASK cells, neither vacuolation nor cell rounding was observed during the 24-h incubation (Fig. 6, panels vah1 rtxA double mutant 4h and vah1 rtxA double mutant o/n). The same result was obtained when uninoculated LB20 cells (negative control) were added to ASK cells (Fig. 6, panels LB20 4h and LB20 o/n). Taken together, these observations strongly suggest that vah1 and rtxA encode secreted cytotoxins that have different effects upon target cells.
FIG. 6.
Morphological changes to ASK cells caused by culture supernatants from the V. anguillarum wild-type M93Sm and hemolysin mutant strains observed by inverted microscopy (magnification, ×100). ASK cells were exposed to overnight (o/n) culture supernatants for 4 h (top row) and for 24 h (bottom row). The V. anguillarum strains from which the supernatants were obtained are indicated at the top of each photo.
RtxAVa is a major virulence factor to Atlantic salmon.
Juvenile Atlantic salmon were infected by i.p. injection with V. anguillarum M93Sm and its hemolysin mutants (Table 4) and observed for 21 days. Fish inoculated with ∼3 × 106 CFU of the wild-type strain M93Sm suffered 100% mortality by 3 days, while fish inoculated with ∼3 × 105 CFU suffered 60% mortality by 5 days and 40% mortality by 9 days with an inoculation of ∼3 × 104 CFU. Similar levels of killing were observed with fish infected with S171 (the vah1 mutant), with 100% mortality at a dose of 2.9 × 106 CFU, 60% mortality at 2.9 × 105 CFU, and 20% mortality when inoculated with 2.9 × 104 CFU. The data indicated that the vah1 mutant showed no significant change in virulence compared with the wild-type strain M93Sm. In contrast, no deaths by vibriosis were observed when fish were inoculated with either the rtxA mutant S123 or the vah1 rtxA double mutant S183. Taken together, these observations strongly suggest that the RtxA hemolysin is a major virulence factor of V. anguillarum and that the mutation in rtxA results in avirulence to Atlantic salmon.
TABLE 4.
Virulence of V. anguillarum strains in juvenile Atlantic salmon
| Strain | Dose/fish (CFU) | Total % of mortality | No. of days until death (no. of fish/total fish)a |
|---|---|---|---|
| M93Sm | 3.01 × 106 | 100 | 2 (2/5), 3 (5/5) |
| 3.01 × 105 | 60 | 3 (2/5), 5 (3/5) | |
| 3.01 × 104 | 40 | 6 (1/5), 9 (2/5) | |
| S171 (vah1 mutant) | 2.9 × 106 | 100 | 2 (3/5), 3 (5/5) |
| 2.9 × 105 | 60 | 4 (1/5), 5 (3/5) | |
| 2.9 × 104 | 20 | 2 (1/5) | |
| S123 (rtxA mutant) | 1.0 × 106 | 0 | NAb |
| 1.0 × 105 | 0 | NAb | |
| 1.0 × 104 | 0 | NAb | |
| S183 (vah1 rtxA | 4.7 × 106 | 0 | NAc |
| double mutant) | 4.7 × 105 | 0 | NAc |
| 4.7 × 104 | 0 | NAb | |
| Control (NSS) | 0 | NAb |
NA, not applicable.
No fish deaths occurred during the 21-day experiment.
One fish in the 4.7 × 106 cells/dose group died at day 8, and two fish in the 4.7 × 105 cells/dose group died at day 12; however, no V. anguillarum cells could be isolated on LB20-Sm200 plates from the dead fish, and no clinical symptoms of vibriosis were observed.
DISCUSSION
The hemolytic activity of V. anguillarum cells has been suggested to be a virulence factor during the infection of fish. The vah1 gene was the first hemolysin gene identified in V. anguillarum (27). Studies demonstrated that vah1 is distributed widely in V. anguillarum strains and is found in serotypes A to I (27); however, additional genes, besides vah1, have been found to contribute to the hemolytic activity of V. anguillarum cells. Rodkhum et al. (41) demonstrated that there are four additional hemolysin genes (vah2 to vah5) in V. anguillarum strain H775-3. Rock and Nelson (40) showed that the mutation of vah1 had no effect on the hemolytic activity of V. anguillarum M93Sm on sheep blood agar, implying that there was an additional hemolysin that contributed to the hemolytic activity of this strain. Additionally, mutations that knocked out the activity of the adjacent divergently transcribed gene (plp) increased hemolytic activity by increasing the transcription of vah1. In this study, mini-Tn10Km mutagenesis was performed with V. anguillarum JR1, a vah1 knock-out mutant (40) to obtain the hemolysin-negative mutant JR7 (Fig. 1). The regions surrounding the mini-Tn10Km insertion were cloned and sequenced, revealing an rtx operon with six genes, rtxACHBDE (Fig. 2), where rtxA encodes the toxin, rtxC encodes the toxin activator (acylase), rtxH encodes a conserved hypothetical protein, and rtxBDE encode three secretion proteins.
