Abstract
Toxoplasma gondii is one of the most successful protozoan parasites of warm-blooded animals. Stage-specific expression of its surface molecules is thought to be key to its ability to establish chronic infection in immunocompetent animals. The rapidly dividing tachyzoite stage displays a different subset of family of surface antigen 1 (SAG1)-related sequences (SRSs) from that displayed by the encysted bradyzoite stage. It is possible that this switch is necessary to protect the bradyzoites against an immune response raised against the tachyzoite stage. Alternatively, it might be that bradyzoite SRSs evolved to facilitate invasion of different cell types, such as those found in the brain, where cysts develop, or the small intestine, where bradyzoites must enter after oral infection. Here we studied the function of a cluster of four tandem genes, encoding bradyzoite SRSs called SAG2C, -D, -X, and -Y. Using bioluminescence imaging of mice infected with parasites expressing firefly luciferase (FLUC) driven by the SAG2D promoter, we show stage conversion for the first time in living animals. A truncated version of the SAG2D promoter (SAG2Dmin) gave efficient expression of FLUC in both tachyzoites and bradyzoites, indicating that the bradyzoite specificity of the complete SAG2D promoter is likely due to an element(s) that normally suppresses expression in tachyzoites. Comparing mice infected with the wild type or a mutant where the SAG2CDXY cluster of genes has been deleted (ΔSAG2CDXY), we demonstrate that whereas ΔSAG2CDXY parasites are less capable of maintaining a chronic infection in the brain, they do not show a defect in oral infectivity.
The Toxoplasma gondii surface is dominated by a family of glycosylphosphatidylinositol-anchored proteins related to surface antigen 1 (SAG1) (12). The majority of SAG1-related sequence (SRS) proteins are expressed in a stage-specific manner, such that the tachyzoite surface is dominated by SAG1, SAG2A, SAG3, SRS1, SRS2, SRS3, and several less highly expressed SRSs (16), while the bradyzoite surface is dominated by SAG2C, SRS9, and SAG4, a molecule not related to the SRS family. Sequence analysis demonstrated that the SRS family is divided into two major branches, the SAG1-like sequence family and the SAG2-like sequence family (12). The precise function of these SRS molecules is unknown, although it is thought that they play an important role in modulating the immune response. SAG1 and SAG2A are immunodominant within the superfamily and induce a high antibody response early after infection (2, 18). It is unknown if the bradyzoite-specific SRSs evolved just to be different from their tachyzoite counterparts and hence not recognizable by the strong immune response generated against the tachyzoite SRSs or if they have a more active role. Some of the tachyzoite SRSs have been shown to be involved in attachment (invasion) (7, 11, 17, 20), and so it is possible that bradyzoite SRSs have a role in attachment to cells in the small intestine, as this is the site where bradyzoites invade after ingestion of a cyst by a host, or attachment to cells in the brain, as this is the site where many Toxoplasma cysts can be found in a chronic infection. Recently, it was reported that one of the major bradyzoite surface antigens belonging to the SAG1 family, SRS9, plays a role in maintaining parasite persistence in the brain (14). In this study, we sought to determine the function of a cluster of genes, SAG2CDXY, encoding bradyzoite surface molecules belonging to the SAG2 family. In vivo stage switching was visualized using bioluminescence imaging (BLI) of mice infected with parasites engineered to express firefly luciferase (FLUC) from the full SAG2D promoter. The function of SAG2CDXY was studied by generating knockout parasites expressing FLUC from a constitutive promoter. We concluded that stage switching begins around 9 days after infection and that the SAG2CDXY cluster is important for persistence of cysts in the host.
MATERIALS AND METHODS
Parasites.
All Toxoplasma strains used in this work were derived from the type II Prugniaud (Pru) strain, which lacks the hypoxanthine-xanthine-guanine-phosphoribosyltransferase gene (HPT) and was a gift from D. Soldati (University of Geneva, Geneva, Switzerland). For the in vitro luciferase assays, we used the engineered Prugniaud strain BSG-4, which lacks HPT and expresses green fluorescent protein (GFP) under the control of the LDH2 bradyzoite-specific promoter (24). Parasites were maintained in vitro by serial passage on monolayers of human foreskin fibroblasts (HFFs) at 37°C in 5% CO2 as previously described (21). HFFs were grown in Dulbecco's modified Eagle's medium (Gibco BRL) supplemented with 10% NuSerum (Collaborative Biomedical Products), 2 mM glutamine, 50 μg/ml each of penicillin and streptomycin, and 20 μg/ml gentamicin.
Generation of bioluminescent ΔSAG2CDXY strain.
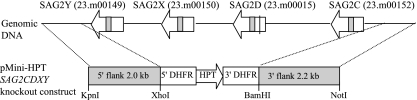
Generation of the Pru Δhpt/GFP/FLUC strain, expressing GFP from the GRA2 promoter and FLUC from the TUB1 promoter (Pru Δhpt/GFP/FLUC), has been described previously (14). A ΔSAG2CDXY strain lacking the entire protein coding region for SAG2C, SAG2D, SAG2X, and SAG2Y was created from the Pru Δhpt/GFP/FLUC strain by double homologous recombination with the pMini-HPT knockout vector (kindly provided by D. S. Roos, University of Pennsylvania, Philadelphia), in which the 2-kb sequence upstream of the SAG2C 5′ untranslated region (5′UTR) and the 2.2-kb sequence downstream of the SAG2Y 3′UTR were placed flanking the HPT-selectable marker, with the latter in inverted orientation relative to the SAG2CDXY genes (see Fig. 2). The primers (including restriction sites) used to PCR clone these flanking sequences from Prugniaud genomic DNA were 5′-CCGCTCGAGCTCGAAGTGCTAATGAGTGACGTT-3′ and 5′-GGGGTACCGGTCCACTCTTCTGTTAGCCTGTC-3′ for the HPT 5′-flanking sequence (from downstream of SAG2Y) and 5′-ATAAGAATGCGGCCGCCAGGGAGATAATTAAAGCCACACA-3′ and 5′-CGGGATCCTTCCGCACACTACCAGTAGAAAAG-3′ for the HPT 3′-flanking sequence (from upstream of SAG2C). The SAG2CDXY knockout construct was linearized with NotI, and 10 μg, 25 μg, and 50 μg of DNA were used to transform 5 × 106 Pru Δhpt/GFP/FLUC parasites by electroporation (26). After three rounds of lysis of HFFs in selection medium (mycophenolic acid [MPA] [100 μg/ml] and xanthine [50 μg/ml]) in a T25 flask, parasites were cloned by limiting dilution. Clones that resulted from a heterologous insertion of the knockout vector were also obtained and used as the heterologous control in subsequent studies.
