Abstract
We reported previously that low concentrations of sodium citrate strongly promote biofilm formation by Staphylococcus aureus laboratory strains and clinical isolates. Here, we show that citrate promotes biofilm formation via stimulating both cell-to-surface and cell-to-cell interactions. Citrate-stimulated biofilm formation is independent of the ica locus, and in fact, citrate represses polysaccharide adhesin production. We show that fibronectin binding proteins FnbA and FnbB and the global regulator SarA, which positively regulates fnbA and fnbB gene expression, are required for citrate's positive effects on biofilm formation, and citrate also stimulates fnbA and fnbB gene expression. Biofilm formation is also stimulated by several other tricarboxylic acid (TCA) cycle intermediates in an FnbA-dependent fashion. While aconitase contributes to biofilm formation in the absence of TCA cycle intermediates, it is not required for biofilm stimulation by these compounds. Furthermore, the GraRS two-component regulator and the GraRS-regulated efflux pump VraFG, identified for their roles in intermediate vancomycin resistance, are required for citrate-stimulated cell-to-cell interactions, but the GraRS regulatory system does not impact the expression of the fnbA and fnbB genes. Our data suggest that distinct genetic factors are required for the early steps in citrate-stimulated biofilm formation. Given the role of FnbA/FnbB and SarA in virulence in vivo and the lack of a role for ica-mediated biofilm formation in S. aureus catheter models of infection, we propose that the citrate-stimulated biofilm formation pathway may represent a clinically relevant pathway for the formation of these bacterial communities on medical implants.
Microorganisms commonly adhere to surfaces in multilayered groupings referred to as biofilms (29, 54). Growth in a biofilm imparts particular properties to member organisms, including elevated levels of resistance to antibiotics and host defenses. Staphylococcus aureus, a common nosocomial pathogen known for its antibiotic resistance and its ability to cause a wide range of infections (43), can form biofilms on a number of medically relevant surfaces such as catheters and intraocular lenses (56, 71, 75). Biofilm formation by this microbe is thought to contribute to its ability to cause persistent infections (19, 71, 74).
Most genetic studies of biofilm formation by S. aureus have involved the search for biofilm-defective mutants using laboratory medium, typically tryptic soy broth (TSB) supplemented with glucose. Such an approach has identified a number of genetic loci required for biofilm formation, including sarA, agr, dlt, hla, clp, and ica, which codes for the polysaccharide component of an extracellular biofilm matrix (9, 25, 27, 45, 55, 66, 72). The ica locus, its gene products, and the polysaccharide produced by the Ica proteins have been studied extensively in vitro (17, 44-46, 50, 53). A recent study reported a role for the ica locus of S. aureus in models of systemic infection and renal abscess infections (37). In contrast, S. aureus strains lacking the ica locus appear to colonize to the same extent as the wild type (WT) in animal models of implant infections (16, 21, 23, 36), although Fluckiger et al. did show a defect in growth for the ica mutant in an implant infection model when competed against the WT (21). Also, several recent studies have shown that the presence of icaA was not predictive of pathogenicity among staphylococci (4, 24, 42, 52, 59). In contrast, a clear role for the ica locus has been demonstrated in biofilm formation and infection models for Staphylococcus epidermidis (6, 42, 53, 70). Taken together, these data call into question the importance of genes identified in vitro using standard laboratory growth conditions in the context of medical device infections in vivo. It is still unclear what pathways are utilized by S. aureus to form biofilms on medical implants in vivo; therefore, given the importance of staphylococci in causing implant infections (19, 57), a better understanding of this process is sorely needed.
We previously showed that low concentrations of citrate strongly stimulate S. aureus biofilm formation by laboratory strains and clinical isolates in vitro (62). Sodium citrate is used as an anticoagulant in blood banks and catheter locks and is an effective antimicrobial at high concentrations (40, 60). Citrate is thought to exert its antimicrobial effects through the chelation of divalent cations necessary for diverse cellular processes (31, 41). However, the mechanism by which citrate stimulates biofilm formation is unknown. Furthermore, molecular components of the citrate-dependent biofilm formation pathway have not yet been identified. In this study, we show that citrate stimulates both cell-to-surface and cell-to-cell interactions and uses distinct sets of genes to mediate these effects. We also discuss the possible relevance of citrate-stimulated biofilm formation in vivo.
MATERIALS AND METHODS
Strains and medium.
Bacterial strains used in this study are listed in Table 1. Unless otherwise noted, all strains used were derived from RN6390 or MZ100. MZ100, like RN6390, is a derivative of 8325-4 and was generated in the same way as RN6390; therefore, these two strains should be isogenic (61, 62). We maintain the different strain designations as a formality. Bacterium inocula for all studies were grown overnight in TSB (Bacto; Becton Dickinson, Sparks, MD) or on tryptic soy agar at 37°C. For all biofilm, aggregation, and expression experiments, 66% TSB-0.2% glucose was used, as this growth medium promotes robust biofilm formation. Henceforth, in this paper, “TSB” will signify medium with 66% TSB. Some cultures were supplemented with 0.2% trisodium citrate (catalog number S279-500; Fisher Scientific, Fair Lawn, NJ), as indicated in each experiment. Isocitrate, α-ketoglutarate, succinate, fumarate, and malate were used at 2.0%, oxaloacetate was used at 0.25%, and glyoxylate was used at 0.003%. Oxaloacetate and glyoxylate were obtained from Sigma Chemical Company (St. Louis, MO), and isocitrate, succinate, fumarate, and malate were obtained from Fisher Scientific. Plasmids were maintained with chloramphenicol at 10 μg/ml at all times.
TABLE 1.
Strains, plasmids, and primers
| Strain, plasmid, or primer | Genotype, description, or sequence | Reference or source |
|---|---|---|
| Strains | ||
| RN6390 | WT laboratory strain | 35 |
| ALC42 | DB clinical isolate | 12 |
| ALC32 | DB sarA::Tn917 | 12 |
| SMC1062 | MZ100 laboratory strain isogenic to RN6390 | 61 |
| SMC2715 | MZ100 sarA::aphA-3 | 61 |
| ALC136 | RN6390 sarA::Tn917 | 13 |
| ALC600 | RN6390 sarA::Tn917 pCL84-sarA | 11 |
| SMC2713 | MZ100 ica::tetK | 61 |
| ALC2629 | RN6390 fnbA::tet | This study |
| ALC2634 | RN6390 fnbB::erm | This study |
| CMS392 | RN6390 ΔgraR | 51 |
| CMS399 | RN6390 ΔgraS | 51 |
| CMS400 | RN6390 ΔvraG | 51 |
| ALC4301 | Mu50 (VISA) | 39 |
| ALC5908 | Mu50 ΔgraR | 51 |
| ALC960 | Col (methicillin-resistant S. aureus) | 26 |
| ALC5542 | Col ΔgraR | 51 |
| SH1000 | rsbU+ | 33 |
| SH1000 acn | acn | Simon Foster |
| ALC1745 | RN6390 pALC1484 | 47 |
| ALC1747 | RN6390 pALC1747 | 73 |
| Plasmids | ||
| pEPSa5 | pC194-based expression vector | 22 |
| pEPSAgraR | pEPSA5 with graR gene | 51 |
| pEPSAgraRS | pEPSA5 with graRS genes | 51 |
| pALC1484 | pSK236 plasmid with promoterless gfp | 47 |
| pALC1747 | fnbA-gfp transcription fusion derived from pALC1484 | 73 |
| Primersa | ||
| gyrBsybrF | GGTGCTGGGCAAATACAAGT | |
| gyrBsybrR | TGGGATACCACGTCCGTTAT | |
| F-fnbA-rt | ACAAGTTGAAGTGGCACAGCC | |
| R-fnbA-rt | CCGCTACATCTGCTGATCTTGTC | |
| F-fnbB-rt | CACCGAAAACTGTGCAAGCA | |
| R-fnbB-rt | TTCCTGTAGTTTCCTTATCAGCAACTT |
Primers were used for qRT-PCR.