Single-insertion mutations created in rtx genes did not eliminate hemolytic activity. These single mutants exhibited hemolytic activity similar to that of the wild-type strain M93Sm (Fig. 1). However, double mutations in vah1 and any of the rtx genes resulted in a hemolysis-negative phenotype (Fig. 1), demonstrating that the rtx operon is a second hemolysin gene cluster in V. anguillarum M93Sm. These data, consistent with that of Rock and Nelson (40), demonstrate that hemolytic activity in V. anguillarum M93Sm is the result of two clusters of hemolysin genes, the vah1 cluster and the rtx cluster.
RTX toxins are a diverse group of protein toxins synthesized by many gram-negative bacteria. Members of the RTX toxin family have been identified as cytolytic toxins, metalloproteases, lipases, and adenylate cyclases. They include E. coli hemolysin HlyA (35), V. cholerae cytotoxin RtxA (32), V. vulnificus cytotoxin RtxA (31), and B. pertussis adenylate cyclase CyaA (4, 26). Most RTX toxins are proteins with a molecular mass ranging from 100 kDa to >400 kDa and are posttranslationally activated by acylation via a specific acyltransferase. The repeated structure of RTX toxin proteins, which gave them their name, is composed typically of repeated glycine-rich nonapeptides binding Ca2+ on the C-terminal half of the protein (20). The N-terminal sequence of RTX toxins is thought to contain sequences that are responsible for binding to target cells and promoting the formation of cation-selective pores (4, 35). It is interesting that while RtxAVa has typical glycine-rich nonapeptide repeats, closer inspection revealed that the repeats may actually be 18-mer repeats. That is, instead of the usual 9-residue (GGXGXDXXX) repeats, an extra 9 amino acid residues are added to each repeat, resulting in an 18-residue consensus motif, GGXGXDXXVXXGXXNXXX. For RtxAVa, these repeats are found at the C-terminal end of the protein (amino acid residues 4031 to 4165) (Fig. 7B). Lin et al. (32) also found 18-residue GD-rich repeats at the C-terminal end of the RtxA toxin of V. cholerae. Additionally, we found that RtxAVa contains a novel 19-amino-acid repeat with the consensus motif GX(A/G)N(I/V)XT(K/H)VGDGXXXXXXX (RtxAVa, amino acids 784 to 1354) (Fig. 7A). This novel 19-amino-acid repeat is very similar to the 19-amino-acid repeat found in V. cholerae, with the consensus sequence of GXAN(I/V)XT(K/H)VGDGXTVAVMX (32). Recently, Satchell (42) demonstrated that MARTX toxins are distinguished from other RTX toxins by the large number of primary sequences composed of glycine-rich repeats. The author noted that there are three conserved repeat regions in the MARTX toxin family, termed the A, B, and C repeats. The A repeats are 20-amino-acid repeats located near the N terminus. The B repeats (originally novel repeats) are 19-amino-acid repeats just downstream of the A repeats. The C repeats are 18-amino-acid GD-rich repeats of the C-terminal region. Similar repeat regions in the rtxA gene indicate that RtxAVa also is a member of MARTX toxin family. We also found two conserved domains by using a search of the CDD (34). One domain is located at amino acid residues 2728 to 2808 and appears to be an alpha/beta hydrolase fold (NCBI Conserved Domains database no. pfam00561). The other domain is located at amino acid residues 1609 to 1868. This domain is similar to a TolA-like protein (pfam06519). The function of these domains in RtxAVa toxin is unknown.
FIG. 7.
Features of RtxAVa toxin. (A) Conserved domains found in RtxAVa. NR, novel repeat or B-repeat (amino acids 784 to 1354); Tol-A like, domain (amino acids 1609 to 1868); Hydrolase fold, amino acids 2728 to 2808; GD-R, GD-rich repeat or C repeat (amino acids 4031 to 4265). (B) The GD-rich repeats and consensus sequences found in the C-terminal portion of RtxAVa. Gray highlights indicate the consensus repeat residues.
