Abstract
A main feature of acute infection with Trypanosoma cruzi is the presence of immunological disorders. A previous study demonstrated that acute infection with the virulent RA strain downregulates the expression of major histocompatibility complex class II (MHC-II) on antigen-presenting cells and impairs the T-cell stimulatory capacity of splenic dendritic cells (DC). In the present work, we assessed the ability of trypomastigotes (Tp) to modulate the differentiation stage and functionality of bone marrow-derived DC in vitro. We observed that the Tp stage of T. cruzi failed to activate DC, which preserved their low expression of MHC-II and costimulatory molecules, as well as their endocytic activity. We also show that Tp induced transforming growth factor β (TGF-β) secretion by DC and enhanced the gap between interleukin-10 (IL-10) and IL-12p70 production, showing a higher IL-10/IL-12p70 ratio upon lipopolysaccharide (LPS) treatment. In addition, we observed that Tp prevented DC full activation induced by LPS, thereby downregulating their MHC-II surface expression and inhibiting their capacity to stimulate lymphocyte proliferation. In vitro IL-10 neutralization during the differentiation process of DC with Tp+LPS showed a reversion of their inhibitory effect during mixed lymphocyte reaction. In contrast, only simultaneous neutralization of IL-10 and TGF-β, after DC differentiation, was involved in the partial restitution of lymphocyte proliferation. Since both TGF-β and IL-10 are immunosuppressive cytokines essential in the modulation of the immune response and important in the induction of tolerance, our results suggest for the first time that Tp are responsible for the generation of regulatory DC in vitro.
Trypanosoma cruzi, the etiological agent of Chagas' disease, is a protozoan parasite that affects over 20 million people in Latin America. The onset of human pathology may be extremely diverse and depends on the parasite biology as well as its relationship with the host. Infection may be acute or chronic, and the last one frequently involves long-lasting inflammatory lesions and immune system disorders with progressive pathology in the heart, esophagus, or colon. In the mammalian host, the parasite life cycle includes the nondividing, blood-circulating trypomastigotes (Tp), which infect nucleated cells, and the replicating intracellular amastigotes (Am), which reside in the cytoplasm of the infected cell (10).
Several reports showed that T. cruzi induces both Th1-type response and nonspecific immunosuppression during the acute phase of the infection (2, 38, 51, 53). However, the mechanisms that control parasite replication and maintain low but persistent numbers of circulating parasites during the chronic phase are not well understood (25). In the murine model, the inability to produce gamma interferon (IFN-γ) is lethal. Various studies have shown that IFN-γ, tumor necrosis factor alpha (TNF-α), and interleukin-12 (IL-12) are important for the control of this infection by ensuring the induction of an efficient adaptive host response (1, 7, 18, 35, 55, 62).
Dendritic cells (DC) possess special features that allow them to act as professional antigen-presenting cells (APC) (8) and are central in the initiation and development of immunity and tolerance (27, 47). Immature DC residing in nonlymphoid tissues capture and process antigen via their high endocytic capacity (40, 48) and undergo an activation and maturation process after recognition of conserved pathogen-associated molecular patterns present in microorganisms through pattern-recognition receptors. Lipopolysaccharide (LPS) is a well-defined component of gram-negative bacteria wall that induces DC maturation (14, 39) and IL-6, IL-8, IL-12, and TNF-α production (59). DC maturation is paralleled by upregulation of major histocompatibility complex class II (MHC-II) and costimulatory molecules, as well as the secretion of both pro-and anti-inflammatory molecules (26). Besides the nature of the DC maturation stimuli, the density and quality of DC present in the T-cell areas of secondary lymphoid organs determine the magnitude and class of the T-cell response (41). The induction of immune response or tolerance may be explained by DC at different developmental stages, characterized by specialized functions. Immature DC have been shown to be able to induce tolerance (15) and, recently, subsets of regulatory DC have been described to be important in maintaining immune homeostasis (5, 46).
In vitro infection of human DC with T. cruzi has been previously reported to reduce both the secretion of cytokines and the upregulation of costimulatory molecules (57). Moreover, Planelles et al. (34) demonstrated the impaired function of macrophages and DC following T. cruzi infection and its association with high susceptibility in a mouse strain-dependent manner. Previously, we have demonstrated that T. cruzi acute infection in mice, with the virulent strain RA, downregulates in vivo expression of MHC-II on APC, as well as impairs T-cell stimulatory activity of splenic DC (6).
In the present study, we further investigated possible DC alterations induced by T. cruzi, following a coculture of bone marrow-derived DC (BM-DC) with Tp. First, we analyzed DC infection by Tp and characterized their maturation profile, describing the expression of relevant surface markers, as well as DC endocytic capacity. Finally, we analyzed whether coculture of DC with Tp affects their cytokine production and functionality, thereby limiting their capacity to stimulate lymphocytes proliferation. Our results suggest that even though Tp are deficient inducers of DC maturation, they are efficient modulators of their differentiation, probably toward a regulatory phenotype.
MATERIALS AND METHODS
Mice.
Eight- to ten-week-old male BALB/c and C3H/HeNk mice and 2- to 3-week-old male CF1 mice were obtained from the animal facilities of our department. Animal care was in accordance with institutional guidelines.
Parasite purification and TCM.
Bloodstream forms (Tp) of the highly virulent RA strain of T. cruzi were used in all of the experiments. Tp were maintained by weekly intraperitoneal inoculation of CF1 mice (105 parasites/mouse). Tp were obtained from whole blood at the peak of parasitemia (7 days postinfection) and purified by differential centrifugation or by density gradient, using Histopaque-1083 (Sigma, St. Louis, MO), as previously reported (52). The mononuclear fraction was collected, and Tp were separated from mononuclear cells by centrifugation (3,000 × g, 10 min, 20°C). Parasites were obtained from the supernatant by centrifugation (10,000 × g, 30 min, 20°C) and resuspended in fresh Iscove modified Dulbecco medium (IMDM; Sigma). The Histopaque-1083 was used when a highly purified Tp suspension totally free of platelets was needed. This situation was assessed in each assay with the corresponding controls. TCM (T. cruzi medium enriched in parasite secretion products), was obtained from suspensions of 107 Tp/ml, which were cultured in IMDM supplemented with 10% heat-inactivated fetal bovine serum (FBS; Natocor, Córdoba, Argentina) at 37°C and 5% CO2 for 24 h, and were filtered as previously described (52).
Culture of BM-DC.
Briefly, bone marrow from femurs and tibias from C3H/HeNk mice were flushed with IMDM supplemented with 10% FBS, 100 U of penicillin/ml, and 100 mg of streptomycin/ml (referred to below as medium) by using syringes and 25-gauge needles. The tissue was resuspended, and DC were obtained by culturing bone marrow cells, supplemented with 50% supernatant from a granulocyte-macrophage colony-stimulating factor (GM-CSF)-expressing cell line (J558 GM-CSF), as previously described (28). Cells were cultured at 37°C and 5% CO2 for 3 days. The supernatant was then removed without disturbing the monolayer and replaced with fresh medium containing 50% of the GM-CSF cell supernatant medium. Cells obtained after 7 days of culture with GM-CSF displayed a myeloid phenotype (>95% CD11b) and were highly enriched in DC (>70% CD11c). Cultures showed a low percentage of cells expressing CD11b+ GR-1+ (myeloid precursors) and were B220− CD8α−.