FIG. 2.
Generation of Pru ΔSAG2CDXY strain. The figures shows a schematic of the SAG2C locus in type II strains. The four closely related genes SAG2C, -D, -X, and -Y are tandemly located on chromosome X. Gene identification numbers (Toxodb release 4.2) are given in parentheses. The premature stop codon in the SAG2D gene is indicated, and introns are shown as shaded boxes. The map is not drawn to scale. Double homologous recombination between the SAG2CDXY knockout construct and genomic DNA replaces SAG2CDXY with the HPT gene, which was used for positive selection.
Generation of DNA constructs with FLUC under the regulation of the SAG2D promoter.
The FLUC constructs were designed using the plasmid pTgEX2 (23), which has a multicloning site flanked by the promoter and downstream regions of the SAG1 gene. To introduce the HPT marker, a SacII-blunted fragment, pMini-HPT (5), was cloned into the BsaAI site. To the resulting plasmid, pHEX2, we inserted a PCR fragment of the FLUC coding sequence (GenBank accession no. P08659) lacking the final three codons into the NcoI site of the multicloning site, which created the vector PHEX2LUC. As a further modification, the SAG1 3′UTR was replaced by the T. gondii TUB1 3′UTR, using the flanking BamHI sites. The resulting vector, PHEX2LUCtub, was digested with XhoI for 2 h and with NcoI for 10 min to eliminate the SAG1 promoter and to retain FLUC sequences. In place of the SAG1 promoter, we introduced either of two different PCR fragments that included various lengths of the presumptive promoter region of the SAG2D gene. SAG2Dfull includes sequences from nucleotide −1684 (where nucleotide +1 is the start of transcription, as determined by 5′ rapid amplification of cDNA ends) to the nucleotide immediately 5′ of the start codon (nucleotide +155) and was amplified using the primers 5′-GGGCCGCCTCGAGGTCACATTGAGATGCAGAGACC-3′ and 5′-GGGCATGCCATGGCGCCAGTAGCGCCAGAAGGAG-3′. The SAG2Dmin promoter fragment includes sequences from nucleotide −113 to the nucleotide immediately 5′ of the start codon and was expanded using primer 5′-GGGCCGCCTCGAGCAAGGACGCGCACGCCCCCATTCC-3′ in combination with the same antisense primer used for SAG2Dfull. The two resulting plasmids were named psag2DfullFLUCtub and psag2DminFLUCtub, respectively. To create plasmids for FLUC and Renilla reniformis luciferase (RLUC) under the control of the constitutive TUB1 promoter, we first digested PHEX2LUCtub with KpnI and NcoI and ligated in a PCR product with the TUB1 promoter sequences (as defined in reference 25). The resulting plasmid, ptubMCStub, has the TUB1 promoter followed by a multicloning site and the TUB1 3′UTR. An NcoI fragment from PHEX2LUCtub that includes FLUC was introduced to produce ptubFLUCtub. To create ptubRLUCtub, ptubMCStub was digested with NcoI and PacI to introduce a PCR fragment carrying RLUC (GenBank accession no. M63501.1).
In vitro luciferase expression assay.
To compare the activities of the various promoters, we transformed BSG-4 parasites with 50 μg of psag2DfullFLUCtub, psag2DminFLUCtub, or ptubFLUCtub, using electroporation. So we could compare the results across transformations, 50 μg ptubRLUCtub was included in all reaction mixtures. After electroporation, each transformation reaction mix was divided into two T25 tissue culture flasks containing human fibroblasts in Dulbecco's modified Eagle's growth medium (described above) and placed in a 37°C incubator for 6 h. At this point, the medium in one of the two T25 flasks was changed to fresh medium and returned to the 37°C incubator. The medium in the other T25 flask was changed to RPMI-1 tissue culture medium (Gibco) supplemented with 1% fetal bovine serum and 50 mM HEPES, pH 8.1, and the culture was placed in a 37°C incubator at ambient CO2. In this manner, one-half of each transformation mix was grown under tachyzoite conditions, and the other half was grown under conditions that promote bradyzoite development. The expression of GFP was used to visually confirm bradyzoite development. Seventy-two hours after transformation, both the tachyzoite and the bradyzoite cultures were processed to measure the expression of both FLUC and RLUC. To do this, cultures were scraped, spun down at 2,000 × g for 10 min at room temperature, and resuspended in 50 μl of the passive lysis buffer provided with a dual-luciferase assay system (Promega). The lysate was treated with three freeze-thaw cycles and spun down in a microcentrifuge at full speed for 5 min at 4°C. Twenty-five microliters of the supernatant was assayed in a Monolight 2010 luminometer (Analytical Luminescence Laboratory) sequentially for FLUC activity and then for RLUC activity, using a dual-luciferase assay system (Promega). The result for each lysate was expressed as the ratio of FLUC activity to that of RLUC. The ratio for the bradyzoite sample over the ratio for the tachyzoite sample was used to assess whether the promoter expressing FLUC had stage-specific activity.
Growth competition assay.
To determine if ΔSAG2CDXY luciferase-expressing strains were impaired in their in vitro growth capacity, equal numbers of the ΔSAG2CDXYFLUC/GFP strain and its wild-type counterpart were mixed and inoculated into T25 flasks with HFFs. At the same time, 100 parasites from the mixture were inoculated in triplicate onto a 24-well plate with HFFs for plaque (viability) assay. After lysis of the T25 monolayer by the parasites, 1/20 of the culture was transferred to another T25 containing HFFs. After 10 passages, another plaque assay was performed and the ratio between the different parasite strains (as determined by the presence or absence of GFP fluorescence) was calculated.
Infection of mice and reactivation of Toxoplasma.
Female BALB/c mice aged 5 to 10 weeks (Jackson Laboratories, Bar Harbor, ME) and female CD-1 mice aged 5 to 10 weeks (Charles River Laboratories, Wilmington, MA) were used. For intraperitoneal (i.p.) infections, tachyzoites were grown in vitro, extracted from host cells by passage through a 27-gauge needle, and quantified using a hemocytometer. Parasites were diluted in phosphate-buffered saline (PBS), and mice were injected i.p. with tachyzoites (in a volume of 200 μl), using a 27-gauge needle. Since viability of parasites isolated in this manner can vary from batch to batch, plaque assays were performed on these preparations to determine the number of viable tachyzoites injected. Dexamethasone was used to reactivate T. gondii as described previously (22). Briefly, dexamethasone (dexamethasone 21-phosphate disodium salt; Sigma) was dissolved at a concentration of 30 mg/liter drinking water, and treatment was started 100 days after infection.
Cyst counts and oral feeding.