In V. cholerae, RtxA functions as cytotoxin that causes cell rounding and depolymerization of actin stress fibers in a broad range of cell types. The depolymerized actin monomers are covalently cross-linked into polymers (22, 32). In our studies, V. anguillarum strains containing an intact rtxA gene (M93Sm or S171) caused ASK cells to round, detach, and die (Fig. 5). No rounding was observed with ASK cells treated with strains lacking a functional rtxA gene (S123 or S183). It has been shown for RtxA of V. cholerae that an ACD is responsible for actin cross-linking but not for cell rounding (11, 43). Furthermore, analysis of RtxA sequences from both V. vulnificus (43) and V. anguillarum reveals that neither protein contains an ACD. These analyses are consistent with those of Sheahan and Satchell (44; unpublished data in Cordero et al. [12]), who were unable to detect actin cross-linking by V. vulnificus RtxA. Similarly, we were also unable to detect actin cross-linking by V. anguillarum RtxA (data not shown). However, as noted above, the RtxA toxin of both V. vulnificus (31) and V. anguillarum M93Sm (Fig. 5 and 6) causes target cell rounding, which implies that cell rounding caused by the RtxA of Vibrio species is triggered by a domain common to all Vibrio RtxA toxins. Interestingly, deletion of the ACD domain from the RtxA of V. cholerae does not completely eliminate the ability to cause cell rounding in target cells. The resulting slow cell rounding is thought to occur due to the inactivation of small Rho GTPases by the Rho inactivation domain (RID) in RtxA of V. cholerae (44). A homologue of the RID domain was also identified in RtxAVa; however, the contribution of the RID to cell rounding and cytotoxicity by RtxAVa needs to be investigated further.
The cytotoxicity assay using ASK cells revealed that Vah1 and RtxAVa are both exotoxins and each has a distinct effect on ASK cells. Our data demonstrate that the culture supernatant from an overnight culture of S171 (the vah1 mutant) caused ASK cell rounding, while the culture supernatant from S123 (the rtxA mutant) caused ASK cell vacuolation (Fig. 6). Culture supernatants from M93Sm caused both rounding and vacuolation, while supernatants from S183 (the rtxA vah1 double mutant) had no effect on ASK cells. Previously, it was found that the HlyA hemolysin of V. cholerae causes vacuolation in many types of eukaryotic cells (10, 19, 38). HlyA shares strong homology (76%) with Vah1 of V. anguillarum. Chakraborty et al. (8) also indentified a cytotoxin in V. fluvialis which has 81% homology to HlyA of V. cholerae and also causes the vacuolation of HeLa cells. Taken together, the data strongly suggest that Vah1 of V. anguillarum M93Sm causes vacuolation of ASK cells. The data also suggest that the two hemolysins/cytotoxins Vah1 and RtxA have different mechanisms for cytotoxic activity and that the two toxins work synergistically to increase the cytotoxicity of M93Sm for ASK cells (Fig. 5A).
Our fish infection studies (Table 4) revealed that rtxA is a major contributor of virulence. Strains of V. anguillarum that lacked a functional rtxA gene were avirulent. In contrast, the vah1 mutant strain S171 exhibited no decrease in virulence compared to the wild-type strain M93Sm. These results are similar to those of Fullner et al. (21) for V. cholerae in which RtxA was found to be a major accessory toxin. However, the deletion of hlyA (a homologue of vah1) from V. cholerae did not affect virulence in the murine pulmonary model, but hlyA contributed predominately to the virulence in the adult mouse intestinal infection model, with rtxA playing a secondary role (39). In contrast, the Vvh toxin of V. vulnificus, also a homologue of Vah1, is thought to contribute directly to virulence by causing vasodilation and hypotensive septic shock (30). Our data clearly show that RtxAVa is a major virulence factor in fish infection by i.p. injection. Additionally, RtxAVa appears to have a strong cytotoxic effect against fish erythrocyte and macrophage cells (unpublished data). It will be interesting to discover whether RtxA plays a role during the initial invasion of the fish across the intestinal epithelium during infections of fish by immersion or anal intubation.
