Abstract
Factor H-binding protein (fHbp) is a novel meningococcal vaccine candidate that elicits serum antibodies that activate classical complement pathway bacteriolysis and also inhibit binding of the complement down-regulatory protein, factor H, to the bacterial surface. One limitation of fHbp as a vaccine candidate is antigenic variability, since antibodies to fHbp in the variant 1 (v.1) antigenic group do not protect against strains expressing v.2 or v.3 proteins, and vice versa. We have identified amino acid residues of epitopes recognized by bactericidal anti-fHbp monoclonal antibodies prepared against fHbp from each of the variant groups. One epitope expressed by nearly all v.1 proteins mapped to the B domain, while epitopes expressed by fHbp v.2 or v.3 mapped to the C domain. The results provided the rationale for engineering chimeric fHbp molecules containing the A domain (which is conserved across all variant groups), a portion of the B domain of a v.1 protein, and the carboxyl-terminal portion of the B domain and the C domain of a v.2 protein. By enzyme-linked immunosorbent assay, the resulting recombinant chimeric proteins expressed epitopes from all three variant groups. In mice, the chimeric vaccines elicited serum antibodies with bactericidal activity against a panel of genetically diverse strains expressing fHbp v.1, v.2, or v.3. The data demonstrate the feasibility of preparing a meningococcal vaccine from a single recombinant protein that elicits broad bactericidal activity, including group B strains, which account for 50 percent of cases of meningococcal disease and for which there currently is no broadly protective vaccine.
Neisseria meningitidis is a gram-negative bacterium that colonizes the nasopharynxes of 10 to 20 percent of healthy humans (1, 9, 10, 23, 26, 41). Rarely, an encapsulated strain invades the bloodstream, which can lead to sepsis or meningitis, both of which can cause death or permanent sequelae. N. meningitidis strains can be divided into different capsular groups based on the presence of structurally and antigenically distinctive polysaccharides.
Conjugated capsular polysaccharide-protein vaccines are available for prevention of disease caused by strains with capsular group A, C, W-135, or Y (reviewed in reference 19) but not that caused by group B strains, which elaborate a polysaccharide capsule that is an autoantigen (15), which is poorly immunogenic (12). Group B strains currently are responsible for 40 to 50 percent of cases of sporadic meningococcal disease in the United States (22) and up to 90 percent of those in certain European countries (20, 40). Group B strains also have a propensity to cause epidemics such as those reported in Norway in the 1970s (5, 6), Cuba in the 1980s (38), and New Zealand in the 1990s and early 2000s (2, 13). Without vaccination, these epidemics can be difficult to control and can last for more than a decade. Outer membrane vesicle (OMV) vaccines have been effective in controlling epidemics caused by a dominant strain (24). However, OMV vaccines are of limited use for prevention of endemic disease caused by genetically diverse strains since the bactericidal antibody responses are directed largely against PorA protein, which is antigenically variable (30). Therefore, the serum bactericidal responses to OMV vaccines tend to be strain specific (39).
Recent genomic studies identified several protein antigens that are promising vaccine candidates for prevention of group B disease (8, 11, 31, 34). One of the most promising candidates is factor H-binding protein (fHbp) (previously referred as genome-derived neisserial antigen 1870 [GNA1870] [31] or lipoprotein 2086 [16]). The fHbp gene encodes a surface-exposed lipoprotein that is present in all N. meningitidis strains tested to date (4, 16, 31). The protein is a unique vaccine antigen since it elicits serum antibodies that both activate classical complement pathway bacteriolysis (43) and inhibit binding of the complement down-regulatory protein factor H (fH) to the bacterial surface (27, 43). Without bound fH, the organism becomes more susceptible to complement-mediated bactericidal activity, particularly by the alternative pathway (27, 37). However, one limitation of fHbp as a vaccine candidate is antigenic variability; the protein can be classified into three major antigenic variant groups (31). In general, antibodies prepared against fHbp from the variant 1 (v.1) group were bactericidal only against other strains expressing fHbp from the v.1 group, and the same is true for anti-v.2 or -v.3 antibodies, which are bactericidal only against strains expressing fHbp in the v.2 or v.3 group (4, 16, 31).
In a recent study, we identified the amino acid residues involved in the epitopes recognized by a panel of murine bactericidal monoclonal antibodies (MAbs) prepared against fHbp from each of three variant groups (P. T. Beernink et al., submitted for publication). One epitope that was present in nearly all fHbp v.1 proteins mapped to G121 of the B domain, while other epitopes associated with fHbp v.2 or v.3 proteins mapped to residues between positions 174 and 216 of the C domain. The results provided the rationale for engineering chimeric fHbp molecules that contained epitopes from all three antigenic variant groups. The purpose of the present study was to investigate the immunogenicity in mice of two prototype chimeric fHbp vaccines for their ability to elicit serum bactericidal antibody responses against N. meningitidis strains expressing fHbp from different variant groups.
MATERIALS AND METHODS
Gene cloning and protein expression and purification.
Wild-type fHbp v.1 and v.2 expression plasmids were constructed by PCR amplification of the genes from genomic DNAs from strains MC58 and 8047, respectively, as described previously (31). The PCR products were cloned into a T/A plasmid (pGEM-T-Easy; Promega), and the approximately 800-base-pair NdeI-XhoI fragment was subcloned into pET21b (Novagen). The encoded protein lacked 26 amino acid residues at the N terminus (including the signal sequence and seven presumably flexible residues) and included a hexahistidine tag at the C terminus.
The fHbp chimera I was constructed by amplifying the region encoding residues 8 to 135 from strain MC58 (fHbp v.1) and that encoding residues 136 to 255 from strain 8047 (fHbp v.2). Note that the numbering is based on the mature protein sequence of fHbp from strain MC58. Primers for the junction point between the v.1 and v.2 gene fragments included 24 bp from each sequence. The two PCR fragments, which shared an overlapping region of 48 bp, were gel purified, assembled by PCR amplification using a 5′ primer for the v.1 gene and a 3′ primer for the v.2 gene, and cloned as described above for the wild-type genes. fHbp chimera II was constructed by site-specific mutagenesis of the plasmid encoding fHbp chimera I to introduce the A174K substitution (associated with expression of the JAR 32 epitope [see below]) using the QuikChange II site-directed mutagenesis kit (Stratagene). The fHbp genes in the expression plasmids were confirmed by DNA sequencing.