A single mouse brain was homogenized over a 100-μm cell strainer, washed in 15 ml of PBS by centrifugation at 1,500 × g for 5 min, and resuspended in 1 ml of PBS. One hundred microliters of this suspension was added to 900 μl of cold methanol and incubated for 5 min. This suspension was washed again by centrifugation and resuspended in 500 μl of a 1:150 dilution of fluorescein-conjugated Dolichos biflorus agglutinin (Vector Laboratories) in PBS to stain the cyst wall. This was then rotated at 4°C for 2 to 24 h, after which it was washed and resuspended in 1 ml of PBS. Fifty-microliter aliquots of this were added to the wells of a 96-well plate containing 100 μl of PBS. Cysts were counted in all 20 wells by use of an inverted fluorescence microscope. For oral infections, cysts from 1/10 of the brain of a chronically infected mouse were counted and ∼150 cysts from the rest of the brain suspension were fed to naïve mice as previously described (3).
In vivo BLI.
An in vivo imaging system (IVIS; Xenogen, Alameda, CA) procedure for imaging Toxoplasma gondii-infected mice has been described in detail previously (22). In short, mice were injected with 200 μl of luciferin (3 mg) and immediately anesthetized in an oxygen-rich induction chamber with 2% isofluorane. Mice were maintained for at least 10 min to allow for adequate dissemination of the injected substrate and for the mice to become fully anesthetized. Mice were imaged in dorsal, ventral, and sometimes lateral positions by collecting two images, a grayscale reference image obtained under low-level illumination and an image of light emission from luciferase expressed in parasites within the animal and transmitted through the tissue. Images were collected with 1- to 5-min integration times depending on the intensity of the bioluminescent signal, and pseudocolor representations of light intensity (with red being the most intense and blue being the least intense) were superimposed over the grayscale reference image. In certain cases, mice were sacrificed after imaging, and individual organs were excised from the mice and imaged ex vivo. Data acquisition and analysis were performed using LivingImage (Xenogen) software with the IgorPro image analysis package (WaveMetrics, Seattle, WA).
rSAG2C, rSAG2X, and rSAG2Y.
Recombinant SAG2C (rSAG2C), rSAG2X, and rSAG2Y were produced in insect cells as secreted proteins by using pAcGP67, a baculovirus expression vector previously described for rSAG1 production (10). Protein coding regions lacking the N-terminal signal sequence and C-terminal glycosylphosphatidylinositol anchor signal were PCR cloned from Prugniaud strain genomic DNA, using the following primers: for SAG2C, 5′-ATCGGATCCCAAGGCGAACTCACAGTCTATTTC-3′ and 5′-CTATCTAGACTAGTGATGGTGATGGTGATGTTGTTCCCCTCTGGAAGGTT-3′; for SAG2X, 5′-ATCGGATCCCTCGGTCCCACAGCAACCAGTT-3′ and 5′-CTATCTAGACTAGTGATGGTGATGGTGATGTCGTTCACCACCCGAAGGTG-3′; and for SAG2Y, 5′-ATCGGATCCCAAGGCATCCGAAGTCCAGTC-3′ and 5′-CTATCTAGACTAGTGATGGTGATGGTGATGTCGTTCACCACCCGAAGGTG-3′. All reverse primers encoded a six-His tag. Highly pure proteins were obtained by using nickel agarose beads.
Antisera against SAG2C, SAG2X, and SAG2Y.
Production of mouse antibodies against SAG2C has been described previously (15). Rabbit polyclonal anti-SAG2Y antisera were raised by intradermal immunization (100 μg of rSAG2Y in complete Freund's adjuvant) followed by four subcutaneous boosts spaced 3 weeks apart (50 μg of rSAG2Y in incomplete Freund's adjuvant [Covance Research Products]). Peptide-specific antibodies against SAG2X and SAG2Y were raised by immunization with 20 μg of peptide (Ac-CDEGLELHKKPTEDG-CONH2 and Ac-CAGLQLRAKPENDEM-CONH2, respectively) per mouse in 200 μl of Ribi adjuvant, composed of purified monophosphoryl lipid A and synthetic trehalose dicorynomycolate (Corixa). Mice were given four boosts spaced 3 weeks apart with the same Ag preparations. Tail vein blood was allowed to coagulate overnight at 4°C and spun for 5 min at 5,000 × g in a microcentrifuge to collect serum.
Immunofluorescence microscopy.
Infected HFFs grown on coverslips were fixed with 3% formaldehyde, permeabilized with 100% ethanol, and blocked with PBS-5% bovine serum albumin-0.2% Triton X-100. Samples were incubated in PBS-3% bovine serum albumin with the indicated anti-parasite antigen antibodies followed by appropriate goat immunoglobulin G (IgG) coupled with Alexa Fluor 488 or Alexa Fluor 594 (Molecular Probes). Coverslips were mounted on a glass slide with Vectashield (Vector Laboratories), and photographs were taken using Image Pro Plus software and a 35-mm digital camera (model C4742-95; Hamamatsu) connected to an upright (model BX60; Olympus) (magnification, ×1,000) or inverted (model Eclipse TE300; Nikon) (magnification, ×400) fluorescence microscope.
In vitro bradyzoite differentiation.
To switch parasites, HFFs were infected with freshly lysed parasites, and after 4 hours, the medium was changed to medium with high pH (8.1) and low serum (1%) and the flask was placed at 37°C at ambient (low) CO2. After 4 days, lysates were prepared as described below.
Protein gel electrophoresis and Western blotting.
Infected monolayers of HFFs were washed with ice-cold PBS, lysed by addition of lysis buffer, boiled for 5 min, and subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were transferred to a polyvinylidene difluoride membrane, which was blocked in PBS-5% nonfat dry milk and incubated with antisera followed by peroxidase-conjugated goat anti-rabbit or anti-mouse IgG (Kirkegaard & Perry Laboratories). Detection was done with enhanced chemiluminescence reagents (Amersham Biosciences). A rabbit polyclonal antibody against GRA7 was used as a parasite loading control.
Statistics.
Student's t test was performed to compare cyst loads in the different experimental groups.
RESULTS
SAG2C, -D, -X, and -Y are members of the SAG2 branch of SRS proteins and are expressed in bradyzoites.