Biofilm studies.
Studies of biofilm formation on polystyrene were performed similarly to previously described methods (61, 62). Cultures of staphylococci were grown overnight in TSB. Cultures were diluted to an optical density at 600 nm of 0.01 in TSB plus glucose (0.2%) and supplemented with citrate to 0.2% as indicated. One hundred microliters of cells plus medium were added to individual wells of tissue culture-treated 96-well polystyrene microtiter dishes (Costar 3595; Corning Inc., Corning, NY). These plates were incubated in a closed, humidified plastic container for 8 h at 37°C and then assayed for biofilm formation as described previously (61). Nonadherent cells were removed, and adherent cells were stained with 0.1% crystal violet. Relative biofilm formation was assayed by reading the absorbance at 550 nm using a Molecular Devices (Sunnyvale, CA) Vmax kinetic microplate reader. Each experiment was repeated at least twice and consisted of two to three independent cultures, tested in at least four replicates, under each condition for each experiment.
Genetic manipulations.
Mutations of the fnbA and fnbB genes were mobilized by phage transductions from previously described mutant strains (15, 73) into strain RN6390 using previously described methods (61).
Cell-cell and cell-surface interactions.
Assays of cell-cell and cell-surface interactions were performed as previously described (61).
Aggregation assay.
Aggregation of cells was determined by growing 1.5-ml cultures of TSB plus 0.2% glucose with or without 0.2% citrate overnight on a rotating drum incubator at 37°C. Twenty microliters of the culture was removed from the top of the culture and placed into 80 μl of phosphate-buffered saline in a 96-well plate; the optical density (A600) was determined. The culture tube was then vortexed for 30 to 60 s to resuspend aggregated cells, 20 μl of this suspension was removed and mixed with 80 μl of phosphate-buffered saline, and the optical density was then determined as described above. To determine the “percent aggregation,” we used the following formula: 100 × (A600 postvortex − A600 prevortex)/A600 postvortex. Three single colonies were grown under each condition for each experiment and tested in triplicate. Each experiment was repeated on different days at least twice.
Measurement of PIA/PNAG levels.
Polysaccharide levels were measured by dot blot assay using antibodies to polysaccharide intracellular adhesion/polymeric N-acetyl-glucosamine (PIA/PNAG), as reported previously (17).
RNA extraction and quantitative real-time PCR analysis.
RNA was extracted from cultures grown overnight in TSB, diluted 1:100 into TSB and grown to mid-logarithmic phase, subcultured to an A600 of 0.1 and grown to mid-logarithmic phase an A600 of 0.6, and split into two aliquots. One half was grown in TSB with 0.2% sodium citrate, and the other half was grown in TSB with an equal volume of saline. After 30 min, five cultures of each genotype were pooled for each replicate, and each experiment was done with three replicates. RNA was extracted from cells as previously described (51).
Quantitative real-time PCR was performed as previously described (38) using primers for fnbA and fnbB as reported previously for real-time analysis of fnbA and fnbB transcription (68), and gyrB served as an internal control using primers gyrBsybrF and gyrBsybrR as previously described (51). The experiment was performed on three different days with independent samples.
Green fluorescent protein expression studies.
The expression of fnbA was assessed using a plasmid-borne fnbA-gfp transcriptional fusion. Fluorescence of mid-log-phase TSB-grown cells (at 4 h postinoculation) supplemented with saline or the indicated tricarboxylic acid (TCA) cycle intermediate was measured with a Bio-Tek Synergy 2 apparatus (Bio-Tek Corporation, Winooski, VT) equipped with a 485/20-nm excitation filter and a 516/20-nm emission filter. Sensitivity was set at 100 for fluorescence readings, and the A600 was used for culture turbidity. Expression is presented as relative fluorescent units and normalized to culture optical density.
Statistical analysis.
Microsoft Excel software was used to determine P values using a Student's t test. Error bars are shown as one standard deviation.
RESULTS
Citrate stimulates cell-to-surface interactions during biofilm formation.
We previously showed that low concentrations of citrate strongly stimulate S. aureus biofilm formation (62). Because staphylococcal biofilm formation is dependent upon both cell-to-surface interactions and cell-to-cell interactions (27), we assessed the effects of citrate on both of these steps in biofilm formation.
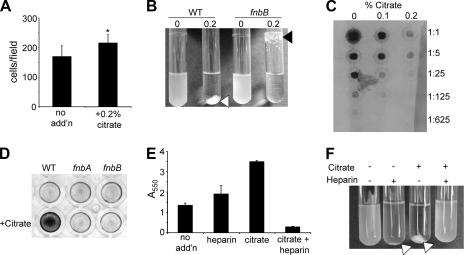
We first determined the effect of 0.2% sodium citrate upon cell-to-surface interactions with polystyrene by S. aureus at 10 min and 45 min. No increase was observed at 10 min, but at 45 min, citrate-treated cultures showed a small but statistically significant increase in the number of adherent bacteria per microscopic field (Fig. 1A and data not shown).
FIG. 1.
Citrate stimulates cell-to-surface and cell-to-cell interactions. (A) The number of cells per microscopic field was determined at 45 min postinoculation by phase-contrast microscopy in the absence (no add'n) and presence of 0.2% citrate. *, P < 0.01 compared to no addition. (B) Visual aggregation assay with the WT and the fnbB mutant grown overnight with 0% or 0.2% citrate. The white arrow indicates the typical WT aggregate, and the black arrow indicates the altered aggregate formed by the fnbB mutant. (C) PIA/PNAG levels measured by dot blot assay. Extracts of the WT strain grown in the absence or presence of citrate were prepared as described in Materials and Methods and spotted in a series of fivefold dilutions. (D) Biofilm formation of the WT and fnb mutants was assessed in a 96-well plate assay in the absence or in the presence of 0.2% citrate (+Citrate). (E and F) Biofilm formation (E) and aggregation (F) phenotypes were assessed for the WT strain grown in the presence and absence of citrate and/or heparin. Biofilm formation and aggregation assays were performed as described in Materials and Methods.
Citrate stimulates cell-to-cell interactions during biofilm formation.
A dramatic representation of citrate promotion of cell-to-cell interactions was observed when cultures were grown overnight in the presence versus in the absence 0.2% sodium citrate (Fig. 1B) (62). The addition of 0.2% citrate caused the formation of a large aggregate, while the remaining culture surrounding the aggregate was optically clear. In contrast, cultures grown in the absence of citrate grew as uniformly turbid cell suspensions. This effect was quantified as described in Materials and Methods, and the percent aggregation in the absence of citrate was calculated as <1%; this value increased to >90% when 0.2% citrate was included in the culture medium (Table 2). This phenotype was exhibited by several other S. aureus strains including N315 and SH1000 (data not shown).
TABLE 2.