Wild-type and chimeric fHbps were expressed in Escherichia coli BL21(DE3) (Novagen). Mid-exponential-phase cultures (1 liter) were grown at 37°C in Super Broth (30 g/liter Bacto-tryptone, 20 g/liter yeast extract, 10 g/liter MOPS [morpholinepropanesulfonic acid]; pH adjusted to 7.0 with NaOH). When the cultures reached an optical density at 600 nm (OD600) of 0.5 to 0.6, gene expression was induced by the addition of 0.5 mM β-d-isopropylthiogalactoside and the cultures were incubated for an additional 3 to 4 h. The bacteria were harvested by centrifugation, resuspended in phosphate-buffered saline (PBS) containing a protease inhibitor cocktail (Complete EDTA-free; Roche), and lysed by incubation with chicken egg white lysozyme (Sigma) and two freeze/thaw cycles. Bacterial lysates were treated with DNase and RNase (Sigma) and were clarified by centrifugation at 13,000 × g. Recombinant fHbps (rfHbps) were purified by nickel chelate chromatography using Ni-nitrilotriacetic acid agarose (Qiagen) and buffers recommended by the supplier. Fractions containing purified fHbp were pooled and dialyzed against PBS (Roche) containing 5% (wt/vol) sucrose and 1 mM dithiothreitol, filter sterilized, and stored at 4°C.
ELISA.
Binding of the MAbs to wild-type and chimeric rfHbps was measured by enzyme-linked immunosorbent assay (ELISA). The wells of a microtiter plate (Immulon 2B; Thermo Electron Corp.) were coated with 1 μg/ml of rfHbp in PBS and incubated overnight at 4°C. The plates were blocked with PBS containing 0.1% Tween-20 (Sigma) (PBST) and 1% (wt/vol) bovine serum albumin (BSA) (Sigma). Concentration-dependent binding of the anti-fHbp MAbs was measured at 0.008 to 50 μg/ml in PBST-BSA. After incubation (2 h at room temperature), the plates were washed and rabbit anti-mouse immunoglobulin G (IgG)-alkaline phosphatase (Zymed; 1:5,000 diluted in PBST/BSA) was added. After 1 h at room temperature, alkaline phosphatase substrate (Sigma) was added, and the absorbance at 405 nm was measured after 30 min. Binding of purified human fH (Complement Technologies, Inc.) to wild-type and chimeric fHbps was measured by a similar procedure, except that the primary antibody was goat polyclonal anti-fH (Bethyl Laboratories) diluted 1:2,000 and the secondary antibody was mouse anti-goat IgG-alkaline phosphatase conjugate (Santa Cruz Biotech) also diluted 1:2,000.
Immunization.
Groups (five mice each) of five-week-old CD-1 female mice (Charles River) were immunized intraperitoneally with four doses of wild-type or chimeric fHbp vaccines, which were given at 2-week intervals. Each 100-μl dose contained 20 μg of recombinant protein mixed with an equal volume of Freund's adjuvant (FA) (Sigma) (complete FA for the first dose and incomplete FA for subsequent doses). At 2 1/2 weeks after the last dose, blood samples were obtained by cardiac puncture and the animals were sacrificed. Aliquots of serum were stored at −70°C for assay. The animal procedures were performed under a protocol approved by the Institutional Animal Care and Use Committee of the Children's Hospital Oakland Research Institute.
Serology.
IgG antibody titers were measured by ELISA as previously described using rfHbp v.1 (strain MC58) or v.2 (strain 8047) as the antigen on the plate. For measurement of serum bactericidal activity, test sera were heated for 30 min at 56°C to inactivate complement. The group B strains used to measure bactericidal antibody were selected based on diversity of their sequence type complexes and PorA and PorB variable regions (Table 1). An additional criterion was diversity in fHbp based on the extent of amino acid sequence identity and reactivity with a panel of murine anti-fHbp MAbs (Table 2). Serum bactericidal activity was measured as previously described using washed, exponential-growth-phase bacteria grown in Mueller-Hinton broth supplemented with 0.25% glucose and 0.02 mM CMP-N-acetylneuraminic acid to an OD620 of 0.6 as previously described (42). The buffer was Dulbecco's PBS containing 0.9 mM CaCl2 and 0.5 mM MgCl2·6H2O (Mediatech, Inc.) with 1% (wt/vol) BSA. The complement source was human serum from a donor lacking bactericidal activity against N. meningitidis group B strains. The serum bactericidal titer was defined as the reciprocal dilution that gave a 50% decrease in the number of CFU after 60 min of incubation at 37°C compared with the CFU at time zero in the negative control reactions.
TABLE 1.
Characteristics of strains used in this study
| Strain | Origin | ST complex (ST)a | Porin protein typeb
|
|
|---|---|---|---|---|
| PorB | PorA | |||
| H44/76 | Norway | ST-32 (32) | 15 | 1.7,16 |
| SK080 | California | ST-162 (162) | 3-73 | 1.22,14 |
| SK084 | California | ST-32 (32) | 3-24 | 1.7,16 |
| NZ98/254 | New Zealand | ST-41/44 (42) | 4 | 1.7-2,4 |
| SK141 | Tennessee | ST-213 (213) | 3-14 | 1.22,14 |
| 8047 | United States | ST-8 (8) | 2b | 1.5-1,2-2 |
| MD1435 | Maryland | ST-35 (35) | 339 | 1.22-1,14 |
| MD1321 | Maryland | ST-41/44 (44) | 3-45 | 1.7-1,1 |
| 03S-0658 | California | ST-32 (1364) | 3-38 | 1.7-2,13-1 |
| M1239 | United States | ST-41/44 (437) | 14 | P1.23,14 |
| SK104 | North Carolina | ST-162 (5748) | 3-73 | 1.22,14 |
TABLE 2.