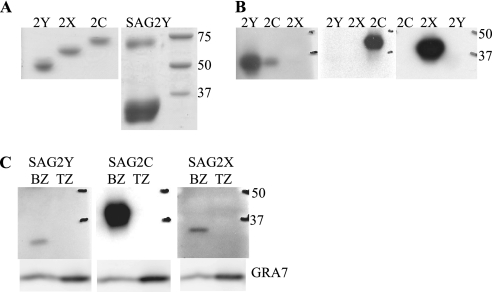
SAG2C (23.m00152) was predicted to be one of the most highly expressed bradyzoite-specific SRS antigens, based on the relative abundance of expressed sequence tags encoding it. Three closely related genes (named SAG2D [23.m00015], SAG2X [23.m00150], and SAG2Y [23.m00149]) are positioned next to it on chromosome X. In type II strains, such as the Prugniaud strain used here, but not in type I (RH) or III (CTg/CEP) strains, SAG2D has an insertion of an adenosine, causing a frameshift and a premature stop codon, two codons after the insertion, presumably causing this protein to be truncated prematurely (data not shown, but see toxodb.org). To investigate if SAG2Y and SAG2X are expressed, we made antigen-specific antibodies by injecting mice with peptides from SAG2X or -Y corresponding to regions with the least sequence similarity. Western blot analysis demonstrated that antibodies against rSAG2C (15), rSAG2X, and rSAG2Y (Fig. 1A) reacted only to the proteins to which they were raised, with little, if any, detectable cross-reactivity to the other two (Fig. 1B). Although the predicted molecular masses of SAG2Y, -X, and -C are 30, 31, and 32 kDa, they migrated at ∼28, 34, and 38 kDa, respectively. This could be due to protein differences in glycosylation by the insect cells, and indeed, they have one, two, and three predicted N-glycosylation sites, respectively. To determine if SAG2X and SAG2Y are also bradyzoite specific, we performed Western blotting on lysates of switched parasites and indeed detected expression of SAG2X and SAG2Y in bradyzoites but not in tachyzoites (Fig. 1C). To determine if SAG2C, -X, and -Y are expressed at even low levels in tachyzoites, we deliberately loaded considerably more material in those lanes than in the bradyzoite lanes (see GRA7 loading control in Fig. 1C). Even with this, we were unable to detect any SAG2C, -X, or -Y in the tachyzoite lysates, indicating that expression of these proteins is tightly down-regulated in this developmental stage. This established that besides SAG2C, SAG2Y and SAG2X are also expressed and are specific for the bradyzoite stage of Toxoplasma. Interestingly, comparing the SAG2CDXY region from type II strains to the sequence predicted by genomic sequencing of type I or III strains (www.toxodb.org), there is evidence for selection of these proteins, as they have a high nonsynonymous/synonymous single-nucleotide polymorphism ratio, especially the SAG2C protein (a ratio of 4 for the type II to the type III strain).
FIG. 1.
Surface antigens SAG2C, SAG2X, and SAG2Y are all expressed in bradyzoites. (A) Recombinant His-tagged SAG2C, -X, and -Y were made in insect cells by using a baculovirus expression vector system, purified using nickel agarose beads, and run in a 10% reducing acrylamide gel. On a nonreduced gel, typical dimer formation was seen, as reported for other SRS antigens. (B) Antibodies raised against peptides (SAG2X and SAG2Y) or against bacterially expressed SAG2C (15) were used to detect rSAG2Y, rSAG2C, and rSAG2X. Because rSAG2C had a small amount of contamination with rSAG2Y, the antibody against the SAG2Y peptide also recognizes a band in the SAG2C lane (but with the molecular weight of SAG2Y). (C) Specific antibodies (see panel B) were used to detect SAG2Y, SAG2C, and SAG2X in lysates from bradyzoites (BZ) or tachyzoites (TZ). A rabbit polyclonal antibody against GRA7 was used as a parasite loading control.
Generation of a bioluminescent Toxoplasma Pru ΔSAG2CDXY strain.
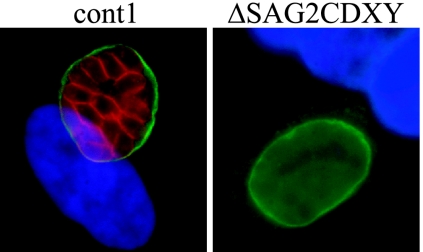
Because of the similarity of SAG2C, -X, and -Y, it is possible that knocking out one of them would have little, if any, effect because the others would still provide full function. We therefore decided to remove the complete SAG2CDXY region by homologous recombination. This was done in a type II strain expressing FLUC and GFP (Pru Δhpt/GFP/FLUC) to allow for comparison of the knockout and parental strains by BLI. Pru Δhpt/GFP/FLUC parasites were therefore transformed with the homologous recombination construct (Fig. 2) and selected with MPA and xanthine. Clones were screened by PCR, using primer sets designed to detect homologous recombination at both sides of the SAG2C and SAG2Y gene and the lack of the genes themselves. Several clones were confirmed to be deleted for the entire SAG2CDXY locus, and two of these clones (Pru ΔSAG2CDXY 1 and Pru ΔSAG2CDXY 2, derived from independent transformations) were used throughout this study. Also two clones that had the knockout vector inserted, but not into the SAG2CDXY locus, were used as controls (cont1 and cont2). Using a rabbit polyclonal antibody that reacts with SAG2X and SAG2Y an immunofluorescence assay (not shown), we confirmed that the Pru ΔSAG2CDXY parasites expressed no SAG2X or -Y after switching them to bradyzoites (Fig. 3). Successful switching was confirmed by staining with Dolichos agglutinin-fluorescein isothiocyanate, which stains the cyst wall, and no obvious differences in switching efficiency were observed between wild-type and knockout parasites (not shown). In the wild type, the rabbit polyclonal antibody that reacts against SAG2X and SAG2Y stained only switched parasites (bradyzoites), not tachyzoites. Similarly, with Western blotting we confirmed that the Pru ΔSAG2CDXY parasites did not express SAG2XY but were able to switch, as indicated by expression of normal levels of the bradyzoite-specific antigen SRS9 (not shown).
FIG. 3.
Verification of Pru ΔSAG2CDXY knockout. HFFs, grown on glass coverslips in a 24-well plate or in T25 flasks, were infected with control or Pru ΔSAG2CDXY parasites. Parasites were switched to bradyzoites by use of pH 8.1 switch medium for 4 days. Coverslips were fixed, permeabilized, and stained with a polyclonal rabbit antibody against SAG2Y (red), with Dolichos agglutinin-FITC (green) reacting with the cyst wall, and with the nuclear stain Hoechst (blue). In tachyzoites, no staining with the antibody against SAG2Y was seen (not shown).
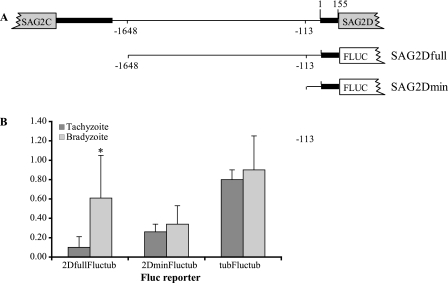
The SAG2D promoter is active and confers stage specificity.