Effects of citrate on clinical, laboratory, and genetically defined strains
| Strain (description)a | Biofilm formation (A550) ± SDb
|
% Aggregationc | |
|---|---|---|---|
| Without citrate | With 0.2% citrate | ||
| WT | 0.65 ± 0.12 | 3.06 ± 0.04 | 94.5 ± 3.2 |
| sarA::aphA-3 | 0.28 ± 0.06 | 0.12 ± 0.03 | 0.8 ± 2.0 |
| sarA::Tn917 | 0.41 ± 0.17 | 0.315 ± 0.04 | ND |
| sarA::Tn917/pCL84-sarA | 0.19 ± 0.064 | 5.49 ± 0.24 | ND |
| DB clinical isolate | 0.92 ± 0.20 | 3.40 ± 0.08 | 70.2 ± 9.2 |
| DB sarA::Tn917 | 0.07 ± 0.01 | 0.08 ± 0.04 | 1.6 ± 3.0 |
| ica::tetK | 0.40 ± 0.1 | 3.50 ± 0.1 | ND |
| fnbA | 0.65 ± 0.06 | 0.66 ± 0.04 | 98.2 ± 2.0 |
| fnbB | 0.72 ± 0.03 | 0.70 ± 0.07 | 97.5 ± 2.6 |
| ΔgraR + pEPSa5 | 1.20 ± 0.14 | 1.26 ± 0.11 | 13.6 ± 2.8 |
| ΔgraR + pEPSAgraR | 0.86 ± 0.04 | 2.83 ± 0.04 | 97.8 ± 0.8 |
| ΔgraS + pEPSa5 | 0.97 ± 0.06 | 0.80 ± 0.04 | 30.1 ± 3.6 |
| ΔgraS + pEPSAgraRS | 0.89 ± 0.07 | 2.15 ± 0.05 | 99.9 ± 0.04 |
| ΔvraG | 1.28 ± 0.23 | 1.00 ± 0.06 | 25.8 ± 1.8 |
| Mu50 (VISA) | 0.12 ± 0.02 | 1.18 ± 0.16 | 25.8 ± 2.1 |
| Mu50 ΔgraR | 0.24 ± 0.03 | 0.30 ± 0.04 | 3.1 ± 0.5 |
| Mu50 ΔvraG | 0.19 ± 0.03 | 0.21 ± 0.02 | <1 |
Unless otherwise indicted, the strain is a derivative of RN6390.
Biofilm formation of biofilms grown for 6 to 8 h assayed by the microtiter biofilm assay. Each strain was tested at least twice, six to seven replicates were assayed per measurement, and one standard deviation is shown.
Aggregation was determined after 12 to 14 h in the presence of 0.2% sodium citrate. In the absence of citrate, aggregation was less than 7% for all strains. ND, not determined.
Cell-to-cell interactions were also quantified in the planktonic phase by measuring the degrees of cell clustering in the absence and in the presence of citrate. Microscopic foci were characterized as either nonclustered (one to two cells per foci) or clustered (at least three cells per foci). We found that in the presence of citrate, there was a significant increase in cell-cell interactions. At 2 h, in the absence of citrate, 55% ± 1% of foci were clustered, compared to 67.3% ± 3.2% of foci that were clustered in the presence of citrate (P < 0.02; n > 300/strain).
Citrate-stimulated biofilm formation is independent of many global regulators and known biofilm factors.
To determine the mechanism by which S. aureus increases biofilm production in the presence of citrate, we screened mutations in global regulators, including several genes known to have a role in biofilm formation, with TSB-grown bacteria. Mutations in the following genes had no impact on citrate-stimulated biofilm formation: the quorum-sensing regulator agr; the regulators rot, mgrA, sigB, and sae; the sarA homologs sarS, sarT, sarU, sarV, sarX, and sarZ; the toxins hla and hlb; and the ssaA homolog SA0620 (data not shown).
The stimulation of S. aureus biofilm formation by low concentrations of sodium citrate is dependent upon the sarA regulator but independent of icaADBC.
As mentioned above, a number of genes coding for global regulators previously reported to be important for biofilm formation were tested to determine if they were required for citrate-stimulated biofilm formation. Only a mutation in the sarA gene, which codes for a global virulence regulator (5, 14, 37, 66), blocked the ability of citrate to promote biofilms, implicating SarA in the response to citrate. The data using two different sarA alleles are shown in Table 2. Providing a WT copy of the sarA gene in a single copy using vector pCL84 complements the citrate stimulation phenotype of a sarA mutant (Table 2). The positive role of SarA in citrate-stimulated biofilm stimulation was also observed for S. aureus clinical isolate DB (Table 2).
SarA is thought to exert a positive effect on staphylococcal biofilm formation through its role in regulating the ica operon (66). We found that our laboratory strain exhibits high levels of PIA/PNAG production that are undetectable in the isogenic sarA mutant, as assessed by immunoassays, and this defect can be complemented by sarA on a plasmid (data not shown). The ica genes code for a polysaccharide component of the biofilm matrix, which is an important adhesin in cell-cell and cell-surface interactions (27). Therefore, we tested the ability of citrate to stimulate the biofilm formed by an ica mutant and showed that the mutant strain was stimulated to the same degree as the WT (Table 2). To further address a role of the PIA/PNAG polysaccharide produced by the ica gene products, we assessed PIA/PNAG levels, as reported previously (17), in the absence and in the presence of citrate. The addition of increasing citrate, up to 0.2%, decreased the level of PIA/PNAG produced by the WT by ∼25-fold (Fig. 1C), arguing against a role for the ica-dependent polysaccharide in citrate-stimulated biofilm formation.
Citrate-dependent cell-to-surface interactions, but not cell-cell aggregation, are dependent upon the fibronectin binding proteins FnbA and FnbB.
Because we had evidence suggesting that the citrate-dependent biofilm formation phenotype is independent of PIA/PNAG, we sought to determine whether other genes regulated by SarA had an effect on citrate-stimulated biofilm formation.
Previously, Cheung and colleagues and Wolz et al. showed that purified SarA binds to a sequence motif found upstream of the fnbA gene (15, 73). This observation has been corroborated by a previously published array study that showed that SarA does indeed positively regulate the fnbA and fnbB genes (20). FnbA and FnbB are large adhesive proteins that are important for virulence and that have previously reported roles in promoting adherence to biotic and abiotic surfaces (30, 34). Strains carrying either the fnbA or the fnbB mutation were assessed for citrate-associated phenotypes. Both were found to be necessary for citrate-dependent biofilm stimulation (Fig. 1D and Table 2). Similar results were observed for fnbA and fnbB mutants in the SH1000 background (data not shown).
To assess whether the fnb mutations impacted cell-to-cell interactions, we performed a visual aggregation assay. The fnbA mutant (not shown) and the fnbB mutant (Fig. 1B) still aggregated to a degree similar to that observed for the WT strain (Table 2); however, the aggregation pattern was altered compared to that of the WT, with most fnb mutant cells binding to the air-liquid interface rather than being tightly associated in an aqueous aggregate. These data suggest that the primary defect of the fnb mutants is in cell-to-surface interactions.
S. aureus fibronectin binding proteins have heparin binding domains, and it has been reported that the addition of exogenous heparin results in the inability of these proteins to participate in adherence to fibronectin-coated coverslips (3, 67). We predicted that if fibronectin binding proteins are required for citrate-stimulated biofilm formation, then the addition of heparin could disrupt the ability of the cells to aggregate. The WT strain grown with either citrate (0.2%) or heparin (1,000 U/ml) was stimulated for biofilm formation (Fig. 1E). The addition of both heparin and citrate resulted in an approximately fivefold decrease in biofilm formation with respect to biofilms formed by the WT in the absence of any additions (P > 0.01). Heparin also inhibited the ability of citrate to promote aggregate formation (Fig. 1F).
Finally, strain Newman has been reported to have nonsense mutations in the fnbA and fnbB genes, resulting in FnbA/FnbB protein truncations and reduced adherence to fibronectin (28). Newman would therefore be predicted to have reduced citrate-stimulated biofilm formation, and consistent with this prediction, Newman was previously shown to not demonstrate a statistically significant increase in biofilm formation upon citrate treatment (62).
Sodium citrate stimulates fnbA and fnbB expression.
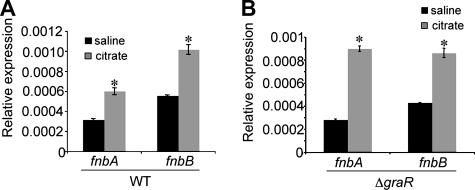
As demonstrated above, the fnbA and fnbB genes are required for citrate-dependent biofilm formation. To assess whether citrate impacts the regulation of these genes, quantitative reverse transcription-PCR (qRT-PCR), as reported previously (38), was performed on strains grown in the absence and those grown in presence of citrate. An approximately twofold increase in fnbA and fnbB gene expression was reproducibly observed when exponential-phase cultures were exposed to 0.2% citrate for 1 h (Fig. 2A).