Characteristics of fHbps expressed by strains
| Strain | Varianta | % Amino acid identityb to:
|
Reactivity with MAb JARc:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| v.1 | v.2 | v.3 | 1 | 4 | 5 | 10 | 11 | 13 | 32 | 33 | 36 | ||
| H44/76 | 1 | 100 | 72 | 59 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| SK080 | 1 | 93 | 69 | 61 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| SK084 | 1 | 96 | 72 | 61 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| NZ98/254 | 1 | 91 | 74 | 61 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| SK141 | 1 | 89 | 71 | 63 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| 8047 | 2 | 72 | 100 | 85 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 |
| MD1435 | 2 | 72 | 99 | 86 | 0 | NDd | 0 | 1 | 1 | 1 | 0 | 0 | 1 |
| MD1321 | 2 | 72 | 94 | 84 | 0 | ND | 0 | 1 | 1 | 0 | 0 | 0 | 1 |
| 03S-0658 | 2 | 69 | 94 | 88 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| M1239 | 3 | 59 | 85 | 100 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| SK104 | 3 | 59 | 85 | 97 | 0 | ND | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
Determined by quantitative PCR as described previously (3).
The amino acid sequence of the mature protein was compared with that of prototype fHbp v.1 (strain MC58), v.2 (strain 8047), or v.3 (strain M1239).
MAb reactivity as determined by ELISA (see Materials and Methods). In measuring reactivity, the background OD405 was <0.05. An OD >10-fold above background was considered positive (designated “1”), and an OD <5-fold above background was considered negative (designated “0”). JAR 1, 4, and 5 were derived from a mouse immunized with fHbp v.1 (strain MC58); JAR 10, 11, and 13 were from a mouse immunized with fHbp v.2 (strain 2996); and JAR 32, 33, and 36 were from a mouse immunized with fHbp v.3 (strain M1239).
ND, not determined.
Statistical analysis.
The geometric mean titers (GMTs) were calculated from the inverse of the mean log10. For calculation of confidence limits about the GMT, 2 times the standard error (SE) of the mean log10 was added or subtracted from the mean log10 values. The confidence limits are reported as the inverse log10 of these values. Differences in the GMTs for two groups of mice were determined by a t test (two tailed) performed on the log10-transformed data using Prism software (GraphPad). Differences between GMTs for the same group of mice were determined by a paired t test.
RESULTS
Expression of MAb epitopes by chimeric fHbp molecules.
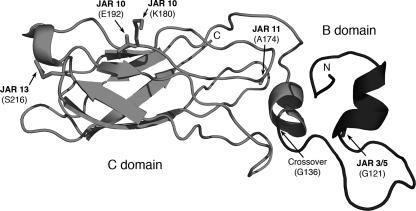
To generate chimera I, we engineered a recombinant protein that combined the A domain, which is highly conserved across fHbps from all three variant groups, and a portion of the B domain of fHbp v.1 from strain MC58 up to amino acid G136 with the remainder of the B domain and the C domain of fHbp v.2 from strain 8047 (Fig. 1). Chimera I expressed the JAR 11 epitope, which is expressed by about one-third of disease-producing group B strains in the United States that have fHbp in the v.2 or v.3 antigenic group (4). Among the JAR 11-negative fHbp v.2 or v.3 strains, about 50 percent express the JAR 32 epitope (4). JAR 11-positive fHbp has an alanine at residue 174 (A174), while JAR 32-positive fHbp has a lysine at that position. In an attempt to increase coverage against JAR 11-negative strains, we prepared a second chimeric vaccine, designated chimera II, which was identical to chimera I except that chimera II had a single amino acid substitution, A174K, associated with JAR 32 reactivity.
FIG. 1.
Schematic model of fHbp chimera I. The A domain (not shown) and N-terminal portion of the B domain (right, dark gray) are encoded by the fHbp gene from strain MC58 and express the v.1 epitopes recognized by anti-fHbp MAbs, JAR 3 and JAR 5. The carboxy-terminal portion of the B domain and the C domain (left, light gray) are encoded by the gene from strain 8047 and contain epitopes recognized by JAR 10, 11, 13, and 36 (v.2 and v.3). The junction point between the two portions of the chimera is residue G136, which immediately precedes the alpha-helix. Chimera II differs from chimera I in having the A174K substitution, which eliminated the JAR 11 epitope and introduced the JAR 32 and JAR 35 epitopes. The coordinates were from the nuclear magnetic resonance solution structure of the combined B and C domains of fHbp v.1 (7). The amino terminus (residue 109) and carboxyl terminus (residue 255) are labeled N and C, respectively.
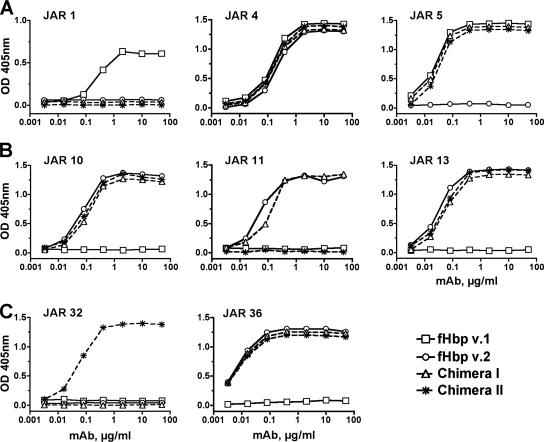
The reactivities of the two chimeric proteins with the different MAbs were measured by concentration-dependent binding in an ELISA (Fig. 2). As expected, JAR 1, which binds to a v.1 epitope in the C domain (R204) (our unpublished results), did not bind to either of the chimeric proteins (Fig. 2A). JAR 5, which is specific for an epitope on the B domain of fHbp v.1, and JAR 4, which cross-reacted with an epitope that is not yet defined but was expressed by most strains expressing fHbp v.1 or v.2, showed identical respective concentration-dependent binding with the two chimeric proteins compared with the respective wild-type v.1 and/or v.2 protein.
FIG. 2.