It is interesting to note that while rtx gene clusters in Vibrio species are highly conserved, retaining strong protein homologies and gene arrangements (Fig. 2 and Table 3), the flanking genes surrounding the rtx genes differ among V. cholerae, V. vulnificus, and V. anguillarum (Fig. 2). Lin et al. (32) demonstrated that the rtx operon in V. cholerae is adjacent to the CTX prophage and is considered part of a pathogenicity island. However, they also found that the 5′ end of rtxA, all of rtxC, and the 5′ end of rtxB were deleted in classical biotypes of V. cholerae. The authors suggested that the acquisition of the rtx operon predated the acquisition of the CTX element. In contrast, the rtx operons in both V. anguillarum and V. vulnificus are flanked by different sets of housekeeping genes and are not associated with obvious pathogenicity islands. The similarity of rtx operons and the distinct flanking sequences among Vibrio species suggests that the rtx operon was probably transferred horizontally between Vibrio species.
Rtx toxins are secreted by a TISS (6), which consists of an ATP-binding protein (i.e., RtxBVa and RtxEVa) and a membrane-fusion protein (RtxDVa). In V. anguillarum, the deduced amino acid sequences of the three secretion proteins (encoded by rtxBDEVa) of the rtx operon show high degrees of amino acid sequence similarity with other rtx operons. For example, for rtxBVa the deduced encoded protein sequence has a 63% similarity to that of E. coli HlyB and an 81% similarity to that of V. vulnificus RtxB (Table 3). Comparison of the rtx secretion genes from various bacterial species revealed an interesting difference among the secretion systems regarding the presence or absence of rtxE. We found, using BLASTx, that rtxE is broadly distributed among rtx operons that contain the larger versions of RtxA (>3,000 amino acid residues long), such as those found in Vibrio, Yersinia, and Aeromonas species (Table 3), while rtxE is not found in rtx operons with smaller RtxA proteins, such as those in E. coli, B. pertussis, and Pasteurella species. Boardman and Satchell (6) suggested that the secretion system containing rtxE is an atypical TISS and thought that RtxB and RtxE might form a heterodimer during the secretion of RtxA in V. cholerae. Our data suggest that a similar secretion mechanism exists in V. anguillarum. Furthermore, secretion of RtxA also requires an additional unlinked gene, tolC (5). However, we have not yet identified a tolC homologue in V. anguillarum M93Sm.
Finally, our data indicate that rtxC and rtxA are cotranscribed, as are rtxB, rtxD, and rtxE. We also found an rtxH homologue, which Liu et al. (33) demonstrated was cotranscribed with rtxC and rtxA in V. vulnificus. The regulation of rtx transcription by rtxH or other factors is currently under investigation.
Acknowledgments
This work was supported by the National Research Initiative of the USDA Cooperative State Research, Education, and Extension Service, grant no. 2005-35204-16294, awarded to D.R.N.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 31 March 2008.
REFERENCES
- 1.Austin, B., and D. A. Austin. 1999. Characteristics of the pathogens. Bacterial fish pathogens: disease of farmed and wild fish, 3rd ed. Praxis Publishing Co., London, United Kingdom.
- 2.Bauer, M. E., and R. A. Welch. 1996. Characterization of an RTX toxin from enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 64167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann, U., S. Wu, K. M. Flaherty, and D. B. McKay. 1993. Three-dimensional structure of the alkaline protease of Pseudomonas aeruginosa: a two-domain protein with a calcium binding parallel beta roll motif. EMBO J. 123357-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benz, R., E. Maier, D. Ladant, A. Ullmann, and P. Sebo. 1994. Adenylate cyclase toxin (CyaA) of Bordetella pertussis. Evidence for the formation of small ion-permeable channels and comparison with HlyA of Escherichia coli. J. Biol. Chem. 26927231-27239. [PubMed] [Google Scholar]
- 5.Bina, J. E., and J. J. Mekalanos. 2001. Vibrio cholerae tolC is required for bile resistance and colonization. Infect. Immun. 694681-4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boardman, B. K., and K. J. Satchell. 2004. Vibrio cholerae strains with mutations in an atypical type I secretion system accumulate RTX toxin intracellularly. J. Bacteriol. 1868137-8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll, J. A., P. E. Stewart, P. Rosa, A. F. Elias, and C. F. Garon. 2003. An enhanced GFP reporter system to monitor gene expression in Borrelia burgdorferi. Microbiology 1491819-1828. [DOI] [PubMed] [Google Scholar]