FIG. 2.
Citrate-mediated gene expression. (A) qRT-PCR analysis of fnb gene expression in the WT strain in the absence (black bar) and presence (gray bar) of citrate. *, P < 0.05. (B) qRT-PCR analysis of fnb gene expression in the ΔgraR mutant in the absence (black bar) and presence (gray bar) of citrate. *, P < 0.05. The expression of the fnb genes is normalized to gyrB expression.
Citrate stimulation of aggregation is dependent upon a putative two-component regulatory system.
As described above, SarA and the SarA-regulated fibronectin binding proteins are required for the early cell-to-surface interaction events during biofilm formation in the presence of citrate. In an effort to identify other genes in the process, including functions required for the downstream aggregation events promoted by citrate, we screened strains with mutations in a number of putative two-component system regulators for their responses to citrate. In-frame deletions in the SA0215, graR (SA0614), SA1159, SA2151, and SA2478 genes, all of which encode predicted two-component system response regulators, were subjected to biofilm and aggregation assays to determine whether citrate's phenotypic influence upon staphylococci is dependent, directly or indirectly, upon the proteins encoded by these genes.
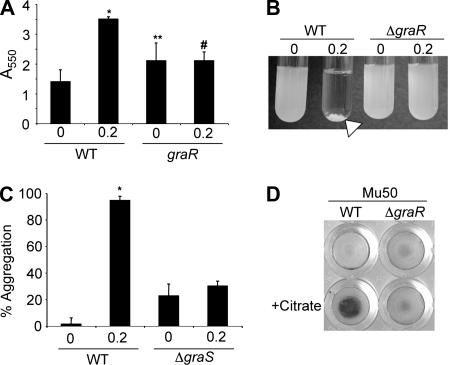
Biofilm formation was strongly stimulated by citrate in all of these strains except the ΔgraR (SA0614) strain, which encodes a two-component response regulator with a role in resistance to glycopeptides and lysozyme (18, 32, 51) The ΔgraR mutant showed no stimulation of biofilm formation by 0.2% sodium citrate (Fig. 3A). Interestingly, the basal level of biofilm formation was significantly increased by the deletion of graR (P = 0.026), suggesting that GraR may play a role in modulating the adherence of S. aureus cells in the absence of citrate.
FIG. 3.
The GraRS two-component regulatory system is required for citrate-stimulated biofilm formation and aggregation. (A) Biofilm formation in the absence and presence of 0.2% citrate for the indicated strain. *, P < 0.001 compared to WT with no citrate; **, P = 0.026 compared to WT with no citrate; #, P = 0.98 compared to the graR mutant without citrate. (B) Aggregation assay after overnight growth for the indicated strains without and with 0.2% citrate. The arrow indicates the aggregate formed by the WT grown with citrate. (C) Quantification of an aggregation assay for citrate-stimulated aggregation by the WT and the ΔgraS mutant. *, P < 0.001 compared to the WT with no citrate. (D) VISA isolate Mu50 requires a functional graR gene for citrate-stimulated biofilm formation in a 96-well plate assay. Biofilm and aggregation assays were performed as described in Materials and Methods.
GraR was also found to be necessary for increased cell-to-cell interactions in the presence of citrate. Aggregation assays revealed that the ΔgraR mutant did not aggregate in response to citrate (Fig. 3B and Table 2).
The open reading frame SA0615 (designated graS) encodes the cognate sensor kinase of the graR (SA0614) response regulator. The graS mutant, like the graR mutant, does not show citrate-stimulated biofilm formation (data not shown) or aggregation. In the absence of citrate, basal levels of aggregation in the planktonic culture were increased 16-fold in the graS deletion strain relative to the levels of the WT strain (P < 0.01) (Fig. 3C). In the presence of citrate, the ΔgraS mutant showed a small but significant increase in aggregation (P < 0.01 for ΔgraS versus ΔgraS plus citrate), while aggregation by the WT was stimulated 67-fold in the presence of citrate versus in the absence of citrate. The expression of WT copies of graR or graRS on multicopy plasmids was able to complement the phenotypic defects of the corresponding mutant strains (Table 2).
Because both fnbAB and graRS are required for citrate-dependent biofilm stimulation, we predicted that GraRS might be important for the regulation of fnbA and fnbB. Using qRT-PCR, we observed that fnbA and fnbB expression levels are similarly increased in the ΔgraRS cultures exposed to citrate (Fig. 2B). Similar results were observed in the ΔgraR mutant bearing WT graR on a multicopy plasmid (data not shown). These data indicate that GraRS does not regulate fnbA or fnbB transcription.
GraR is necessary for the citrate responses in clinically relevant S. aureus strains.
Over 95% of tested S. aureus strains have exhibited a robust response to citrate (n > 25) (62). We sought to determine the requirement of GraRS for this response in other laboratory strains and clinically relevant isolates. Biofilm formation of methicillin-resistant S. aureus strain Col is significantly stimulated by 0.2% sodium citrate at 16 h, with the biofilm A550 reading increasing from 0.20 ± 0.06 without citrate to 0.32 ± 0.08 in the presence of 0.2% citrate (P = 0.026). The Col ΔgraR deletion strain is not stimulated by the presence of 0.2% sodium citrate (A550 of 0.36 ± 0.04 without citrate and 0.25 ± 0.05 with 0.2% citrate).
The importance of graR was also tested in vancomycin-intermediate S. aureus (VISA) strain Mu50 to determine whether these genes were important for the citrate response in diverse strain backgrounds. Mu50 was induced to aggregate in the presence of sodium citrate from 6.7% ± 2.0% to 25.8% ± 3.1%. However, the isogenic graR mutant was not stimulated (4.6% ± 0.5% versus 3.1% ± 0.5%). A similar pattern was observed with biofilm formation. WT Mu50 exhibited an 853% increase in biofilm formation in the presence of citrate, while the isogenic ΔgraR mutant was stimulated only 26% (Fig. 3D and Table 2).
VraFG is required for citrate responses in laboratory and clinical S. aureus strains.
The VraFG ABC transporter has been shown to be required for full levels of vancomycin resistance, and the transcription of vraFG is controlled by GraRS (18, 32, 51). We questioned whether this putative pump plays a role in citrate-stimulated cell-to-cell and cell-to-surface phenomena. RN6390 and Mu50 with clean deletions of the vraG gene were compared to their respective WT strains for biofilm formation and aggregation in the presence and absence of citrate. The mutation of vraG conferred phenotypes identical to those mutations of genes coding for the GraRS regulatory system (Table 2), that is, a loss of citrate-stimulated biofilm formation and aggregation.
Role for TCA cycle intermediates in biofilm formation by S. aureus.
In addition to its role as a chelator, citrate is an important intermediate in the TCA cycle (Fig. 4A). A previous study by Vuong and colleagues (69) showed that in S. epidermidis, TCA cycle stress stimulated the production of the ica-dependent polysaccharide, suggesting a role for the TCA cycle in biofilm formation. Furthermore, a mutation in the clpC gene reduced the expression of citB, which codes for the TCA cycle enzyme aconitase, and resulted in a loss of biofilm formation in S. aureus (10, 25). Again, these data suggested a link between the TCA cycle and biofilm formation, thus prompting us to examine the roles of other TCA cycle intermediates in biofilm formation.
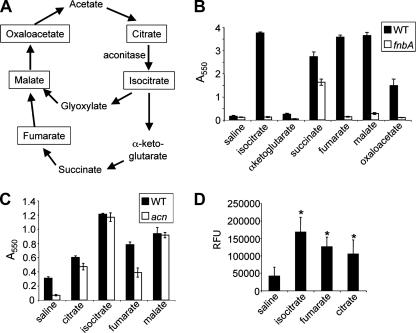
FIG. 4.