At day 7, cells were harvested by gentle pipetting, washed, plated (106 cells/ml) in 24-well plates (Nunc), and cultured in medium with or without 10 μg/ml of LPS (Escherichia coli O26:B6; Sigma)/ml and/or Tp (parasite/cell ratio of 1/1, 4/1, or 8/1) for 6, 12, 18, or 24 h, depending on the assay. In some experiments the DC were cultured with different %TCM (10, 50, and 100). Control DC were cultured only with medium.
T. cruzi infection of DC.
In order to analyze the infection and also the rate of infection, Tp and DC were cocultured at different Tp/DC ratios (1/1, 4/1, and 8/1) for 24 h. Thereafter, DC were washed to remove free parasites and were further incubated for another 24 h. Then, the DC were washed and stained with anti-mouse CD11c phycoerythrin (PE)-conjugated monoclonal antibody (BD Pharmingen, San Diego, CA). Finally, they were attached to positively charged glass slides (Fisherbrand, Pittsburg, PA), fixed in methanol, and stained with anti-parasite rabbit serum or appropriate controls and fluorescein isothiocyanate (FITC)-conjugated secondary antibody (Sigma). DC infection was assessed by indirect immunofluorescence. The percentages of infected cells at the different Tp/DC ratios were defined after microscopic examination (×400 magnification) of at least 400 cells.
Surface and intracellular staining.
For surface staining, cells were washed twice in ice-cold phosphate-buffered saline (PBS) supplemented with 1% bovine serum albumin and 0.1% NaN3. To minimize nonspecific staining, cells were incubated with 2% normal mouse serum. The cells were then incubated for 30 min at 4°C with a previously optimized amount of one or more of the following anti-mouse PE-, FITC-, or biotin (Biot)-conjugated monoclonal antibodies: CD11b-PE (M1/70), CD11c-PE or -Biot (HL3), I-Ak-FITC (11-5.2), CD40-Biot (3/23), CD80-Biot (16-10A1), and CD86-Biot (GL-1). When necessary, streptavidin-PE or Cy-chrome was used as a second reagent. For intracellular cytokine staining, cells were incubated with brefeldin A (Sigma) for the last 4 h of culture. After surface staining, the cells were fixed in 4% paraformaldehyde (20 min), permeabilized, and stained intracellularly with anti-IL-10-PE (JES5-16E3) in permeabilization buffer (0.1% saponin from Sigma and 10% FBS in PBS). Cells were also stained with corresponding isotype-matched control monoclonal antibodies. Finally, cells were fixed with 1% paraformaldehyde. All monoclonal antibodies and second reagents were purchased from Pharmingen. Samples were acquired on FACSCalibur (Becton Dickinson), and data were analyzed with WinMDI 2.8 software.
Dextran uptake.
FITC-dextran (Sigma) uptake was analyzed as described by Sallusto et al. (40). Briefly and after different treatments, cells were resuspended in medium. FITC-dextran was added at a final concentration of 250 μg/ml, and cells were incubated at 37°C for 40 min. To estimate the fluorescence background, cells were also incubated at 0°C (negative controls). Then, DC were washed three times in ice-cold PBS with 1% bovine serum albumin and 0.1% NaN3 and stained with anti-mouse CD11c-PE (30 min, 4°C), washed, and fixed with 1% paraformaldehyde. FITC-dextran uptake was analyzed by flow cytometry on a FACSCalibur using WinMDI 2.8 software.
Cytokine assay.
Cells were harvested at different time points or coculture conditions. The culture supernatants were then collected and stored at −80°C until used. Mouse IL-10, TGF-β1, IL-12p70, and IFN-γ levels were assayed by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN) according to the manufacturer's protocol.
Tp labeling with carboxyfluorescein succinimidyl ester (CFSE) and DC infection.
Tp labeling was carried out by using a 5 μM solution of CFDA-SE (Sigma) diluted in PBS from a stock of 5 mM dimethyl sulfoxide. After Tp were washed two times in PBS, 2 × 107 Tp were incubated at 37°C for 15 min with 500 μl of CFDA-SE solution as described by Wang et al. (61). After labeling, Tp were washed three times in PBS and cocultured at a Tp/DC ratio of 4/1 for 12 h. The DC were then washed and stained for flow cytometric analysis.
MLR assay.
To test the response of allogenic lymphocytes to stimulation, C3H/HeNk DC were cultured with different stimuli (medium, LPS, Tp, and Tp+LPS) for 24 h, harvested, washed, irradiated (30 Gy), and plated with single-cell suspension enriched in T cells prepared from lymph nodes of 8-week-old female BALB/c mice (90% CD3+ cells analyzed by flow cytometry [data not shown]). Cells were plated at DC/lymphocyte ratios of 1/5 and 1/10 using 105 lymphocytes/well and cultured at 37°C and 5% CO2 in 10% FBS-RPMI 1640 medium (Gibco) supplemented with 2 mM l-glutamine, 100 U of penicillin/ml, 100 mg of streptomycin/ml, and 50 μM 2-mercaptoethanol. For neutralization, anti-IL-10 and/or transforming growth factor β (TGF-β; JES052A5 and 9016; R&D Systems) or isotype-matched control antibody were used at 10 μg/ml. Neutralization was performed during DC differentiation with Tp+LPS (cells were then washed and used as accessory cells in the mixed lymphocyte reaction [MLR] assay), during MLR after DC treatment with Tp+LPS, and in control DC. MLR assay was performed in 96-well microplates (Nunc) in triplicate and cultured for 3 days. Cultures were pulsed with 1 μCi of [3H]thymidine/well for the last 18 h. Finally, cells were harvested and counted by using a Rack beta scintillation counter (Pharmacia).
Statistical analysis.
Analysis of variance and Bonferroni's or Dunnett's multiple-comparison tests were performed in order to analyze statistical significance. The median fluorescence intensity (MFI) was analyzed by using nonparametric Friedman's and Dunn's multiple-comparison tests. All analyses were carried out with GraphPad Prism 4 software for Windows. A P value of <0.05 was assumed to be significant.
RESULTS
Tp infect DC.
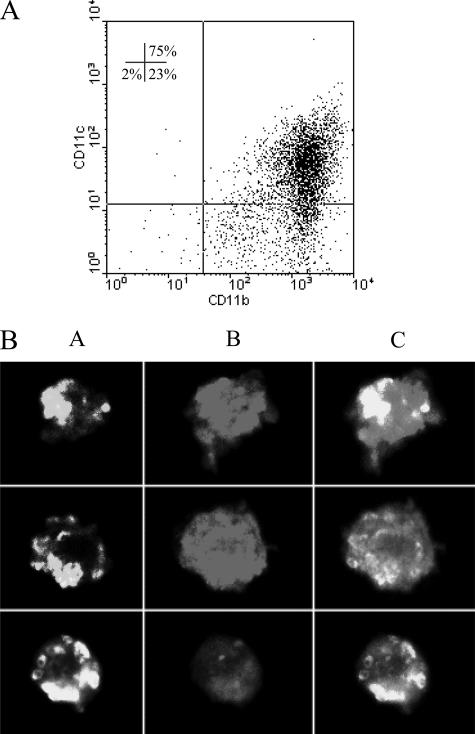
As a first step, we assessed whether Tp affect the phenotype of BM-DC obtained after 7 days of culture with GM-CSF conditioned medium. Both control and Tp-cocultured cells displayed a myeloid phenotype (>95% CD11b) and were highly enriched in DC (>70% CD11c) (Fig. 1A). There were no significant differences between them in any of the markers described in Materials and Methods (data not shown).
FIG. 1.