To assess whether the promoter regions of the SAG2CDXY gene cluster were sufficient to confer bradyzoite-specific expression, we studied the ability of the SAG2D promoter to drive expression of FLUC in parasites. The promoter of SAG2D was chosen for these assays since it presumably must lie within the segment delimited by the 3′ end of the 3′UTR of SAG2C and the start codon of SAG2D (SAG2Dfull) (Fig. 4A). A construct of FLUC driven by the SAG2Dfull promoter was transiently introduced into Prugniaud parasites, and half of the transformed parasites were grown as tachyzoites, while the other half were induced to develop into bradyzoites in tissue culture. Seventy-two hours after the transformation, the cultures were lysed and FLUC activity in both the tachyzoite and bradyzoite samples was measured. To be able to compare the levels of FLUC activity across samples, we included a second reporter gene driven by the constitutive Toxoplasma TUB1 promoter in all of our transformation mixtures. This second reporter was RLUC, which requires a different substrate than FLUC, and thus both FLUC and RLUC activity can be measured from the same sample. As shown in Fig. 4B, the relative expression (i.e., the ratio of FLUC activity to RLUC activity) of the SAG2DfullFLUC construct was significantly higher in the bradyzoite sample than in the tachyzoite one. Indeed, the ratio of relative expression in bradyzoites to that in tachyzoites was 6.1 on average. These results indicate that the SAG2CD intergenic region is sufficient to drive expression of a reporter gene and confer stage specificity. When the FLUC reporter was driven by the constitutive TUB1 promoter as a control experiment, the relative expression of FLUC was not significantly different in the bradyzoite and tachyzoite samples (Fig. 4B). To further characterize the SAG2D promoter, we used a truncated version of SAG2Dfull that includes only 268 base pairs upstream of the SAG2D start codon (SAG2Dmin) (Fig. 4A). Unlike the situation with the SAG2Dfull construct, the relative expression of SAG2DminFLUC was indistinguishable in tachyzoites and bradyzoites (Fig. 4B). Thus, while this minimal promoter can drive the expression of FLUC in parasites, it is not sufficient to drive the bradyzoite-specific expression that is seen with the larger region.
FIG. 4.
In vitro activity of the SAG2D promoter. (A) The intergenic region between SAG2C and SAG2D is shown at the top. Black bars represent the 3′UTR of SAG2C and the 5′UTR of SAG2D. The numbers indicate the base numbers, where 1 is the transcription start of SAG2D according to the results of rapid amplification of cDNA ends. The lower constructs are the two fragments (SAG2Dfull and SAG2Dmin) used to drive expression of FLUC. (B) Parasites were transformed with FLUC under the control of either the SAG2Dfull, SAG2Dmin, or Tub1 promoter and with RLUC under the control of the Tub1 promoter and grown as tachyzoites or bradyzoites. The data are expressed as the ratio of FLUC activity to RLUC activity in each population at 72 h posttransformation. Each bar represents the average for at least four independent experiments, and the error bars indicate standard deviations. The asterisk indicates a significant difference (P = 0.0089).
The SAG2D promoter is active 9 days after infection in the lungs.
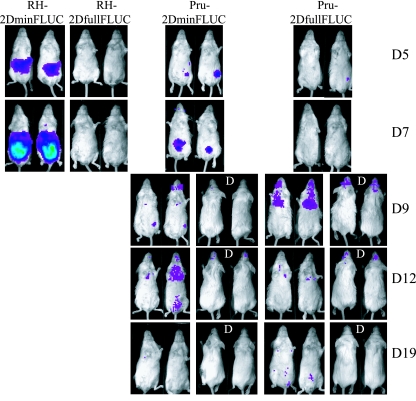
Very little is known about when and where after infection Toxoplasma tachyzoites begin to differentiate into the bradyzoite stage. To answer these questions, we created stable parasite lines expressing FLUC from the SAG2D promoter so we could monitor stage conversion in vivo by BLI. Both RH (type I) parasites and Prugniaud parasites were transfected with either a linearized psag2DfullFLUCtub or psag2DminFLUCtub plasmid, and after selection with MPA and xanthine, several clones stably expressing the introduced plasmid were obtained. When mice were infected i.p. with either RH-2DminFLUC or Pru-2DminFLUC tachyzoites, a strong light signal could be detected in the peritoneal area by BLI by 5 days postinfection (dpi) (Fig. 5). Mice infected with Pru-2DminFLUC survived infection, and the signal spread from the peritoneal area (∼5 to 7 dpi) to the chest (∼9 to 12 dpi) and later to the brain (∼9 to 19 dpi) (Fig. 5). Mice infected with RH-2DFullFLUC, however, never showed any signal, and these mice died 8 days after infection (Fig. 5). This indicated that the SAG2Dfull promoter has relatively little (if any) activity in tachyzoites in vivo, consistent with the in vitro data (Fig. 4B). For mice infected with Pru-2DFullFLUC, no signal was seen until 9 dpi, when signals were detected in the chest area (probably coming from the lungs) and the brain (Fig. 5). Thus, at least in this i.p. model of infection, Toxoplasma appears to initiate the switch from tachyzoite to bradyzoite at ∼9 dpi, predominantly in the lungs and brain.
FIG. 5.
Detection of Toxoplasma tachyzoite-to-bradyzoite conversion by BLI. Six-week-old BALB/c female mice were infected i.p. with 500 tachyzoites of the RH or Prugniaud strain expressing FLUC from a full (2DfullFLUC) or minimal (2DminFLUC) SAG2D promoter. For all images shown here, the color scale ranges from blue (just above background noise; set to 6,000 photons/s/cm2/steradian) to red (at least 1 × 105 photons/s/cm2/steradian). Mice were imaged using BLI with an IVIS imaging system (Xenogen Corporation, Alameda, CA) on the days indicated on the right side of the images. For some days, dorsal (D) images are also shown. Mice infected with the RH strain died 8 days after infection. The course of infection revealed by BLI is shown for two representative mice per strain.
Pru ΔSAG2CDXY parasites form fewer cysts in the brain.