TCA cycle intermediates stimulate biofilm formation. (A) Diagram of the TCA cycle. Boxed compounds stimulate biofilm formation in an FnbA-dependent manner. (B) Biofilm formation by the WT (black bars) and the fnbA mutant (white bars) in the presence of TCA cycle intermediates. Saline served as a control in these studies. The biofilm assay was performed as described in Materials and Methods. (C) Biofilm formation by the WT (black bars) and the acn mutant (white bars) in the presence of TCA cycle intermediates. These assays were performed as described above (B). (D) Expression of the fnbA-gfp fusion was assessed in a solution containing TSB plus 0.2% glucose supplemented with saline or the indicated TCA cycle intermediates. Expression is presented as relative fluorescence units (RFU).
The TCA cycle intermediates isocitrate, succinate, fumarate, malate, and oxaloacetate all stimulated biofilm formation by S. aureus compared to growth on TSB medium (Fig. 4B, black bars). Interestingly, for all of these compounds, with the exception of succinate, the stimulation of biofilm formation was strongly dependent on a functional fnbA gene (Fig. 4B, white bars), a finding identical to that observed for the addition of citrate. These data indicate that isocitrate, fumarate, malate, and oxaloacetate may stimulate biofilm formation via the same pathway utilized by citrate. The addition of glyoxylate and α-ketoglutarate had no impact on biofilm formation (data not shown). Also, the addition of glucose at up to 2% did not stimulate biofilm formation, suggesting that the effects on biofilm formation mediated by the addition of TCA cycle intermediates are somewhat specific.
We next examined the effect of mutating the gene coding for aconitase on biofilm formation in the presence of TCA cycle intermediates. In TSB medium, a loss of aconitase function did result in a decrease in biofilm formation. This observation is consistent with previous work showing that a decrease in aconitase activity in a clpC mutant results in decreased biofilm formation (10, 25). However, in all cases, a functional aconitase was not shown to be required for the stimulation of biofilm formation by TCA cycle intermediates (Fig. 4C).
Finally, we demonstrated above that citrate was able to stimulate the expression of fibronectin binding proteins (Fig. 2) and, furthermore, that the stimulation of biofilm formation by TCA cycle intermediates, including isocitrate and fumarate, was dependent upon the fibronectin binding proteins (Fig. 4B). Further supporting a role for fibronectin binding proteins in the stimulation of biofilm formation by TCA cycle intermediates, we demonstrated that isocitrate and fumarate, like citrate, can stimulate the expression of fnbA (Fig. 4D).
DISCUSSION
Previous work from our group showed that while a high concentration of citrate (2%) is bactericidal, a low citrate concentration (0.2%) stimulates biofilm formation (62). Work presented here extends this finding by showing that citrate stimulates two distinct steps in biofilm formation: cell-to-surface interactions and cell-to-cell interactions. Interestingly, most of the known factors that have previously been reported to be important for biofilm formation in S. aureus appear to play no role in citrate-stimulated biofilm formation, including the ica genes, the agr-encoded global regulator, and the hla toxin (9, 17, 72). The studies here did identify several genetic factors that are required for citrate-stimulated biofilm formation by S. aureus, including the global regulator SarA, the SarA-regulated fibronectin binding proteins, the GraRS two-component regulatory system, and the GraRS-regulated VraFG efflux pump.
The following observations support the conclusion that fibronectin binding proteins are required for citrate's positive effect on biofilm formation: (i) the fnbA and fnbB mutants in different strains are insensitive to citrate with respect to increased biofilm formation, (ii) exogenous citrate increases the transcription of fnbA and fnbB, and (iii) heparin, which is known to inhibit fibronectin binding protein function, can prevent citrate-dependent biofilm stimulation. Furthermore, our data are consistent with fibronectin binding proteins mediating enhanced cell-to-surface interactions that lead to increased biofilm formation in the presence of sodium citrate. The fnb mutants appear to still be capable of aggregation, although the pattern of aggregation is altered compared to that of the WT strain, indicating that the fibronectin binding proteins, while not required for this process, may also be involved in some aspect(s) of cell-to-cell interactions during biofilm formation. Finally, given the role of fibronectin binding proteins in adherence to abiotic surfaces, the range of surfaces to which these proteins can bind may be broader than their name implies.
We also showed that biofilm formation by the ΔsarA mutant is not stimulated by citrate. This finding is consistent with the previously reported role of SarA as a regulator of fnb gene expression. Indeed, previous studies identified a SarA binding site, and such a site is found upstream of the fnbA gene, implicating SarA as a being direct regulator of expression of the fnb genes. Therefore, our data suggest that the early steps in citrate-stimulated biofilm formation are controlled as part of a larger network of virulence factors under the control of SarA.
Citrate can serve as a chelator of divalent cations. Previous studies from our laboratory indicate that the chelation activity of citrate cannot explain the ability of this compound to stimulate biofilm formation. For example, the addition of 0.2% citrate altered free Ca2+ by less than 3%. Furthermore, the addition of additional divalent cations to the medium (including Mn and Mg) still resulted in citrate-mediated stimulation of biofilm formation (62). However, both vancomycin and bacitracin require divalent cations to be effective, and the antimicrobial capacity of bacitracin is reported to be inhibited by citrate, suggesting that citrate inhibits these antibiotics through the sequestration of cations (1, 7, 8, 58). Furthermore, since emerging glycopeptide resistance in S. aureus is an important concern for human health (2), we chose to test glycopeptide resistance determinants as potential regulators of citrate-stimulated biofilm formation. A number of genes have been shown by microarray to be up-regulated in the presence of glycopeptides, such as vancomycin, and in strains that exhibit inherent elevated levels of vancomycin resistance (18, 48, 49). Among these is a set of two-component regulators including graRS (18, 48).
We observed that a mutation of graRS leads to elevated levels of biofilm formation compared to that of the WT in the absence of citrate. It has recently been reported that a mutation of graRS leads to a net increase in the negative charge of the cell surface (42). Furthermore, GraRS has been shown to positively regulate dlt expression (26). Dlt is required for the processing of cell surface lipoteichoic acid molecules, and dlt mutants exhibit elevated negative charges and increased levels of biofilm formation (21, 26). Therefore, it is possible that the increased basal levels of biofilm formation on positively charged plastic surfaces, exhibited by graRS mutants, is a manifestation of reduced Dlt levels, leading to an alteration in surface charges that favors attachment.
The GraRS two-component regulatory system appears to be required specifically for citrate stimulation of cell-to-cell interactions, as the graR and graS mutants do not form aggregates in the presence of citrate. Our expression studies show that GraRS does not regulate the expression of the fnb genes, consistent with our model that these genes impact different steps during citrate-stimulated biofilm formation. The vraFG ABC transporter genes are both controlled transcriptionally by GraRS and are required for citrate-associated phenotypes (18, 32, 51). Although it is not clear how a molecular pump is involved in citrate-stimulated biofilm formation, our data suggest that the mechanism underlying the ΔgraRS mutant phenotypes is the reduced levels of vraFG expression. Taken together, our findings are consistent with a model wherein the stimulation of cell-to-surface interactions and the stimulation of cell-to-cell interactions are distinct processes that are genetically separable. This model will be tested more rigorously in future studies.
We showed that other TCA cycle intermediates, in addition to citrate, including isocitrate, succinate, fumarate, malate, and oxaloacetate, also stimulated biofilm formation. These findings have several important implications. First, the fact that these other TCA cycle intermediates with no known role as chelators stimulate biofilm formation is consistent with our conclusion that citrate's effects on biofilm formation are not mediated via its chelation activity. Furthermore, the observation that biofilm stimulation by isocitrate, fumarate, malate, and oxaloacetate requires a functional fnbA gene and also stimulates the expression of this gene, as was observed for citrate, suggests that all of these compounds act through a common pathway. Interestingly, succinate still stimulates biofilm formation even in the fnbA mutant, indicating that this carbon source may have an additional impact on the cell's ability to form a biofilm. Also of interest is the observation that a functional aconitase is not required for stimulation by these TCA cycle intermediates, arguing against the need for a functional TCA cycle when biofilm formation is stimulated by intermediates in the TCA cycle, a point which merits further investigation.