Concentration-dependent binding of anti-fHbp MAbs to epitopes on wild-type and chimeric fHbps. The chimera I and II vaccines are identical except for a single amino acid substitution in chimera II (A174K). (A) Binding of MAbs prepared against fHbp v.1. JAR 1 and 5 are specific for v.1 proteins, while JAR 4 cross-reacts with v.1, v.2, and, to a lesser extent, v.3 (44). (B) Binding of MAbs prepared against fHbp v.2. JAR 10 cross-reacts with fHbp v.1, v.2, and v.3 (4), and JAR 11 and 13 cross-react with v.2 and v.3. (C) Binding of MAbs prepared against fHbp v.3. JAR 32 and 36 cross-react with v.2 and v.3.
JAR 10, 11, and 13 recognized epitopes on the C domain of fHbp v.2 of strain 8047. All three MAbs showed similar respective concentration-dependent binding with the chimera I protein as they did with the wild-type rfHbp v.2 control protein (Fig. 2B). As expected, JAR 11 did not bind to chimera II, since this protein had lysine substituted for alanine at position 174 (A174K), which eliminated the JAR 11 epitope and introduced the JAR 32 epitope (Fig. 2C). JAR 36, which cross-reacted with an epitope not yet defined but present on most strains expressing fHbp v.2 or v.3 fHbp, bound to both of the chimeric proteins and to the wild-type rfHbp v.2 control but not to the fHbp v.1 control (Fig. 2C). Collectively, the data showed that the two chimeric fHbps expressed epitopes associated with fHbp v.1, v.2, or v.3 proteins and reacted as expected with the various MAbs in accordance with our previous studies localizing the epitopes (Beernink et al., submitted). By ELISA, both chimeric proteins also showed similar concentration-dependent binding with purified human fH compared with binding by the two control v.1 and v.2 rfHbps (data not shown).
Immunogenicity.
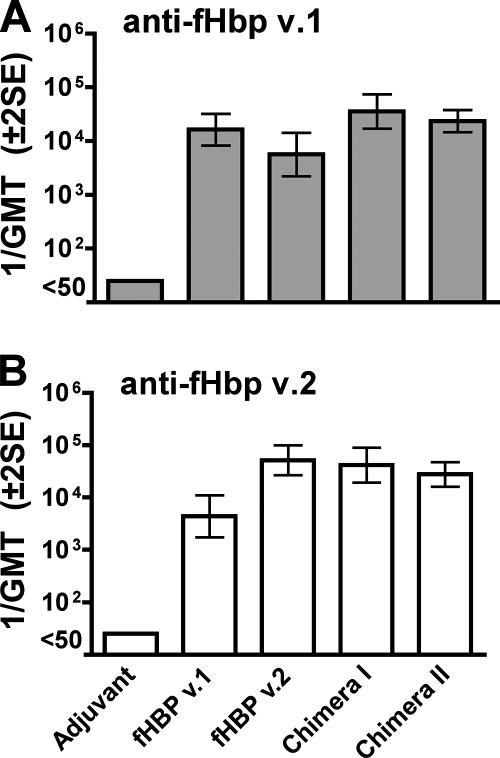
The IgG anti-fHbp v.1 antibody response of control mice immunized with wild-type fHbp v.1 was higher than that of the control mice immunized with fHbp v.2 (Fig. 3), but the difference was not statistically significant (P = 0.097). The IgG anti-fHbp v.2 antibody response of the control mice immunized with rfHbp v.2 was higher than that of mice immunized with fHbp v.1 (P = 0.005). In contrast, mice given either of the chimeric vaccines had anti-v.1 responses similar to those of control mice immunized with rfHbp v.1 and had anti-v.2 responses similar to those of the control mice immunized with rfHbp v.2 (Fig. 3).
FIG. 3.
IgG anti-fHbp antibody responses (1/GMT ± 2 SE) of mice (n = 5 per group), measured by ELISA using rfHbp v.1 (gene from strain MC58, top panel) or fHbp v.2 (gene from strain 8047, bottom panel) as the antigen on the plates. Mice immunized with wild-type rfHbp v.1 had a higher anti-rfHbp v.1 reciprocal GMT than mice immunized with fHbp v.2 (P = 0.097). Mice immunized with rfHbp v.2 had a higher anti-fHbp v.2 reciprocal GMT than mice immunized with fHbp v.1 (P = 0.005). There were no significant differences between the respective anti-v.1 antibody responses of mice given the chimera I or II vaccine and those of control mice immunized with the wild-type v.1 protein, and there were no significant differences between the respective anti-v.2 antibody responses of mice given the chimera I or II vaccine and those of control mice immunized with the wild-type v.2 protein.
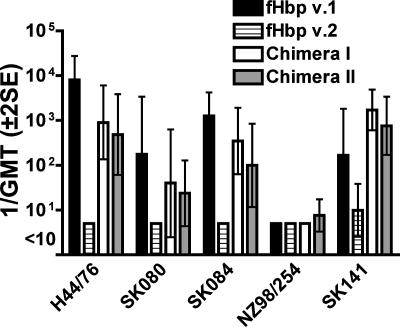
The GMTs of the bactericidal antibody measured with human complement in sera from the different groups of mice as measured against different strains expressing fHbp v.1 or subvariants of v.1 are summarized in Fig. 4. As expected, the control mice immunized with wild-type rfHbp v.1 had high responses when measured against strain H44/76, which expressed fHbp with an amino acid sequence identical to that of the recombinant v.1 protein vaccine (GMT of ∼1:10,000). The GMTs against the other test strains that expressed subvariants of fHbp v.1 ranged from <1:10 (strain NZ98/254) to >1:1,000 (strain SK084). The corresponding titers of the sera from control mice immunized with rfHbp v.2 were either negative (GMT of <1:10) (four strains) or low (GMT of 1:10) (strain SK141). The mice immunized with either chimeric recombinant protein vaccine developed serum bactericidal antibodies against all four v.1 strains that were susceptible to bactericidal activity of sera from the control mice immunized with the wild-type fHbp v.1. For three of the four test strains, the respective GMTs of the chimeric vaccine groups were ∼1 log unit lower than those of control mice immunized with the fHbp v.1 vaccine. Against the fourth strain, SK141, the responses to both chimeric vaccines were as high as or higher than those of the positive control mice that received fHbp v.1.
FIG. 4.