DC characterization after Tp coculture. (A) CD11b and CD11c surface expression on nonadherent cell derived from bone marrow after 7 days of culture in the presence of GM-CSF. After 24 h of coculture with or without Tp (4/1, Tp/DC), the cells were harvested, washed, and stained with anti-mouse monoclonal antibody. Quadrant gates were set on appropriate isotype controls. The data are representative of seven independent experiments with no differences between treatments. (B) Indirect immunofluorescence of infected DC with Am. After 24 h of coculture with Tp (8/1, Tp/DC), the cells were harvested, washed, and cultured for another 24 h. They were then stained with anti-mouse CD11c-PE, fixed, and stained with anti-parasite rabbit serum and anti-rabbit immunoglobulin G FITC-conjugated secondary antibody. Intracellular Am (A), CD11c+ cells (B), and merge (C) images are shown. Original magnification, ×400. Data are representative of three independent experiments.
In order to test the infection of DC with Tp, cocultures were performed at different parasite/cell ratios, as described above. As expected, intracellular Am were detected in CD11c+ cells by indirect immunofluorescence (Fig. 1B). The mean percentages of infected cells (± the standard errors of the mean) at Tp/DC ratios of 1, 4, and 8 were 3.2 ± 0.2, 11.9 ± 0.2, and 16.3 ± 1.2, respectively. Cell viability was not altered by the infection in relation to controls, as assessed by propidium iodide staining using flow cytometry analysis (data not shown).
Tp fail to upregulate MHC-II and costimulatory molecules and reduce LPS-induced upregulation of MHC-II on DC.
There is growing evidence that pathogens have evolved and developed multiple strategies to evade the immune system to persist in an immunocompetent host. Several reports have demonstrated that although some viruses and intracellular microorganisms induce the activation of DC due to the development of cell-mediated immunity (17, 19, 21), others display mechanisms that interfere with DC full activation, possibly affecting the first steps in the initiation of an effective immune response (20, 56).
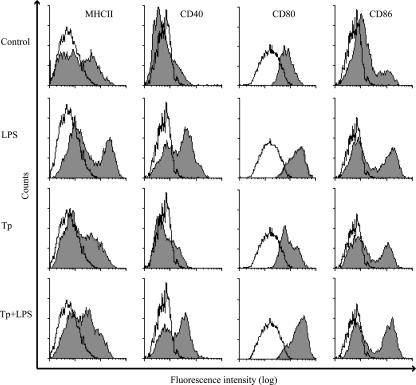
To test the impact of Tp on DC maturation in vitro, BM-DC were cultured in medium with or without LPS and/or Tp for 24 h. We then analyzed by flow cytometry the expression of the surface markers described above on CD11c+ cells. LPS enhanced the expression of MHC-II, CD40, CD80, and CD86. In contrast, DC cocultured with Tp displayed low levels of these surface markers with no significant differences in MFI with control cells. Moreover, the coculture with Tp reduced the MFI of MHC-II surface expression induced by LPS but maintained high levels of costimulatory molecules (Fig. 2 and Table 1).
FIG. 2.
Tp fail to increase the MFI of maturation markers and reduce LPS-induced upregulation of MHC-II on DC. Cells (106/ml) were incubated for 24 h in medium, LPS (10 μg/ml), Tp (4 × 106 Tp/ml), or Tp+LPS. The cells were harvested, and the surface expression levels of the indicated markers were measured on CD11c+ cells by flow cytometry. Gray histograms represent the fluorescence-activated cell sorting (FACS) profiles of the indicated markers. Open histograms represent the FACS profiles after staining with the isotype-matched control antibodies. The results are representative of seven independent experiments.
TABLE 1.
Effects of Tp and LPS on the phenotype of mice DCa
| Treatment | Mean CD11c+ surface molecule expression ± SEMb
|
|||||||
|---|---|---|---|---|---|---|---|---|
| MHC-II
|
CD40
|
CD80
|
CD86
|
|||||
| MFI | % | MFI | % | MFI | % | MFI | % | |
| Control | 119 ± 27 | 20 ± 12 | 35 ± 5 | 16 ± 4 | 57 ± 15 | 63 ± 10 | 178 ± 48 | 17 ± 4 |
| Tp | 111 ± 22 | 27 ± 16 | 45 ± 6 | 38 ± 8 | 63 ± 15 | 64 ± 9 | 187 ± 45 | 43 ± 6† |
| LPS | 186 ± 40* | 38 ± 7* | 60 ± 10* | 62 ± 5† | 105 ± 27† | 81 ± 7* | 229 ± 55* | 51 ± 5† |
| Tp+LPS | 131 ± 25 | 44 ± 15† | 70 ± 14† | 64 ± 5† | 103 ± 31* | 72 ± 9 | 210 ± 49* | 58 ± 5† |
DC were cultured for 24 h in medium, LPS, Tp, or Tp+LPS. Only LPS treatment modified the maturation profile of DC, observed as an increase in the expression of the surface markers evaluated. Tp+LPS together induced a high level of expression of costimulatory molecules but not a significant increase in the expression of MHC-II. DC were stained with different monoclonal antibodies or isotypic controls, and surface expression was analyzed by FACS.
Results are expressed as the MFI or the percentage of positive cells for each marker, calculated by subtracting the corresponding value for the isotype controls. Data are given as means of seven independent experiments. * and †, Significantly different (P < 0.05 [*] and P < 0.01 [†]) from the value for control DC as calculated by a nonparametric Friedman's test.
Dextran uptake.
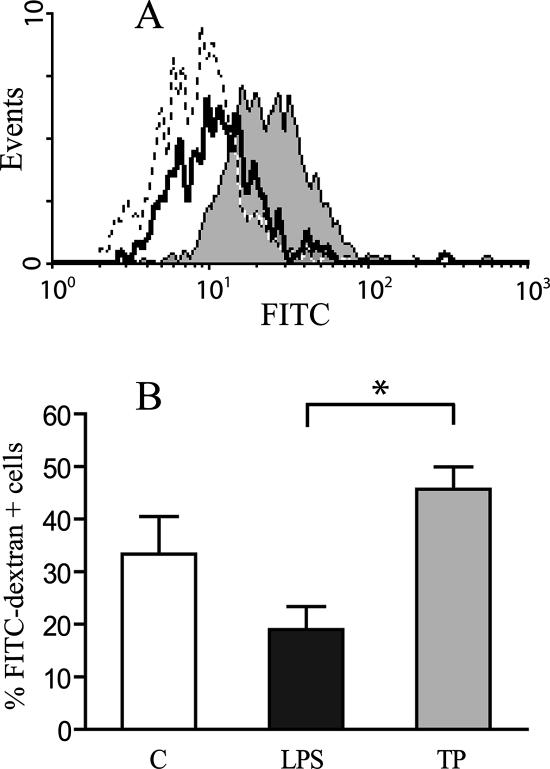
Immature DC are extremely efficient in antigen capture, but they lose the endocytic capacity during their maturation process (8, 40, 48). To further analyze the maturation state of Tp-treated DC, FITC-dextran uptake experiments were performed.
As shown in Fig. 3, FITC-dextran uptake was strongly reduced in DC after LPS exposure. Few or no changes were detected in the MFI after Tp coculture compared to the control at 37°C (data not shown). FITC-dextran intake was higher in Tp than in LPS-treated DC (Fig. 3A). Tp also induced a slight increase in the number of cells that incorporated FITC-dextran compared to the control, showing a significant difference between Tp and LPS-treated DC (P < 0.05; Fig. 3B). When Tp+LPS were present, FITC-dextran uptake was reduced to the levels of LPS treatment (data not shown).
FIG. 3.