- 8.Chakraborty, R., S. Chakraborty, K. De, S. Sinha, A. K. Mukhopadhyay, J. Khanam, T. Ramamurthy, Y. Takeda, S. K. Bhattacharya, and G. B. Nair. 2005. Cytotoxic and cell vacuolating activity of Vibrio fluvialis isolated from paediatric patients with diarrhoea. J. Med. Microbiol. 54707-716. [DOI] [PubMed] [Google Scholar]
- 9.Chen, C. Y., K. M. Wu, Y. C. Chang, C. H. Chang, H. C. Tsai, T. L. Liao, Y. M. Liu, H. J. Chen, A. B. Shen, J. C. Li, T. L. Su, C. P. Shao, C. T. Lee, L. I. Hor, and S. F. Tsai. 2003. Comparative genome analysis of Vibrio vulnificus, a marine pathogen. Genome Res. 132577-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coelho, A., J. R. Andrade, A. C. Vicente, and V. J. Dirita. 2000. Cytotoxic cell vacuolating activity from Vibrio cholerae hemolysin. Infect. Immun. 681700-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cordero, C. L., D. S. Kudryashov, E. Reisler, and K. J. Satchell. 2006. The actin cross-linking domain of the Vibrio cholerae RTX toxin directly catalyzes the covalent cross-linking of actin. J. Biol. Chem. 28132366-32374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cordero, C. L., S. Sozhamannan, and K. J. Satchell. 2007. RTX toxin actin cross-linking activity in clinical and environmental isolates of Vibrio cholerae. J. Clin. Microbiol. 452289-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denkin, S. M., and D. R. Nelson. 1999. Induction of protease activity in Vibrio anguillarum by gastrointestinal mucus. Appl. Environ Microbiol. 653555-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denkin, S. M., and D. R. Nelson. 2004. Regulation of Vibrio anguillarum empA metalloprotease expression and its role in virulence. Appl. Environ. Microbiol. 704193-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denkin, S. M., P. Sekaric, and D. R. Nelson. 2004. Gel shift analysis of the empA promoter region in Vibrio anguillarum. BMC Microbiol. 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Lorenzo, M., M. Stork, M. E. Tolmasky, L. A. Actis, D. Farrell, T. J. Welch, L. M. Crosa, A. M. Wertheimer, Q. Chen, P. Salinas, L. Waldbeser, and J. H. Crosa. 2003. Complete sequence of virulence plasmid pJM1 from the marine fish pathogen Vibrio anguillarum strain 775. J. Bacteriol. 1855822-5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eggers, C. H., M. J. Caimano, M. L. Clawson, W. G. Miller, D. S. Samuels, and J. D. Radolf. 2002. Identification of loci critical for replication and compatibility of a Borrelia burgdorferi cp32 plasmid and use of a cp32-based shuttle vector for the expression of fluorescent reporters in the Lyme disease spirochaete. Mol. Microbiol. 43281-295. [DOI] [PubMed] [Google Scholar]
- 18.Egidius, E. 1987. Vibriosis: pathogenicity and pathology. Aquaculture 715-28. [Google Scholar]
- 19.Figueroa-Arredondo, P., J. E. Heuser, N. S. Akopyants, J. H. Morisaki, S. Giono-Cerezo, F. Enriquez-Rincon, and D. E. Berg. 2001. Cell vacuolation caused by Vibrio cholerae hemolysin. Infect. Immun. 691613-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frey, J., and P. Kuhnert. 2002. RTX toxins in Pasteurellaceae. Int. J. Med. Microbiol. 292149-158. [DOI] [PubMed] [Google Scholar]
- 21.Fullner, K. J., J. C. Boucher, M. A. Hanes, G. K. Haines, 3rd, B. M. Meehan, C. Walchle, P. J. Sansonetti, and J. J. Mekalanos. 2002. The contribution of accessory toxins of Vibrio cholerae O1 El Tor to the proinflammatory response in a murine pulmonary cholera model. J. Exp. Med. 1951455-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fullner, K. J., and J. J. Mekalanos. 2000. In vivo covalent cross-linking of cellular actin by the Vibrio cholerae RTX toxin. EMBO J. 195315-5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia, T., K. Otto, S. Kjelleberg, and D. R. Nelson. 1997. Growth of Vibrio anguillarum in salmon intestinal mucus. Appl. Environ. Microbiol. 631034-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gish, W., and D. J. States. 1993. Identification of protein coding regions by database similarity search. Nat. Genet. 3266-272. [DOI] [PubMed] [Google Scholar]