Serum bactericidal antibody responses (1/GMT ± 2 SE) of mice (n = 5 per group) immunized with chimeric rfHbp vaccines as measured against strains expressing fHbp in the v.1 antigenic group. Strain H44/76 expresses fHbp v.1 with an amino acid sequence identical to that of the fHbp v.1 control vaccine. The remaining strains express subvariants of fHbp v.1 (Table 2).
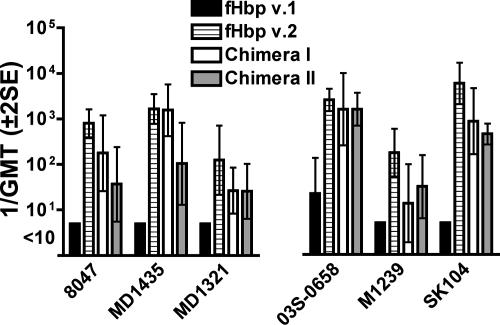
The serum bactericidal antibody responses measured against the six strains expressing fHbp in the v.2 or v.3 variant group (Tables 1 and 2) are shown in Fig. 5. Three of the test strains were JAR 11 positive (left panel), and three were JAR 32 positive (right panel). Sera from the control mice immunized with rfHbp v.2 were bactericidal against all six strains. With one exception, strain 03S-0658, the serum bactericidal titers of control mice immunized with rfHbp v.1 were <1:10. The sera from the mice immunized with either chimeric vaccine were bactericidal against all six strains. For four of the strains (8047, MD1321, M1239, and SK104), the respective GMTs of the chimeric groups were ∼1 log unit lower than those of the control mice immunized with rfHbp v.2. For the remaining two strains (03S-0658 and MD1435), the serum GMTs of one or both chimeric vaccine groups were similar to those of the mice given the positive control rfHbp v.2 vaccine. Thus, in contrast to the control rfHbp v.1 and v.2 vaccines, which elicited strain-specific bactericidal antibody responses, the chimeric vaccines elicited bactericidal antibody responses against strains expressing fHbp from each of the three antigenic variant groups.
FIG. 5.
Serum bactericidal antibody responses (1/GMT ± 2 SE) of mice (n = 5 per group) immunized with rfHbp vaccines against strains expressing fHbp in the v.2 or v.3 antigenic group. Strain 8047 expresses fHbp v.2 identical to that of control rfHbp v.2 vaccine. The remaining strains express subvariants of fHbp v.2 or v.3 (Table 2). The data are grouped by JAR 11 (left panel) or JAR 32 (right panel) reactivity. The chimera I and II vaccines are identical except that chimera I is JAR 11 positive and JAR 32 negative, whereas chimera II is JAR 11 negative and JAR 32 positive (see text).
Although the chimera I vaccine expressed the JAR 11 epitope (A174) (Fig. 1) and the chimera II vaccine expressed the JAR 32 epitope (K174), the respective serum bactericidal responses of the groups of mice immunized with either chimeric vaccine were not significantly different when measured against strains expressing JAR 11-positive or JAR 32-positive fHbp (Fig. 5). The only exception was against the JAR 11-positive strain MD1435, where there was a trend for a higher GMT in the group of mice immunized with chimera I than in those immunized with chimera II (P = 0.06).
DISCUSSION
Many of the new protein antigens being investigated as vaccine candidates for prevention of group B meningococcal disease show antigenic variability and/or variable expression by different N. meningitidis strains (25, 29, 31-33, 43-45). Achievement of broad protection by a recombinant protein-based vaccine, therefore, will likely require the use of multiple antigens (17, 45). However, there may be limits to the number of different recombinant proteins that can be combined into one vaccine without loss of immunogenicity. Therefore, for a multicomponent vaccine containing fHbp, it would be desirable to use a single rfHbp capable of eliciting serum bactericidal antibodies against strains expressing fHbps from the different major antigenic variant groups.
Chimeric proteins previously have been used for vaccine development in a variety of ways. One strategy employed a genetic fusion of two different antigens from the same organism to enhance cross-protection against strains with antigenic diversity (18, 46). An example is a multicomponent meningococcal group B vaccine that contains two fusion proteins, GNA2091 fused with fHbp v.1 and GNA2132 fused with GNA1030, along with a third antigen, rNadA (17). In mice each of the individual fusion proteins elicited serum bactericidal antibody responses that were as high as or higher than those elicited by the respective individual recombinant protein antigens. Further, the multicomponent vaccine containing five meningococcal antigens elicited broader bactericidal responses than any of the respective individual antigens.
Another strategy has been to construct a fusion of different serologic variants (“serovars”) of an individual antigen to induce cross-protection against strains with antigenic diversity. An example is a tetravalent OspC chimeric Lyme disease vaccine that induced bactericidal antibody responses against spirochete strains expressing each of the OspC types that were incorporated into the construct (14). In another example, four different serologic variants of domain III of the dengue fever virus envelope protein were fused together for use as a diagnostic protein (21). These chimeric antigens consisted of repeats of an individual domain with antigenic variability. The respective variants of the domain were expressed in tandem in one protein (i.e., the same domain from different strains, A1-A2-A3-A4, etc.). In some cases, these recombinant tandem proteins can be convenient for manufacturing and quality control. However, they also can be very large and subject to improper folding or degradation.
The chimeric fHbp antigens that we investigated differed from the fusion proteins described above in that we combined different individual domains from two proteins (domain A and part of domain B from a v.1 protein with the remainder of domains B and C from a v.2 protein, i.e., A1-B1/B2-C2) to form chimeric proteins that expressed antigenically unrelated epitopes that were specific for all three fHbp v. groups. There are few examples of combining epitopes expressed by different domains into a single chimeric antigen that resulted in an effective immunogen. In one previous study, the investigators demonstrated that a truncated meningococcal rfHbp consisting only of the B and C domains elicited bactericidal titers similar to those elicited by the respective rfHbp v.1 protein containing the A domain (18). They then constructed a hybrid of the B domain of an fHbp v.3 protein with the C domain of an fHbp v.1 protein. However, in contrast to the A1-B1/2-C2 chimeric proteins investigated in the present study, the B3-C1 chimeric fHbp investigated previously did not elicit serum bactericidal antibody responses in mice against strains expressing fHbp from either v.1 or v.3. In retrospect, this chimeric vaccine may not have worked because the chimeric protein contained the B domain from fHbp v.3, which lacks the region of the JAR 3/5 epitope (including G121) that may be critical for eliciting high serum bactericidal antibody titers to strains expressing fHbp v.1. Also, the C domain of the v.1 protein did not contain the JAR 10, 11, 13, or 32 epitopes, which interact with bactericidal antibodies directed at fHbp v.2 or v.3. In contrast, both of the chimeric fHbps that we investigated contained the portion of the B domain of the v.1 protein with the JAR 3/5 epitope and the C domain of a v.2 protein that expressed multiple epitopes shown to interact with bactericidal MAbs against strains with fHbp from the v.2 or v.3 variant group (Fig. 2).