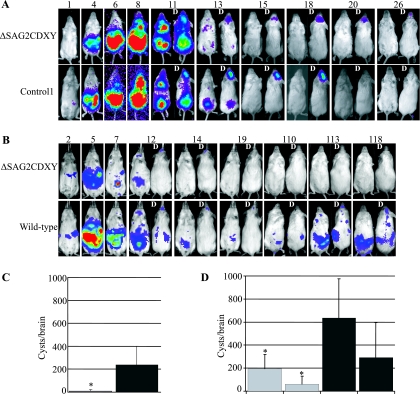
To learn more about the function of the SAG2CDXY cluster, we compared Pru ΔSAG2CDXY with control and wild-type parasites by using BLI. This enabled us to directly compare knockout parasites with heterologous controls and parental parasites in a side-by-side manner. No significant differences were seen in the acute phase of infection between the Pru ΔSAG2CDXY, wild-type, or control parasites (both of the independent knockout clones and both controls were assessed in this way, but only data for Pru ΔSAG2CDXY 1 and cont1 are shown) (Fig. 6A). All infected mice showed very similar signals until 8 dpi, after which most mice died (between ∼9 and 10 dpi), except for two mice infected with Pru ΔSAG2CDXY 1 and one mouse infected with cont1 (these are the mice shown in Fig. 6A). Both mice infected with Pru ΔSAG2CDXY 1 that survived the acute phase developed a chronic infection with abundant signal in the brain, suggesting that this strain has no defect in reaching this organ.
FIG. 6.
Growth, dissemination, reactivation, and cyst counts of luminescent Pru ΔSAG2CDXY or control parasites. Mice were imaged at the days indicated at the top of the images. For some days, dorsal (D) images are also shown. Data for one representative mouse per strain are shown. (A) Eight-week-old BALB/c females were infected i.p. with 2,500 tachyzoites of the ΔSAG2CDXY strain or with control parasites. (B) Eight-week-old CD-1 females were infected i.p. with 500 tachyzoites of the ΔSAG2CDXY strain or with wild-type parasites. One hundred days after infection, dexamethasone (30 mg/ml) was added to the drinking water to suppress the immune system and reactivate parasites. (C) Average cyst numbers 9 weeks after infection for mice infected with 50 or 5,000 Pru ΔSAG2CDXY (ΔSAG2CDXY) parasites or with 50 or 5,000 Pru Δhpt/GFP/FLUC or cont2 parasites (control). Because no significant differences were seen in signal intensities during infection or in cyst numbers between the mice infected with 50 or 5,000 parasites or between wild-type and control parasites, the results were pooled. (D) Average cyst numbers 4 and 9 weeks after infection of mice infected with 5,000 Pru ΔSAG2CDXY 1/2 (ΔSAG2CDXY), Pru Δhpt/GFP/FLUC, cont1, or cont2 (control) parasites. The error bars indicate the standard deviations. Asterisks indicate significant differences compared to the control (same time point).
Because mortality in BALB/c mice was so high after i.p. infection with these strains, it was difficult to determine whether there are differences in cyst levels between the different strains. We therefore infected outbred CD-1 mice, which are more resistant. Groups of five mice were infected with 5,000, 500, and 50 Pru ΔSAG2CDXY 1, cont1, and wild-type parasites. In the acute phase, no significant differences were seen in signal intensities, tissue tropism, or dissemination kinetics (Fig. 6C; results are shown only for i.p. infection with 500 parasites of the Pru ΔSAG2CDXY 1 and wild-type strains). Nine weeks after infection, the groups of mice infected with 5,000 and 50 parasites were sacrificed for counts of cysts in their brains. All mice, except one, infected with the Pru ΔSAG2CDXY 1 strain had no detectable cysts, while the mice infected with the control or wild-type parasites had many cysts (Fig. 6C; the one mouse showing cysts with Pru ΔSAG2CDXY 1 had 60 cysts in the brain, compared to the mean for infections with the wild type of 236 cysts/brain). The absence of detectable cysts in the brain did not preclude the possibility that cysts were still present in other organs. We therefore immunosuppressed the mice that were infected with 500 parasites by adding dexamethasone to the drinking water 100 days after the initial infection. As previously described (22), this is a very effective method of reactivating parasites. None of the four surviving mice infected with Pru ΔSAG2CDXY 1 showed any sign of reactivation, while all nine surviving mice infected with cont1 or the wild type showed reactivation of infection, as demonstrated by luminescent signals coming from various body regions (Fig. 6B).
Two months after infection, cysts were found in the brain in only one of five mice infected with Pru ΔSAG2CDXY 1. To investigate if we could detect this difference even earlier, we infected groups of five mice with Pru ΔSAG2CDXY, control, and wild-type parasites and did cyst counts 1 and 2 months after infection. Although at 1 month there were still significant cyst numbers in the brains of mice infected with Pru ΔSAG2CDXY, these numbers were already significantly lower than those for mice infected with wild-type or control parasites (Fig. 6D). Two months after infection, mice infected with Pru ΔSAG2CDXY parasites had significantly fewer cysts in the brain than did mice infected with wild-type and control parasites, although the difference was less pronounced than that in the first experiment. No differences were seen in cyst size (not shown). It is believed that cysts are not static but occasionally rupture during a chronic infection. The majority of the released bradyzoites will be eliminated by the immune system, but some will reinvade and form new cysts. To investigate if bradyzoites released from Pru ΔSAG2CDXY cysts were defective in the ability to invade new cells, we put cysts from mice infected with Pru ΔSAG2CDXY or wild-type parasites into HFF cultures and counted plaques 1 week later. Both strains were able to form plaques, with no significant differences in efficiency (not shown).
Pru ΔSAG2CDXY cysts are still orally infective.
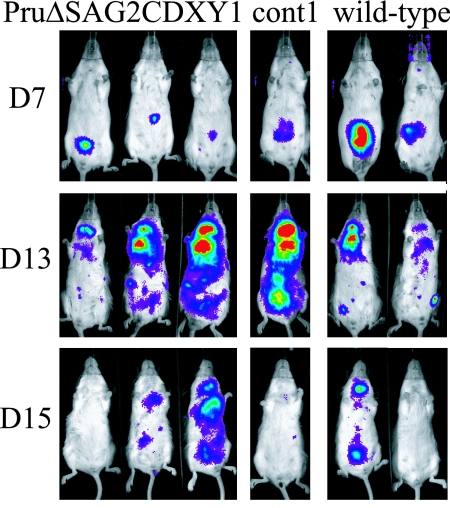
To determine if bradyzoites use SAG2CDXY for invasion of cells in the small intestine, we orally infected mice with Pru ΔSAG2CDXY cysts. Equal numbers of cysts isolated after 1 month from the brains of mice infected with Pru ΔSAG2CDXY, control, or wild-type parasites were fed to BALB/c mice, and infection was followed using BLI. At 7 dpi, a signal could be seen in the peritoneal area, after which infection progressed to the lungs, cervical lymph nodes, and brain (Fig. 7). No obvious differences were seen in comparing Pru ΔSAG2CDXY parasites with wild-type or control parasites, demonstrating that SAG2CDXY proteins do not appear to be essential for invasion of gut epithelial cells (Fig. 7).