In S. aureus, the TCA cycle is typically induced in postexponential growth after glucose is exhausted and acetate has accumulated (63-65). The observation that an aconitase mutant strain is defective in biofilm formation when grown on TSB plus glucose indicates that the TCA cycle does indeed contribute to biofilm formation under these laboratory conditions. Interestingly, a study of S. epidermidis indicated that TCA cycle stress stimulates the production of the ica-encoded polysaccharide (69), although biofilm formation was not assessed in this study. In contrast, we observed that mutating a component of the TCA cycle results in a loss of biofilm formation in TSB medium, suggesting that the interaction between TCA cycle function, polysaccharide production, and biofilm formation is complex. However, a functional TCA cycle does not appear to impact the ability of TCA cycle intermediates to stimulate biofilm formation. At this point, we cannot fully explain the link between citrate and the other TCA cycle intermediates with regard to their abilities to stimulate biofilm formation and, in particular, how these compounds mediate SarA-, FnbA/B-, and GraRS-dependent biofilm stimulation. Additional studies will be required to fully dissect how these carbon sources can act to promote biofilm formation.
In a previous study, we showed that heparin enhanced S. aureus biofilm formation (61). Heparin was shown to rescue the biofilm-deficient status of a number of mutant strains including a ΔsarA mutant, and additionally, while heparin does stimulate cell-cell interactions, it does not initiate the dramatic aggregation phenotype displayed by cells grown in the presence of 0.2% sodium citrate. Taken together, these data indicate that heparin and citrate stimulate biofilm formation through independent mechanisms. Furthermore, the relationship between citrate and heparin with regard to biofilm formation appears to be complex. While individually, both compounds stimulate biofilm formation, together, they cause a modest decrease in the formation of biofilms and aggregates. At this point, it is not clear if heparin and citrate impact the same biofilm formation pathway or, alternatively, that the effects of heparin on citrate-stimulated biofilm formation are distinct from those of the heparin-mediated stimulation of biofilm formation. We are currently investigating these questions.
The possibility of multiple independent mechanisms required for biofilm formation by S. aureus has important implications both in the clinic and for drug discovery programs targeted at antibiofilm therapeutics. Simply put, at this point, it is not clear which pathway or pathways should be targeted to block biofilm formation in vivo. For example, while the ica locus may be important for biofilm formation in vitro and for systemic infections, previous studies using three different implant models concluded that the ica locus was not important for biofilm formation by S. aureus on abiotic surfaces in vivo (16, 36). It is possible that there are redundant pathways by which S. aureus forms biofilms on medical implants or, alternatively, that the citrate-dependent and/or heparin-dependent pathways reported by our groups are utilized by S. aureus when adhering to medical implants in vivo. It will be important to distinguish between these models if efforts to develop antibiofilm therapies are to be fruitful.
Acknowledgments
We thank members of the O'Toole laboratory for helpful discussions, Simon Foster for kindly sharing the acn mutant strain, and Kristi Frank for critical reading of the manuscript.
This work was supported by T32-DF007301 (B.A. Stanton, P.I.) to N.P.D., AI47441 to A.L.C., GM66658 to R.M.Q.S., and R21-AI055774 and AI51360 to G.A.O.
Editor: A. Camilli
Footnotes
Published ahead of print on 17 March 2008.
REFERENCES
- 1.Adler, R. H., and J. E. Snoke. 1962. Requirement of divalent metal ions for bacitracin activity. J. Bacteriol. 831315-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelbaum, P. C. 2006. The emergence of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 12(Suppl. 1)16-23. [DOI] [PubMed] [Google Scholar]
- 3.Arciola, C. R., Y. Bustanji, M. Conti, D. Campoccia, L. Baldassarri, B. Samori, and L. Montanaro. 2003. Staphylococcus epidermidis—fibronectin binding and its inhibition by heparin. Biomaterials 243013-3019. [DOI] [PubMed] [Google Scholar]
- 4.Arciola, C. R., D. Campoccia, and L. Montanaro. 2002. Detection of biofilm-forming strains of Staphylococcus epidermidis and S. aureus. Expert Rev. Mol. Diagn. 2478-484. [DOI] [PubMed] [Google Scholar]
- 5.Beenken, K. E., P. M. Dunman, F. McAleese, D. Macapagal, E. Murphy, S. J. Projan, J. S. Blevins, and M. S. Smeltzer. 2004. Global gene expression in Staphylococcus aureus biofilms. J. Bacteriol. 1864665-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Begun, J., J. M. Gaiani, H. Rohde, D. Mack, S. B. Calderwood, F. M. Ausubel, and C. D. Sifri. 2007. Staphylococcal biofilm exopolysaccharide protects against Caenorhabditis elegans immune defenses. PLoS Pathog. 3e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Best, G. K., and N. N. Durham. 1964. Effect of vancomycin on Bacillus subtilis. Arch. Biochem. Biophys. 105120-125. [DOI] [PubMed] [Google Scholar]
- 8.Best, G. K., and N. N. Durham. 1965. Vancomycin adsorption to Bacillus subtilis cell walls. Arch. Biochem. Biophys. 111685-692. [DOI] [PubMed] [Google Scholar]
- 9.Caiazza, N. C., and G. A. O'Toole. 2003. Alpha-toxin is required for biofilm formation by Staphylococcus aureus. J. Bacteriol. 1853214-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee, I., P. Becker, M. Grundmeier, M. Bischoff, G. A. Somerville, G. Peters, B. Sinha, N. Harraghy, R. A. Proctor, and M. Herrmann. 2005. Staphylococcus aureus ClpC is required for stress resistance, aconitase activity, growth recovery, and death. J. Bacteriol. 1874488-4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung, A. L., M. G. Bayer, and J. H. Heinrichs. 1997. sar genetic determinants necessary for transcription of RNAII and RNAIII in the agr locus of Staphylococcus aureus. J. Bacteriol. 1793963-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung, A. L., J. M. Koomey, C. A. Butler, S. J. Projan, and V. A. Fischetti. 1992. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc. Natl. Acad. Sci. USA 896462-6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung, A. L., and S. J. Projan. 1994. Cloning and sequencing of sarA of Staphylococcus aureus, a gene required for the expression of agr. J. Bacteriol. 1764168-4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung, A. L., and G. Zhang. 2002. Global regulation of virulence determinants in Staphylococcus aureus by the SarA protein family. Front. Biosci. 7d1825-d1842. [DOI] [PubMed] [Google Scholar]
- 15.Chien, Y., A. C. Manna, S. J. Projan, and A. L. Cheung. 1999. SarA, a global regulator of virulence determinants in Staphylococcus aureus, binds to a conserved motif essential for sar-dependent gene regulation. J. Biol. Chem. 27437169-37176. [DOI] [PubMed] [Google Scholar]
- 16.Chokr, A., D. Leterme, D. Watier, and S. Jabbouri. 2007. Neither the presence of ica locus, nor in vitro-biofilm formation ability is a crucial parameter for some Staphylococcus epidermidis strains to maintain an infection in a guinea pig tissue cage model. Microb. Pathog. 4294-97. [DOI] [PubMed] [Google Scholar]