Mice immunized with either chimeric protein vaccine I or II developed serum anti-fHbp antibodies with bactericidal activity against N. meningitidis strains that expressed fHbp v.1, v.2, or v.3, whereas the respective control wild-type rfHbp v.1 or v.2 vaccines elicited bactericidal responses predominantly against strains that expressed fHbp from the respective variant group homologous to that of the vaccine. Although the magnitudes of the serum bactericidal titers of the mice immunized with the chimeric vaccines were lower than those of control mice immunized with the corresponding wild-type rfHbp v.1 or v.2 vaccine, the data from the groups given the chimeric vaccines provide proof of concept that an individual chimeric protein can elicit serum antibodies that are bactericidal with human complement against strains expressing fHbps from all three antigenic variant groups. One limitation of our study was that we used FA, which is not suitable for use in humans. Therefore, additional studies are needed to investigate the immunogenicity of the chimeric proteins given with adjuvants that are more suitable for use in humans.
Despite engineering expression of the JAR 11 epitope in chimera I and of the JAR 32 epitope in chimera II, we found no statistically significant differences in the respective serum bactericidal antibody responses of mice immunized with either vaccine when measured against JAR 11-positive strains or JAR 32-positive strains. At the time we designed the chimeric vaccines, we did not know that binding of antibody to an epitope located near residue 174 (i.e., JAR 11 in some strains or JAR 32 in others) was not sufficient to elicit complement-mediated bactericidal activity in the absence of a second antibody that binds to an epitope associated with an ion pair at residues 180 and 192 (such as JAR 10 in some strains or JAR 33 in others) (Beernink et al., submitted). Among wild-type strains expressing fHbp v.2 or v.3, expression of JAR 32 is usually associated with expression of JAR 33, while expression of JAR 11 is usually associated with expression of JAR 10 (see, for example, our strain panel [Table 2]). Therefore, in the future it may be possible to generate more effective chimeric fHbp vaccines against JAR 32-positive strains by engineering a chimeric protein that also expresses the JAR 33 epitope.
Acknowledgments
We thank Ray Chen and Ryan Palapaz for providing expert technical assistance. We are indebted to Jo Anne Welsch, who prepared the anti-fHbp MAbs used in this study.
This study was supported by Public Health Service grant R01 AI46464 from the National Institute of Allergy and Infectious Diseases, NIH. The laboratory work was performed in a facility funded by Research Facilities Improvement Program grant C06 RR16226 from the National Center for Research Resources, NIH.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 24 March 2008.
REFERENCES
- 1.Amadou Hamidou, A., S. Djibo, A. Elhaj Mahamane, A. Moussa, H. Findlow, F. Sidikou, R. Cisse, A. Garba, R. Borrow, S. Chanteau, and P. Boisier. 2006. Prospective survey on carriage of Neisseria meningitidis and protective immunity to meningococci in schoolchildren in Niamey (Niger): focus on serogroup W135. Microbes Infect. 82098-2104. [DOI] [PubMed] [Google Scholar]
- 2.Baker, M. G., D. R. Martin, C. E. Kieft, and D. Lennon. 2001. A 10-year serogroup B meningococcal disease epidemic in New Zealand: descriptive epidemiology, 1991-2000. J. Paediatr. Child Health 37S13-S19. [DOI] [PubMed] [Google Scholar]
- 3.Beernink, P. T., A. Leipus, and D. M. Granoff. 2006. Rapid genetic grouping of factor H-binding protein (genome-derived neisserial antigen 1870), a promising group B meningococcal vaccine candidate. Clin. Vaccine Immunol. 13758-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beernink, P. T., J. A. Welsch, L. H. Harrison, A. Leipus, S. L. Kaplan, and D. M. Granoff. 2007. Prevalence of factor H-binding protein variants and NadA among meningococcal group B isolates from the United States: implications for the development of a multicomponent group B vaccine. J. Infect. Dis. 1951472-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berild, D., T. W. Gedde-Dahl, and T. Abrahamsen. 1980. Meningococcal disease in the Norwegian Armed Forces 1967-1979. Some epidemiological aspects. NIPH Ann. 323-30. [PubMed] [Google Scholar]
- 6.Bovre, K., E. Holten, H. Vik-Mo, A. Brondbo, D. Bratlid, P. Bjark, and P. J. Moe. 1977. Neisseria meningitidis infections in northern Norway: an epidemic in 1974-1975 due mainly to group B organisms. J. Infect. Dis. 135669-672. [DOI] [PubMed] [Google Scholar]
- 7.Cantini, F., S. Savino, M. Scarselli, V. Masignani, M. Pizza, G. Romagnoli, E. Swennen, D. Veggi, L. Banci, and R. Rappuoli. 2006. Solution structure of the immunodominant domain of protective antigen GNA1870 of Neisseria meningitidis. J. Biol. Chem. 2817220-7227. [DOI] [PubMed] [Google Scholar]
- 8.Capecchi, B., J. Adu-Bobie, F. Di Marcello, L. Ciucchi, V. Masignani, A. Taddei, R. Rappuoli, M. Pizza, and B. Arico. 2005. Neisseria meningitidis NadA is a new invasin which promotes bacterial adhesion to and penetration into human epithelial cells. Mol. Microbiol. 55687-698. [DOI] [PubMed] [Google Scholar]