- 25.Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 1726557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hewlett, E. L., K. J. Kim, S. J. Lee, and M. C. Gray. 2000. Adenylate cyclase toxin from Bordetella pertussis: current concepts and problems in the study of toxin functions. Int. J. Med. Microbiol. 290333-335. [DOI] [PubMed] [Google Scholar]
- 27.Hirono, I., T. Masuda, and T. Aoki. 1996. Cloning and detection of the hemolysin gene of Vibrio anguillarum. Microb. Pathog. 21173-182. [DOI] [PubMed] [Google Scholar]
- 28.Jansen, R., J. Briaire, E. M. Kamp, A. L. Gielkens, and M. A. Smits. 1993. Cloning and characterization of the Actinobacillus pleuropneumoniae-RTX-toxin III (ApxIII) gene. Infect. Immun. 61947-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jansen, R., J. Briaire, E. M. Kamp, A. L. Gielkens, and M. A. Smits. 1993. Structural analysis of the Actinobacillus pleuropneumoniae-RTX-toxin I (ApxI) operon. Infect. Immun. 613688-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kook, H., S. E. Lee, Y. H. Baik, S. S. Chung, and J. H. Rhee. 1996. Vibrio vulnificus hemolysin dilates rat thoracic aorta by activating guanylate cyclase. Life Sci. 59PL41-PL47. [DOI] [PubMed] [Google Scholar]
- 31.Lee, J. H., M. W. Kim, B. S. Kim, S. M. Kim, B. C. Lee, T. S. Kim, and S. H. Choi. 2007. Identification and characterization of the Vibrio vulnificus rtxA essential for cytotoxicity in vitro and virulence in mice. J. Microbiol. 45146-152. [PubMed] [Google Scholar]
- 32.Lin, W., K. J. Fullner, R. Clayton, J. A. Sexton, M. B. Rogers, K. E. Calia, S. B. Calderwood, C. Fraser, and J. J. Mekalanos. 1999. Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc. Natl. Acad. Sci. USA 961071-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu, M., A. F. Alice, H. Naka, and J. H. Crosa. 2007. The HlyU protein is a positive regulator of rtxA1, a gene responsible for cytotoxicity and virulence in the human pathogen Vibrio vulnificus. Infect. Immun. 753282-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marchler-Bauer, A., and S. H. Bryant. 2004. CD-search: protein domain annotations on the fly. Nucleic Acids Res. 32(Web server issue)W327-W331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menestrina, G., C. Moser, S. Pellet, and R. Welch. 1994. Pore-formation by Escherichia coli hemolysin (HlyA) and other members of the RTX toxins family. Toxicology 87249-267. [DOI] [PubMed] [Google Scholar]
- 36.Milton, D. L., A. Norqvist, and H. Wolf-Watz. 1992. Cloning of a metalloprotease gene involved in the virulence mechanism of Vibrio anguillarum. J. Bacteriol. 1747235-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milton, D. L., R. O'Toole, P. Horstedt, and H. Wolf-Watz. 1996. Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 1781310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitra, R., P. Figueroa, A. K. Mukhopadhyay, T. Shimada, Y. Takeda, D. E. Berg, and G. B. Nair. 2000. Cell vacuolation, a manifestation of the El Tor hemolysin of Vibrio cholerae. Infect. Immun. 681928-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olivier, V., G. K. Haines III, Y. Tan, and K. J. Satchell. 2007. Hemolysin and the multifunctional autoprocessing RTX toxin are virulence factors during intestinal infection of mice with Vibrio cholerae El Tor O1 strains. Infect. Immun. 755035-5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rock, J. L., and D. R. Nelson. 2006. Identification and characterization of a hemolysin gene cluster in Vibrio anguillarum. Infect. Immun. 742777-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodkhum, C., I. Hirono, J. H. Crosa, and T. Aoki. 2005. Four novel hemolysin genes of Vibrio anguillarum and their virulence to rainbow trout. Microb. Pathog. 39109-119. [DOI] [PubMed] [Google Scholar]
- 42.Satchell, K. J. 2007. MARTX, multifunctional autoprocessing repeats-in-toxin toxins. Infect. Immun. 755079-5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheahan, K. L., C. L. Cordero, and K. J. Satchell. 2004. Identification of a domain within the multifunctional Vibrio cholerae RTX toxin that covalently cross-links actin. Proc. Natl. Acad. Sci. USA 1019798-9803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheahan, K. L., and K. J. Satchell. 2007. Inactivation of small Rho GTPases by the multifunctional RTX toxin from Vibrio cholerae. Cell. Microbiol. 91324-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toranzo, A. E., and J. L. Barja. 1993. Virulence factors of bacteria pathogenic for coldwater fish. Annu. Rev. Fish Dis. 35-36. [Google Scholar]