FITC-dextran uptake. DC preserve their endocytic capacity after Tp coculture. After different treatments (medium, LPS, Tp, and Tp+LPS), the cells were resuspended in medium. FITC-dextran was added at a final concentration of 250 μg/ml, and DC were incubated at 37°C with 5% CO2 for 40 min or at 0°C. The cells were then washed three times in ice-cold PBS with 1% bovine serum albumin and 0.1% NaN3, stained with anti-mouse CD11c-PE, and analyzed by flow cytometry. (A) The open histogram corresponds to the FACS profile of DC treated with LPS, whereas the gray histogram displays the profile of DC cocultured with Tp. The dashed line represents the fluorescence intensity of the negative control, kept at 0°C. (B) Percentage of positive FITC-dextran cells. There is a significant increase in the number of cells that incorporate FITC-dextran after Tp coculture compared to LPS (P < 0.05), expressed as the percentage of positive cells ± the standard error of the mean (SEM). The fluorescence background (control at 0°C) was always subtracted. The results are representative of three independent experiments.
Tp regulate cytokine production by DC.
Cytokine production by DC is subject to a tight regulation, being generally transient and restricted to a narrow temporal window after the induction of DC maturation (26, 41). In light of our results, the attention was focused on the analysis of cytokines that might modulate the activation process of DC. Both IL-10 and TGF-β are well-known immunosuppressive cytokines (4, 31, 58), and both have been reported to be produced during T. cruzi infection (37, 44, 45).
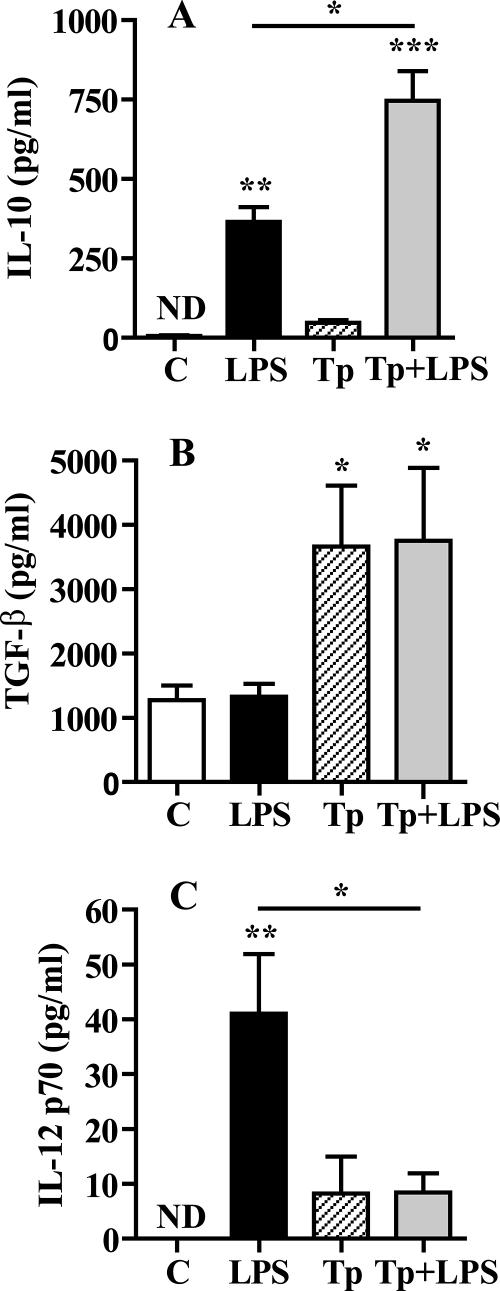
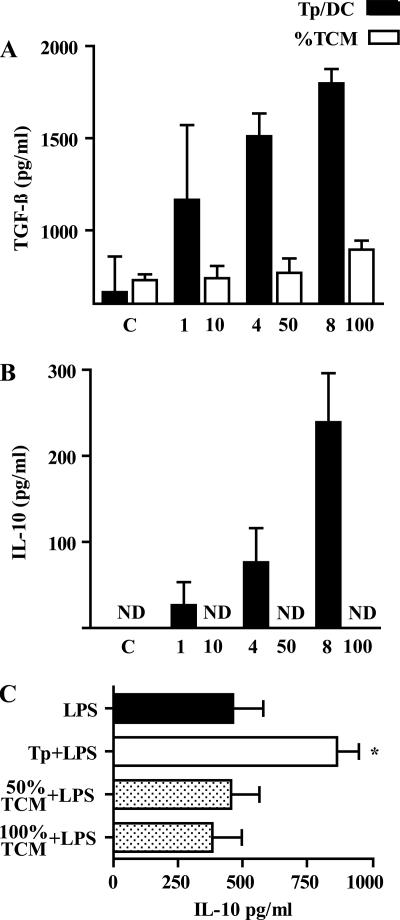
In order to assess whether Tp modulate the production of these cytokines, DC with or without LPS were incubated in the presence or absence of Tp for 24 h. Then, cytokine secretion was measured in culture supernatants by ELISA. As shown in Fig. 4, Tp increased twofold the IL-10 production induced by LPS (P < 0.05), whereas there was no significant production of this cytokine with Tp alone (Fig. 4A). Tp also increased TGF-β production by DC (P < 0.05, Fig. 4B). On the other hand, Tp significantly reduced the levels of IL-12p70 induced by LPS (P < 0.05, Fig. 4C), while IFN-γ levels were below the detection limit of the assay (data not shown).
FIG. 4.
Tp regulate IL-10 and IL-12 production in LPS-treated DC and induce TGF-β production by DC. Cells (106/ml) were incubated in medium or stimulated with LPS (10 μg/ml), Tp at a Tp/cell ratio of 4/1, and Tp+LPS for 24 h. In culture supernatants the IL-10 (A), TGF-β (B), and IL-12p70 (C) levels were determined by ELISA. ND, not detected. The results are represented as the means ± the SEM of seven independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared to the respective controls).
Dose-response secretion of TGF-β and IL-10 induced by Tp but not by Tp secretion products.
Increasing numbers of parasites induced an enhanced secretion of both TGF-β and IL-10 by DC in a dose-dependent manner (Fig. 5A and B). Interestingly, these findings were correlated with an increasing percentage of infected cells with an active intracellular multiplication of Am at different parasite/cell ratios (see above). We then investigated whether secretion of TGF-β and IL-10 was induced by Tp secretion products, so DC were cultured at different %TCM. Neither TGF-β nor IL-10 was induced by TCM (Fig. 5A and B). Furthermore, TCM did not enhance the IL-10 secretion induced by LPS as Tp did (Fig. 5C).
FIG. 5.
An increasing number of Tp cocultured with DC, not their secretion products (TCM), induce a greater secretion of TGF-β and IL-10. Cells (106/ml) were cultured in medium at different Tp/cell ratios (1/1, 4/1, or 8/1) or %TCM concentrations (10, 50, or 100%) with or without LPS for 24 h. Both TGF-β (A) and IL-10 (B and C) levels were determined by ELISA. ND, not detected. The results are represented as the means ± the SEM of three independent experiments.
These findings suggest that both TGF-β and IL-10 secretion requires the presence of Tp in culture and possibly cell-to-cell contact and/or early infection.
Secretion of IL-10 and TGF-β by DC occurs early in time, and IL-10 secretion does not require DC infection.