- 17.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Gotz. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 675427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui, L., J. Lian, H. Neoh, E. Reyes, and K. Hiramatsu. 2005. DNA microarray-based identification of genes associated with glycopeptide resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 493404-3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.del Pozo, J. L., and R. Patel. 2007. The challenge of treating biofilm-associated bacterial infections. Clin. Pharmacol. Ther. 82204-209. [DOI] [PubMed] [Google Scholar]
- 20.Dunman, P. M., E. Murphy, S. Haney, D. Palacios, G. Tucker-Kellogg, S. Wu, E. L. Brown, R. J. Zagursky, D. Shlaes, and S. J. Projan. 2001. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J. Bacteriol. 1837341-7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fluckiger, U., M. Ulrich, A. Steinhuber, G. Doring, D. Mack, R. Landmann, C. Goerke, and C. Wolz. 2005. Biofilm formation, icaADBC transcription, and polysaccharide intercellular adhesin synthesis by staphylococci in a device-related infection model. Infect. Immun. 731811-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forsyth, R. A., R. J. Haselbeck, K. L. Ohlsen, R. Yamamoto, T. H. Xu, J. D. Trawick, D. Wall, L. Wang, V. Brown-Driver, J. M. Froelich, G. C. Kedar, P. King, M. McCartney, C. Malone, B. Misiner, D. Robbins, Z. Tan, Z. Zhu, G. Carr, D. A. Mosca, C. Zamudio, J. G. Foulkes, and J. W. Zyskind. 2002. A genome-wide strategy for the identification of essential genes in Staphylococcus aureus. Mol. Microbiol. 431387-1400. [DOI] [PubMed] [Google Scholar]
- 23.Francois, P., P. H. T. Quoc, C. Bisognano, W. L. Kelley, D. P. Lew, J. Schrenzel, S. E. Cramton, F. Gotz, and P. Vaudaux. 2003. Lack of biofilm contribution to bacterial colonisation in an experimental model of foreign body infection by Staphylococcus aureus and Staphylococcus epidermidis. FEMS Immunol. Med. Microbiol. 35135-140. [DOI] [PubMed] [Google Scholar]
- 24.Frank, K. L., A. D. Hanssen, and R. Patel. 2004. icaA is not a useful diagnostic marker for prosthetic joint infection. J. Clin. Microbiol. 424846-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frees, D., A. Chastanet, S. Qazi, K. Sorensen, P. Hill, T. Msadek, and H. Ingmer. 2004. Clp ATPases are required for stress tolerance, intracellular replication and biofilm formation in Staphylococcus aureus. Mol. Microbiol. 541445-1462. [DOI] [PubMed] [Google Scholar]
- 26.Gill, S. R., D. E. Fouts, G. L. Archer, E. F. Mongodin, R. T. Deboy, J. Ravel, I. T. Paulsen, J. F. Kolonay, L. Brinkac, M. Beanan, R. J. Dodson, S. C. Daugherty, R. Madupu, S. V. Angiuoli, A. S. Durkin, D. H. Haft, J. Vamathevan, H. Khouri, T. Utterback, C. Lee, G. Dimitrov, L. Jiang, H. Qin, J. Weidman, K. Tran, K. Kang, I. R. Hance, K. E. Nelson, and C. M. Fraser. 2005. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 1872426-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gotz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 431367-1378. [DOI] [PubMed] [Google Scholar]
- 28.Grundmeier, M., M. Hussain, P. Becker, C. Heilmann, G. Peters, and B. Sinha. 2004. Truncation of fibronectin-binding proteins in Staphylococcus aureus strain Newman leads to deficient adherence and host cell invasion due to loss of the cell wall anchor function. Infect. Immun. 727155-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall-Stoodley, L., J. W. Costerton, and P. Stoodley. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 295-108. [DOI] [PubMed] [Google Scholar]
- 30.Hauck, C. R., and K. Ohlsen. 2006. Sticky connections: extracellular matrix protein recognition and integrin-mediated cellular invasion by Staphylococcus aureus. Curr. Opin. Microbiol. 95-11. [DOI] [PubMed] [Google Scholar]
- 31.Helander, I. M., and T. Mattila-Sandholm. 2000. Fluorometric assessment of gram-negative bacterial permeabilization. J. Appl. Microbiol. 88213-219. [DOI] [PubMed] [Google Scholar]
- 32.Herbert, S., A. Bera, C. Nerz, D. Kraus, A. Peschel, C. Goerke, M. Meehl, A. Cheung, and F. Gotz. 2007. Molecular basis of resistance to muramidase and cationic antimicrobial peptide activity of lysozyme in staphylococci. PLoS Pathog. 3e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horsburgh, M. J., J. L. Aish, I. J. White, L. Shaw, J. K. Lithgow, and S. J. Foster. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 1845457-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joh, H. J., K. House-Pompeo, J. M. Patti, S. Gurusiddappa, and M. Hook. 1994. Fibronectin receptors from gram-positive bacteria: comparison of active sites. Biochemistry 336086-6092. [DOI] [PubMed] [Google Scholar]
- 35.Kornblum, J., B. Kreiswirth, S. J. Projan, H. Ross, and R. P. Novick. 1990. agr: a polycistronic locus regulating exoprotein synthesis in Staphylococcus aureus, p. 373-402. In R. P. Novick (ed.), Molecular biology of the staphylococci. VCH Publishers, New York, NY.
- 36.Kristian, S. A., T. Golda, F. Ferracin, S. E. Cramton, B. Neumeister, A. Peschel, F. Gotz, and R. Landmann. 2004. The ability of biofilm formation does not influence virulence of Staphylococcus aureus and host response in a mouse tissue cage infection model. Microb. Pathog. 36237-245. [DOI] [PubMed] [Google Scholar]
- 37.Kropec, A., T. Maira-Litran, K. K. Jefferson, M. Grout, S. E. Cramton, F. Gotz, D. A. Goldmann, and G. B. Pier. 2005. Poly-N-acetylglucosamine production in Staphylococcus aureus is essential for virulence in murine models of systemic infection. Infect. Immun. 736868-6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuchma, S. L., K. M. Brothers, J. H. Merritt, N. T. Liberati, F. M. Ausubel, and G. A. O'Toole. 2007. BifA, a cyclic-di-GMP phosphodiesterase, inversely regulates biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J. Bacteriol. 1898165-8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuroda, M., T. Ohta, I. Uchiyama, T. Baba, H. Yuzawa, I. Kobayashi, L. Cui, A. Oguchi, K. Aoki, Y. Nagai, J. Lian, T. Ito, M. Kanamori, H. Matsumaru, A. Maruyama, H. Murakami, A. Hosoyama, Y. Mizutani-Ui, N. K. Takahashi, T. Sawano, R. Inoue, C. Kaito, K. Sekimizu, H. Hirakawa, S. Kuhara, S. Goto, J. Yabuzaki, M. Kanehisa, A. Yamashita, K. Oshima, K. Furuya, C. Yoshino, T. Shiba, M. Hattori, N. Osgasawara, H. Hayashi, and K. Hiramatsu. 2001. Whole genome sequencing of methicillin-resistant Staphylococcus aureus. Lancet 3571225-1240. [DOI] [PubMed] [Google Scholar]
- 40.Lee, Y.-L., T. Cesario, J. Owens, E. Shanbrom, and L. D. Thrupp. 2002. Antibacterial activity of citrate and acetate. Nutrition 18665-666. [DOI] [PubMed] [Google Scholar]
- 41.Lee, Y.-L., L. D. Thrupp, T. Owens, T. Cesario, and E. Shanbrom. 2001. Bactericidal activity of citrate against gram-positive cocci. Lett. Appl. Microbiol. 33349-351. [DOI] [PubMed] [Google Scholar]
- 42.Li, H., L. Xu, J. Wang, Y. Wen, C. Vuong, M. Otto, and Q. Gao. 2005. Conversion of Staphylococcus epidermidis strains from commensal to invasive by expression of the ica locus encoding production of biofilm exopolysaccharide. Infect. Immun. 733188-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339520-532. [DOI] [PubMed] [Google Scholar]