- 9.Caugant, D. A., C. Fogg, F. Bajunirwe, P. Piola, R. Twesigye, F. Mutebi, L. O. Froholm, E. Rosenqvist, V. Batwala, I. S. Aaberge, J. A. Rottingen, and P. J. Guerin. 2006. Pharyngeal carriage of Neisseria meningitidis in 2-19-year-old individuals in Uganda. Trans. R Soc. Trop. Med. Hyg. 1001159-1163. [DOI] [PubMed] [Google Scholar]
- 10.Claus, H., M. C. Maiden, D. J. Wilson, N. D. McCarthy, K. A. Jolley, R. Urwin, F. Hessler, M. Frosch, and U. Vogel. 2005. Genetic analysis of meningococci carried by children and young adults. J. Infect. Dis. 1911263-1271. [DOI] [PubMed] [Google Scholar]
- 11.Comanducci, M., S. Bambini, B. Brunelli, J. Adu-Bobie, B. Arico, B. Capecchi, M. M. Giuliani, V. Masignani, L. Santini, S. Savino, D. M. Granoff, D. A. Caugant, M. Pizza, R. Rappuoli, and M. Mora. 2002. NadA, a novel vaccine candidate of Neisseria meningitidis. J. Exp. Med. 1951445-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devi, S. J., W. D. Zollinger, P. J. Snoy, J. Y. Tai, P. Costantini, F. Norelli, R. Rappuoli, and C. E. Frasch. 1997. Preclinical evaluation of group B Neisseria meningitidis and Escherichia coli K92 capsular polysaccharide-protein conjugate vaccines in juvenile rhesus monkeys. Infect. Immun. 651045-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dyet, K. H., and D. R. Martin. 2006. Clonal analysis of the serogroup B meningococci causing New Zealand's epidemic. Epidemiol. Infect. 134377-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Earnhart, C. G., E. L. Buckles, and R. T. Marconi. 2007. Development of an OspC-based tetravalent, recombinant, chimeric vaccinogen that elicits bactericidal antibody against diverse Lyme disease spirochete strains. Vaccine 25466-480. [DOI] [PubMed] [Google Scholar]
- 15.Finne, J., M. Leinonen, and P. H. Makela. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet ii355-357. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher, L. D., L. Bernfield, V. Barniak, J. E. Farley, A. Howell, M. Knauf, P. Ooi, R. P. Smith, P. Weise, M. Wetherell, X. Xie, R. Zagursky, Y. Zhang, and G. W. Zlotnick. 2004. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect. Immun. 722088-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giuliani, M. M., J. Adu-Bobie, M. Comanducci, B. Arico, S. Savino, L. Santini, B. Brunelli, S. Bambini, A. Biolchi, B. Capecchi, E. Cartocci, L. Ciucchi, F. Di Marcello, F. Ferlicca, B. Galli, E. Luzzi, V. Masignani, D. Serruto, D. Veggi, M. Contorni, M. Morandi, A. Bartalesi, V. Cinotti, D. Mannucci, F. Titta, E. Ovidi, J. A. Welsch, D. Granoff, R. Rappuoli, and M. Pizza. 2006. A universal vaccine for serogroup B meningococcus. Proc. Natl. Acad. Sci. USA 10310834-10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giuliani, M. M., L. Santini, B. Brunelli, A. Biolchi, B. Arico, F. Di Marcello, E. Cartocci, M. Comanducci, V. Masignani, L. Lozzi, S. Savino, M. Scarselli, R. Rappuoli, and M. Pizza. 2005. The region comprising amino acids 100 to 255 of Neisseria meningitidis lipoprotein GNA 1870 elicits bactericidal antibodies. Infect. Immun. 731151-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granoff, D. M., L. Harrison, and R. Borrow. 2008. Meningoccal vaccines, p. 399-434. In S. A. Plotkin, P. Offit, and W. A. Orenstein (ed.), Vaccines, 5th ed. Saunders Elsevier, Philadelphia, PA.
- 20.Gray, S. J., C. L. Trotter, M. E. Ramsay, M. Guiver, A. J. Fox, R. Borrow, R. H. Mallard, and E. B. Kaczmarski. 2006. Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the Meningococcal Reference Unit. J. Med. Microbiol. 55887-896. [DOI] [PubMed] [Google Scholar]
- 21.Hapugoda, M. D., G. Batra, W. Abeyewickreme, S. Swaminathan, and N. Khanna. 2007. Single antigen detects both immunoglobulin M (IgM) and IgG antibodies elicited by all four dengue virus serotypes. Clin. Vaccine Immunol. 141505-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan, S. L., G. E. Schutze, J. A. Leake, W. J. Barson, N. B. Halasa, C. L. Byington, C. R. Woods, T. Q. Tan, J. A. Hoffman, E. R. Wald, K. M. Edwards, and E. O. Mason, Jr. 2006. Multicenter surveillance of invasive meningococcal infections in children. Pediatrics 118e979-984. [DOI] [PubMed] [Google Scholar]
- 23.Kellerman, S. E., K. McCombs, M. Ray, W. Baughman, M. W. Reeves, T. Popovic, N. E. Rosenstein, M. M. Farley, P. Blake, and D. S. Stephens. 2002. Genotype-specific carriage of Neisseria meningitidis in Georgia counties with hyper- and hyposporadic rates of meningococcal disease. J. Infect. Dis. 18640-48. [DOI] [PubMed] [Google Scholar]
- 24.Kelly, C., R. Arnold, Y. Galloway, and J. O'Hallahan. 2007. A prospective study of the effectiveness of the New Zealand meningococcal B vaccine. Am. J. Epidemiol. 166817-823. [DOI] [PubMed] [Google Scholar]
- 25.Kortekaas, J., A. Pettersson, J. van der Biezen, V. E. Weynants, P. van der Ley, J. Poolman, M. P. Bos, and J. Tommassen. 2007. Shielding of immunogenic domains in Neisseria meningitidis FrpB (FetA) by the major variable region. Vaccine 2572-84. [DOI] [PubMed] [Google Scholar]