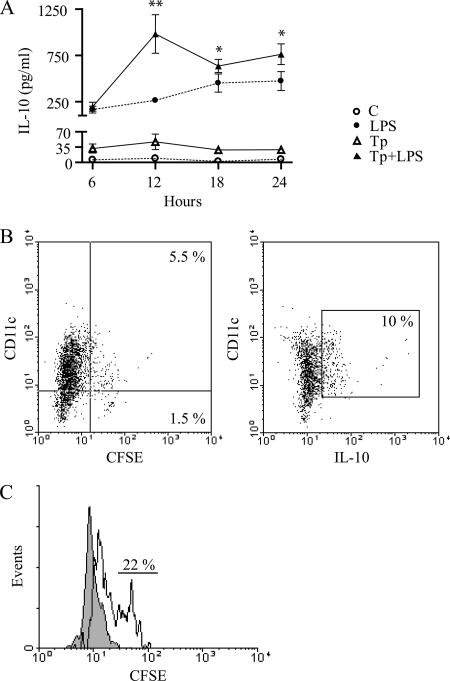
We studied the kinetics of IL-10 and TGF-β production over 24 h. Tp alone induced a slight increase of IL-10 secretion, which was enhanced at 12 h but did not modify the basal secretion of IL-10 in a significant manner. As expected, it was enhanced after LPS addition, at 6 h after stimulation, and was sustained for at least 24 h. Tp addition exerted a synergistic effect on the IL-10 secretion induced by LPS, increasing >3-fold the production induced by LPS alone at 12 h after stimulation (P < 0.01). The difference remained significant up to the end of the experimental observations (P < 0.05) (Fig. 6A). TGF-β levels only increased in the presence of Tp with or without LPS and were detectable since 6 h after Tp coculture. The levels then remained unchanged (data not shown).
FIG. 6.
Kinetics of IL-10 secretion by DC. Secretion occurs early and is not correlated with DC infection. (A) Cells (106/ml) were cultured in medium with or without LPS (10 μg/ml) and/or Tp at a Tp/cell ratio of 4/1 for 6, 12, 18, or 24 h. The concentrations of IL-10 in culture supernatants were measured by ELISA. The data show the means of three independent experiments ± the SEM. *, P < 0.05; **, P < 0.01 (compared to LPS treatment values). (B) Cells were also cocultured with Tp labeled with CFSE (4/1, Tp/DC) for 12 h. The cells were then washed, and the percentage of infection and IL-10 production were determined by flow cytometry. (C) Percentages of infected DC that were actively producing IL-10. The gray histogram represents the FACS profile of control cells. The open histogram represents the FACS profile of Tp-treated cells gated in the IL-10 positive quadrant. Quadrant gates were set on appropriate controls or isotype-matched controls. The data are representative of two independent experiments.
In a second round of experiments, we analyzed whether the IL-10 production was associated with DC infection. Initially, we measured the percentage of infected cells using Tp labeled with CFSE as described above and characterized the percentage of DC that produced IL-10 after 12 h of coculture (Fig. 6B). When we analyzed the gate of IL-10 producers-DC, we observed that only 22% were infected (CFSE) (Fig. 6C).
MLR.
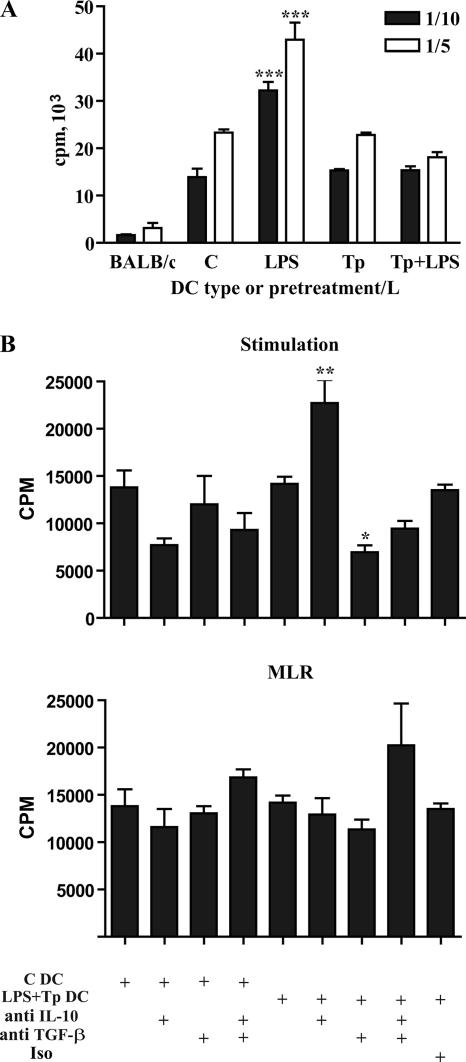
Fully mature DC can be characterized by their ability to induce immunity, which can be reflected in their capacity to initiate strong lymphocyte responses (8, 27). Our results show that Tp downregulate the MHC-II expression on LPS-treated DC and also that Tp modulate the cytokine profile of DC toward a regulatory one (Fig. 2 and 4). To test the stimulatory capacity of DC preactivated with LPS in the presence or absence of Tp, we performed an allogeneic MLR using two different doses of pretreated and/or cocultured DC. LPS-mature DC gained a strong stimulatory capacity (P < 0.001) that was not observed in DC that were prestimulated with Tp. During LPS pretreatment, the addition of Tp inhibited DC-stimulatory capacity, taking it to the levels shown by control DC (Fig. 7A).
FIG. 7.
Tp inhibit the lymphocyte stimulatory capacity of DC pretreated with LPS, and the effect is reversed by IL-10 neutralization during the differentiation process of DC. (A) Control BALB/c DC were cultured with medium, and C3H/HeNk DC were cultured with medium or different stimuli for 24 h. The cells were then washed, irradiated, and cocultured with BALB/c lymph node cells enriched in T cells at 1/5 (□) or 1/10 (▪) DC/lymphocyte ratios for 3 days. Cell proliferation was assessed by determining the [3H]thymidine incorporation during the last 18 h of culture. Cultures were set up in triplicate, and the results are expressed as means ± the SEM. The data are representative of four independent experiments. ***, P < 0.001 (compared to the rest of the treatments). (B) Effect of anti-IL-10 and/or anti-TGF-β antibodies on the regulatory function of DC pretreated with Tp+LPS or control DC during their differentiation process (stimulation) or during MLR. Cell proliferation and culture set up was assessed as described above. The data are representative of three independent experiments. *, P < 0.05; **, P < 0.01 (compared to Tp+LPS treatment or isotype-matched controls).
In order to get deeper in the analysis of the possible role of both TGF-β and IL-10 in the inhibition of the LPS-induced response by Tp, we carried out experiments with neutralizing antibody simultaneously with LPS+Tp treatment during the differentiation process of DC or during MLR. We observed a clear reversion of the inhibitory effect induced by Tp on LPS treatment during MLR when IL-10 was neutralized during the stimulation process of DC (P < 0.01). As expected, this reversion was not displayed in control DC. Surprisingly, the neutralization of TGF-β during DC stimulation increased the inhibitory effect induced by Tp observed in the MLR (P < 0.05; Fig. 7B). Simultaneous addition of IL-10 and TGF-β neutralizing antibody during the MLR partially increased lymphoproliferation in a nonsignificant manner (Fig. 7B). These results showed that both cytokines were probably important in the described inhibitory event during the MLR. However, only IL-10 seems to play a critical role in DC differentiation induced by Tp because the inhibitory capacity of DC can be reversed by the use of neutralizing antibody during that differentiation process. Indeed, it was previously reported that IL-10 modulates T-cell responses mainly via its suppressive effects on APC (43, 60).