- 44.Mack, D., W. Fischer, A. Krokotsch, K. Leopold, R. Hartmann, H. Egge, and R. Laufs. 1996. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol. 178175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mack, D., M. Nedelmann, A. Krokotsch, A. Schwarzkopf, J. Heesemann, and R. Laufs. 1994. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infect. Immun. 623244-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mack, D., J. Riedewald, H. Rohde, T. Magnus, H. H. Feucht, H. A. Elsner, R. Laufs, and M. E. Rupp. 1999. Essential functional role of the polysaccharide intercellular adhesin of Staphylococcus epidermidis in hemagglutination. Infect. Immun. 671004-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manna, A., and A. L. Cheung. 2001. Characterization of sarR, a modulator of sar expression in Staphylococcus aureus. Infect. Immun. 69885-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mascher, T., N. G. Margulis, T. Wang, R. W. Ye, and J. D. Helmann. 2003. Cell wall stress responses in Bacillus subtilis: the regulatory network of the bacitracin stimulon. Mol. Microbiol. 501591-1604. [DOI] [PubMed] [Google Scholar]
- 49.McAleese, F., S. W. Wu, K. Sieradzki, P. Dunman, E. Murphy, S. Projan, and A. Tomasz. 2006. Overexpression of genes of the cell wall stimulon in clinical isolates of Staphylococcus aureus exhibiting vancomycin-intermediate-S. aureus-type resistance to vancomycin. J. Bacteriol. 1881120-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKenney, D., J. Hubner, E. Muller, Y. Wang, D. A. Goldmann, and G. B. Pier. 1998. The ica locus of Staphylococcus epidermidis encodes production of the capsular polysaccharide/adhesin. Infect. Immun. 664711-4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meehl, M., S. Herbert, F. Gotz, and A. Cheung. 2007. Interaction of the GraRS two-component system with the VraFG ABC transporter to support vancomycin-intermediate resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 512679-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ninin, E., N. Caroff, E. Espaze, J. Maraillac, D. Lepelletier, N. Milpied, and H. Richet. 2006. Assessment of ica operon carriage and biofilm production in Staphylococcus epidermidis isolates causing bacteraemia in bone marrow transplant recipients. Clin. Microbiol. Infect. 12446-452. [DOI] [PubMed] [Google Scholar]
- 53.O'Gara, J. P. 2007. ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol. Lett. 270179-188. [DOI] [PubMed] [Google Scholar]
- 54.O'Toole, G., H. B. Kaplan, and R. Kolter. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 5449-79. [DOI] [PubMed] [Google Scholar]
- 55.Pratten, J., S. J. Foster, P. F. Chan, M. Wilson, and S. P. Nair. 2001. Staphylococcus aureus accessory regulators: expression within biofilms and effect on adhesion. Microbes Infect. 3633-637. [DOI] [PubMed] [Google Scholar]
- 56.Raad, I. 1998. Intravascular-catheter-related infections. Lancet 351893-898. [DOI] [PubMed] [Google Scholar]
- 57.Raad, I. I., and H. A. Hanna. 2002. Intravascular catheter-related infections. Arch. Intern. Med. 162871-878. [DOI] [PubMed] [Google Scholar]
- 58.Russell, A. D., and I. L. Thomas. 1966. Effect of Mg++ on the activity of vancomycin against Escherichia coli. Appl. Microbiol. 14902-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Satorres, S. E., and L. E. Alcaráz. 2007. Prevalence of icaA and icaD genes in Staphylococcus aureus and Staphylococcus epidermidis strains isolated from patients and hospital staff. Cent. Eur. J. Public Health 1587-90. [DOI] [PubMed] [Google Scholar]
- 60.Shah, C. B., M. W. Mittelman, J. W. Costerton, S. Parenteau, M. Pelak, R. Arsenault, and L. A. Mermel. 2002. Antimicrobial activity of a novel catheter lock solution. Antimicrob. Agents Chemother. 461674-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shanks, R. M., N. P. Donegan, M. L. Graber, S. E. Buckingham, M. E. Zegans, A. L. Cheung, and G. A. O'Toole. 2005. Heparin stimulates Staphylococcus aureus biofilm formation. Infect. Immun. 734596-4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shanks, R. M., J. L. Sargent, R. M. Martinez, M. L. Graber, and G. A. O'Toole. 2006. Catheter lock solutions influence staphylococcal biofilm formation on abiotic surfaces. Nephrol. Dial. Transplant. 212247-2255. [DOI] [PubMed] [Google Scholar]
- 63.Somerville, G. A., M. S. Chaussee, C. I. Morgan, J. R. Fitzgerald, D. W. Dorward, L. J. Reitzer, and J. M. Musser. 2002. Staphylococcus aureus aconitase inactivation unexpectedly inhibits post-exponential-phase growth and enhances stationary-phase survival. Infect. Immun. 706373-6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Somerville, G. A., B. Said-Salim, J. M. Wickman, S. J. Raffel, B. N. Kreiswirth, and J. M. Musser. 2003. Correlation of acetate catabolism and growth yield in Staphylococcus aureus: implications for host-pathogen interactions. Infect. Immun. 714724-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strasters, K. C., and K. C. Winkler. 1963. Carbohydrate metabolism of Staphylococcus aureus. J. Gen. Microbiol. 33213-229. [DOI] [PubMed] [Google Scholar]
- 66.Valle, J., A. Toledo-Arana, C. Berasain, J. M. Ghigo, B. Amorena, J. R. Penades, and I. Lasa. 2003. SarA and not sigmaB is essential for biofilm development by Staphylococcus aureus. Mol. Microbiol. 481075-1087. [DOI] [PubMed] [Google Scholar]
- 67.Vaudaux, P., T. Avramoglou, D. Letourneur, D. P. Lew, and J. Jozefonvicz. 1992. Inhibition by heparin and derivatized dextrans of Staphylococcus aureus adhesion to fibronectin-coated biomaterials. J. Biomater. Sci. Polym. Ed. 489-97. [PubMed] [Google Scholar]
- 68.Vaudaux, P., P. Francois, C. Bisognano, W. L. Kelley, D. P. Lew, J. Schrenzel, R. A. Proctor, P. J. McNamara, G. Peters, and C. Von Eiff. 2002. Increased expression of clumping factor and fibronectin-binding proteins by hemB mutants of Staphylococcus aureus expressing small colony variant phenotypes. Infect. Immun. 705428-5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vuong, C., J. B. Kidder, E. R. Jacobson, M. Otto, R. A. Proctor, and G. A. Somerville. 2005. Staphylococcus epidermidis polysaccharide intercellular adhesin production significantly increases during tricarboxylic acid cycle stress. J. Bacteriol. 1872967-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vuong, C., S. Kocianova, J. M. Voyich, Y. Yao, E. R. Fischer, F. R. Deleo, and M. Otto. 2004. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J. Biol. Chem. 27954881-54886. [DOI] [PubMed] [Google Scholar]
- 71.Vuong, C., and M. Otto. 2002. Staphylococcus epidermidis infections. Microbes Infect. 4481-489. [DOI] [PubMed] [Google Scholar]
- 72.Vuong, C., H. L. Saenz, F. Gotz, and M. Otto. 2000. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J. Infect. Dis. 1821688-1693. [DOI] [PubMed] [Google Scholar]
- 73.Wolz, C., P. Pohlmann-Dietze, A. Steinhuber, Y. T. Chien, A. Manna, W. van Wamel, and A. Cheung. 2000. Agr-independent regulation of fibronectin-binding protein(s) by the regulatory locus sar in Staphylococcus aureus. Mol. Microbiol. 36230-243. [DOI] [PubMed] [Google Scholar]
- 74.Yarwood, J. M., and P. M. Schlievert. 2003. Quorum sensing in Staphylococcus infections. J. Clin. Investig. 1121620-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zegans, M. E., R. M. Q. Shanks, and G. A. O'Toole. 2005. Bacterial biofilms and ocular infections. Ocul. Surface 312-19. [DOI] [PubMed] [Google Scholar]