- 26.MacLennan, J., G. Kafatos, K. Neal, N. Andrews, J. C. Cameron, R. Roberts, M. R. Evans, K. Cann, D. N. Baxter, M. C. Maiden, and J. M. Stuart. 2006. Social behavior and meningococcal carriage in British teenagers. Emerg. Infect. Dis. 12950-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madico, G., J. A. Welsch, L. A. Lewis, A. McNaughton, D. H. Perlman, C. E. Costello, J. Ngampasutadol, U. Vogel, D. M. Granoff, and S. Ram. 2006. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J. Immunol. 177501-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maiden, M. C., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 953140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin, D., N. Cadieux, J. Hamel, and B. R. Brodeur. 1997. Highly conserved Neisseria meningitidis surface protein confers protection against experimental infection. J. Exp. Med. 1851173-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin, D. R., N. Ruijne, L. McCallum, J. O'Hallahan, and P. Oster. 2006. The VR2 epitope on the PorA P1.7-2,4 protein is the major target for the immune response elicited by the strain-specific group B meningococcal vaccine MeNZB. Clin. Vaccine Immunol. 13486-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masignani, V., M. Comanducci, M. M. Giuliani, S. Bambini, J. Adu-Bobie, B. Arico, B. Brunelli, A. Pieri, L. Santini, S. Savino, D. Serruto, D. Litt, S. Kroll, J. A. Welsch, D. M. Granoff, R. Rappuoli, and M. Pizza. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J. Exp. Med. 197789-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moe, G. R., S. Tan, and D. M. Granoff. 1999. Differences in surface expression of NspA among Neisseria meningitidis group B strains. Infect. Immun. 675664-5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moe, G. R., P. Zuno-Mitchell, S. S. Lee, A. H. Lucas, and D. M. Granoff. 2001. Functional activity of anti-neisserial surface protein A monoclonal antibodies against strains of Neisseria meningitidis serogroup B. Infect. Immun. 693762-3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pizza, M., V. Scarlato, V. Masignani, M. M. Giuliani, B. Arico, M. Comanducci, G. T. Jennings, L. Baldi, E. Bartolini, B. Capecchi, C. L. Galeotti, E. Luzzi, R. Manetti, E. Marchetti, M. Mora, S. Nuti, G. Ratti, L. Santini, S. Savino, M. Scarselli, E. Storni, P. Zuo, M. Broeker, E. Hundt, B. Knapp, E. Blair, T. Mason, H. Tettelin, D. W. Hood, A. C. Jeffries, N. J. Saunders, D. M. Granoff, J. C. Venter, E. R. Moxon, G. Grandi, and R. Rappuoli. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 2871816-1820. [DOI] [PubMed] [Google Scholar]
- 35.Russell, J. E., K. A. Jolley, I. M. Feavers, M. C. Maiden, and J. Suker. 2004. PorA variable regions of Neisseria meningitidis. Emerg. Infect. Dis. 10674-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sacchi, C. T., A. P. Lemos, M. E. Brandt, A. M. Whitney, C. E. Melles, C. A. Solari, C. E. Frasch, and L. W. Mayer. 1998. Proposed standardization of Neisseria meningitidis PorA variable-region typing nomenclature. Clin. Diagn. Lab. Immunol. 5845-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider, M. C., R. M. Exley, H. Chan, I. Feavers, Y. H. Kang, R. B. Sim, and C. M. Tang. 2006. Functional significance of factor H binding to Neisseria meningitidis. J. Immunol. 1767566-7575. [DOI] [PubMed] [Google Scholar]
- 38.Sierra, G. V., H. C. Campa, N. M. Varcacel, I. L. Garcia, P. L. Izquierdo, P. F. Sotolongo, G. V. Casanueva, C. O. Rico, C. R. Rodriguez, and M. H. Terry. 1991. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 14195-210. [PubMed] [Google Scholar]
- 39.Tappero, J. W., R. Lagos, A. M. Ballesteros, B. Plikaytis, D. Williams, J. Dykes, L. L. Gheesling, G. M. Carlone, E. A. Hoiby, J. Holst, H. Nokleby, E. Rosenqvist, G. Sierra, C. Campa, F. Sotolongo, J. Vega, J. Garcia, P. Herrera, J. T. Poolman, and B. A. Perkins. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 2811520-1527. [DOI] [PubMed] [Google Scholar]
- 40.Trotter, C. L., M. Chandra, R. Cano, A. Larrauri, M. E. Ramsay, C. Brehony, K. A. Jolley, M. C. Maiden, S. Heuberger, and M. Frosch. 2007. A surveillance network for meningococcal disease in Europe. FEMS Microbiol. Rev. 3127-36. [DOI] [PubMed] [Google Scholar]
- 41.Trotter, C. L., N. J. Gay, and W. J. Edmunds. 2006. The natural history of meningococcal carriage and disease. Epidemiol. Infect. 134556-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welsch, J. A., and D. Granoff. 2007. Immunity to Neisseria meningitidis group B in adults despite lack of serum bactericidal activity. Clin. Vaccine Immunol. 141596-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welsch, J. A., S. Ram, O. Koeberling, and D. M. Granoff. 2008. Complement-dependent synergistic bactericidal activity of antibodies against factor H-binding protein, a sparsely distributed meningococcal vaccine antigen. J. Infect. Dis. 1971053-1061. [DOI] [PubMed] [Google Scholar]
- 44.Welsch, J. A., R. Rossi, M. Comanducci, and D. M. Granoff. 2004. Protective activity of monoclonal antibodies to genome-derived neisserial antigen 1870, a Neisseria meningitidis candidate vaccine. J. Immunol. 1725606-5615. [DOI] [PubMed] [Google Scholar]
- 45.Weynants, V. E., C. M. Feron, K. K. Goraj, M. P. Bos, P. A. Denoel, V. G. Verlant, J. Tommassen, I. R. Peak, R. C. Judd, M. P. Jennings, and J. T. Poolman. 2007. Additive and synergistic bactericidal activity of antibodies directed against minor outer membrane proteins of Neisseria meningitidis. Infect. Immun. 755434-5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, P., C. Jespersgaard, L. Lamberty-Mallory, J. Katz, Y. Huang, G. Hajishengallis, and S. M. Michalek. 2002. Enhanced immunogenicity of a genetic chimeric protein consisting of two virulence antigens of Streptococcus mutans and protection against infection. Infect. Immun. 706779-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]