DISCUSSION
In a previous study, we showed that T. cruzi infection can inhibit APC activation, modulating the expression of MHC-II and downregulating T-cell stimulatory activity of splenic DC (6). In addition, independent studies have shown the ability of parasite molecules or the whole parasite to trigger different activities in cells from the innate immune system that are important determinants of host resistance to T. cruzi infection (9, 11-13, 33). However, whether the parasite itself can modulate and/or switch the DC responses modifying their role in the development of innate and/or adaptative immunity remains unknown.
In the present study we demonstrated that T. cruzi fails to activate DC in vitro, as reflected by their low expression of MHC-II and costimulatory molecules after coculture with Tp. We also observed that DC preserve their endocytic capacity, a feature of immaturity. Interestingly, Tp induce DC secretion of TGF-β and IL-10, both in a dose-dependent manner. TGF-β is a cytokine with immunoregulatory properties (4, 49), and both, IL-10 and TGF-β were shown to be important in the induction of tolerance (31, 43, 50, 63).
Immature DC have been shown to induce tolerance (15). Recently, a new regulatory subset of DC, displaying a Th-2 cytokine profile with an increased secretion of IL-10 and less IL-12 and that also inhibits antigen-specific T-cell proliferation, has been reported in vivo (64). Moreover, LPS or other Toll-like receptor (TLR) agonist treatment could not reverse the inhibitory function of these regulatory DC (36). TLR-mediated recognition of microbial components by immature DC induces a reprogramming of cellular functions (29, 42). LPS treatment induces DC maturation, a phenomenon that involves increased expression of MHC-II, CD40, and costimulatory molecules such as CD80 and CD86 (14, 39, 59). Therefore, using LPS to activate DC, we further analyzed whether this treatment affected the apparent regulatory profile of our cells obtained after coculture with Tp. As we demonstrated here, Tp modulate the maturation process of DC currently induced by LPS, downregulating the expression of MHC-II on their surface. Moreover, we found that during Tp coculture and LPS treatment, DC secrete high levels of TGF-β and IL-10 and also that Tp have a synergistic effect on IL-10 secretion induced by LPS. Both cytokines were reported to be produced during T. cruzi infection (37, 44). Besides, a previous study showed that the addition of IL-10 and TGF-β inhibits the anti-parasite response by IFN-γ-activated macrophages in vitro (16).
IL-12 is the major Th-1-polarizing cytokine produced by DC (24), and its production can be modulated by cytokines such as IL-10 and mediators present during induction of maturation that can exert an inhibitory effect (41). Interestingly, IL-12 has been reported to mediate a mechanism of resistance to T. cruzi in an IFN-γ- and TNF-α-dependent way (23), and previous studies have associated the production of IL-10 with the suppression of a protective cell-mediated immune response to T. cruzi (3, 22, 37). In contrast to the reported induction of IL-12 by Tp in macrophages (7), our results showed that Tp inhibit the upregulation of IL-12 by LPS in DC. Previous works have also reported different responses displayed by DC and macrophages after the stimulus with parasitic protozoa. One example is Leishmania donovani, which settles the infection via macrophage invasion (19). In light of these results, we assume that T. cruzi might be displaying an evasion mechanism, polarizing the DC response to an immunoregulatory one. We also evidence that the regulatory profile induced by Tp is strong enough to counteract TLR-4 activation.
The regulatory role of T. cruzi has been previously reported in an in vivo study (53), where it was shown that experimental autoimmune encephalomyelitis is prevented and cured by infection with T. cruzi. A regulatory profile induced by T. cruzi may limit DC capacity to initiate a strong protective response, affecting the first-line mechanisms involved in the control of parasite burden and finally resulting in a failure to control the infection during acute Chagas' disease.
All our results indicate for the first time that Tp may be responsible for the induction of regulatory DC in vitro. The regulatory DC subsets reported until now have similar functions but exhibit different phenotypes, and their hallmark is the inhibition of T-cell proliferation (54). Several studies have shown that a certain population of phenotypical mature DC, expressing high levels of costimulatory molecules and/or MHC-II molecules, may induce T-cell unresponsiveness and also possess tolerogenic properties (5, 30, 60). A previous study reported that accessory cells in contact with T. cruzi in vitro became incompetent as APC, being unable to induce a normal T-cell proliferative response (32). Here, we demonstrated that the presence of Tp during LPS pretreatment of DC makes them lose their capacity to induce lymphocyte proliferation, in spite of their upregulated expression levels of CD40, CD80, and CD86. Furthermore, Tp even enhance the gap between IL-10 and IL-12p70 production, inducing a high IL-10/IL-12p70 ratio during LPS treatment. It was previously reported that IL-10 modulates T-cell responses mainly via its suppressive effects on APC (43, 60). In vitro neutralization assays evidenced that IL-10, and not TGF-β, is one of the responsible for DC differentiation to a regulatory phenotype by Tp. Thus, we can clearly confirm that Tp exert an inhibitory effect, affecting DC functionality even in a context of activation using LPS, and that this phenomenon involves at least IL-10 during DC differentiation.
Since our experimental condition makes it impossible to block infection without affecting DC functionality, we cannot assume whether early infection of DC or just Tp-DC contact is required for the induction of a regulatory phenotype. However, the early secretion of immunoregulatory cytokines, the results showing that a low percentage of IL-10 producing-DC are infected after 12 h of coculture, and the identical kinetic of IL-10 secretion induced by K98 (a strain with low rate of infection) (data not shown) support the idea that the differentiation process is possibly dependent on DC-Tp contact. Another possibility is that very early infection of some DC may induce the early secretion of immunoregulatory cytokine, influencing the response and differentiation of neighboring cells in an autocrine and/or paracrine manner.
On the other hand, these data strongly suggest that the mechanisms involved in that regulation were totally independent of parasite multiplication because Tp of RA require 6 h after cell invasion to complete the first multiplicative cycle, whereas K98 requires more than 24 h (G. A. Mirkin, unpublished data). However, additional studies are necessary in order to understand the mechanisms of that phenotype induction.
Further investigations about the molecular mechanisms involved in T. cruzi induction of regulatory DC in vitro would permit a better understanding of how the parasite can evade or regulate the immune system in order to persist. Future studies would also provide additional tools for developing new strategies for effective immunotherapy.
Acknowledgments
We thank Eduardo Gimenez and Federico Boucar for technical assistance and Marcela Cucher and Susana Fink for critical reading of the manuscript.
This study was supported by Agencia Nacional de Promoción Científica y Tecnológica, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), and the Universidad de Buenos Aires, Buenos Aires, Argentina. C.V.P. and C.A.S. are fellows of CONICET. S.M.G.C. is a member of the research career of CONICET.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 17 March 2008.