FIG. 7.
BLI of BALB/c mice orally infected with Pru ΔSAG2CDXY 1, cont1, or wild-type cysts showing that removal of the SAG2CDXY genes has no effect on oral infectivity.
DISCUSSION
One of the Toxoplasma protein families with the most members is the SRS family. Most of the SRS proteins that have been examined are expressed in a stage-specific manner, and many of their cognate genes are located in stage-specific clusters. Besides evidence that some of the tachyzoite-specific SRSs play a role in attachment to the host cell surface, little is known about their function and why Toxoplasma needs so many SRS proteins. Here we studied a cluster of four genes encoding some of the most abundantly expressed bradzyoite-specific SRS proteins, SAG2C, -D, -X, and -Y.
Using specific antibodies, we were able to determine that SAG2X and SAG2Y are surface antigens that are also expressed only in the bradyzoite stage (or, at least, they do not appear to be expressed in tachyzoites, as determined by immunofluorescence assay and Western blotting). This expands the set of SRS proteins that are now known to be stage specific and further reinforces the trend of physical clustering of genes that are similarly regulated during development. By comparing the SAG2Dfull promoter with the SAG2Dmin promoter, it is clear that the bradyzoite-specific expression of the former is likely due to an element(s) that negatively regulates expression in tachyzoites. It is unknown if a protein binds to this putative element(s), if this element(s) is somehow modified, or if its accessibility is regulated (e.g., by histone modifications). Recent studies by others have shown that histone modification is likely to be at least one mechanism by which this sort of regulation is achieved (9, 27). To identify the putative element(s) involved, the antibodies described in this study could be used in a screening strategy, after mutagenesis of Toxoplasma, to obtain mutants that do not express SAG2CDXY after stage switching.
Using BLI, we were able to assess in living animals when and where after infection Toxoplasma switches from the rapidly dividing tachyzoite stage to the encysted bradyzoite stage. From mice infected with these parasites, it is clear that switching occurs as early as ∼9 dpi and possibly earlier, because signal can be detected only when sufficient parasites are present. The change from being a tachyzoite to a bradyzoite is likely to be a continuum, as the best evidence of existence of “functional” bradyzoites in tissue is that functional bradyzoites are highly infectious to cats and give rise to oocyst shedding with a short prepatent period (10 days or less), while tachyzoites are less infectious and give rise to oocysts with a long prepatent period (>14 days). Using this assay, it has been demonstrated that mice infected with Toxoplasma contain functional bradyzoites (short prepatent period) in the lungs and brain as early as 7 days after infection (6), which is consistent with our results. The SAG2D promoter is an appropriate promoter to drive a reporter gene because SAG2D has been reported to be one of the earliest genes expressed when parasites start switching from the tachyzoite to the bradyzoite stage (4). However, from the small amount of signal detected at 9 dpi (Fig. 5) and the absence of signal in the chronic stage, it is clear that improved sensitivity of detection and/or the use of an even stronger promoter will be necessary for more refined analyses of differentiation. Even with such improvements, it may be that the low metabolic activity of bradyzoites and the scarcity of cysts in the brain represent major challenges to the detection by BLI of the established, chronic stages of Toxoplasma infection.
To our knowledge, this is the first time that a cluster of four genes has been knocked out, although there was no a priori reason to suppose that such would be any more difficult (ignoring the issue of deleterious phenotypes) than knocking out a single gene of similar overall size as the cluster. In fact, the Pru ΔSAG2CDXY parasites were easy to obtain, possibly because they have a slight growth advantage compared to control parasites (not shown). It is unknown if this is an advantage in doubling time, invasion, reinvasion, or survivability outside host cells.
As expected, no differences between mice infected with Pru ΔSAG2CDXY and wild-type parasites were seen in the acute phase of infection (Fig. 6). However, a significant difference was seen in the numbers of cysts formed 1 and 2 months after infection. Remarkably, in one experiment, most mice had completely cleared all parasites, as demonstrated by the absence of reactivation when these mice were immunosuppressed. The lower cyst numbers in mice infected with Pru ΔSAG2CDXY parasites could be due to a defect of these parasites in attachment or (re)invasion of brain cells. However, it cannot be excluded that removal of bradyzoite SRS antigens leads to a stronger immune response because bradyzoites could deliver variant T-cell epitopes that antagonize the development of effector T-cell responses against other SRS antigens (altered peptide ligands) (19). This type of immune evasion has been suggested to facilitate the survival of several parasites and viruses (1, 8, 13). However, this hypothesis cannot be tested until we know which peptides are presented to T cells. Similarly, until we know which exact brain cells Toxoplasma bradyzoites invade, we will not be able to test if bradyzoite surface antigens are involved in this process. The surface antigens SAG2CDXY appear not to be involved in facilitating invasion of the small intestine, as no defect was seen after oral feeding of cysts isolated from the brains of mice infected with Pru ΔSAG2CDXY.
Overall, as previously reported for the bradyzoite-specific surface antigen SRS9, belonging to the SAG1 subfamily, the surface antigens SAG2CDXY, belonging to the SAG2 subfamily, are important for persistence of cysts in the brain. Because parasites deficient in either of these proteins seem to have a partial phenotype, it would be interesting to make a parasite strain deficient in both SRS9 and SAG2CDXY. If this strain were unable to invade after switching to the bradyzoite stage, it would make an excellent beginning for a recombinant vaccine.
Acknowledgments
This work was supported by National Institutes of Health grants AI21423 and AI41014 and a grant from The Ellison Medical Foundation (a Senior Scholar Award) to J.C.B., by University of California Universitywide AIDS Research Program grant F04-ST-216 to J.P.J.S., and by National Institutes of Health grant P20 RR15587 to G.A.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 17 March 2008.