REFERENCES
- 1.Abrahamsohn, I. A. 1998. Cytokines in innate and acquired immunity to Trypanosoma cruzi infection. Braz. J. Med. Biol. Res. 31117-121. [DOI] [PubMed] [Google Scholar]
- 2.Abrahamsohn, I. A., and R. L. Coffman. 1995. Cytokine and nitric oxide regulation of the immunosuppression in Trypanosoma cruzi infection. J. Immunol. 1553955-3963. [PubMed] [Google Scholar]
- 3.Abrahamsohn, I. A., and R. L. Coffman. 1996. Trypanosoma cruzi: IL-10, TNF, IFN-γ, and IL-12 regulate innate and acquired immunity to infection. Exp. Parasitol. 84231-244. [DOI] [PubMed] [Google Scholar]
- 4.Ahuja, S. S., F. Paliogianni, H. Yamada, J. E. Balow, and D. T. Boumpas. 1993. Effect of transforming growth factor-β on early and late activation events in human T cells. J. Immunol. 1503109-3118. [PubMed] [Google Scholar]
- 5.Akbari, O., R. H. De Kruyff, and D. T. Umetsu. 2001. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat. Immunol. 2725-731. [DOI] [PubMed] [Google Scholar]
- 6.Alba Soto, C. D., G. A. Mirkin, M. E. Solana, and S. M. González Cappa. 2003. Trypanosoma cruzi infection modulates in vivo expression of major histocompatibility complex class II molecules on antigen-presenting cells and T-cell stimulatory activity of dendritic cells in a strain-dependent manner. Infect. Immun. 711194-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aliberti, J. C., M. A. Cardoso, G. A. Martins, R. T. Gazzinelli, L. Q. Vieira, and J. S. Silva. 1996. Interleukin-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infect. Immun. 641961-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392245-252. [DOI] [PubMed] [Google Scholar]
- 9.Brodskyn, C., J. Patricio, R. Oliveira, L. Lobo, A. Arnholdt, L. Mendonca-Previato, A. Barral, and M. Barral-Netto. 2002. Glycoinositol phospholipids from Trypanosoma cruzi interfere with macrophages and dendritic cell responses. Infect. Immun. 703736-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burleigh, B. A., and N. W. Andrews. 1995. The mechanisms of Trypanosoma cruzi invasion of mammalian cells. Annu. Rev. Microbiol. 49175-200. [DOI] [PubMed] [Google Scholar]
- 11.Campos, M. A., I. C. Almeida, O. Takeuchi, S. Akira, E. P. Valente, D. O. Procopio, L. R. Travassos, J. A. Smith, D. T. Golenbock, and R. T. Gazzinelli. 2001. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J. Immunol. 167416-423. [DOI] [PubMed] [Google Scholar]
- 12.Celentano, A. M., and S. M. González Cappa. 1992. Induction of macrophage activation and opsonizing antibodies by Trypanosoma cruzi subpopulations. Parasite Immunol. 14155-167. [DOI] [PubMed] [Google Scholar]
- 13.de Diego, J., C. Punzón, M. Duarte, and M. Fresno. 1997. Alteration of macrophage function by a Trypanosoma cruzi membrane mucin. J. Immunol. 1594983-4989. [PubMed] [Google Scholar]
- 14.De Smedt, T., B. Pajak, E. Muraille, L. Lespagnard, E. Heinen, P. De Baetselier, J. Urbain, O. Leo, and M. Moser. 1996. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J. Exp. Med. 1841413-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhodapkar, M. V., R. M. Steinman, J. Krasovsky, C. Munz, and N. Bhardwaj. 2001. Antigen-specific inhibition of effector T-cell functions in humans after injection of immature dendritic cells. J. Exp. Med. 193233-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gazzinelli, R. T., I. P. Oswald, S. Hieny, S. L. James, and A. Sher. 1992. The microbicidal activity of interferon-gamma-treated macrophages against Trypanosoma cruzi involves an l-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-beta. Eur. J. Immunol. 222501-2506. [DOI] [PubMed] [Google Scholar]
- 17.Ghanekar, S., L. Zheng, A. Logar, J. Navratil, L. Borowski, P. Gupta, and C. Rinaldo. 1996. Cytokine expression by human peripheral blood dendritic cells stimulated in vitro with HIV-1 and herpes simplex virus. J. Immunol. 1574028-4036. [PubMed] [Google Scholar]
- 18.Golden, J. M., and R. L. Tarleton. 1991. Trypanosoma cruzi: cytokine effects on macrophage trypanocidal activity. Exp. Parasitol. 72391-402. [DOI] [PubMed] [Google Scholar]
- 19.Gorak, P. M., C. R. Engwerda, and P. M. Kaye. 1998. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur. J. Immunol. 28687-695. [DOI] [PubMed] [Google Scholar]
- 20.Grosjean, I., C. Caux, C. Bella, I. Berger, F. Wild, J. Banchereau, and D. Kaiserlian. 1997. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J. Exp. Med. 186801-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson, R. A., S. C. Watkins, and J. L. Flynn. 1997. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J. Immunol. 159635-643. [PubMed] [Google Scholar]
- 22.Hunter, C. A., L. A. Ellis-Neyes, T. Slifer, S. Kanaly, G. Grunig, M. Fort, D. Rennick, and F. G. Araujo. 1997. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J. Immunol. 1583311-3316. [PubMed] [Google Scholar]
- 23.Hunter, C. A., T. Slifer, and F. Araujo. 1996. Interleukin-12-mediated resistance to Trypanosoma cruzi is dependent on tumor necrosis factor alpha and gamma interferon. Infect. Immun. 642381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaliński, P., C. M. Hilkens, E. A. Wierenga, and M. L. Kapsenberg. 1999. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol. Today 20561-567. [DOI] [PubMed] [Google Scholar]
- 25.Kotner, J., and R. Tarleton. 2007. Endogenous CD4+ CD25+ regulatory T cells have a limited role in the control of Trypanosoma cruzi infection in mice. Infect. Immun. 75861-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langenkamp, A., M. Messi, A. Lanzavecchia, and F. Sallusto. 2000. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat. Immunol. 1311-316. [DOI] [PubMed] [Google Scholar]
- 27.Lanzavecchia, A., and F. Sallusto. 2000. Regulation of T-cell immunity by dendritic cells. Cell 106263-266. [DOI] [PubMed] [Google Scholar]
- 28.Loscher, C. E., E. Draper, O. Leavy, D. Kelleher, K. H. Mills, and H. M. Roche. 2005. Conjugated linoleic acid suppresses NF-κB activation and IL-12 production in dendritic cells through ERK-mediated IL-10 induction. J. Immunol. 1754990-4998. [DOI] [PubMed] [Google Scholar]
- 29.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1135-145. [DOI] [PubMed] [Google Scholar]
- 30.Menges, M., S. Rössner, C. Voigtlander, H. Schindler, N. A. Kukutsch, C. Bogdan, K. Erb, G. Schuler, and M. B. Lutz. 2002. Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J. Exp. Med. 19515-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19683-765. [DOI] [PubMed] [Google Scholar]
- 32.Motran, C., A. Gruppi, C. M. Vullo, M. C. Pistoresi-Palencia, and H. M. Serra. 1996. Involvement of accessory cells in the Trypanosoma cruzi-induced inhibition of the polyclonal response of T lymphocytes. Parasite Immunol. 1843-48. [DOI] [PubMed] [Google Scholar]
- 33.Oliveira, A. C., J. R. Peixoto, L. B. de Arruda, M. A. Campos, R. T. Gazzinelli, D. T. Golenbock, S. Akira, J. O. Previato, L. Mendonca-Previato, A. Nobrega, and M. Bellio. 2004. Expression of functional TLR4 confers proinflammatory responsiveness to Trypanosoma cruzi glycoinositolphospholipids and higher resistance to infection with T. cruzi. J. Immunol. 1735688-5696. [DOI] [PubMed] [Google Scholar]
- 34.Planelles, L., M. C. Thomas, C. Marañón, M. Morell, and M. C. López. 2003. Differential CD86 and CD40 co-stimulatory molecules and cytokine expression pattern induced by Trypanosoma cruzi in APCs from resistant or susceptible mice. Clin. Exp. Immunol. 13141-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plata, F., J. Wietzerbin, F. G. Pons, E. Falcoff, and H. Eisen. 1984. Synergistic protection by specific antibodies and interferon against infection by Trypanosoma cruzi in vitro. Eur. J. Immunol. 14930-935. [DOI] [PubMed] [Google Scholar]
- 36.Qian, C., X. Jiang, H. An, Y. Yu, Z. Guo, S. Liu, H. Xu, and X. Cao. 2006. TLR agonists promote ERK-mediated preferential IL-10 production of regulatory dendritic cells (diffDCs), leading to NK-cell activation. Blood 1082307-2315. [DOI] [PubMed] [Google Scholar]
- 37.Reed, S. G., C. E. Brownell, D. M. Russo, J. S. Silva, K. H. Grabstein, and P. J. Morrissey. 1994. IL-10 mediates susceptibility to Trypanosoma cruzi infection. J. Immunol. 1533135-3140. [PubMed] [Google Scholar]
- 38.Reed, S. G., S. B. Roters, and E. A. Goidl. 1983. Spleen cell-mediated suppression of IgG production to a non-parasite antigen during chronic Trypanosoma cruzi infection in mice. J. Immunol. 1311978-1982. [PubMed] [Google Scholar]
- 39.Roake, J. A., A. S. Rao, P. J. Morris, C. P. Larsen, D. F. Hankins, and J. M. Austyn. 1995. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J. Exp. Med. 1812237-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sallusto, F., M. Cella, C. Danieli, and A. Lanzavecchia. 1995. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sallusto, F., and A. Lanzavecchia. 2002. The instructive role of dendritic cells on T-cell responses. Arthritis Res. 4127-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schnare, M., G. M. Barton, A. C. Holt, K. Takeda, S. Akira, and R. Medzhitov. 2001. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2947-950. [DOI] [PubMed] [Google Scholar]
- 43.Schröder, M., C. Meisel, K. Buhl, N. Profanter, N. Sievert, H. D. Volk, and G. Grütz. 2003. Different modes of IL-10 and TGF-β to inhibit cytokine-dependent IFN-γ production: consequences for reversal of lipopolysaccharide desensitization. J. Immunol. 1705260-5267. [DOI] [PubMed] [Google Scholar]
- 44.Silva, J. S., D. R. Twardzik, and S. G. Reed. 1991. Regulation of Trypanosoma cruzi infections in vitro and in vivo by transforming growth factor beta (TGF-β). J. Exp. Med. 174539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Souza, P. E., M. O. Rocha, C. A. Menezes, J. S. Coelho, A. C. Chaves, K. J. Gollob, and W. O. Dutra. 2007. Trypanosoma cruzi infection induces differential modulation of costimulatory molecules and cytokines by monocytes and T cells from patients with indeterminate and cardiac Chagas' disease. Infect. Immun. 751886-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinbrink, K., E. Graulich, S. Kubsch, J. Knop, and A. H. Enk. 2002. CD4+ and CD8+ anergic T cells induced by interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood 992468-2476. [DOI] [PubMed] [Google Scholar]
- 47.Steinman, R. M., D. Hawiger, and M. C. Nussenzweig. 2003. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21685-711. [DOI] [PubMed] [Google Scholar]
- 48.Steinman, R. M., and J. Swanson. 1995. The endocytic activity of dendritic cells. J. Exp. Med. 182283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strobl, H., and W. Knapp. 1999. TGF-β1 regulation of dendritic cells. Microbes Infect. 11283-1290. [DOI] [PubMed] [Google Scholar]
- 50.Sun, W., Q. Wang, L. Zhang, J. Pan, M. Zhang, G. Lu, H. Yao, J. Wang, and X. Cao. 2002. TGF-β1 gene modified immature dendritic cells exhibit enhanced tolerogenicity but induce allograft fibrosis in vivo. J. Mol. Med. 80514-523. [DOI] [PubMed] [Google Scholar]
- 51.Sztein, M. B., and F. Kierszenbaum. 1993. Mechanisms of development of immunosuppression during Trypanosoma infections. Parasitol. Today 9424-428. [DOI] [PubMed] [Google Scholar]
- 52.Sztein, M. B., and F. Kierszenbaum. 1992. Suppression by Trypanosoma cruzi of T-cell receptor expression by activated human lymphocytes. Immunology 77277-283. [PMC free article] [PubMed] [Google Scholar]
- 53.Tadokoro, C. E., A. L. Vallochi, L. S. Rios, G. A. Martins, D. Schlesinger, T. Mosca, V. K. Kuchroo, L. V. Rizzo, and I. A. Abrahamsohn. 2004. Experimental autoimmune encephalomyelitis can be prevented and cured by infection with Trypanosoma cruzi. J. Autoimmun. 23103-115. [DOI] [PubMed] [Google Scholar]
- 54.Tang, H., Z. Guo, M. Zhang, J. Wang, G. Chen, and X. Cao. 2006. Endothelial stroma programs hematopoietic stem cells to differentiate into regulatory dendritic cells through IL-10. Blood 1081189-1197. [DOI] [PubMed] [Google Scholar]
- 55.Torrico, F., H. Heremans, M. T. Rivera, E. Van Marck, A. Billiau, and Y. Carlier. 1991. Endogenous IFN-γ is required for resistance to acute Trypanosoma cruzi infection in mice. J. Immunol. 1463626-3632. [PubMed] [Google Scholar]
- 56.Urban, B. C., D. J. Ferguson, A. Pain, N. Willcox, M. Plebanski, J. M. Austyn, and D. J. Roberts. 1999. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 40073-77. [DOI] [PubMed] [Google Scholar]
- 57.Van Overtvelt, L., N. Vanderheyde, V. Verhasselt, J. Ismaili, L. De Vos, M. Goldman, F. Willems, and B. Vray. 1999. Trypanosoma cruzi infects human dendritic cells and prevents their maturation: inhibition of cytokines, HLA-DR, and costimulatory molecules. Infect. Immun. 674033-4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Veldhoen, M., H. Moncrieffe, R. J. Hocking, C. J. Atkins, and B. Stockinger. 2006. Modulation of dendritic cell function by naive and regulatory CD4+ T cells. J. Immunol. 1766202-6210. [DOI] [PubMed] [Google Scholar]
- 59.Verhasselt, V., C. Buelens, F. Willems, D. De Groote, N. Haeffner-Cavaillon, and M. Goldman. 1997. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J. Immunol. 1582919-2925. [PubMed] [Google Scholar]
- 60.Wakkach, A., N. Fournier, V. Brun, J. P. Breittmayer, F. Cottrez, and H. Groux. 2003. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity 18605-617. [DOI] [PubMed] [Google Scholar]
- 61.Wang, X. Q., X. M. Duan, L. H. Liu, Y. Q. Fang, and Y. Tan. 2005. Carboxyfluorescein diacetate succinimidyl ester fluorescent dye for cell labeling. Acta Biochim. Biophys. Sin. 37379-385. [DOI] [PubMed] [Google Scholar]
- 62.Wirth, J. J., F. Kierszenbaum, G. Sonnenfeld, and A. Zlotnik. 1985. Enhancing effects of gamma interferon on phagocytic cell association with and killing of Trypanosoma cruzi. Infect. Immun. 4961-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zeller, J. C., A. Panoskaltsis-Mortari, W. J. Murphy, F. W. Ruscetti, S. Narula, M. G. Roncarolo, and B. R. Blazar. 1999. Induction of CD4+ T-cell alloantigen-specific hyporesponsiveness by IL-10 and TGF-β. J. Immunol. 1633684-3691. [PubMed] [Google Scholar]
- 64.Zhang, M., H. Tang, Z. Guo, H. An, X. Zhu, W. Song, J. Guo, X. Huang, T. Chen, J. Wang, and X. Cao. 2004. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat. Immunol. 51124-1133. [DOI] [PubMed] [Google Scholar]