REFERENCES
- 1.Bertoletti, A., A. Sette, F. V. Chisari, A. Penna, M. Levrero, M. De Carli, F. Fiaccadori, and C. Ferrari. 1994. Natural variants of cytotoxic epitopes are T-cell receptor antagonists for antiviral cytotoxic T cells. Nature 369407-410. [DOI] [PubMed] [Google Scholar]
- 2.Bessieres, M. H., S. Le Breton, and J. P. Seguela. 1992. Analysis by immunoblotting of Toxoplasma gondii exo-antigens and comparison with somatic antigens. Parasitol. Res. 78222-228. [DOI] [PubMed] [Google Scholar]
- 3.Boyle, J. P., J. P. Saeij, and J. C. Boothroyd. 2007. Toxoplasma gondii: inconsistent dissemination patterns following oral infection in mice. Exp. Parasitol. 116302-305. [DOI] [PubMed] [Google Scholar]
- 4.Cleary, M. D., U. Singh, I. J. Blader, J. L. Brewer, and J. C. Boothroyd. 2002. Toxoplasma gondii asexual development: identification of developmentally regulated genes and distinct patterns of gene expression. Eukaryot. Cell 1329-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donald, R. G., D. Carter, B. Ullman, and D. S. Roos. 1996. Insertional tagging, cloning, and expression of the Toxoplasma gondii hypoxanthine-xanthine-guanine phosphoribosyltransferase gene. Use as a selectable marker for stable transformation. J. Biol. Chem. 27114010-14019. [DOI] [PubMed] [Google Scholar]
- 6.Dubey, J. P. 1997. Bradyzoite-induced murine toxoplasmosis: stage conversion, pathogenesis, and tissue cyst formation in mice fed bradyzoites of different strains of Toxoplasma gondii. J. Eukaryot. Microbiol. 44592-602. [DOI] [PubMed] [Google Scholar]
- 7.Dzierszinski, F., M. Mortuaire, M. F. Cesbron-Delauw, and S. Tomavo. 2000. Targeted disruption of the glycosylphosphatidylinositol-anchored surface antigen SAG3 gene in Toxoplasma gondii decreases host cell adhesion and drastically reduces virulence in mice. Mol. Microbiol. 37574-582. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert, S. C., M. Plebanski, S. Gupta, J. Morris, M. Cox, M. Aidoo, D. Kwiatkowski, B. M. Greenwood, H. C. Whittle, and A. V. Hill. 1998. Association of malaria parasite population structure, HLA, and immunological antagonism. Science 2791173-1177. [DOI] [PubMed] [Google Scholar]
- 9.Gissot, M., K. A. Kelly, J. W. Ajioka, J. M. Greally, and K. Kim. 2007. Epigenomic modifications predict active promoters and gene structure in Toxoplasma gondii. PLoS Pathog. 3e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He, X. L., M. E. Grigg, J. C. Boothroyd, and K. C. Garcia. 2002. Structure of the immunodominant surface antigen from the Toxoplasma gondii SRS superfamily. Nat. Struct. Biol. 9606-611. [DOI] [PubMed] [Google Scholar]
- 11.Jacquet, A., L. Coulon, J. De Neve, V. Daminet, M. Haumont, L. Garcia, A. Bollen, M. Jurado, and R. Biemans. 2001. The surface antigen SAG3 mediates the attachment of Toxoplasma gondii to cell-surface proteoglycans. Mol. Biochem. Parasitol. 11635-44. [DOI] [PubMed] [Google Scholar]
- 12.Jung, C., C. Y. Lee, and M. E. Grigg. 2004. The SRS superfamily of Toxoplasma surface proteins. Int. J. Parasitol. 34285-296. [DOI] [PubMed] [Google Scholar]
- 13.Kahn, S. J., and M. Wleklinski. 1997. The surface glycoproteins of Trypanosoma cruzi encode a superfamily of variant T cell epitopes. J. Immunol. 1594444-4451. [PubMed] [Google Scholar]
- 14.Kim, S. K., A. Karasov, and J. C. Boothroyd. 2007. Bradyzoite-specific surface antigen SRS9 plays a role in maintaining Toxoplasma gondii persistence in the brain and in host control of parasite replication in the intestine. Infect. Immun. 751626-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lekutis, C., D. J. P. Ferguson, and J. C. Boothroyd. 2000. Toxoplasma gondii: identification of a developmentally regulated family of genes related to SAG2. Exp. Parasitol. 9689-96. [DOI] [PubMed] [Google Scholar]
- 16.Manger, I. D., A. B. Hehl, and J. C. Boothroyd. 1998. The surface of Toxoplasma tachyzoites is dominated by a family of glycosylphosphatidylinositol-anchored antigens related to SAG1. Infect. Immun. 662237-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mineo, J. R., and L. H. Kasper. 1994. Attachment of Toxoplasma gondii to host cells involves major surface protein, SAG-1 (P30). Exp. Parasitol. 7911-20. [DOI] [PubMed] [Google Scholar]
- 18.Partanen, P., H. J. Turunen, R. T. Paasivuo, and P. O. Leinikki. 1984. Immunoblot analysis of Toxoplasma gondii antigens by human immunoglobulin G, M, and A antibodies at different stages of infection. J. Clin. Microbiol. 20133-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plebanski, M., E. A. Lee, C. M. Hannan, K. L. Flanagan, S. C. Gilbert, M. B. Gravenor, and A. V. Hill. 1999. Altered peptide ligands narrow the repertoire of cellular immune responses by interfering with T-cell priming. Nat. Med. 5565-571. [DOI] [PubMed] [Google Scholar]
- 20.Robinson, S. A., J. E. Smith, and P. A. Millner. 2004. Toxoplasma gondii major surface antigen (SAG1): in vitro analysis of host cell binding. Parasitology 128391-396. [DOI] [PubMed] [Google Scholar]
- 21.Roos, D. S., R. G. Donald, N. S. Morrissette, and A. L. Moulton. 1994. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 4527-63. [DOI] [PubMed] [Google Scholar]
- 22.Saeij, J. P., J. P. Boyle, M. E. Grigg, G. Arrizabalaga, and J. C. Boothroyd. 2005. Bioluminescence imaging of Toxoplasma gondii infection in living mice reveals dramatic differences between strains. Infect. Immun. 73695-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seeber, F., and J. C. Boothroyd. 1996. Escherichia coli beta-galactosidase as an in vitro and in vivo reporter enzyme and stable transfection marker in the intracellular protozoan parasite Toxoplasma gondii. Gene 16939-45. [DOI] [PubMed] [Google Scholar]
- 24.Singh, U., J. L. Brewer, and J. C. Boothroyd. 2002. Genetic analysis of tachyzoite to bradyzoite differentiation mutants in Toxoplasma gondii reveals a hierarchy of gene induction. Mol. Microbiol. 44721-733. [DOI] [PubMed] [Google Scholar]
- 25.Soldati, D., and J. C. Boothroyd. 1995. A selector of transcription initiation in the protozoan parasite Toxoplasma gondii. Mol. Cell. Biol. 1587-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soldati, D., and J. C. Boothroyd. 1993. Transient transfection and expression in the obligate intracellular parasite Toxoplasma gondii. Science 260349-352. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan, W. J., Jr., and M. A. Hakimi. 2006. Histone mediated gene activation in Toxoplasma gondii. Mol. Biochem. Parasitol. 148109-116. [DOI] [PubMed] [Google Scholar]